Single and Combined Effects of Meropenem, Valproic Acid, and Ketoprofen on Adult Zebrafish Behavior, Oxidative Stress, and Acetylcholinesterase Activity
Abstract
1. Introduction
2. Results
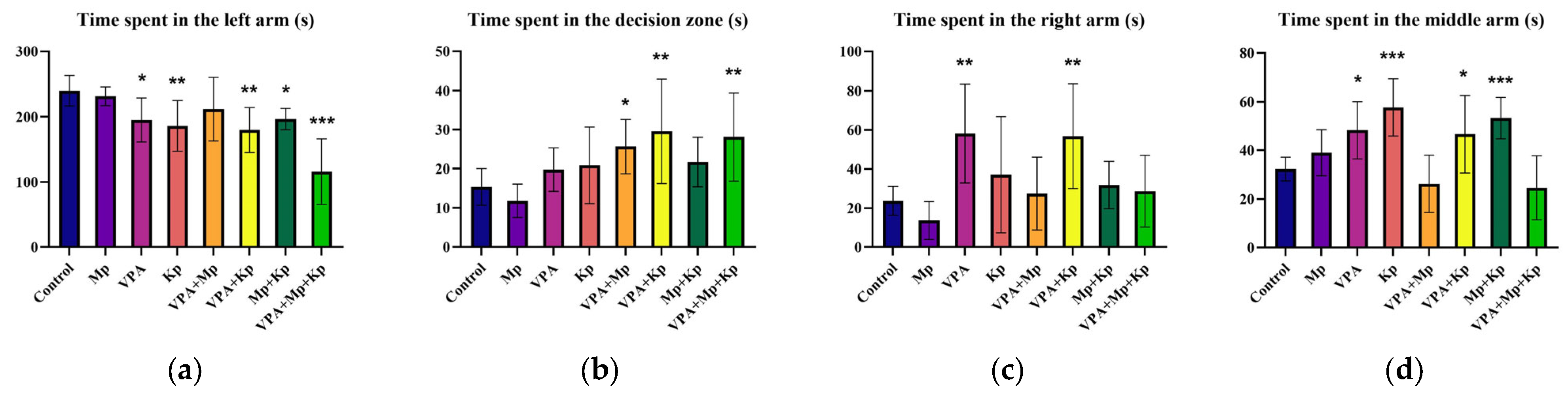
2.1. Behavior Analysis
2.1.1. Novel Tank Test
2.1.2. Social Behavior Test
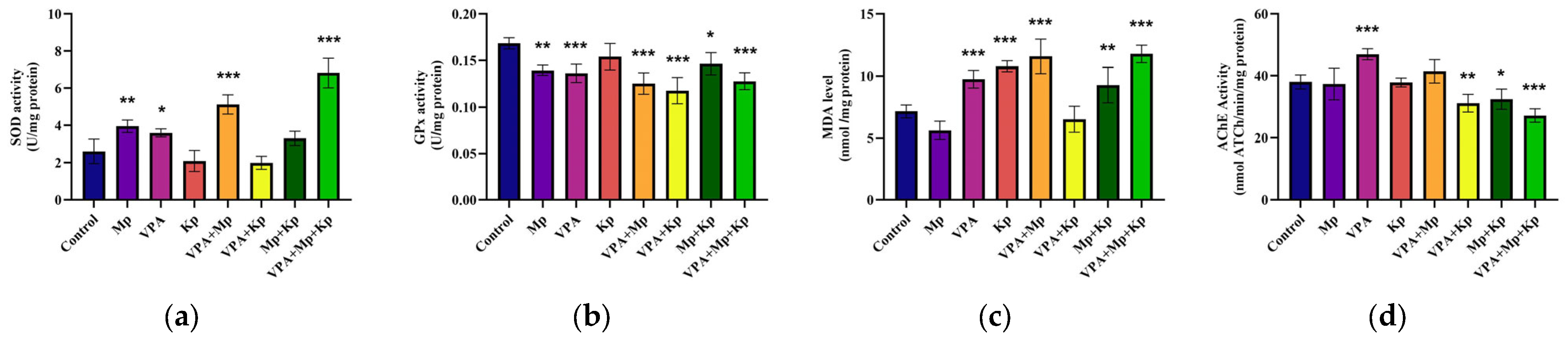
2.2. Oxidative Stress Status and Acetylcholinesterase Activity
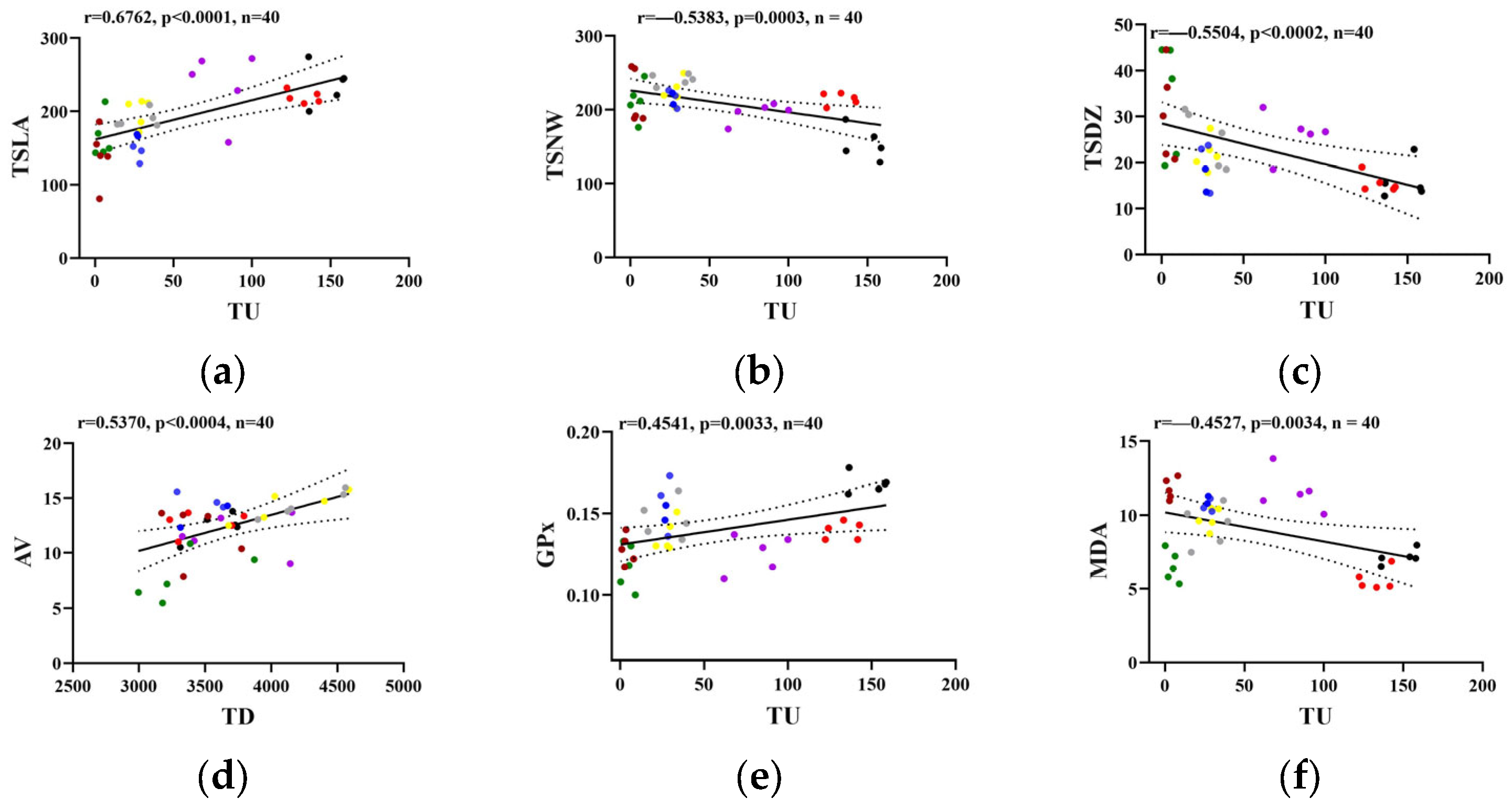
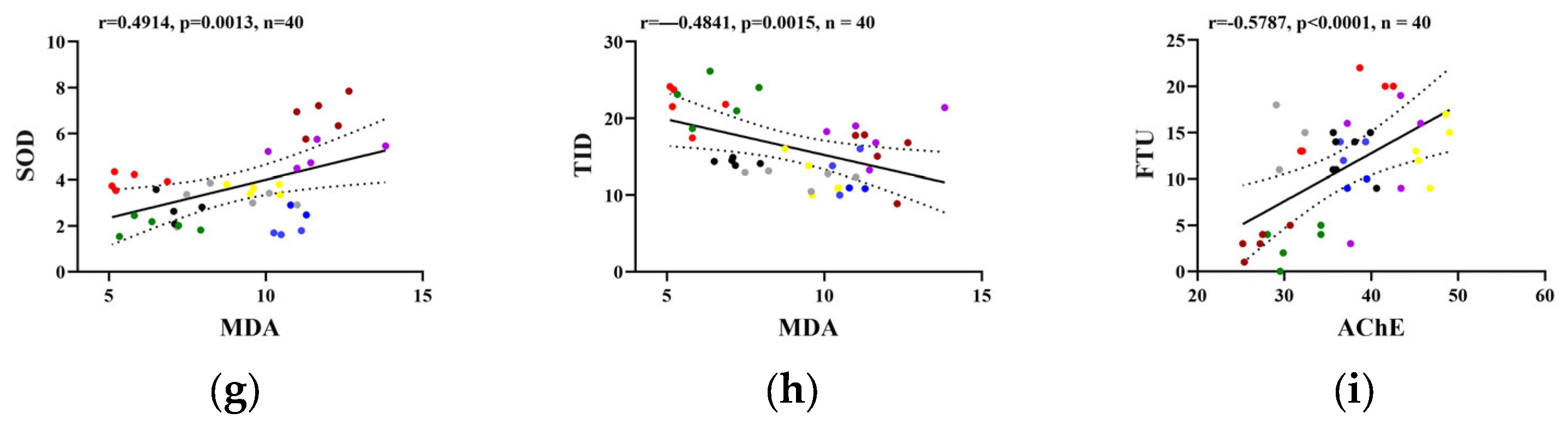
2.3. Pearson’s Correlation Analysis Among Biochemical and Behavioral Parameters
3. Discussion
4. Materials and Methods
4.1. Chemicals and Test Substances
4.2. Zebrafish Maintenance
4.3. Experimental Design for Acute Toxicity in Adult Zebrafish
4.4. Behavioral Analysis
4.4.1. Three-Dimensional Novel Tank Test
4.4.2. Social Preference Test
4.5. Biochemical Analysis
4.5.1. Assessment of Enzymatic Antioxidants and of Lipid Peroxidation
4.5.2. Evaluation of Acetylcholinesterase Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Mp | Meropenem |
| Kp | Ketoprofen |
| VPA | Valproic acid |
| LD | Linear dichroism |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| GPx | Glutathione peroxidase |
| AChE | Acetylcholinesterase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| TSLA | Time spent in the left arm |
| TDS | Total distance |
| AV | Average velocity |
| TID | Total immobilization duration |
| CCRS | Counter-clockwise rotations |
| TU | Time in upper half |
| FTU | Frequency of transitions to upper half |
| TSNW | Time spent near the wall |
| FTW | Frequency of transitions to walls |
| Cd | Cadmium |
| hpf | Hours post-fertilization |
| dpf | Days post-fertilization |
| WWTPs | Wastewater treatment plants |
| COX | Cyclooxygenase |
| AST | Glutamic oxaloacetic transaminase |
| ALT | Glutamic pyruvic transaminase |
| LDH | Lactate dehydrogenase |
| APX | Ascorbate peroxidase |
| ASD | Autism spectrum disorder |
References
- Alkimin, G.D.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of ketoprofen toxicity in two freshwater species: Effects on biochemical, physiological and population endpoints. Environ. Pollut. 2020, 265, 114993. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.; Salgado, R.; Pereira, V.; Carvalho, G.; Oehmen, A.; Reis, M.; Noronha, J. Ecotoxicity of ketoprofen, diclofenac, atenolol and their photolysis byproducts in zebrafish (Danio rerio). Sci. Total Environ. 2015, 505, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Spongberg, A.L.; Witter, J.D.; Acuña, J.; Vargas, J.; Murillo, M.; Umaña, G.; Gómez, E.; Perez, G. Reconnaissance of selected PPCP compounds in Costa Rican surface waters. Water Res. 2011, 45, 6709–6717. [Google Scholar] [CrossRef]
- Marsik, P.; Rezek, J.; Židková, M.; Kramulová, B.; Tauchen, J.; Vaněk, T. Non-steroidal anti-inflammatory drugs in the watercourses of Elbe basin in Czech Republic. Chemosphere 2017, 171, 97–105. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef]
- Wiest, L.; Chonova, T.; Bergé, A.; Baudot, R.; Bessueille-Barbier, F.; Ayouni-Derouiche, L.; Vulliet, E. Two-year survey of specific hospital wastewater treatment and its impact on pharmaceutical discharges. Environ. Sci. Pollut. Res. 2018, 25, 9207–9218. [Google Scholar] [CrossRef]
- Santos, J.; Aparicio, I.; Alonso, E. Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ. Int. 2007, 33, 596–601. [Google Scholar] [CrossRef]
