Causal Inference of Adverse Drug Events in Pulmonary Arterial Hypertension: A Pharmacovigilance Study
Abstract
1. Introduction
2. Results
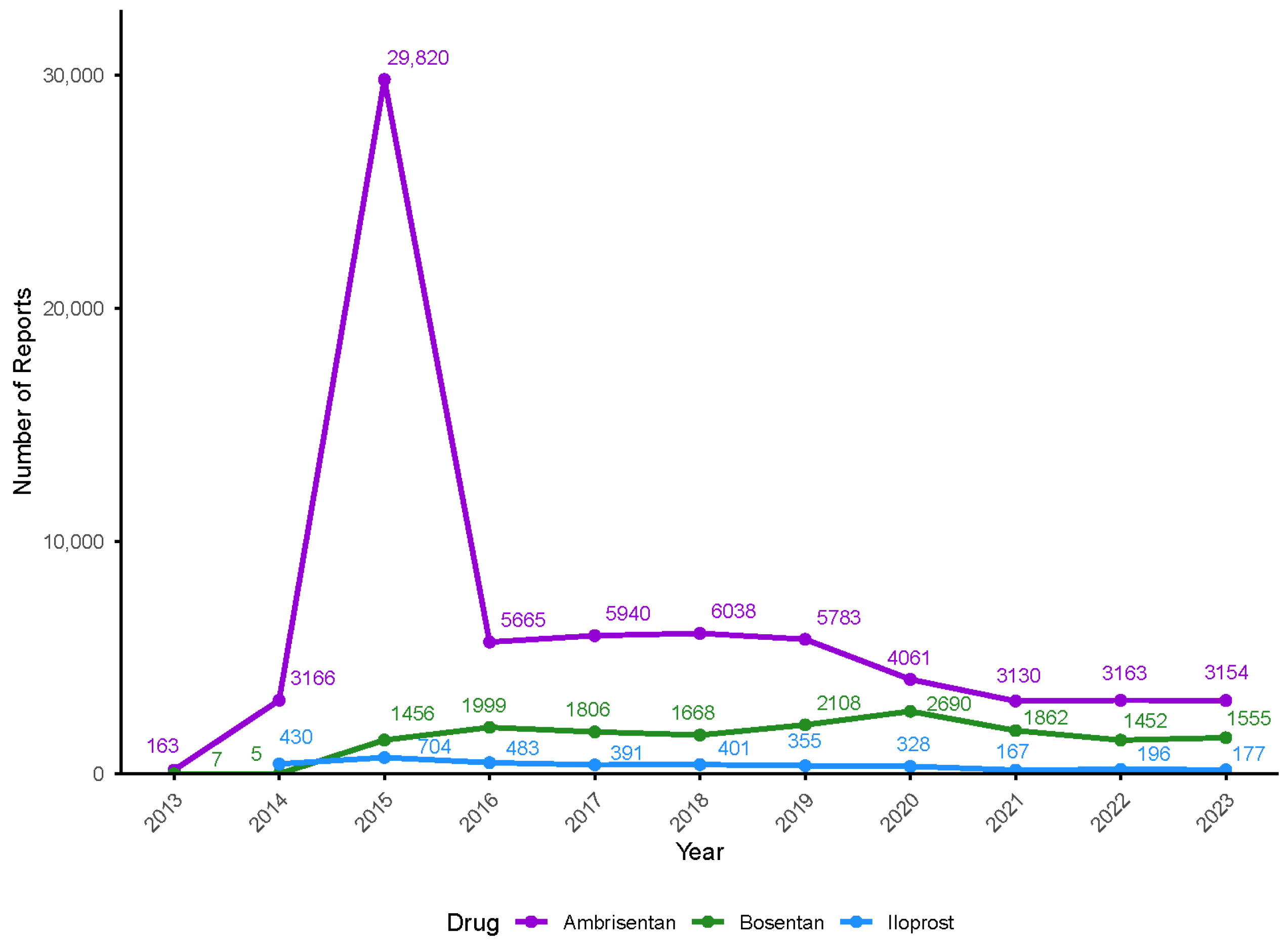
2.1. Basic Characteristics of Report
2.2. Drug Risk Signals
2.3. The Causal Relationship and Effects of ADEs
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Data Processing Procedure
4.3. Drug Signal Detection
4.4. Construction of Bayesian Causal Graph Model
- (1)
- Node X: Represents a random variable. Each node corresponds to a specific drug (Dᵢ), adverse drug event (ADE, Aᵢ), or confounder (eᵢ).
