Acrocomia aculeata Oil-Loaded Nanoemulsion: A Promising Candidate for Cancer and Diabetes Management
Abstract
1. Introduction
2. Result and Discussion
2.1. Physicochemical Characterization of Bocaiuva Oil
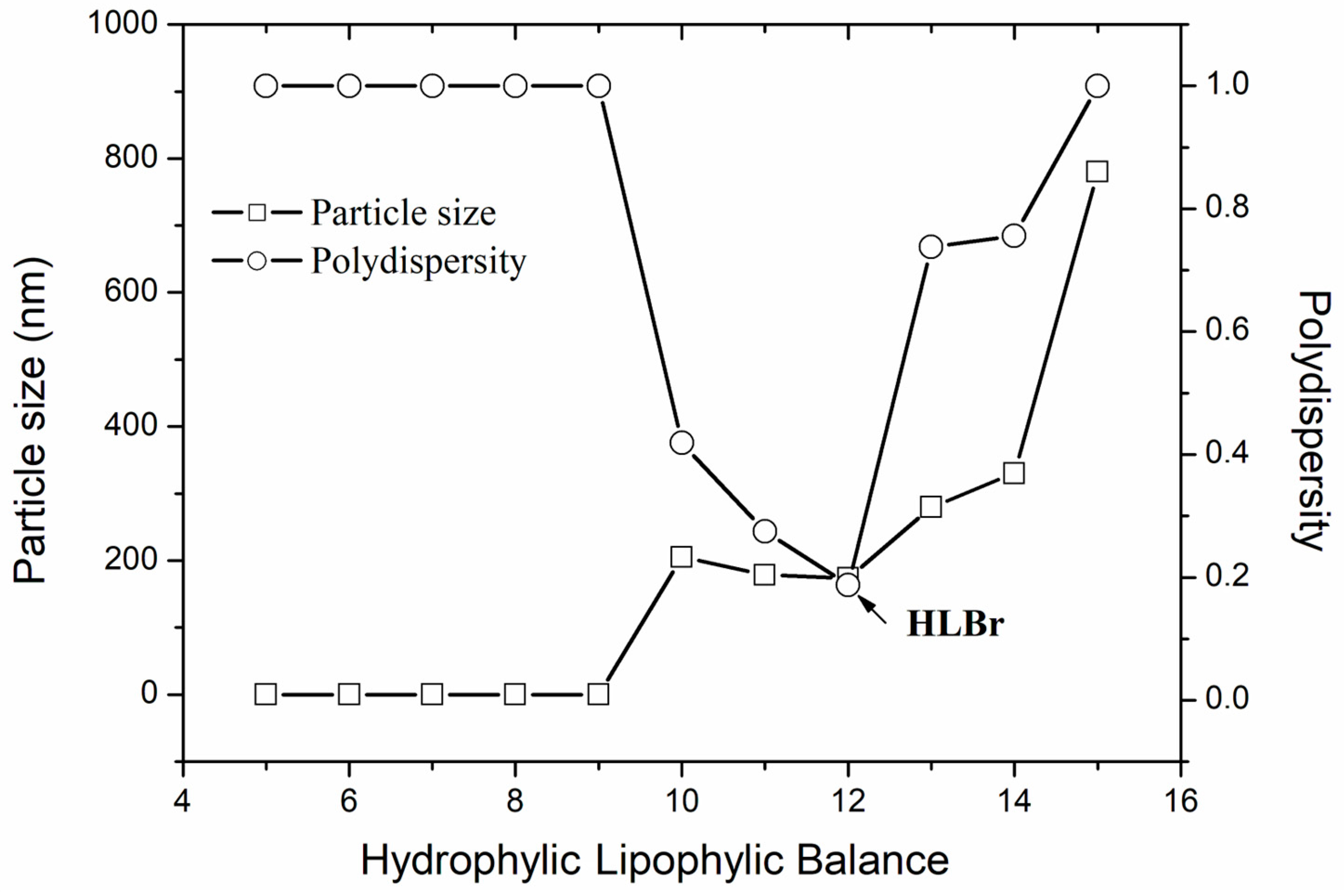
2.2. Required Hydrophilic–Lipophilic Balance (HLBr) of the Bocaiuva Oil
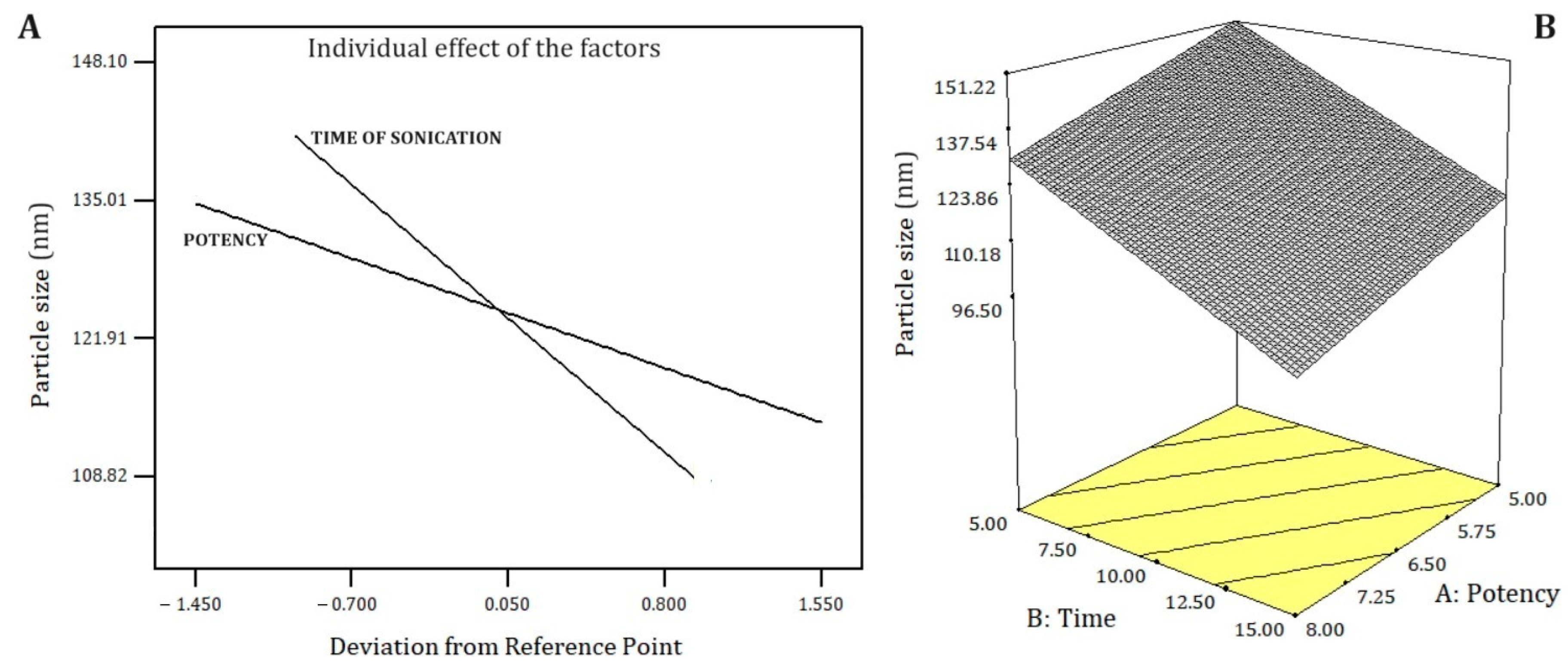
2.3. Optimization Process
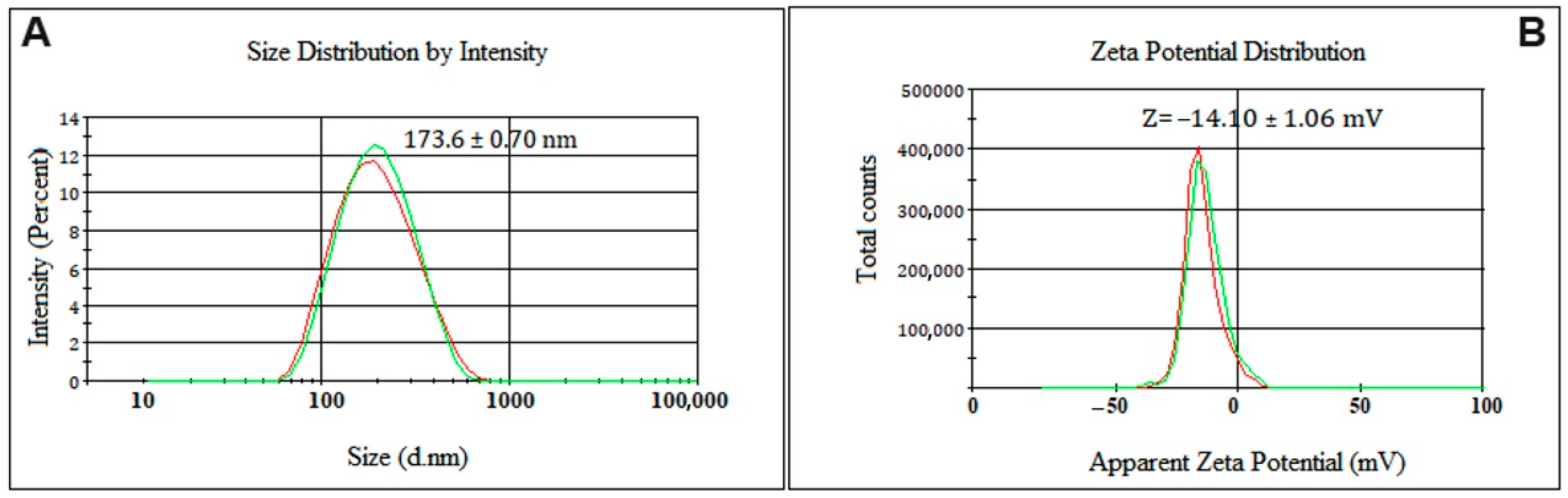
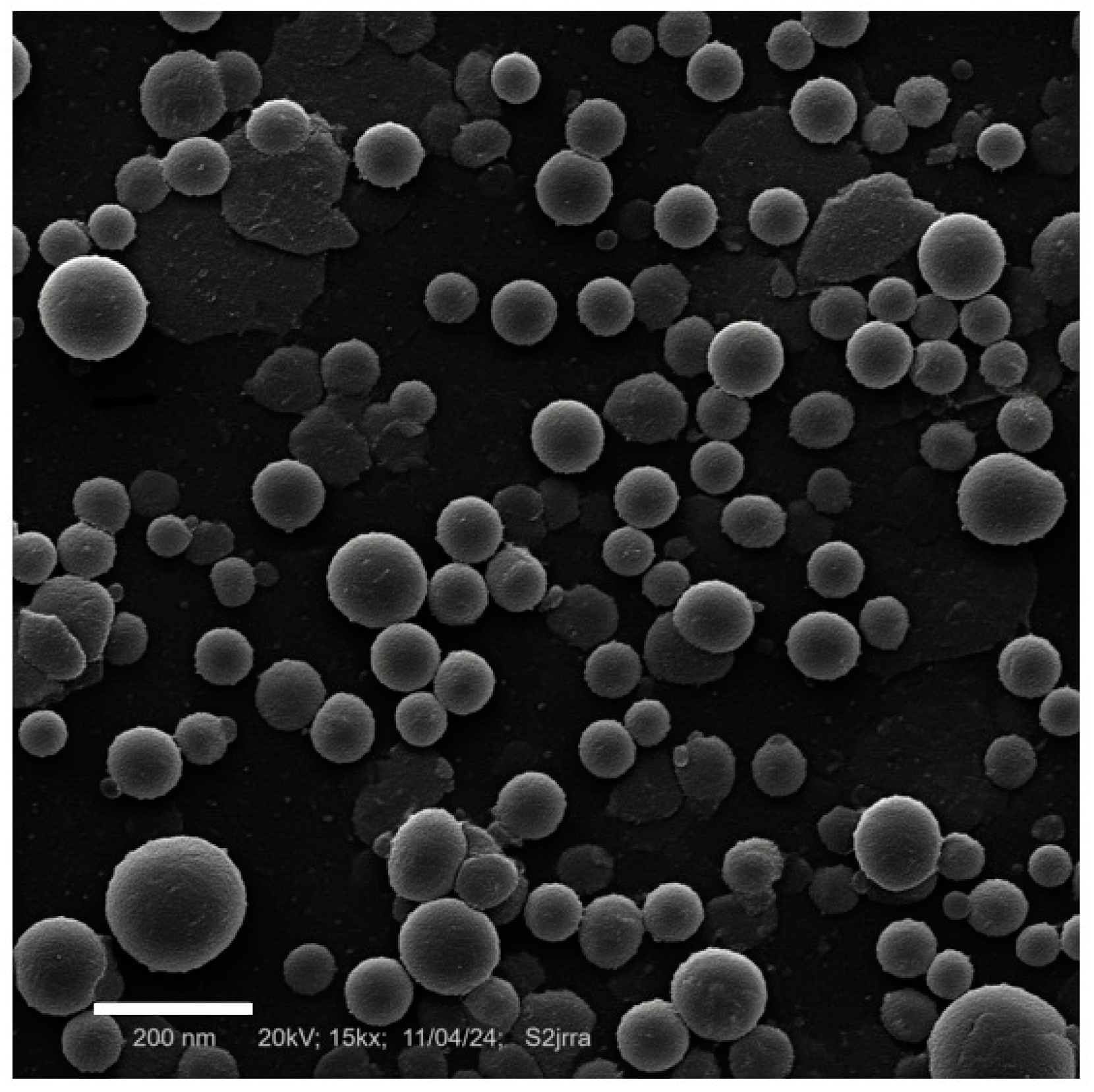
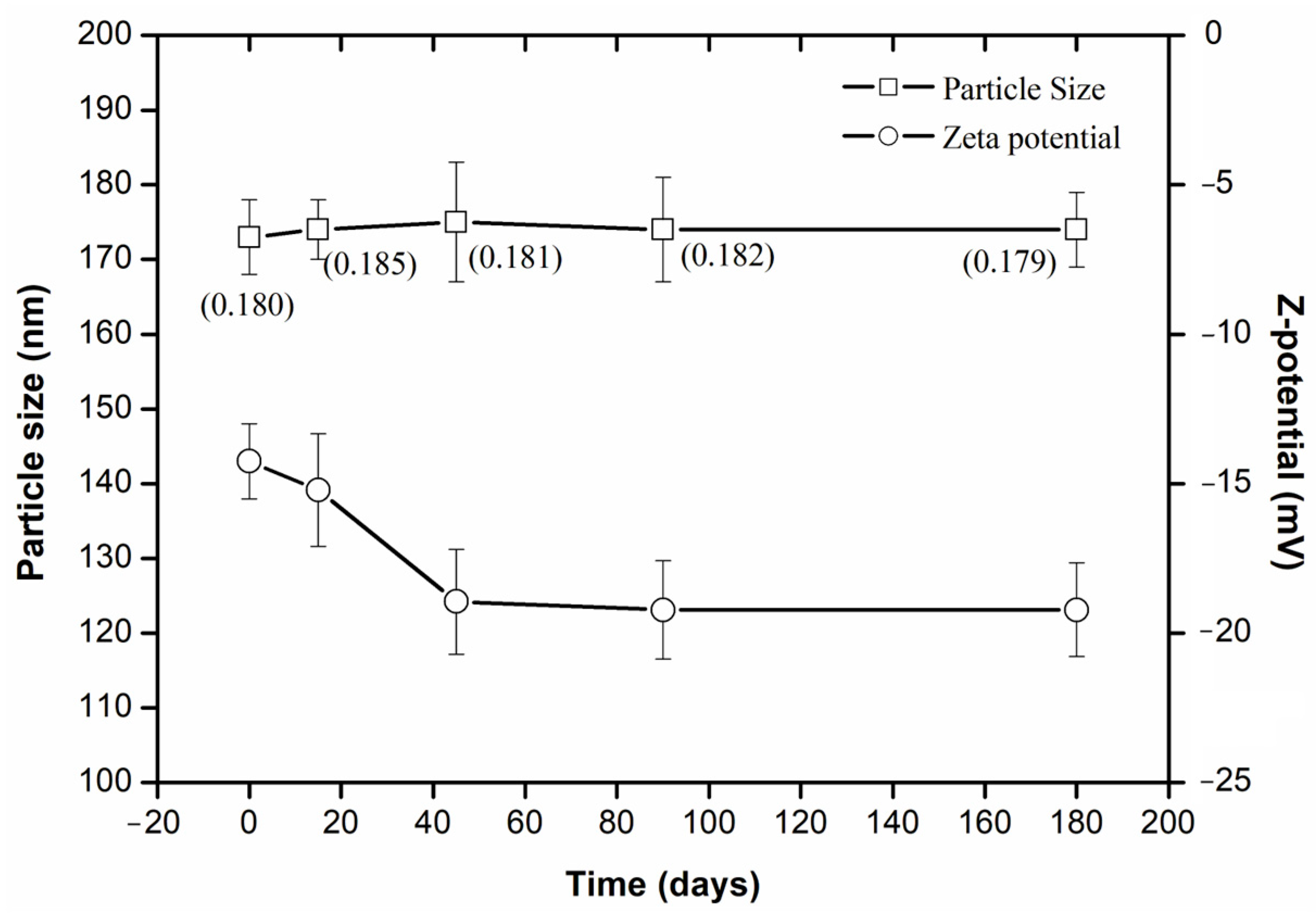
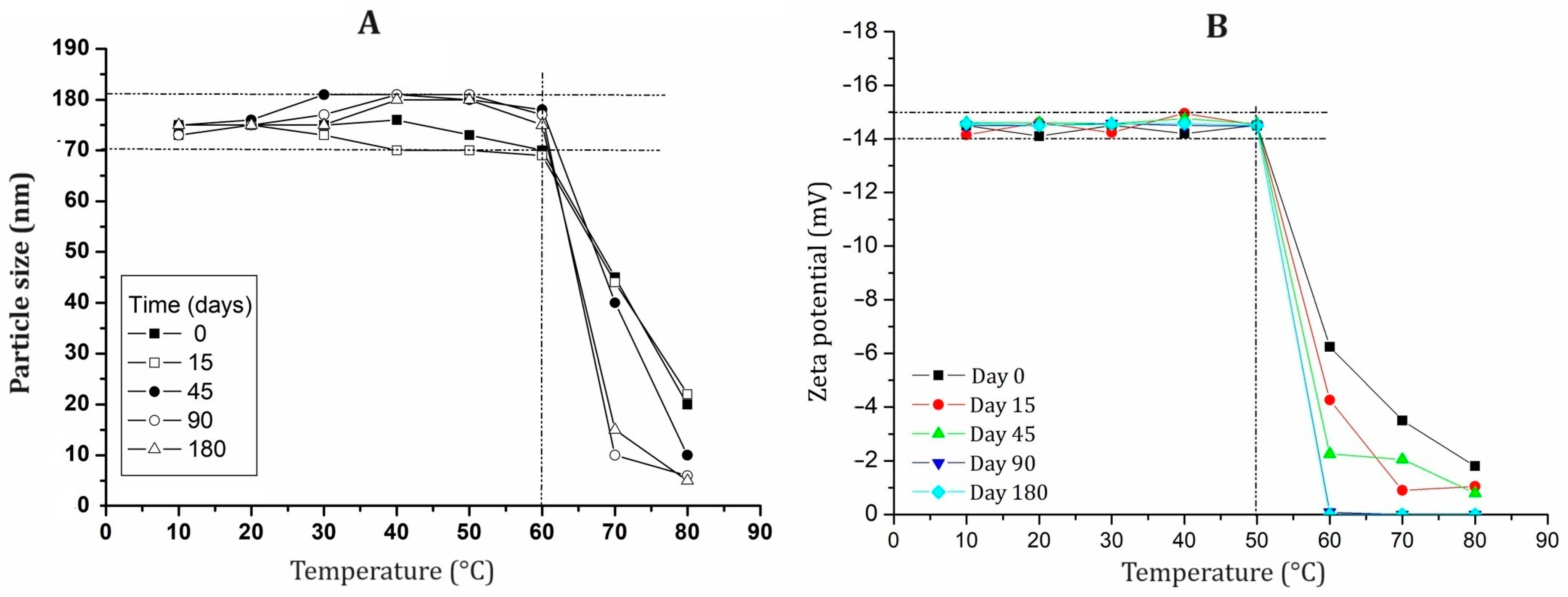
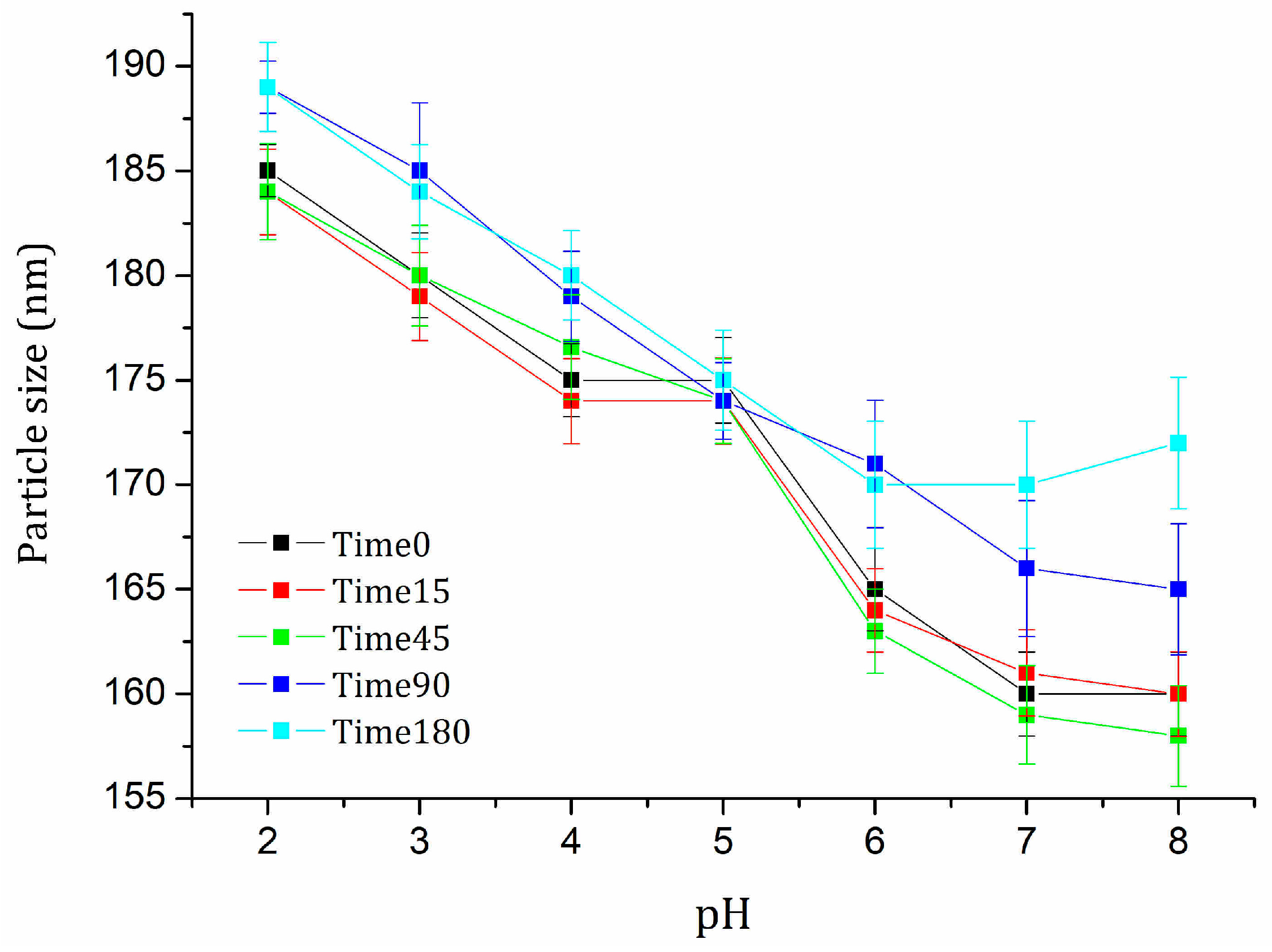
2.4. Stability Evaluation
2.5. Alpha-Glycosidase and Pancreatic Lipase Inhibitory Effect
2.6. Antiglycant Effect
2.7. Cytotoxic Effect
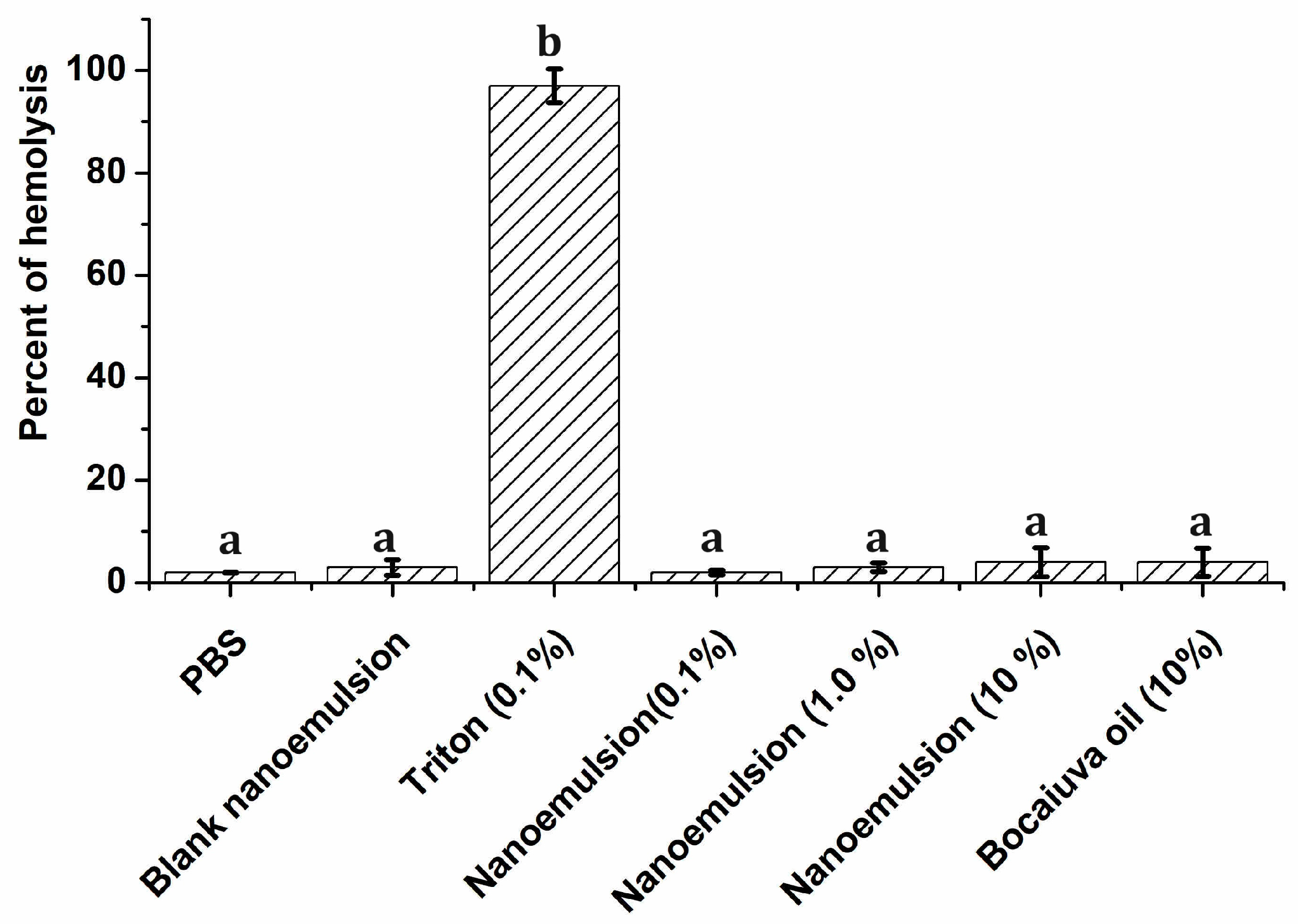
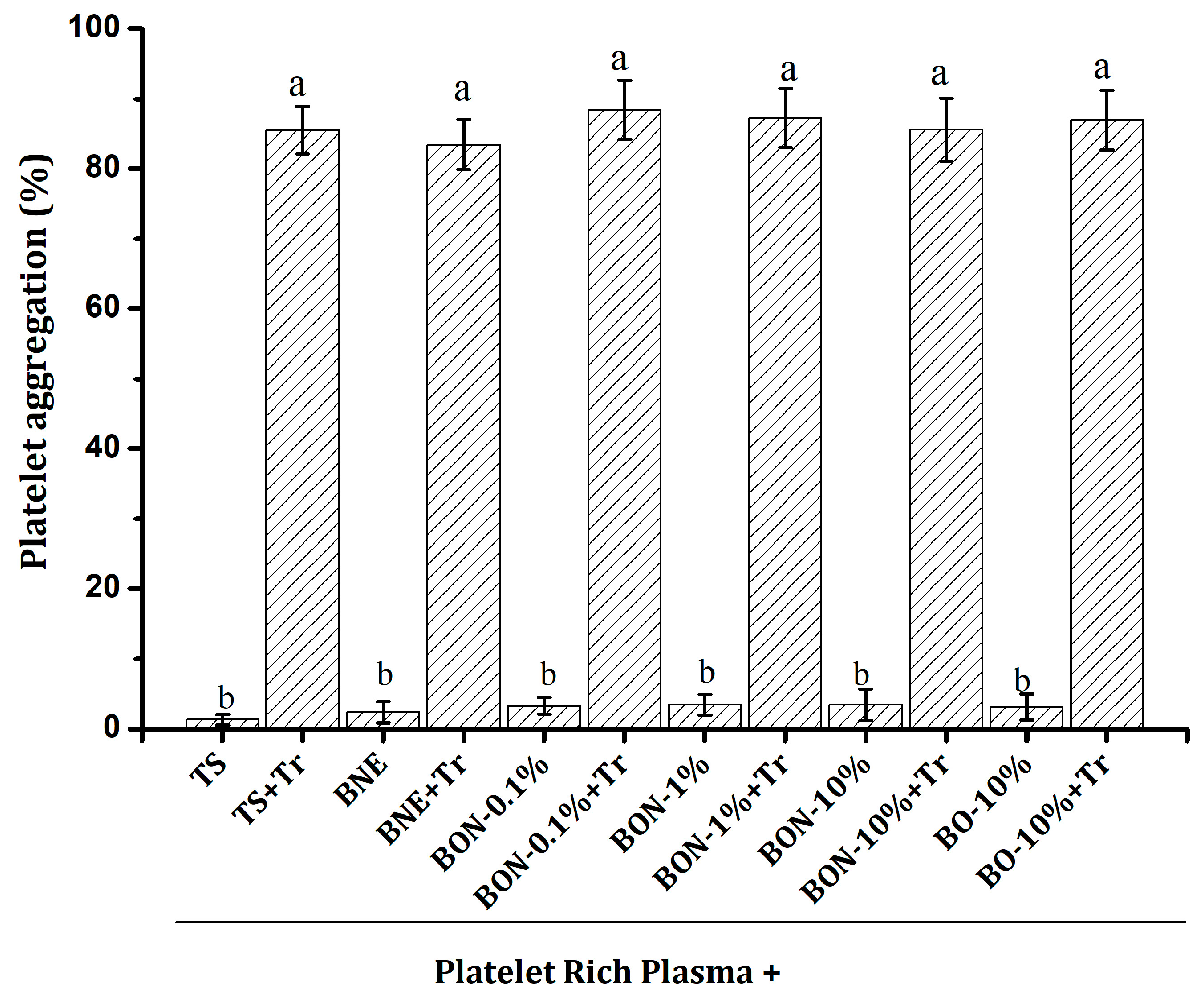
2.8. Hemolytic Effect and Platelet Aggregation
2.9. Future Perspectives
3. Materials and Methods
3.1. Plant Material
3.2. Oil Extraction
3.3. Organoleptic and Physicochemical Characterization
3.4. Phenolic Content
3.5. Carotenoids as β-Carotene
3.6. GC/MS Analysis
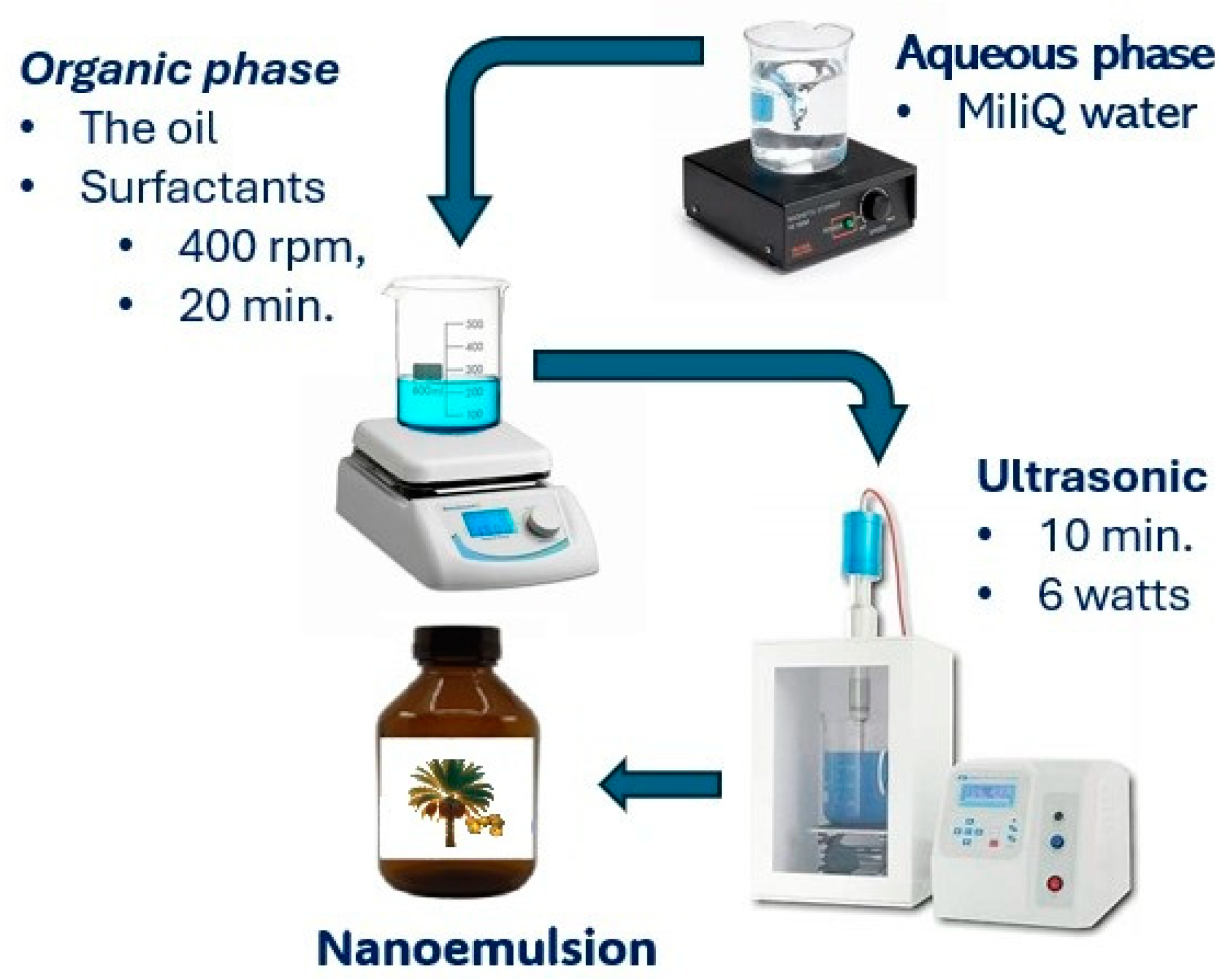
3.7. Nanoemulsion Development
3.8. Required Hydrophilic–Lipophilic Balance
3.9. Particle Size, ζ-Potential, and pH
3.10. Scanning Electronic Microscopy
3.11. pH Evaluation
3.12. Nanoemulsion Optimization
3.13. On-Shelf Physical and Chemical Stability
3.14. Alpha-Glucosidase Inhibition
3.15. Pancreatic Lipase Inhibition
3.16. Antiglycant Activity—Oxidative Pathway
3.17. Antiglycant Activity—Non-Oxidative Pathway
3.18. Antiproliferative Effect
Selectivity Index
3.19. Effect of Bocaiuva Oil and Nanoemulsion on Blood Cells
Hemolytic Effect
3.20. Platelet Aggregation Test
3.21. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahid, R.K.; Ahmed, S.; Le, D.; Yadav, S. Diabetes and Cancer: Risk, Challenges, Management and Outcomes. Cancers 2021, 13, 5735. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Brown, K.; Miksza, J.K.; Howells, L.M.; Morrison, A.; Issa, E.; Yates, T.; Khunti, K.; Davies, M.J.; Zaccardi, F. Risk of Cancer Incidence and Mortality Associated with Diabetes: A Systematic Review with Trend Analysis of 203 Cohorts. Nutr. Metab. Cardiovas Dis. 2021, 31, 14–22. [Google Scholar] [CrossRef]
