Auricularia auricula’s Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin–1-Mediated Immunomodulation and Microbiota Remodeling
Abstract
1. Introduction
2. Results and Discussion
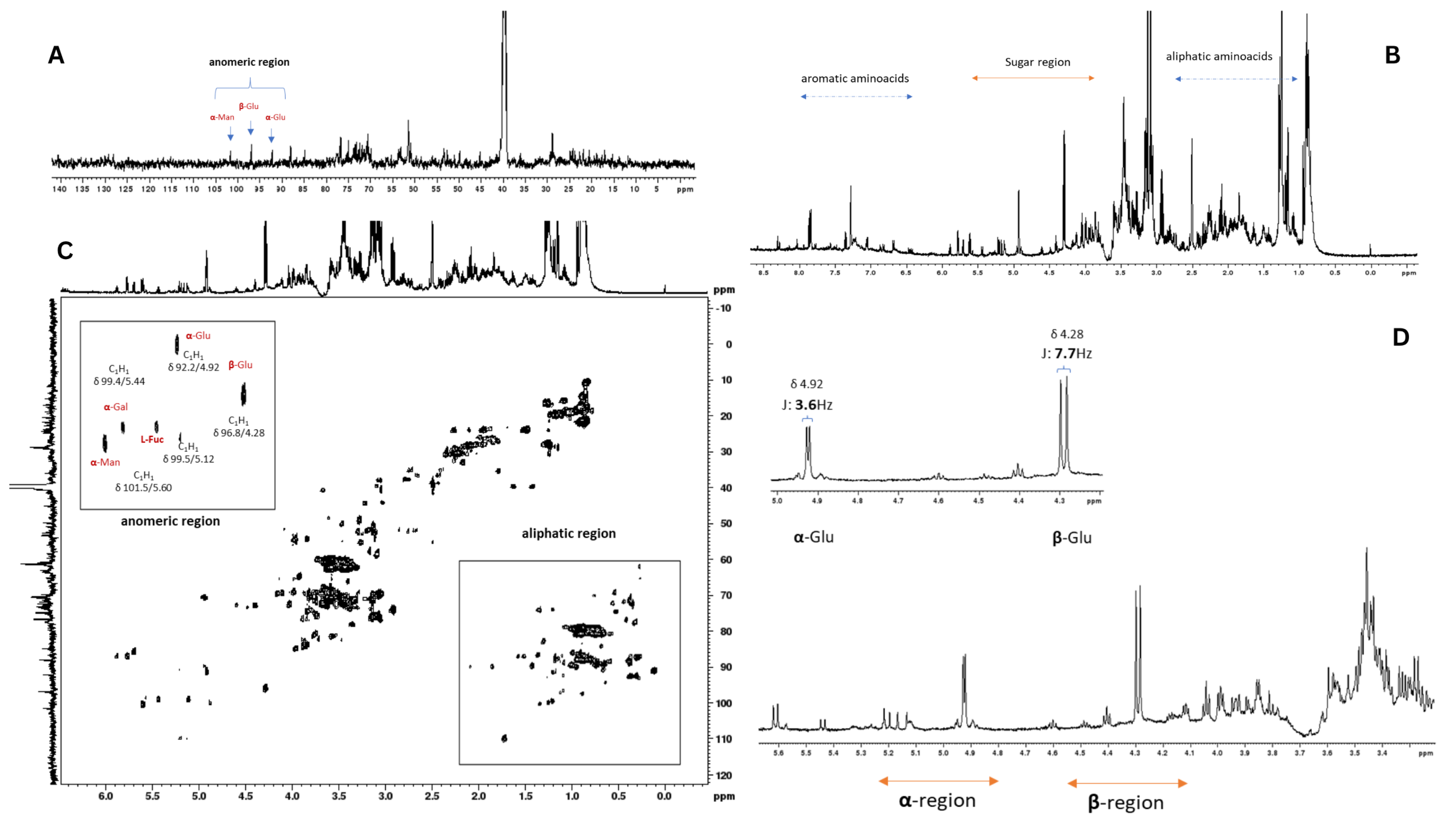
2.1. NMR Analysis of Auricularia auricula Polysaccharides
2.2. A. auricula Polysaccharides’ Activity in the Murine Colitis Model
2.3. Cytokine Production
2.4. Beta Diversity Results
2.5. Alpha Diversity Results
2.6. Differences in Taxonomic Groups
3. Materials and Methods
3.1. Cultivation of Auricularia auricula and Polysaccharide Fraction
3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.3. Colitis Model Experiment
3.4. Cytokine Quantification
3.5. Histopathological Analysis
3.6. 16S rRNA Gene Sequencing and Taxonomic Assignment
3.7. Diversity Analysis
3.8. α-Diversity Statistics
3.9. β-Diversity Statistics
3.10. ASV-Level Modeling
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhu, K.; Zeng, S.; Zheng, Y.; Cao, J.; Li, C. Microbiome–Metabolomics Analysis Reveals the Mechanism of Holothuria Leucospilota Polysaccharides (HLP) in Ulcerative Colitis. Mol. Nutr. Food Res. 2023, 67, 2200633. [Google Scholar] [CrossRef]
- Kumareswaran, A.; Ekeuku, S.O.; Mohamed, N.; Muhammad, N.; Hanafiah, A.; Pang, K.-L.; Wong, S.K.; Chew, D.C.H.; Chin, K.-Y. The Effects of Tocotrienol on Gut Microbiota: A Scoping Review. Life 2023, 13, 1882. [Google Scholar] [CrossRef]
- Törős, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus Ostreatus Mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The Impacts of Natural Polysaccharides on Intestinal Microbiota and Immune Responses—A Review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Zhao, Q.; Patrick Manzi, H.; Su, L.; Liu, D.; Huang, X.; Long, D.; Tang, Z.; Zhang, Y. The Benefits of Edible Mushroom Polysaccharides for Health and Their Influence on Gut Microbiota: A Review. Front. Nutr. 2023, 10, 1213010. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Sharma, D.; Malireddi, R.S.; Guy, C.S.; Chang, T.-C.; Olsen, S.R.; Neale, G.; Vogel, P.; Kanneganti, T.-D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity 2018, 49, 515–530.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ji, Y.; Chen, N.; Dai, L.; Deng, H. Colitis-Associated Carcinogenesis: Crosstalk between Tumors, Immune Cells and Gut Microbiota. Cell Biosci. 2023, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Mouzan, M.I.E.; Korolev, K.S.; Mofarreh, M.A.A.; Menon, R.; Winter, H.S.; Sarkhy, A.A.A.; Dowd, S.E.; Barrag, A.M.A.; Assiri, A.A. Fungal Dysbiosis Predicts the Diagnosis of Pediatric Crohn’s Disease. World J. Gastroenterol. 2018, 24, 4510–4516. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between Intestinal Microbiota and Ulcerative Colitis: Mechanisms and Clinical Application of Probiotics and Fecal Microbiota Transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Sommer, F.; Rühlemann, M.C.; Bang, C.; Höppner, M.; Rehman, A.; Kaleta, C.; Schmitt-Kopplin, P.; Dempfle, A.; Weidinger, S.; Ellinghaus, E.; et al. Microbiomarkers in Inflammatory Bowel Diseases: Caveats Come with Caviar. Gut 2017, 66, 1734–1738. [Google Scholar] [CrossRef]