- (2)
- Directed edge E: Represents a causal relationship. The direction of the edge flows from the drug (Dᵢ) to the ADE (Aᵢ). For example, a directed edge from drug D1 to ADE A1 indicates that D1 is a potential cause of A1.
- (3)
- Three fundamental graphical structures: chain structure, fork structure, and V-structure.
- (1)
- L contains a chain structure D → e → A or a fork structure D ← e → A, where the intermediate node e is in the conditioning set Z;
- (2)
- L contains a V-structure A → e ← C, where the intermediate node e is not in Z, and none of its descendants are in Z.
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maron, B.A. Revised Definition of Pulmonary Hypertension and Approach to Management: A Clinical Primer. J. Am. Heart Assoc. 2023, 12, 8. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.; Humbert, M.; Saxena, A.; Jing, Z.C.; Sliwa, K.; Thienemann, F.; Archer, S.L.; Stewart, S. Pulmonary hypertension. Nat. Rev. Dis. Primers 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, Z.; Yang, Q.; Wang, H.; Zhang, H.; Yan, W.; Wang, P.; Wang, C.; Su, Z.; Thangaraju, P.; et al. The Challenge in Burden of Pulmonary Arterial Hypertension: A Perspective From the Global Burden of Disease Study. MedComm 2025, 6, e70175. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.R.; Elliot, C.G.; Levine, D.J.; Bossone, E.; Duvall, L.; Fagan, K.; Frantsve-Hawley, J.; Kawut, S.M.; Ryan, J.J.; Rosenzweig, E.B.; et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report (vol 155, pg 565, 2019). Chest 2021, 159, 457. [Google Scholar] [CrossRef]
- Chin, K.M.; Gaine, S.P.; Gerges, C.; Jing, Z.C.; Mathai, S.C.; Tamura, Y.; McLaughlin, V.V.; Sitbon, O. Treatment algorithm for pulmonary arterial hypertension. Eur. Respir. J. 2024, 64, 2401325. [Google Scholar] [CrossRef]
- Zolty, R. Advances in the discovery of drugs that treat pulmonary arterial hypertension. Expert. Opin. Drug Dis. 2023, 18, 445–466. [Google Scholar] [CrossRef]
- Tamargo, J.; Agewall, S.; Ambrosio, G.; Borghi, C.; Cerbai, E.; Dan, G.A.; Drexel, H.; Ferdinandy, P.; Grove, E.L.; Klingenberg, R.; et al. New pharmacological agents and novel cardiovascular pharmacotherapy strategies in 2024. Eur. Heart J.-Card. Pha 2025, 11, 292–317. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, N.; Gu, Z.C.; Wei, A.H.; Cheng, A.N.; Fang, S.S.; Du, H.L.; Wang, L.Z.; Zhang, G.Q. A network meta-analysis for safety of endothelin receptor antagonists in pulmonary arterial hypertension. Cardiovasc. Diagn. The 2019, 9, 239. [Google Scholar] [CrossRef]
- Weber, S.; Gerbes, A.L. Challenges and Future of Drug-Induced Liver Injury Research-Laboratory Tests. Int. J. Mol. Sci. 2022, 23, 6049. [Google Scholar] [CrossRef]
- Jeon, K.; Yoo, S.B.; Lee, Y.H.; Lee, E.B.; Kim, H.K.; Chang, H.J.; Chang, S.A. Safety and effectiveness of ambrisentan in real clinical practice in pulmonary arterial hypertension: Results from the Korean post-marketing surveillance. Pharmacoepidem Dr. S 2023, 32, 1387–1394. [Google Scholar] [CrossRef]
- Barnes, H.; Yeoh, H.L.; Fothergill, T.; Burns, A.; Humbert, M.; Williams, T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst. Rev. 2019, 5, CD012785. [Google Scholar] [CrossRef]