- Acácio, B.R.; Prada, A.L.; Neto, S.F.; Gomes, G.B.; Perdomo, R.T.; Nazario, C.E.D.; Neto, E.S.; Martines, M.A.U.; de Almeida, D.A.T.; Gasparotto, A., Jr.; et al. Cytotoxicity, Anti-Inflammatory Effect, and Acute Oral Toxicity of a Novel Attalea phalerata Kernel Oil-Loaded Nanocapsules. Biomed. Pharmacother. 2024, 174, 116308. [Google Scholar] [CrossRef]
- Ramalingam, K.; Shanmugam, R. Biomedical Applications of Lauric Acid: A Narrative Review. Cureus 2024, 16, e62770. [Google Scholar] [CrossRef]
- Ramya, V.; Shyam, K.P.; Kowsalya, E.; Balavigneswaran, C.K.; Kadalmani, B. Dual Roles of Coconut Oil and Its Major Component Lauric Acid on Redox Nexus: Focus on Cytoprotection and Cancer Cell Death. Front. Neurosci. 2022, 16, 833630. [Google Scholar] [CrossRef]
- Bungau, S.G.; Vesa, C.M.; Bustea, C.; Purza, A.L.; Tit, D.M.; Brisc, M.C.; Radu, A.-F. Antioxidant and Hypoglycemic Potential of Essential Oils in Diabetes Mellitus and Its Complications. Int. J. Mol. Sci. 2023, 24, 16501. [Google Scholar] [CrossRef]
- Petersen, K.S.; Maki, K.C.; Calder, P.C.; Belury, M.A.; Messina, M.; Kirkpatrick, C.F.; Harris, W.S. Perspective on the Health Effects of Unsaturated Fatty Acids and Commonly Consumed Plant Oils High in Unsaturated Fat. Br. J. Nutr. 2024, 132, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.V.B.; Ramiro, M.M.; Iriguchi, E.K.K.; Corrêa, W.A.; Lowe, J.; Cardoso, C.A.L.; Arena, A.C.; Kassuya, C.A.L.; Muzzi, R.M. Antidiabetic, Cytotoxic and Antioxidant Activities of Oil Extracted from Acrocomia aculeata Pulp. Nat. Prod. Res. 2019, 33, 2413–2416. [Google Scholar] [CrossRef]
- Lescano, C.H.; Oliveira, I.P.; Silva, L.R.; Baldivia, D.S.; Sanjinez-Argandoña, E.J.; Arruda, E.J.; Moraes, I.C.F.; Lima, F.F. Nutrients Content, Characterization and Oil Extraction from Acrocomia aculeata (Jacq.) Lodd. Fruits. Afr. J. Food Sci. 2015, 9, 113–119. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; dos Santos, J.M.; Antunes, K.Á.; Cunha, J.; da Silva Baldivia, D.; Pires, A.S.; Marques, I.; Abrantes, A.M.; Botelho, M.F.; Monteiro, L.; et al. Acrocomia aculeata Associated with Doxorubicin: Cardioprotection and Anticancer Activity. Front. Pharmacol. 2023, 14, 1223933. [Google Scholar] [CrossRef]