- Praveenkumarreddy, Y.; Vimalkumar, K.; Ramaswamy, B.R.; Kumar, V.; Singhal, R.K.; Basu, H.; Gopal, C.M.; Vandana, K.E.; Bhat, K.; Udayashankar, H.N.; et al. Assessment of non-steroidal anti-inflammatory drugs from selected wastewater treatment plants of Southwestern India. Emerg. Contam. 2021, 7, 43–51. [Google Scholar] [CrossRef]
- Rangasamy, B.; Hemalatha, D.; Shobana, C.; Nataraj, B.; Ramesh, M. Developmental toxicity and biological responses of zebrafish (Danio rerio) exposed to anti-inflammatory drug ketoprofen. Chemosphere 2018, 213, 423–433. [Google Scholar] [CrossRef]
- Chabchoubi, I.B.; Bouchhima, R.A.; Louhichi, N.; Baanannou, A.; Masmoudi, S.; Hentati, O. Short-term effects of various non-steroidal anti-inflammatory drugs (NSAIDs) on Danio rerio embryos. MethodsX 2023, 10, 102215. [Google Scholar] [CrossRef]
- Bownik, A.; Jasieczek, M.; Kosztowny, E. Ketoprofen affects swimming behavior and impairs physiological endpoints of Daphnia magna. Sci. Total Environ. 2020, 725, 138312. [Google Scholar] [CrossRef] [PubMed]
- Prášková, E.; Štěpánová, S.; Chromcová, L.; Plhalová, L.; Voslářová, E.; Pištěková, V.; Prokeš, M.; Svobodová, Z. The effects of subchronic exposure to ketoprofen on early developmental stages of common carp. Acta Vet. Brno 2013, 82, 343–347. [Google Scholar] [CrossRef]
- Hurst, M.; Lamb, H.M. Meropenem. Drugs 2000, 59, 653–680. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Ng, C.; Chen, H.; Yi, X.Z.; Koh, T.H.; Barkham, T.M.S.; Zhou, Z.; Gin, K.Y.-H. Occurrences and characterization of antibiotic-resistant bacteria and genetic determinants of hospital wastewater in a tropical country. Antimicrob. Agents Chemother. 2016, 60, 7449–7456. [Google Scholar] [CrossRef]
- Proia, L.; Anzil, A.; Borrego, C.; Farrè, M.; Llorca, M.; Sanchis, J.; Bogaerts, P.; Balcázar, J.L.; Servais, P. Occurrence and persistence of carbapenemases genes in hospital and wastewater treatment plants and propagation in the receiving river. J. Hazard. Mater. 2018, 358, 33–43. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T.; Ivekovic, D.; Repec, S.; Stipanicev, D.; Ganjto, M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017, 126, 232–239. [Google Scholar] [CrossRef]
- Habjan, E.; Ho, V.Q.; Gallant, J.; Van Stempvoort, G.; Jim, K.K.; Kuijl, C.; Geerke, D.P.; Bitter, W.; Speer, A. An anti-tuberculosis compound screen using a zebrafish infection model identifies an aspartyl-tRNA synthetase inhibitor. Dis. Models Mech. 2021, 14, dmm049145. [Google Scholar] [CrossRef]
- Kato, Y.; Tonomura, Y.; Hanafusa, H.; Nishimura, K.; Fukushima, T.; Ueno, M. Adult Zebrafish Model for Screening Drug-Induced Kidney Injury. Toxicol. Sci. 2020, 174, 241–253. [Google Scholar] [CrossRef]
- Guzman-Tordecilla, M.; Pacheco-Bustos, C.; Coronado-Posada, N.; Pedrosa-Gomes, M.; Martinez-Burgos, W.J.; Mejía-Marchena, R.; Zorman-Marques, R. Exploring the ecotoxicological impact of meropenem on Lemna minor: Growth, photosynthetic activity, and oxidative stress. Environ. Res. 2024, 258, 119409. [Google Scholar] [CrossRef]
- Messina, A.; Sovrano, V.A.; Baratti, G.; Musa, A.; Gobbo, A.; Adiletta, A.; Sgadò, P. Valproic acid exposure affects social visual lateralization and asymmetric gene expression in zebrafish larvae. Sci. Rep. 2024, 14, 4474. [Google Scholar] [CrossRef]
- Muhsen, M.; Youngs, J.; Riu, A.; Gustafsson, J.Å.; Kondamadugu, V.S.; Garyfalidis, E.; Bondesson, M. Folic acid supplementation rescues valproic acid-induced developmental neurotoxicity and behavioral alterations in zebrafish embryos. Epilepsia 2021, 62, 1689–1700. [Google Scholar] [CrossRef]
