Chemical Composition and Biological Activities of Psilocybe Mushrooms: Gaps and Perspectives
Abstract
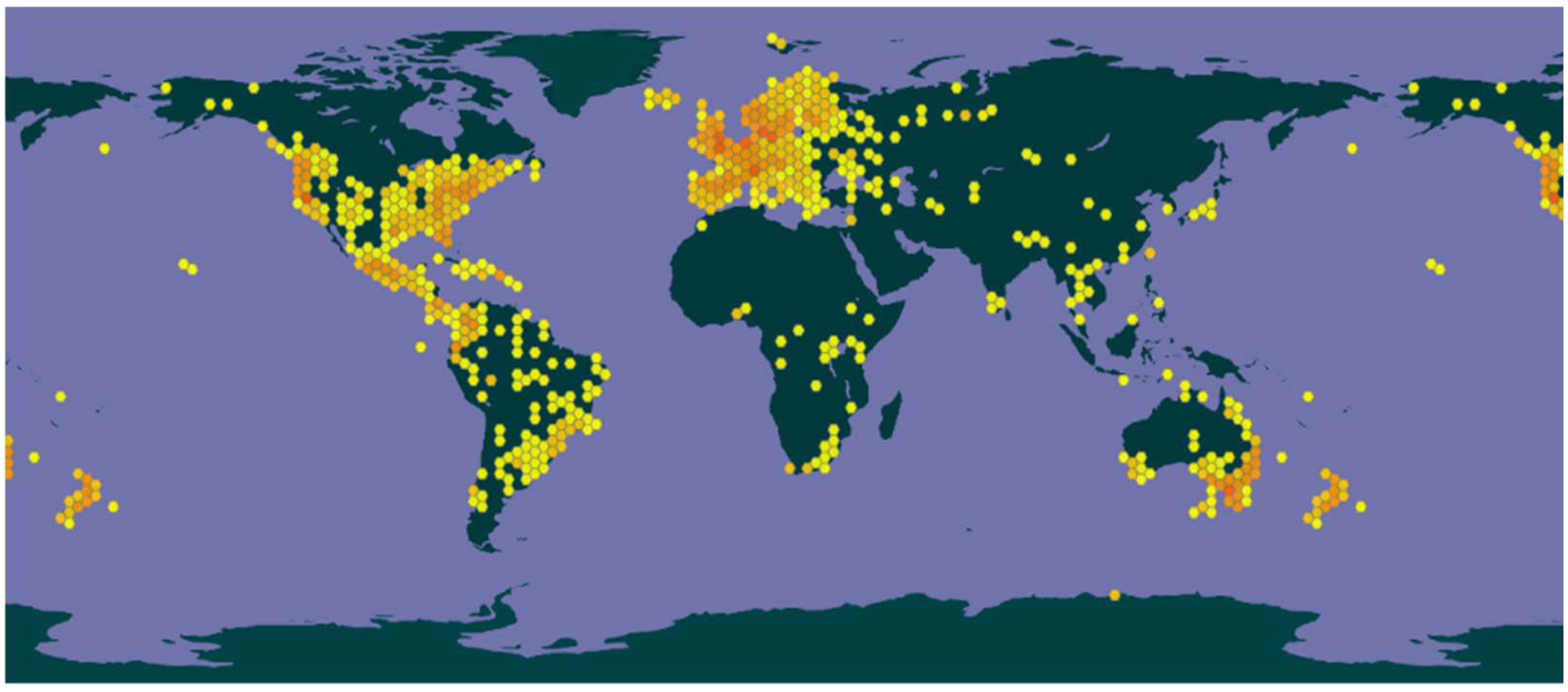
1. Introduction
2. Results and Discussion
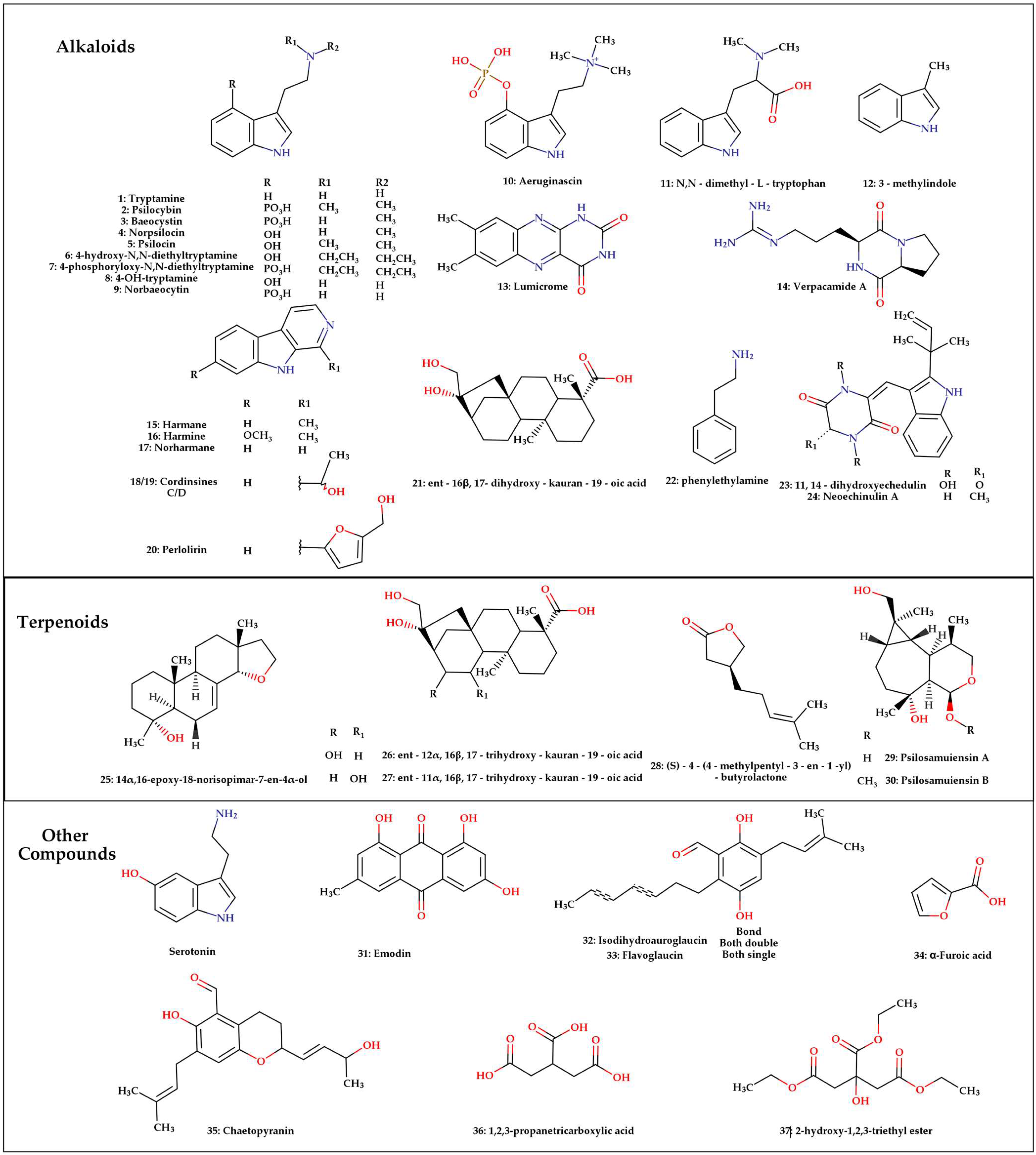
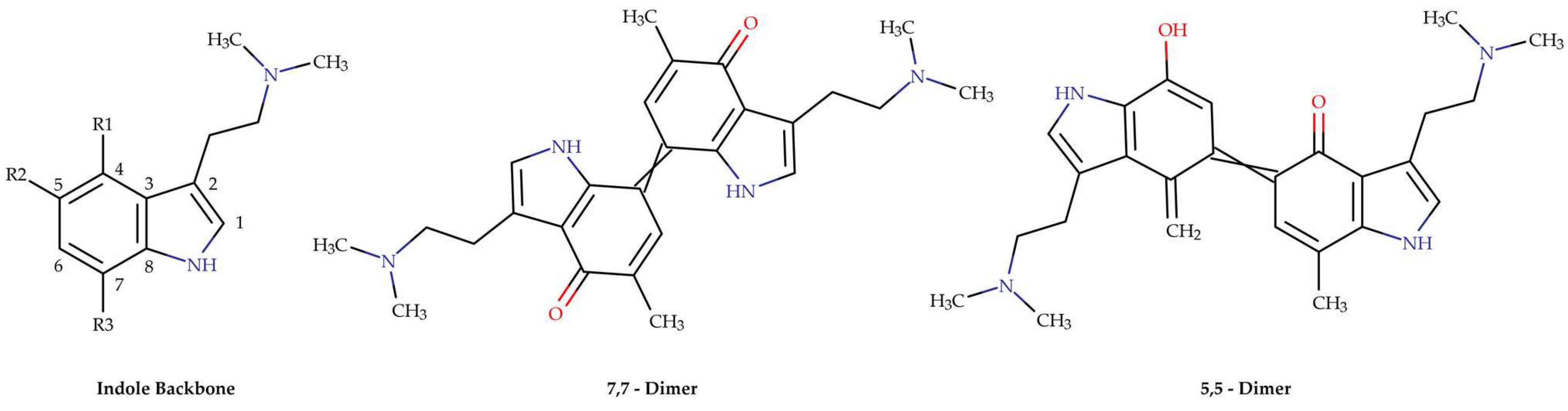
2.1. Secondary Metabolites Found in Mushrooms of the Psilocybe Genus
| Species | Substances | Mushroom Parts (Type of Extract) | Concentration (%) 1 | Refs. |
|---|---|---|---|---|
| P. arcana | 2 and 5 | Whole mushroom (methanolic) | 2 = 0.01–1.15 and 5 = 0.03–0.85 | [31] |