- Freitas de Lima, F.; Lescano, C.H.; Arrigo, J.S.; Cardoso, C.A.L.; Coutinho, J.P.; Moslaves, I.S.B.; Ximenes, T.V.d.N.; Kadri, M.C.T.; Weber, S.S.; Perdomo, R.T.; et al. Anti-Inflammatory, Antiproliferative and Cytoprotective Potential of the Attalea phalerata Mart. ex Spreng. Pulp Oil. PLoS ONE 2018, 13, e0195678. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Â.A.; Buccini, D.F.; Jaques, J.A.S.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; Caldas, R.A.; Carvalho, C.M.E. Effect of Acrocomia aculeata Kernel Oil on Adiposity in Type 2 Diabetic Rats. Plant Foods Hum. Nutr. 2018, 73, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Espinoça, I.T.; Basilio, D.C.L.S.; de Araujo, A.J.P.; Ota, R.S.N.; de Souza, K.F.S.; Cassemiro, N.S.; Lagatta, D.C.; Paredes-Gamero, E.J.; Macedo, M.L.R.; Silva, D.B.; et al. Antithrombotic Effect of Oil from the Pulp of Bocaiúva—Acrocomia aculeata (Jacq.) Lodd. ex Mart. (Arecaceae). Nutrients 2024, 16, 2024. [Google Scholar] [CrossRef]
- Aleixo, D.T.; Gualberto, A.C.M.; Valle, A.B.C.d.S.; da Silva, L.C.; Ferreira, K.C.B.; Lemos, A.S.d.O.; Fabri, R.L.; Tavares, G.D.; Cazarim, M.d.S.; Gameiro, J.; et al. Macauba Oil Carried by Polymeric Micelles Reduces Migration and Proliferation of Triple-Negative Breast Cancer Cells. RSC Pharm. 2024, 1, 524–535. [Google Scholar] [CrossRef]
- Amado, J.R.R.; Prada, A.L.; Diaz, J.G.; Souto, R.N.P.; Arranz, J.C.E.; de Souza, T.P. Development, Larvicide Activity, and Toxicity in Nontarget Species of the Croton linearis Jacq Essential Oil Nanoemulsion. Environ. Sci. Pollut. Res. 2020, 27, 9410–9423. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Karmakar, V.; Sisinthy, S.P.; Pandey, M.; Jain, N.; Gorain, B. Nanoemulsion and Nanoemulgel-Based Carriers as Advanced Delivery Tools for the Treatment of Oral Diseases. Drug Deliv. Transl. Res. 2024, 5, 1139–1155. [Google Scholar] [CrossRef]
- Costa, G.L.A.; Buccini, D.F.; Arruda, A.L.A.; Favaro, S.P.; Moreno, S.E. Phytochemical Profile, Anti-Inflammatory, Antimutagenic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd. Pulp Oil. Food Sci. Technol. 2020, 40, 963–971. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.C. Oleic Acid Coating on the Monodisperse Magnetite Nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, T.E.; Gil, B. Correlation between Refractive Index of Vegetable Oils Measured with Surface Plasmon Resonance and Acid Values Determined with the AOCS Official Method. LWT 2013, 53, 517–521. [Google Scholar] [CrossRef]