- Yu, J.T.; Bisceglia, K.J.; Bouwer, E.J.; Roberts, A.L.; Coelhan, M. Determination of pharmaceuticals and antiseptics in water by solid-phase extraction and gas chromatography/mass spectrometry: Analysis via pentafluorobenzylation and stable isotope dilution. Anal. Bioanal. Chem. 2012, 403, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Rehrl, A.-L. Occurrence and fate of Organic Micropollutants (OMPs) in Lake Mälaren. Master’s Thesis, Swedish University of Agricultural Sciences, Department of Aquatic Sciences and Assessment, Uppsala, Sweden, 2019. Available online: https://stud.epsilon.slu.se/14185/ (accessed on 1 May 2025).
- Borova, V.L.; Maragou, N.C.; Gago-Ferrero, P.; Pistos, C.; Τhomaidis, Ν.S. Highly sensitive determination of 68 psychoactive pharmaceuticals, illicit drugs, and related human metabolites in wastewater by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 4273–4285. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; De Paula Cognato, G.; Bonan, C.D. Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): A developmental behavior analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, Z.; Guven, B.; Kideys, A.E. Microplastic distribution in the surface water and sediment of the Ergene River. Environ. Res. 2023, 234, 116500. [Google Scholar] [CrossRef]
- Tenorio-Chávez, P.; Cerro-López, M.; Castro-Pastrana, L.I.; Ramírez-Rodrigues, M.M.; Orozco-Hernández, J.M.; Gómez-Oliván, L.M. Effects of effluent from a hospital in Mexico on the embryonic development of zebrafish, Danio rerio. Sci. Total Environ. 2020, 727, 138716. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Li, J.; Wang, D.; Bao, Z.; Wang, Q.; Jin, Y. Health risks of chlorothalonil, carbendazim, prochloraz, their binary and ternary mixtures on embryonic and larval zebrafish based on metabolomics analysis. J. Hazard. Mater. 2021, 404, 124240. [Google Scholar] [CrossRef]
- Jijie, R.; Solcan, G.; Nicoara, M.; Micu, D.; Strungaru, S.-A. Antagonistic effects in zebrafish (Danio rerio) behavior and oxidative stress induced by toxic metals and deltamethrin acute exposure. Sci. Total Environ. 2020, 698, 134299. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; Dai, D.; Xu, Z.; Cai, L.; Wang, Q.; Yu, Y. Individual and mixture effects of five agricultural pesticides on zebrafish (Danio rerio) larvae. Environ. Sci. Pollut. Res. 2017, 24, 4528–4536. [Google Scholar] [CrossRef]
- Gomes, S.d.S.; da Silva, J.F.; Padilha, R.M.O.; de Vasconcelos, J.V.A.; Negreiros Neto, L.G.d.; Marrs, J.A.; Cadena, P.G. Behavioral Effects of the Mixture and the Single Compounds Carbendazim, Fipronil, and Sulfentrazone on Zebrafish (Danio rerio) Larvae. Biomedicines 2024, 12, 1176. [Google Scholar] [CrossRef]
- Savuca, A.; Chelaru, I.-A.; Balmus, I.-M.; Curpan, A.-S.; Nicoara, M.N.; Ciobica, A.S. Toxicological response of zebrafish exposed to cocktails of polymeric materials and valproic acid. Sustainability 2024, 16, 2057. [Google Scholar] [CrossRef]
- Madesh, S.; Sudhakaran, G.; Meenatchi, R.; Manikandan, K.; Dhayanithi, N.B.; Almutairi, M.H.; Almutairi, B.O.; Guru, A.; Arockiaraj, J. Neurobehavioral and bioaccumulative toxicity in adult in-vivo zebrafish model due to prolonged cadmium exposure in the presence of ketoprofen. J. Biochem. Mol. Toxicol. 2024, 38, e70005. [Google Scholar] [CrossRef] [PubMed]