- Tang, F.J.; Ma, Q.H.; Liu, Y.H.; Yang, X.J. Risk of respiratory, thoracic, and mediastinal disorders associated with endothelin receptor antagonists and prostacyclin-related drugs in pulmonary hypertension: A disproportionality analysis based on FAERS. Expert. Opin. Drug Saf. 2025, 24, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.T.; Dong, B. Clinical adverse events to letairis: A real-world drug safety study based on FDA Adverse Event Reporting System (FAERS). Expert. Opin. Drug Saf. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Gu, J.J.; Guo, Y.T.; Wu, B.; He, J.H. Liver injury associated with endothelin receptor antagonists: A pharmacovigilance study based on FDA adverse event reporting system data. Int. J. Clin. Pharm-Net. 2024, 46, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.H.; He, W.J.; Li, Y.X.; Chen, Y.X.; Liang, J.Y.; Lei, H.; Fu, L.; Chen, Y.H.; Ren, N.; Jiang, Q.; et al. Efficacy and safety of novel-targeted drugs in the treatment of pulmonary arterial hypertension: A Bayesian network meta-analysis. Drug Deliv. 2021, 28, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Locatelli, I. Comparative effectiveness of pulmonary arterial hypertension drugs in treatment-naive patients: A network meta-analysis. J. Comp. Effect Res. 2020, 9, 7–22. [Google Scholar] [CrossRef]
- Wang, P.W.; Deng, J.X.; Zhang, Q.Y.; Feng, H.Y.; Zhang, Y.H.; Lu, Y.Z.; Han, L.Z.; Yang, P.F.; Deng, Z.J. Additional Use of Prostacyclin Analogs in Patients With Pulmonary Arterial Hypertension: A Meta-Analysis. Front. Pharmacol. 2022, 13, 817119. [Google Scholar] [CrossRef]
- Preston, I.R.; Burger, C.D.; Bartolome, S.; Safdar, Z.; Krowka, M.; Sood, N.; Ford, H.J.; Battarjee, W.F.; Chakinala, M.M.; Gomberg-Maitland, M.; et al. Ambrisentan in portopulmonary hypertension: A multicenter, open-label trial. J. Heart Lung Transpl. 2020, 39, 464–472. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhai, Z.G.; Huang, K.; Xie, W.M.; Wan, J.; Wang, C. Bosentan therapy for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: A systemic review and meta-analysis. Clin. Respir. J. 2018, 12, 2065–2074. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Guo, N.; Chen, J.; Parks, D.; Tian, Z. Comparative assessment of efficacy and safety of ambrisentan and bosentan in patients with pulmonary arterial hypertension: A meta-analysis. J. Clin. Pharm. Ther. 2022, 47, 146–156. [Google Scholar] [CrossRef]
- Weatherald, J.; Hemnes, A.R.; Maron, B.A.; Mielniczuk, L.M.; Gerges, C.; Price, L.C.; Hoeper, M.M.; Humbert, M. Phenotypes in pulmonary hypertension. Eur. Respir. J. 2024, 64, 2301633. [Google Scholar] [CrossRef]
- Wang, L.L.; Mao, Z.Y.; Zheng, P.D.; Zi, G.S.; Zhang, F.Q.; Zhu, X.Y.; Chen, L.X.; Liu, H.G.; Zhou, L.; Wei, S. Assessment of Riociguat-related adverse events: A disproportionality analysis utilizing the FDA adverse event reporting system database. Expert. Opin. Drug Saf. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Chai, S.J.; Xu, H.M.; Xu, G.C.; Cai, C.M. ORENITRAM’s decadal journey: Unveiling safety profiles and adverse event through a real-world pharmacovigilance study of FAERS events. Expert. Opin. Drug Saf. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Ahangaran, M.; Jahed-Motlagh, M.R.; Minaei-Bidgoli, B. A novel method for predicting the progression rate of ALS disease based on automatic generation of probabilistic causal chains. Artif. Intell. Med. 2020, 107, 101879. [Google Scholar] [CrossRef]