- Tanuku, S.; Velisila, D.; Thatraju, D.; Vadaga, A. kumar Nanoemulsion Formulation Strategies for Enhanced Drug Delivery. J. Pharma Insights Res. 2024, 2, 125–138. [Google Scholar] [CrossRef]
- Gabrielsson, J.; Lindberg, N.; Lundstedt, T. Multivariate Methods in Pharmaceutical Applications. J. Chemom. 2002, 16, 141–160. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of Food—Grade Nanoemulsions Using Low—Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Patil, S.; Patil, M.; Maheshwari, V.L.; Patil, R.H. Pancreatic Lipase (PL) Inhibitors from Medicinal Plants and Their Potential Applications in the Management of Obesity. In Natural Products as Enzyme Inhibitors; Springer Nature: Singapore, 2022; pp. 153–167. [Google Scholar]
- Kumar, A.; Chauhan, S. Pancreatic Lipase Inhibitors: The Road Voyaged and Successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Pan, Y.; Chen, S.; Zhao, Y.; Hu, Y. New Insights into the Role of Dietary Triglyceride Absorption in Obesity and Metabolic Diseases. Front. Pharmacol. 2023, 14, 1097835. [Google Scholar] [CrossRef]
- Wang, N.; Manabe, Y.; Sugawara, T.; Paul, N.A.; Zhao, J. Identification and Biological Activities of Carotenoids from the Freshwater Alga Oedogonium intermedium. Food Chem. 2018, 242, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Bhattacharjee, N.; Chakraborty, P.; Bhattacharjee, S. Carotenoids as Antioxidants. In Carotenoids: Structure and Function in the Human Body; Springer International Publishing: Cham, Switzerland, 2021; pp. 447–473. [Google Scholar]
- Li, X.; Morita, S.; Yamada, H.; Koga, K.; Ota, W.; Furuta, T.; Yamatsu, A.; Kim, M. Free Linoleic Acid and Oleic Acid Reduce Fat Digestion and Absorption In Vivo as Potent Pancreatic Lipase Inhibitors Derived from Sesame Meal. Molecules 2022, 27, 4910. [Google Scholar] [CrossRef]
- Tsushima, Y.; Lansang, M.C.; Makin, V. The Role of SGLT-2 Inhibitors in Managing Type 2 Diabetes. Cleve Clin. J. Med. 2021, 88, 47–58. [Google Scholar] [CrossRef]
- Takahashi, M. Glycation of Proteins. In Glycoscience: Biology and Medicine; Springer: Tokyo, Japan, 2015; pp. 1339–1345. [Google Scholar]