- Madesh, S.; Sudhakaran, G.; Murugan, R.; Almutairi, M.H.; Almutairi, B.O.; Kathiravan, M.; Arockiaraj, J. Parental (F0) exposure to Cadmium and Ketoprofen induces developmental deformities in offspring (F1): A transgenerational toxicity assessment in zebrafish model. Sci. Total Environ. 2024, 950, 175319. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Chen, J.; Zhang, C.; Xu, Z.; Li, G.; Cai, L.; Shen, W.; Wang, Q. Single and joint toxicity assessment of four currently used pesticides to zebrafish (Danio rerio) using traditional and molecular endpoints. Chemosphere 2018, 192, 14–23. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Zhao, J.; Zheng, R.; Xing, J.; Li, Z.; Zhang, Y.; Xu, Q. The enhanced hepatotoxicity of isobavachalcone in depigmented zebrafish due to calcium signaling dysregulation and lipid metabolism disorder. J. Appl. Toxicol. 2024, 44, 919–932. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Hu, N.; Long, D.; Cao, Y. The uses of zebrafish (Danio rerio) as an in vivo model for toxicological studies: A review based on bibliometrics. Ecotoxicol. Environ. Saf. 2024, 272, 116023. [Google Scholar] [CrossRef]
- Harini, K.; Girigoswami, K.; Vajagathali, M.; Bose, D.; Thirumalai, A.; Kiran, V.; Durgadevi, P.; Girigoswami, A. Enhanced behavioral impact of optimized bupropion-encapsulated bilosomes over traditional niosomes treating depression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 4373–4392. [Google Scholar] [CrossRef]
- Boopathi, S.; Mendonca, E.; Gandhi, A.; Rady, A.; Darwish, N.M.; Arokiyaraj, S.; Kumar, T.T.A.; Pachaiappan, R.; Guru, A.; Arockiaraj, J. Exploring the combined effect of exercise and apigenin on aluminium-induced neurotoxicity in zebrafish. Mol. Neurobiol. 2024, 61, 5320–5336. [Google Scholar] [CrossRef]
- Swank, A.; Wang, L.; Ward, J.; Schoenfuss, H. Multigenerational effects of a complex urban contaminant mixture on the behavior of larval and adult fish in multiple fitness contexts. Sci. Total Environ. 2021, 791, 148095. [Google Scholar] [CrossRef]
- Liu, H.; Fu, R.; Zhang, Y.; Mao, L.; Zhu, L.; Zhang, L.; Liu, X.; Jiang, H. Integrate transcriptomic and metabolomic analysis reveals the underlying mechanisms of behavioral disorders in zebrafish (Danio rerio) induced by imidacloprid. Sci. Total Environ. 2023, 870, 161541. [Google Scholar] [CrossRef]
- Souza, T.P.; Franscescon, F.; Stefanello, F.V.; Müller, T.E.; Santos, L.W.; Rosemberg, D.B. Acute effects of ethanol on behavioral responses of male and female zebrafish in the open field test with the influence of a non-familiar object. Behav. Process. 2021, 191, 104474. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, M.; Xie, X.; Ru, Y.; Ru, S. Carbofuran induces increased anxiety-like behaviors in female zebrafish (Danio rerio) through disturbing dopaminergic/norepinephrinergic system. Chemosphere 2020, 253, 126635. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Picolo, V.; Domingues, I.; Perillo, V.; Villacis, R.A.; Grisolia, C.K.; Oliveira, M. Effects of environmental concentrations of caffeine on adult zebrafish behaviour: A short-term exposure scenario. Environ. Sci. Pollut. Res. 2023, 30, 63776–63787. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef]