- Yin, Z.Q.; Zhan, Z.; Qiu, Y.J.; Wang, M.H.; Li, J.L.; Song, B.Y.; Chen, Z.Q.; Wu, J.; Wang, Z. Exploring the Relationship Between Antipsychotic Drug Target Genes and Epilepsy: Evidence From Food and Drug Administration Adverse Event Reporting System Database and Mendelian Randomization. Brain Behav. 2025, 15, e70467. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Li, W.C.; Ma, Y.P.; Ma, Z.F.; Wang, X.; Hu, C.Y. Drugs Associated with Urinary Retention Adverse Reactions: A Joint Analysis of FDA Adverse Event Reporting System and Mendelian Randomization. Urology 2024, 194, 99–104. [Google Scholar] [CrossRef]
- Wei, X.H.; Zhang, Y.L.; Wang, C. Bayesian Network Structure Learning Method Based on Causal Direction Graph for Protein Signaling Networks. Entropy 2022, 24, 1351. [Google Scholar] [CrossRef]
- Kalisch, M.; Bühlmann, P. Estimating high-dimensional directed acyclic graphs with the PC-algorithm. J. Mach. Learn. Res. 2007, 8, 613–636. [Google Scholar]


| Characteristics | Ambrisentan | Bosentan | Iloprost |
|---|---|---|---|
| (n = 70,083) | (n = 16,608) | (n = 3632) | |
| Sex (%) | |||
| Female | 52,328 (74.66) | 9562 (57.57) | 2385 (65.67) |
| Male | 16,839 (24.03) | 3399 (20.47) | 1074 (29.57) |
| Missing | 916 (1.31) | 3647 (21.96) | 173 (4.76) |
| Age (years, %) | |||
| ≤17 | 1492 (2.13) | 1623 (9.77) | 71 (1.95) |
| 18~64 | 31,361 (44.75) | 3956 (23.82) | 1517 (41.77) |
| 65~85 | 26,877 (38.35) | 4113 (24.77) | 1221 (33.62) |
| ≥86 | 1802 (2.57) | 509 (3.06) | 58 (1.60) |
| Missing | 8551 (12.20) | 6407 (38.58) | 765 (21.06) |
| Occupation (%) | |||
| Consumer | 42,081 (60.04) | 3857 (23.22) | 234 (6.44) |
| Health-professional | 21,703(30.97) | 10,765(64.82) | 2829(77.89) |
| Other | 4665 (6.66) | 1946 (11.72) | 564 (15.53) |
| Missing | 1634 (2.33) | 40 (0.24) | 5 (0.14) |
| Outcome (%) | n = 93004 | n = 19881 | n = 4782 |
| DE | 8788 (9.45) | 3680 (18.51) | 1840 (38.48) |
| LT | 354 (0.38) | 200 (1.01) | 64 (1.34) |
| HO | 26,569 (28.57) | 7428 (37.36) | 1674 (35.01) |
| DS | 350 (0.38) | 176 (0.89) | 32 (0.67) |
| CA | 19 (0.02) | 18 (0.09) | 2 (0.04) |
| RI | 26 (0.03) | 3 (0.01) | 0(0.00) |
| OT | 29,361 (31.57) | 3292 (16.56) | 924 (19.32) |
| Missing | 27,537 (29.60) | 5084 (25.57) | 246 (5.14) |
| Drug | ADE | a | b | c | d | PRR | |
|---|---|---|---|---|---|---|---|
| Ambrisentan | |||||||
| Dyspnea | 10,591 | 165,188 | 369,466 | 41,212,066 | 6.78 | 51,209.29 | |
| Death | 6538 | 169,241 | 595,980 | 40,985,552 | 2.60 | 6433.15 | |
| Pneumonia | 4113 | 171,666 | 227,925 | 41,353,607 | 4.27 | 10,168.89 | |
| Headache | 3930 | 171,849 | 428,461 | 41,153,071 | 2.17 | 2481.63 | |
| Dizziness | 3177 | 172,602 | 324,768 | 41,256,764 | 2.31 | 2366.34 | |
| Fluid retention | 3097 | 172,682 | 33,975 | 41,547,557 | 21.56 | 55,707.13 | |
| Malaise | 2823 | 172,956 | 317,678 | 41,263,854 | 2.10 | 1629.35 | |
| Peripheral swelling | 2624 | 173,155 | 124,156 | 41,457,376 | 5.00 | 8246.90 | |
| Edema peripheral | 2292 | 173,487 | 59,088 | 41,522,444 | 9.18 | 16,097.13 | |
| Edema | 2180 | 173,599 | 31,858 | 41,549,674 | 16.19 | 29,097.02 | |
| Bosentan | |||||||
| Death | 2142 | 56,143 | 600,376 | 41,098,650 | 2.55 | 2044.94 | |