- Liu, J.; Pan, S.; Wang, X.; Liu, Z.; Zhang, Y. Role of Advanced Glycation End Products in Diabetic Vascular Injury: Molecular Mechanisms and Therapeutic Perspectives. Eur. J. Med. Res. 2023, 28, 553. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research. Profiles Drug Subst. Excip. Relat Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, D.K.; Moon, J.Y.; Lee, M.-Y.; Cho, S.K. Oleic Acid Inhibits the Migration and Invasion of Breast Cancer Cells with Stemness Characteristics through Oxidative Stress-Mediated Attenuation of the FAK/AKT/NF-ΚB Pathway. J. Funct. Foods 2024, 116, 106224. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic Acid, the Main Monounsaturated Fatty Acid of Olive Oil, Suppresses Her-2/Neu (ErbB-2) Expression and Synergistically Enhances the Growth Inhibitory Effects of Trastuzumab (HerceptinTM) in Breast Cancer Cells with Her-2/Neu Oncogene Amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef]
- Arruzazabala, M.d.L.; Molina, V.; Más, R.; Carbajal, D.; Marrero, D.; González, V.; Rodríguez, E. Effects of Coconut Oil on Testosterone-Induced Prostatic Hyperplasia in Sprague-Dawley Rats. J. Pharm. and Pharmacol. 2007, 59, 995–999. [Google Scholar] [CrossRef]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Mar-tínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
- Preeti, R.; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, T.; Jain, K.; Gu, S.X.; Qiu, M.; Gu, V.W.; Melchinger, H.; Rinder, H.; Martin, K.A.; Gardiner, E.E.; Lee, A.I.; et al. A Guide to Molecular and Functional Investigations of Platelets to Bridge Basic and Clinical Sciences. Nat. Cardiovasc. Res. 2022, 1, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Bernier, A.; Tobias, T.; Nguyen, H.; Kumar, S.; Tuga, B.; Imtiaz, Y.; Smith, C.W.; Sunasee, R.; Ckless, K. Vascular and Blood Compatibility of Engineered Cationic Cellulose Nanocrystals in Cell-Based Assays. Nanomaterials 2021, 11, 2072. [Google Scholar] [CrossRef]
- Yedgar, S.; Barshtein, G.; Gural, A. Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility. Micromachines 2022, 13, 2091. [Google Scholar] [CrossRef]
- Abassi, D. A Review of Nanoparticle Hemocompatibility: Exploring Cell Nanoparticle Interactions and Effects on Hemostasis. J. AIDS Clin. Res. 2024, 15, 1–2. [Google Scholar]