- Wu, B.; Yu, H.; Yi, J.; Lei, P.; He, J.; Ruan, J.; Xu, P.; Tao, R.; Jin, L.; Wu, W.; et al. Behavioral Studies of Zebrafish Reveal a New Perspective on the Reproductive Toxicity of Micro- and Nanoplastics. Toxics 2024, 12, 178. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Li, S.; Nie, Y.; Ma, B.; Liu, J. Behavioral and biochemical responses in freshwater fish Carassius auratus exposed to sertraline. Chemosphere 2015, 135, 146–155. [Google Scholar] [CrossRef]
- Vieira, L.R.; Gravato, C.; Soares, A.; Morgado, F.; Guilhermino, L. Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: Linking biomarkers to behaviour. Chemosphere 2009, 76, 1416–1427. [Google Scholar] [CrossRef]
- Heredia-García, G.; Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M.; Islas-Flores, H.; García-Medina, S.; Galar-Martínez, M.; Dublán-García, O. Realistic concentrations of Bisphenol-A trigger a neurotoxic response in the brain of zebrafish: Oxidative stress, behavioral impairment, acetylcholinesterase inhibition, and gene expression disruption. Chemosphere 2023, 330, 138729. [Google Scholar] [CrossRef]
- Costa, F.V.; Gonçalves, F.L.; Borba, J.V.; Sabadin, G.R.; Biasuz, E.; Santos, L.W.; Sneddon, L.U.; Kalueff, A.V.; Rosemberg, D.B. Acetic acid-induced pain elicits stress-, and camouflage-related responses in zebrafish: Modulatory effects of opioidergic drugs on neurobehavioral phenotypes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 270, 109640. [Google Scholar] [CrossRef]
- DePasquale, C.; Franklin, K.; Jia, Z.; Jhaveri, K.; Buderman, F.E. The effects of exploratory behavior on physical activity in a common animal model of human disease, zebrafish (Danio rerio). Front. Behav. Neurosci. 2022, 16, 1020837. [Google Scholar] [CrossRef]
- Popovici, L.-F.; Brinza, I.; Gatea, F.; Badea, G.I.; Vamanu, E.; Oancea, S.; Hritcu, L. Enhancement of Cognitive Benefits and Anti-Anxiety Effects of Phytolacca americana Fruits in a Zebrafish (Danio rerio) Model of Scopolamine-Induced Memory Impairment. Antioxidants 2025, 14, 97. [Google Scholar] [CrossRef]
- Vliegenthart, A.D.; Tucker, C.S.; Del Pozo, J.; Dear, J.W. Zebrafish as model organisms for studying drug-induced liver injury. Br. J. Clin. Pharmacol. 2014, 78, 1217–1227. [Google Scholar] [CrossRef]
- Toni, M.; Arena, C.; Cioni, C.; Tedeschi, G. Temperature- and chemical-induced neurotoxicity in zebrafish. Front. Physiol. 2023, 14, 1276941. [Google Scholar] [CrossRef]
- di Domenico, K.; Lacchetti, I.; Cafiero, G.; Mancini, A.; Carere, M.; Mancini, L. Reviewing the use of zebrafish for the detection of neurotoxicity induced by chemical mixtures through the analysis of behaviour. Chemosphere 2024, 359, 142246. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.D.; Pereira, T.C.B.; Altenhofen, S.; Nabinger, D.D.; Ferreira, P.M.d.A.; Bogo, M.R.; Bonan, C.D. Antibiotic drugs alter zebrafish behavior. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.B. Behavioral profile alterations in zebrafish larvae exposed to environmentally relevant concentrations of eight priority pharmaceuticals. Sci. Total Environ. 2019, 664, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.; Domingues, I.; Faria, M.; Oliveira, M. Chronic Effects of Fluoxetine on Danio rerio: A Biochemical and Behavioral Perspective. Appl. Sci. 2022, 12, 2256. [Google Scholar] [CrossRef]
- Zhou, J.; Yun, X.; Wang, J.; Li, Q.; Wang, Y.; Zhang, W.; Fan, Z. Biological toxicity of sulfamethoxazole in aquatic ecosystem on adult zebrafish (Danio rerio). Sci. Rep. 2024, 14, 9401. [Google Scholar] [CrossRef]
- Šíma, M.; Hartinger, J.; Rulíšek, J.; Šachl, R.; Slanař, O. Meropenem-induced valproic acid elimination: A case report of clinically relevant drug interaction. Prague Med. Rep. 2017, 118, 105–109. [Google Scholar] [CrossRef]