| Dyspnea | 2097 | 56,188 | 377,960 | 41,321,066 | 3.97 | 4674.91 | |
| Product dose omission issue | 1231 | 57,054 | 374,305 | 41,324,721 | 2.35 | 963.12 | |
| Pneumonia | 930 | 57,355 | 231,108 | 41,467,918 | 2.88 | 1142.25 | |
| Hospitalization | 761 | 57,524 | 109,489 | 41,589,537 | 4.97 | 2404.86 | |
| Cough | 613 | 57,672 | 194,764 | 41,504,262 | 2.25 | 427.22 | |
| Pulmonary arterial hypertension | 606 | 57,679 | 10,949 | 41,688,077 | 39.60 | 21,609.63 | |
| Condition aggravated | 570 | 57,715 | 203,730 | 41,495,296 | 2.00 | 286.31 | |
| Chest pain | 478 | 57,807 | 112,108 | 41,586,918 | 3.05 | 657.78 | |
| Fluid retention | 463 | 57,822 | 36,609 | 41,662,417 | 9.05 | 3276.00 | |
| Iloprost | |||||||
| Death | 1194 | 11,878 | 601,324 | 41,142,915 | 6.34 | 5439.16 | |
| Dyspnea | 455 | 12,617 | 379,602 | 41,364,637 | 3.83 | 958.05 | |
| Hospitalization | 208 | 12,864 | 110,042 | 41,634,197 | 6.04 | 874.64 | |
| Cough | 194 | 12,878 | 195,183 | 41,549,056 | 3.17 | 289.96 | |
| Pulmonary arterial hypertension | 368 | 25,776 | 24,544 | 83,463,934 | 47.88 | 16,647.08 | |
| Pneumonia | 151 | 12,921 | 231,887 | 41,512,352 | 2.08 | 85.03 | |
| Product use issue | 140 | 12,932 | 150,565 | 41,593,674 | 2.97 | 183.35 | |
| Inappropriate schedule of product administration | 139 | 12,933 | 184,355 | 41,559,884 | 2.41 | 114.83 | |
| Fluid retention | 129 | 12,943 | 36,943 | 41,707,296 | 11.15 | 1188.95 | |
| Chest pain | 122 | 12,950 | 112,464 | 41,631,775 | 3.46 | 214.19 |
| Drug | ADE | Conditional Set | ACE |
|---|---|---|---|
| Iloprost | Hyperthyroidism | None | 0.048 |
| Ambrisentan | Peripheral swelling | Age, Iloprost | 0.032 |
| Ambrisentan | Anemia | None | 0.021 |
| Bosentan | Therapy change | None | 0.007 |
| Ambrisentan | Blood bilirubin increased | None | −0.005 |
| Ambrisentan | Gamma-glutamyltransferase increased | None | −0.013 |
| Ambrisentan | Aspartate aminotransferase increased | None | −0.016 |
| Ambrisentan | Alanine aminotransferase increased | None | −0.018 |
| Ambrisentan | Hospitalization | None | −0.021 |
| Ambrisentan | Disease progression | None | −0.023 |
| Ambrisentan | Hepatic function abnormal | None | −0.047 |
| Iloprost | Ambrisentan | None | −0.773 |
| Bosentan | Ambrisentan | None | −0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; He, X.; Chen, C.; Ni, Q.; Ni, L.; Zhou, J.; Peng, B. Causal Inference of Adverse Drug Events in Pulmonary Arterial Hypertension: A Pharmacovigilance Study. Pharmaceuticals 2025, 18, 1084. https://doi.org/10.3390/ph18081084
Li H, He X, Chen C, Ni Q, Ni L, Zhou J, Peng B. Causal Inference of Adverse Drug Events in Pulmonary Arterial Hypertension: A Pharmacovigilance Study. Pharmaceuticals. 2025; 18(8):1084. https://doi.org/10.3390/ph18081084
Chicago/Turabian StyleLi, Hongmei, Xiaojun He, Cui Chen, Qiao Ni, Linghao Ni, Jiawei Zhou, and Bin Peng. 2025. "Causal Inference of Adverse Drug Events in Pulmonary Arterial Hypertension: A Pharmacovigilance Study" Pharmaceuticals 18, no. 8: 1084. https://doi.org/10.3390/ph18081084
APA StyleLi, H., He, X., Chen, C., Ni, Q., Ni, L., Zhou, J., & Peng, B. (2025). Causal Inference of Adverse Drug Events in Pulmonary Arterial Hypertension: A Pharmacovigilance Study. Pharmaceuticals, 18(8), 1084. https://doi.org/10.3390/ph18081084