- Tang, C.; Kamiya, T.; Liu, Y.; Kadoki, M.; Kakuta, S.; Oshima, K.; Hattori, M.; Takeshita, K.; Kanai, T.; Saijo, S.; et al. Inhibition of Dectin-1 Signaling Ameliorates Colitis by Inducing Lactobacillus-Mediated Regulatory T Cell Expansion in the Intestine. Cell Host Microbe 2015, 18, 183–197. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions Between Commensal Fungi and the C-Type Lectin Receptor Dectin-1 Influence Colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Yue, B.; Luo, X.; Yu, Z.; Mani, S.; Wang, Z.; Dou, W. Inflammatory Bowel Disease: A Potential Result from the Collusion between Gut Microbiota and Mucosal Immune System. Microorganisms 2019, 7, 440. [Google Scholar] [CrossRef]
- Wang, Y.; Spatz, M.; Da Costa, G.; Michaudel, C.; Lapiere, A.; Danne, C.; Agus, A.; Michel, M.-L.; Netea, M.G.; Langella, P.; et al. Deletion of Both Dectin-1 and Dectin-2 Affects the Bacterial but Not Fungal Gut Microbiota and Susceptibility to Colitis in Mice. Microbiome 2022, 10, 91. [Google Scholar] [CrossRef]
- Limon, J.J.; Tang, J.; Li, D.; Wolf, A.J.; Michelsen, K.S.; Funari, V.; Gargus, M.; Nguyen, C.; Sharma, P.; Maymi, V.I.; et al. Malassezia Is Associated with Crohn’s Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe 2019, 25, 377–388.e6. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.; Gil Castro, A.; Martins, A.C.; Carriche, G.M.; Murigneux, V.; Castro, I.; Cumano, A.; Vieira, P.; Saraiva, M. The Dynamics of Interleukin-10-Afforded Protection during Dextran Sulfate Sodium-Induced Colitis. Front. Immunol. 2018, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Cao, Q.; Ye, L.; Wang, J.; Guo, M. The Function of Natural Polysaccharides in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 927855. [Google Scholar] [CrossRef]
- Ning, K.; Duan, Y.; Tong, W.; Chen, Y.; Zhang, Q.; Xie, Q.; Xiang, H. Protective Effects of Different Molecular Weights of Purslane (Portulaca oleracea L.) Aqueous Extract on DSS-Induced Ulcerative Colitis in Mice. Antioxidants 2023, 12, 1400. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Ren, G.; Chen, X.; Cai, H.; Hong, J.; Kan, J.; Jin, C.; Niu, F.; Zhang, W. Impact of Purple Sweet Potato (Ipomoea batatas L.) Polysaccharides on the Fecal Metabolome in a Murine Colitis Model. RSC Adv. 2022, 12, 11376–11390. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.-C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome—A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Temel, H.Y.; Kaymak, Ö.; Kaplan, S.; Bahcivanci, B.; Gkoutos, G.V.; Acharjee, A. Role of Microbiota and Microbiota-derived Short-chain Fatty Acids in PDAC. Cancer Med. 2023, 12, 5661–5675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, C.; Liu, Y.; Li, B.; Shao, M.; Zhao, W.; Zhou, C. The Role of Polysaccharides in Immune Regulation through Gut Microbiota: Mechanisms and Implications. Front. Immunol. 2025, 16, 1555414. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.; Parkin, K.; Martino, D. The Potential Effects of Short-Chain Fatty Acids on the Epigenetic Regulation of Innate Immune Memory. Challenges 2020, 11, 25. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Dong, N.; Fang, Q.; Zhang, Y.; Chen, C.; Cui, S.W.; Nie, S. Polysaccharides from Natural Cordyceps Sinensis Attenuated Dextran Sodium Sulfate-Induced Colitis in C57BL/6J Mice. Food Funct. 2023, 14, 720–733. [Google Scholar] [CrossRef]