- Johnson, A.; Loh, E.; Verbitsky, R.; Slessor, J.; Franczak, B.C.; Schalomon, M.; Hamilton, T.J. Examining behavioural test sensitivity and locomotor proxies of anxiety-like behaviour in zebrafish. Sci. Rep. 2023, 13, 3768. [Google Scholar] [CrossRef]
- Dhanabal, M.; Durairaj, B. Valproic Acid Induced Autism-Like Behaviour in Zebrafish-Chrysin as a Miracle Cure. Indian J. Pharm. Sci. 2024, 86, 361–368. [Google Scholar] [CrossRef]
- Baronio, D.; Puttonen, H.A.; Sundvik, M.; Semenova, S.; Lehtonen, E.; Panula, P. Embryonic exposure to valproic acid affects the histaminergic system and the social behaviour of adult zebrafish (Danio rerio). Br. J. Pharmacol. 2018, 175, 797–809. [Google Scholar] [CrossRef]
- Li, F.; Lin, J.; Liu, X.; Li, W.; Ding, Y.; Zhang, Y.; Zhou, S.; Guo, N.; Li, Q. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann. Transl. Med. 2018, 6, 173. [Google Scholar] [CrossRef]
- Karimi, Z.; Zarifkar, A.; Dianatpour, M.; Mirzaei, E.; Dara, M.; Aligholi, H. Finding a Proper Valproic Acid-Based Autism Spectrum Disorder Model in Zebrafish: Early and Long-term Neurobehavioral Studies. Iran. J. Psychiatry Behav. Sci./Prog. Psychiatry Behav. Sci. 2023, 17, e137118. [Google Scholar] [CrossRef]
- Praskova, E.; Zivna, D.; Stepanova, S.; Sevcikova, M.; Blahova, J.; Marsalek, P.; Siroka, Z.; Voslarova, E.; Svobodova, Z. Acute toxicity of acetylsalicylic acid to juvenile and embryonic stages of Danio rerio. Neuro Endocrinol. Lett. 2012, 33 (Suppl. 3), 72–76. [Google Scholar] [PubMed]
- Buzenchi Proca, T.M.; Solcan, C.; Solcan, G. Neurotoxicity of Some Environmental Pollutants to Zebrafish. Life 2024, 14, 640. [Google Scholar] [CrossRef] [PubMed]
- Ilie, O.-D.; Duta, R.; Jijie, R.; Nita, I.-B.; Nicoara, M.; Faggio, C.; Dobrin, R.; Mavroudis, I.; Ciobica, A.; Doroftei, B. Assessing Anti-Social and Aggressive Behavior in a Zebrafish (Danio rerio) Model of Parkinson’s Disease Chronically Exposed to Rotenone. Brain Sci. 2022, 12, 898. [Google Scholar] [CrossRef]
- Scatterty, K.R.; Pitman, T.; Eckersley, T.; Schmaltz, R.; Hamilton, T.J. Zebrafish aversion to infrasound in an open field test. Front. Behav. Neurosci. 2023, 16, 1019368. [Google Scholar] [CrossRef] [PubMed]
- Scharf, I.; Farji-Brener, A. Chapter One—Wall-following behavior: Its ultimate and proximate explanations, prevalence, and implications. In Advances in the Study of Behavior; Podos, J., Healy, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 56, pp. 1–49. [Google Scholar]
- Lagunas-Rangel, F.A.; Linnea-Niemi, J.V.; Kudłak, B.; Williams, M.J.; Jönsson, J.; Schiöth, H.B. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. GeoHealth 2022, 6, e2021GH000552. [Google Scholar] [CrossRef]
- Kotova, M.M.; Galstyan, D.S.; Kolesnikova, T.O.; de Abreu, M.S.; Amstislavskaya, T.G.; Strekalova, T.; Petersen, E.V.; Yenkoyan, K.B.; Demin, K.A.; Kalueff, A.V. Understanding CNS Effects of Antimicrobial Drugs Using Zebrafish Models. Vet. Sci. 2023, 10, 96. [Google Scholar] [CrossRef]
- Grossman, L.; Utterback, E.; Stewart, A.; Gaikwad, S.; Chung, K.M.; Suciu, C.; Wong, K.; Elegante, M.; Elkhayat, S.; Tan, J.; et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav. Brain Res. 2010, 214, 277–284. [Google Scholar] [CrossRef]
- Nunes, A.R.; Carreira, L.; Anbalagan, S.; Blechman, J.; Levkowitz, G.; Oliveira, R.F. Perceptual mechanisms of social affiliation in zebrafish. Sci. Rep. 2020, 10, 3642. [Google Scholar] [CrossRef] [PubMed]