- AOAC. AOAC 921.08-1921—Index of Refraction of Oils and Fats. AOAC Official Method; AOAC: Rockville, MD, USA, 2024. [Google Scholar]
- Brazil Brazilian Pharmacopoeia, 6th ed.; ANVISA: Brazilia, Brasil, 2019; Volume 1.
- BP. British Pharmacopoeia (BP), 2024th ed.; Her Majesty Stationary Office, Ed.; The Stationery Office: Norwich, UK, 2024; Volume 1. [Google Scholar]
- Rodriguez-Amaya, D.B. Food Carotenoids; Wiley: Hoboken, NJ, USA, 2015; ISBN 9781118733301. [Google Scholar]
- Igne, B.; Bondi, R.W.; Airiau, C. Multivariate Data Analysis for Enhancing Process Understanding, Monitoring, and Control—Active Pharmaceutical Ingredient Manufacturing Case Studies. In Multivariate Analysis in the Pharmaceutical Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 185–210. [Google Scholar]
- de Araújo, R.L.; de Pinho, C.L.C.; Farias, F.O.; Zanzarini, I.d.S.; Moure, V.R.; Valdameri, G.; Igarashi-Mafra, L.; Mafra, M.R. Anti-Diabetic, Antiglycant, Antioxidant, and Anticancer Activities of Crinum americanum L. Leaves Lipid Fraction. Ind. Crops Prod. 2024, 209, 118012. [Google Scholar] [CrossRef]
| Characteristic | Value (n = 3) |
|---|---|
| Relative density (30 °C) | 0.9000 ± 0.0001 |
| Iodine index (g of I2/100 g) | 74.50 ± 1.50 |
| Refractive index (30 °C) | 1.456 ± 0.001 |
| Peroxide index (mEq/Kg) | 4.50 ± 0.40 |
| Saponification index (mg KOH/g) | 133.00 ± 4.50 |
| Acidity (mg/100 g) | 0.92 ± 0.10 |
| Total carotenoids (mg/100 g) | 0.27 ± 0.01 |
| Polyphenols (mg/100 g) | 12.60 ± 0.30 |
| Fatty Acids | Chain | Rt | Ri | Content (%) |
|---|---|---|---|---|
| Saturated | ||||
| Hexanoic acid (caproic) | C6:0 | 5.15 | 974 | 0.22 ± 0.02 |
| Octanoic acid (caprylic) | C8:0 | 7.26 | 1169 | 0.25 ± 0.02 |
| Decanoic acid (capric) | C10:0 | 11.55 | 1365 | 0.13 ± 0.01 |
| Dodecanoic acid (lauric) | C12:0 | 15.95 | 1548 | 0.85 ± 0.01 |
| Tetradecanoic acid (myristic) | C14:0 | 21.49 | 1747 | 0.70 ± 0.01 |
| Hexadecanoic acid (palmitic) | C16:0 | 27.86 | 1970 | 16.52 ± 0.15 |
| Octadecanoic acid (stearic) | C18:0 | 33.28 | 2164 | 4.11 ± 0.15 |
| Eicosanoic acid (arachidic) | C20:0 | 35.37 | 2369 | 0.20 ± 0.03 |
| Docosanoic acid (behenic) | C22:0 | 41.58 | 2562 | 0.06 ± 0.03 |
| Subtotal | 23.04 | |||
| Monounsaturated | ||||