- Ma, M.; Fu, T.; Wang, Y.; Zhang, A.; Gao, P.; Shang, Q.; Yu, G. Polysaccharide from Edible Alga Enteromorpha clathrata Improves Ulcerative Colitis in Association with Increased Abundance of Parabacteroides Spp. in the Gut Microbiota of Dextran Sulfate Sodium-Fed Mice. Mar. Drugs 2022, 20, 764. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, G.; Cao, H.; Yu, D.; Fang, X.; Vos, W.M.; Wu, H. Gut Dysbacteriosis and Intestinal Disease: Mechanism and Treatment. J. Appl. Microbiol. 2020, 129, 787–805. [Google Scholar] [CrossRef]
- Blackman, A.C.; Thapa, S.; Venkatachalam, A.; Horvath, T.D.; Runge, J.K.; Haidacher, S.J.; Hoch, K.M.; Haag, A.M.; Luna, R.A.; Anagnostou, A. Insights into Microbiome and Metabolic Signatures of Children Undergoing Peanut Oral Immunotherapy. Children 2022, 9, 1192. [Google Scholar] [CrossRef]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Manolis, A.S. Gut Microbiota and Cardiovascular Disease: Symbiosis Versus Dysbiosis. Curr. Med. Chem. 2022, 29, 4050–4077. [Google Scholar] [CrossRef]
- Colombo, A.V.; Sadler, R.K.; Llovera, G.; Singh, V.; Roth, S.; Heindl, S.; Sebastian Monasor, L.; Verhoeven, A.; Peters, F.; Parhizkar, S.; et al. Microbiota-Derived Short Chain Fatty Acids Modulate Microglia and Promote Aβ Plaque Deposition. eLife 2021, 10, e59826. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.M.M.; De Castro, R.J.A.; De Castro, T.B.; Guimarães, H.I.; Polez, V.L.P.; Carbonero, E.R.; Pomin, V.H.; Hoffmann, C.; Grossi-de-Sa, M.F.; Tavares, A.H.; et al. Immunomodulatory Activity of β-Glucan-Containing Exopolysaccharides from Auricularia auricular in Phagocytes and Mice Infected with Cryptococcus neoformans. Med. Mycol. 2020, 58, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N. NMR Analysis of Compositional Heterogeneity in Polysaccharides. Pure Appl. Chem. 2017, 89, 877–883. [Google Scholar] [CrossRef]
- Merkx, D.W.H.; Westphal, Y.; Van Velzen, E.J.J.; Thakoer, K.V.; De Roo, N.; Van Duynhoven, J.P.M. Quantification of Food Polysaccharide Mixtures by 1H NMR. Carbohydr. Polym. 2018, 179, 379–385. [Google Scholar] [CrossRef]
- Speciale, I.; Notaro, A.; Garcia-Vello, P.; Di Lorenzo, F.; Armiento, S.; Molinaro, A.; Marchetti, R.; Silipo, A.; De Castro, C. Liquid-State NMR Spectroscopy for Complex Carbohydrate Structural Analysis: A Hitchhiker’s Guide. Carbohydr. Polym. 2022, 277, 118885. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran Sodium Sulfate Colitis Murine Model: An Indispensable Tool for Advancing Our Understanding of Inflammatory Bowel Diseases Pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Ruder, B.; Atreya, R.; Becker, C. Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Disease. Int. J. Mol. Sci. 2019, 20, 1887. [Google Scholar] [CrossRef]
- Ninnemann, J.; Winsauer, C.; Bondareva, M.; Kühl, A.A.; Lozza, L.; Durek, P.; Lissner, D.; Siegmund, B.; Kaufmann, S.H.E.; Mashreghi, M.-F.; et al. TNF Hampers Intestinal Tissue Repair in Colitis by Restricting IL-22 Bioavailability. Mucosal Immunol. 2022, 15, 698–716. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Aljamaei, H.M.; Stadnyk, A.W. The Production and Function of Endogenous Interleukin-10 in Intestinal Epithelial Cells and Gut Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1343–1352. [Google Scholar] [CrossRef]