- Robea, M.A.; Jijie, R.; Nicoara, M.; Plavan, G.; Ciobica, A.S.; Solcan, C.; Audira, G.; Hsiao, C.-D.; Strungaru, S.-A. Vitamin C Attenuates Oxidative Stress and Behavioral Abnormalities Triggered by Fipronil and Pyriproxyfen Insecticide Chronic Exposure on Zebrafish Juvenile. Antioxidants 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, T.; Lu, W. The effects of valproic acid neurotoxicity on aggressive behavior in zebrafish autism model. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2024, 275, 109783. [Google Scholar] [CrossRef] [PubMed]
- Al Shuraiqi, A.; Abed, R.M.M.; Al-Habsi, A.; Barry, M.J. Personality Affects Zebrafish Response to Sertraline. Environ. Toxicol. Chem. 2023, 43, 132–146. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, A.; Fu, L.; Liu, M.; Li, K.; Chian, S.; Yao, W.; Wang, B.; Wang, J. Fentanyl Induces Novel Conditioned Place Preference in Adult Zebrafish, Disrupts Neurotransmitter Homeostasis, and Triggers Behavioral Changes. Int. J. Environ. Res. Public Health 2022, 19, 13533. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Wu, L.; Ma, T. Effects of 4-epianhydrotetracycline on oxidative stress in zebrafish (Danio rerio) embryos. Sci. Total Environ. 2021, 796, 149047. [Google Scholar] [CrossRef]
- Saputra, F.; Kishida, M.; Hu, S.-Y. Oxidative stress induced by hydrogen peroxide disrupts zebrafish visual development by altering apoptosis, antioxidant and estrogen related genes. Sci. Rep. 2024, 14, 14454. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Li, J.; Lv, X.; Yu, L.; Wu, K.; Yang, Y. Metal contamination, bioaccumulation, ROS generation, and epigenotoxicity influences on zebrafish exposed to river water polluted by mining activities. J. Hazard. Mater. 2021, 405, 124150. [Google Scholar] [CrossRef]
- Shukla, S.; Jhamtani, R.C.; Dahiya, M.S.; Agarwal, R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017, 4, 240–244. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef]
- Asghar, M.A.; Tang, S.; Wan, B.; Chen, Y.; Zhang, X.; Zhao, Q. Valproic acid-induced oxidative stress: Systematic review, meta-analysis and network pharmacology highlights disruption in antioxidant pathways in rodents. Toxicol. Appl. Pharmacol. 2025, 494, 117160. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Alajbegovic, A.; Gomes, A.V. NSAIDs and cardiovascular diseases: Role of reactive oxygen species. Oxidative Med. Cell. Longev. 2015, 2015, 536962. [Google Scholar] [CrossRef] [PubMed]
- Jijie, R.; Paduraru, E.; Simionov, I.A.; Faggio, C.; Ciobica, A.; Nicoara, M. Effects of Single and Combined Ciprofloxacin and Lead Treatments on Zebrafish Behavior, Oxidative Stress, and Elements Content. Int. J. Mol. Sci. 2023, 24, 4952. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Hara, Y.; Kataoka, S.; Kawanai, T.; Maeda, Y.; Watanabe, R.; Takano, E.; Hayata-Takano, A.; Hashimoto, H.; Ago, Y. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol. Biochem. Behav. 2014, 126, 43–49. [Google Scholar] [CrossRef]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, J.-W.; Kim, K.C.; Shin, C.Y. Exploring the validity of valproic acid animal model of autism. Exp. Neurobiol. 2015, 24, 285. [Google Scholar] [CrossRef]
- Basselin, M.; Chang, L.; Chen, M.; Bell, J.M.; Rapoport, S.I. Chronic administration of valproic acid reduces brain NMDA signaling via arachidonic acid in unanesthetized rats. Neurochem. Res. 2008, 33, 2229–2240. [Google Scholar] [CrossRef]