| δ-9-cis-hexadecenoico acid (palmitoleic) | C16:1 | 25.36 | 1939 | 2.54 ± 0.01 |
| cis-9-octadecenoic acid (oleic) | C18:1 | 30.75 | 2241 | 71.25 ± 2.21 |
| Subtotal | 73.79 | |||
| Polyunsaturated | ||||
| cis-9,12-octadecadienoic acid (linoleic) | C18:2 | 32.46 | 2154 | 0.80 ± 0.04 |
| cis-octadeca-9,12,15-trienoic acid (linolenic) | C18:3 | 31.05 | 2176 | 2.20 ± 0.33 |
| Subtotal | 3.00 | |||
| Total fatty acids | 99.83 |
| Run | Factors | Responses | ||||
|---|---|---|---|---|---|---|
| Potency | Time | 24 h | 72 h | |||
| (Watt) | (min.) | Size(nm) | PdI | Size (nm) | PdI | |
| 1 | 8 | 10 | 111.10 ± 0.56 | 0.245 | 374.50 ± 3.23 | 0.386 |
| 2 | 6 | 15 | 121.60 ± 1.27 | 0.235 | - | - |
| 3 | 7 | 15 | 98.31 ± 0.60 | 0.240 | - | - |
| 4 | 5 | 5 | 148.10 ± 0.21 | 0.252 | 428.70 ± 2.58 | 0.345 |
| 5 | 7 | 10 | 119.70 ± 1.24 | 0.244 | 327.10 ± 9.28 | 0.437 |
| 6 | 7 | 10 | 118.10 ± 0.21 | 0.251 | 200.40 ± 11.85 | 0.382 |
| 7 | 8 | 5 | 95.73 ± 1.25 | 0.249 | - | - |
| 8 | 6 | 5 | 145.40 ± 0.07 | 0.217 | 175.50 ± 21.47 | 0.308 |
| 9 | 6 | 15 | 108.70 ± 0.14 | 0.246 | - | - |
| 10 | 7 | 10 | 119.30 ± 0.77 | 0.248 | - | - |
| 11 | 6 | 10 | 125.80 ± 0.56 | 0.248 | 187.10 ± 8.27 | 0.348 |
| 12 | 7 | 5 | 136.80 ± 1.26 | 0.257 | 198.40 ± 8.47 | 0.357 |
| 13 | 7 | 10 | 121.3 ± 0.00 | 0.272 | 171.5 ± 2.58 | 0.472 |
| 14 | 8 | 15 | 136.40 ± 2.4 | 0.235 | 139.40 ± 1.75 | 0.207 |
| 15 | 7 | 15 | 103.30 ± 2.05 | 0.254 | - | - |
| Tested Substances | α-Glucosidase IC50(µg/mL) | Pancreatic Lipase IC50 (µg/mL) |
|---|---|---|
| Blank nanoemulsion | Does not inhibit | Does not inhibit |
| Bocaiuva oil | >250 | >250 |
| Nanoemulsion | 43.21 ± 2.15 a | 41.99 ± 3.48 a |
| Acarbose® | 63.08 ± 3.25 b | Not tested |
| Orlistat® | Not tested | 0.24 ± 0.04 b |
| Substances | Oxidative Pathway | Non-Oxidative Pathway | ||
|---|---|---|---|---|
| Inhibition (%) (at 100 μg/mL) | IC50 (μg/mL) | Inhibition (%) (at 100 μg/mL) | IC50 (μg/mL) | |
| Blank nanoemulsion | No inhibition | No inhibition | Not tested | |
| Nanoemulsion | 42.08 ± 2.73 a | 18.36 ± 2.11 a | 38.29 ± 4.18 a | 16.33 ± 2.85 a |
| Bocaiuva oil | 92.41 ± 3.53 b | 57.33 ± 4.70 b | >250 | Not tested |
| Quercetin | 75.36 ± 3.54 c | 42.37 ± 3.74 c | Not tested | Not tested |
| Aminoguanidine | Not tested | Not tested | 74.50 ± 3.37 b | 37.34 ± 1.71 b |