- Gunasekera, D.C.; Ma, J.; Vacharathit, V.; Shah, P.; Ramakrishnan, A.; Uprety, P.; Shen, Z.; Sheh, A.; Brayton, C.F.; Whary, M.T.; et al. The Development of Colitis in Il10 Mice Is Dependent on IL-22. Mucosal Immunol. 2020, 13, 493–506. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote Th1 Cell IL-10 Production to Maintain Intestinal Homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.L.; Crossen, A.J.; Negoro, P.E.; Harding, H.B.; Ward, R.A.; Vargas-Blanco, D.A.; Timmer, K.D.; Reardon, C.M.; Basham, K.J.; Mansour, M.K.; et al. The C-Type Lectin Receptor Dectin-2 Is a Receptor for Aspergillus fumigatus Galactomannan. mBio 2023, 14, e03184-22. [Google Scholar] [CrossRef] [PubMed]
- Elcombe, S.E.; Naqvi, S.; Van Den Bosch, M.W.M.; MacKenzie, K.F.; Cianfanelli, F.; Brown, G.D.; Arthur, J.S.C. Dectin-1 Regulates IL-10 Production via a MSK1/2 and CREB Dependent Pathway and Promotes the Induction of Regulatory Macrophage Markers. PLoS ONE 2013, 8, e60086. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Verma, R.; Byun, S.; Jeun, E.-J.; Kim, G.-C.; Lee, S.; Kang, H.-J.; Kim, C.J.; Sharma, G.; Lahiri, A.; et al. Structural Specificities of Cell Surface β-Glucan Polysaccharides Determine Commensal Yeast Mediated Immuno-Modulatory Activities. Nat. Commun. 2021, 12, 3611. [Google Scholar] [CrossRef]
- Gold, A.; Zhu, J. Not Just a Gut Feeling: A Deep Exploration of Functional Bacterial Metabolites That Can Modulate Host Health. Gut Microbes 2022, 14, 2125734. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. β-Glucans: A Potential Source for Maintaining Gut Microbiota and the Immune System. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Lin, S.; Mukherjee, S.; Li, J.; Hou, W.; Pan, C.; Liu, J. Mucosal Immunity–Mediated Modulation of the Gut Microbiome by Oral Delivery of Probiotics into Peyer’s Patches. Sci. Adv. 2021, 7, eabf0677. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Jiang, Y.; Chen, M.; Ma, X.; Yu, X.; Ren, D.; Jiang, B. Akkermansia muciniphila ONE Effectively Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis in Mice. Npj Sci. Food 2024, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Schierová, D.; Březina, J.; Mrázek, J.; Fliegerová, K.O.; Kvasnová, S.; Bajer, L.; Drastich, P. Gut Microbiome Changes in Patients with Active Left-Sided Ulcerative Colitis after Fecal Microbiome Transplantation and Topical 5-Aminosalicylic Acid Therapy. Cells 2020, 9, 2283. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria with Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2853–2862. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Lan, R.; Hu, D.; Li, X.; Qiao, L.; Zhang, S.; Lin, X.; Yang, J.; Ren, Z.; et al. Bacteroides vulgatus Attenuates Experimental Mice Colitis through Modulating Gut Microbiota and Immune Responses. Front. Immunol. 2022, 13, 1036196. [Google Scholar] [CrossRef]
- Tolonen, A.C.; Beauchemin, N.; Bayne, C.; Li, L.; Tan, J.; Lee, J.; Meehan, B.M.; Meisner, J.; Millet, Y.; LeBlanc, G.; et al. Synthetic Glycans Control Gut Microbiome Structure and Mitigate Colitis in Mice. Nat. Commun. 2022, 13, 1244. [Google Scholar] [CrossRef]