- Santi, A.; Menezes, C.; Duarte, M.M.F.; Leitemperger, J.; Lópes, T.; Loro, V.L. Oxidative stress biomarkers and acetylcholinesterase activity in human erythrocytes exposed to clomazone (in vitro). Interdiscip. Toxicol. 2011, 4, 149. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Massei, R.; Brack, W.; Seidensticker, S.; Hollert, H.; Muz, M.; Schulze, T.; Krauss, M.; Küster, E. Neurotoxicity in complex environmental mixtures—A case-study at River Danube in Novi Sad (Serbia) using zebrafish embryos. Environ. Sci. Pollut. Res. 2023, 30, 96138–96146. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, V.; Vishali, S.; Elumalai, S.; Perumal, E. Acute exposure to environmentally relevant concentrations of pharmaceutical pollutants induces neurobehavioral toxicity in zebrafish (Danio rerio). Drug Chem. Toxicol. 2025, 48, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Jijie, R.; Simionov, I.-A.; Gavrilescu, C.-M.; Ilie, T.; Iacob, D.; Lupitu, A.; Moisa, C.; Muresan, C.; Copolovici, L.; et al. Honey Enriched with Additives Alleviates Behavioral, Oxidative Stress, and Brain Alterations Induced by Heavy Metals and Imidacloprid in Zebrafish. Int. J. Mol. Sci. 2024, 25, 11730. [Google Scholar] [CrossRef]
- Underwood, W.; Anthony, R. (AVMA) American Veterinary Medical Association, Guidelines for the Euthanasia of Animals, Schaumburg, IL, USA. 2020. Available online: https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals (accessed on 4 April 2025).
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Hritcu, L. Anxiolytic, promnesic, anti-acetylcholinesterase and antioxidant effects of cotinine and 6-hydroxy-L-nicotine in scopolamine-induced zebrafish (Danio rerio) model of Alzheimer’s disease. Antioxidants 2021, 10, 212. [Google Scholar] [CrossRef]



| Compounds | Mp (μg/L) | Kp (μg/L) | VPA (μg/L) |
|---|---|---|---|
| Mp | 1 | 0 | 0 |
| Kp | 0 | 5 | 0 |
| VPA | 0 | 0 | 3 |
| VPA + Mp | 1 | 0 | 3 |
| VPA + Kp | 0 | 5 | 3 |
| Mp + Kp | 1 | 5 | 0 |
| VPA + Mp + Kp | 1 | 5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelaru, I.-A.; Strungaru-Jijie, R.; Nicoara, M.; Mirila, D.; Ciobica, A.; Ureche, D. Single and Combined Effects of Meropenem, Valproic Acid, and Ketoprofen on Adult Zebrafish Behavior, Oxidative Stress, and Acetylcholinesterase Activity. Pharmaceuticals 2025, 18, 1096. https://doi.org/10.3390/ph18081096
Chelaru I-A, Strungaru-Jijie R, Nicoara M, Mirila D, Ciobica A, Ureche D. Single and Combined Effects of Meropenem, Valproic Acid, and Ketoprofen on Adult Zebrafish Behavior, Oxidative Stress, and Acetylcholinesterase Activity. Pharmaceuticals. 2025; 18(8):1096. https://doi.org/10.3390/ph18081096
Chicago/Turabian StyleChelaru, Ionut-Alexandru, Roxana Strungaru-Jijie, Mircea Nicoara, Diana Mirila, Alin Ciobica, and Dorel Ureche. 2025. "Single and Combined Effects of Meropenem, Valproic Acid, and Ketoprofen on Adult Zebrafish Behavior, Oxidative Stress, and Acetylcholinesterase Activity" Pharmaceuticals 18, no. 8: 1096. https://doi.org/10.3390/ph18081096
APA StyleChelaru, I.-A., Strungaru-Jijie, R., Nicoara, M., Mirila, D., Ciobica, A., & Ureche, D. (2025). Single and Combined Effects of Meropenem, Valproic Acid, and Ketoprofen on Adult Zebrafish Behavior, Oxidative Stress, and Acetylcholinesterase Activity. Pharmaceuticals, 18(8), 1096. https://doi.org/10.3390/ph18081096