| F-test, p-value | 168.21, 0.0000 | 62.34, 0.0000 | 351.25, 0.0000 | 28.45, 0.0000 |
| Cell Line | Doxorubicin IC50 (μg/mL) | Bocaiuva Oil | Nanoemulsion | ||
|---|---|---|---|---|---|
| IC50 (μg/mL) | Selectivity Index | IC50 (μg/mL) | Selectivity Index | ||
| HFF1 | 2.45 ± 0.03 a | >250 | - | >250 b | - |
| NIH/3T3 | 3.89 ± 0.41 a | >250 | - | >250 b | - |
| MDA-MB 231 | 1.51 ± 0.03 a | >250 | - | 27.22 ± 0.03 b | 9.18 b |
| MCF-7 | 0.19 ± 0.01 a | >250 | - | 26.99 ± 0.03 b | 9.26 b |
| PC-03 | 0.28 ± 0.11 a | 66.25 | 3.77 | 19.13 ± 0.03 b | 13.07 a |
| 786-0 | 0.26 ± 0.12 a | >250 | - | 82.29 ± 0.03 b | 3.04 c |
| HepG2 | 0.25 ± 0.11 a | >250 | - | 212.32 ± 0.03 b | 1.18 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafourcade Prada, A.; Rodríguez Amado, J.R.; Trentin Perdomo, R.; Gomes, G.B.; Tavares de Almeida, D.A.; Cavalheiro, L.F.; Gasparotto Junior, A.; Florentino Neto, S.; Utrera Martines, M.A. Acrocomia aculeata Oil-Loaded Nanoemulsion: A Promising Candidate for Cancer and Diabetes Management. Pharmaceuticals 2025, 18, 1094. https://doi.org/10.3390/ph18081094
Lafourcade Prada A, Rodríguez Amado JR, Trentin Perdomo R, Gomes GB, Tavares de Almeida DA, Cavalheiro LF, Gasparotto Junior A, Florentino Neto S, Utrera Martines MA. Acrocomia aculeata Oil-Loaded Nanoemulsion: A Promising Candidate for Cancer and Diabetes Management. Pharmaceuticals. 2025; 18(8):1094. https://doi.org/10.3390/ph18081094
Chicago/Turabian StyleLafourcade Prada, Ariadna, Jesus Rafael Rodríguez Amado, Renata Trentin Perdomo, Giovanna Bicudo Gomes, Danielle Ayr Tavares de Almeida, Leandro Fontoura Cavalheiro, Arquimedes Gasparotto Junior, Serafim Florentino Neto, and Marco Antonio Utrera Martines. 2025. "Acrocomia aculeata Oil-Loaded Nanoemulsion: A Promising Candidate for Cancer and Diabetes Management" Pharmaceuticals 18, no. 8: 1094. https://doi.org/10.3390/ph18081094
APA StyleLafourcade Prada, A., Rodríguez Amado, J. R., Trentin Perdomo, R., Gomes, G. B., Tavares de Almeida, D. A., Cavalheiro, L. F., Gasparotto Junior, A., Florentino Neto, S., & Utrera Martines, M. A. (2025). Acrocomia aculeata Oil-Loaded Nanoemulsion: A Promising Candidate for Cancer and Diabetes Management. Pharmaceuticals, 18(8), 1094. https://doi.org/10.3390/ph18081094