- Spigaglia, P. Clostridioides difficile and Gut Microbiota: From Colonization to Infection and Treatment. Pathogens 2024, 13, 646. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut Bacteria from Multiple Sclerosis Patients Modulate Human T Cells and Exacerbate Symptoms in Mouse Models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of Intestinal Parabacteroides in Human Health and Diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Cuffaro, B.; Assohoun, A.L.W.; Boutillier, D.; Súkeníková, L.; Desramaut, J.; Boudebbouze, S.; Salomé-Desnoulez, S.; Hrdý, J.; Waligora-Dupriet, A.-J.; Maguin, E.; et al. In Vitro Characterization of Gut Microbiota-Derived Commensal Strains: Selection of Parabacteroides distasonis Strains Alleviating TNBS-Induced Colitis in Mice. Cells 2020, 9, 2104. [Google Scholar] [CrossRef] [PubMed]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral Administration of Parabacteroides distasonis Antigens Attenuates Experimental Murine Colitis through Modulation of Immunity and Microbiota Composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef]
- Yan, Y.; Lei, Y.; Qu, Y.; Fan, Z.; Zhang, T.; Xu, Y.; Du, Q.; Brugger, D.; Chen, Y.; Zhang, K.; et al. Bacteroides uniformis-Induced Perturbations in Colonic Microbiota and Bile Acid Levels Inhibit TH17 Differentiation and Ameliorate Colitis Developments. Npj Biofilms Microbiomes 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.M.A.; Sundekilde, U.K.; Andersen, H.J.; Nielsen, D.S.; Bertram, H.C. Lactose and Bovine Milk Oligosaccharides Synergistically Stimulate B. longum Subsp. Longum Growth in a Simplified Model of the Infant Gut Microbiome. J. Proteome Res. 2019, 18, 3086–3098. [Google Scholar] [CrossRef]
- Gao, F.; Wang, F.; Wang, D.; Du, G.; Gao, F. Bibliometric Analysis of the S24-7 Family and Its Association with Health. Front. Microbiol. 2025, 16, 1571883. [Google Scholar] [CrossRef]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction Ameliorates Ulcerative Colitis in Mice via Modulating Gut Microbiota and Its Metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, X.; Liu, X.; Li, Y.; Tan, Q.; Dan, Q.; Yuan, T.; Liu, X.; Liu, R.H.; Liu, Z. Ficus carica Polysaccharide Attenuates DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Food Funct. 2020, 11, 6666–6679. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, L.C.; Favilla, L.D.; de Castro, T.B.; Leal, M.C.B.D.M.; Hoffmann, C.; Bocca, A.L. Auricularia auricula’s Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin–1-Mediated Immunomodulation and Microbiota Remodeling. Pharmaceuticals 2025, 18, 1085. https://doi.org/10.3390/ph18081085
Coelho LC, Favilla LD, de Castro TB, Leal MCBDM, Hoffmann C, Bocca AL. Auricularia auricula’s Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin–1-Mediated Immunomodulation and Microbiota Remodeling. Pharmaceuticals. 2025; 18(8):1085. https://doi.org/10.3390/ph18081085
Chicago/Turabian StyleCoelho, Luísa Coutinho, Luísa Dan Favilla, Thais Bergmann de Castro, Maria Carolina B. Di Medeiros Leal, Christian Hoffmann, and Anamélia Lorenzetti Bocca. 2025. "Auricularia auricula’s Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin–1-Mediated Immunomodulation and Microbiota Remodeling" Pharmaceuticals 18, no. 8: 1085. https://doi.org/10.3390/ph18081085
APA StyleCoelho, L. C., Favilla, L. D., de Castro, T. B., Leal, M. C. B. D. M., Hoffmann, C., & Bocca, A. L. (2025). Auricularia auricula’s Exopolysaccharide Mitigates DSS-Induced Colitis Through Dectin–1-Mediated Immunomodulation and Microbiota Remodeling. Pharmaceuticals, 18(8), 1085. https://doi.org/10.3390/ph18081085