| P. argentipes | 2 and 5 | Whole mushroom (methanolic) | 2 = 0.125–0.38 and 5 = 0.069 | [32,33] |
| P. azurescens | 2, 5, 9, 3, 4, 10, 15, 16, 17, 18, 19, 11, 13 and 14 | Whole mushroom (methanolic) | Not reported | [21] |
| P. baeocystis | 2 and 5 | Whole mushroom (hydro-methanolic) | Not reported | [34,35] |
| 2, 5, 3 and 9 | Whole mushroom (methanolic) | 2 = 0.15–0.85, 5 = 0.048–0.3 and 3 = 0.01–0.1 | [36,37,38,39,40,41] | |
| P. bohemica | 2 and 3 | Cap (methanolic) | 2 = 0.31–1.12 and 3 = 0.02–0.03 | [42] |
| 2 and 3 | Stipe (methanolic) | 2 = 0.14–0.5 and 3 = 0.01–0.02 | [42] | |
| 2, 5 and 3 | Whole mushroom (methanolic) | 2 = 0.10–1.34, 5 = 0.01–1.27 and 3 = 0.03 | [31,42,43,44,45,46,47,48] | |
| P. bolivarii | 2 and 5 | Whole mushroom (methanolic) | Not reported | [49] |
| P. bonetii | 2 and 5 | Whole mushroom (methanolic) | Not reported | [49] |
| P. caerulipes | 2 and 5 | Whole mushroom (methanolic) | Not reported | [39] |
| P. coprophila | 2 and 5 | Whole mushroom (methanolic) | Not reported | [36] |
| 11 and 1 | Mushroom mycelium (0.1 M HCl) | Not reported | [50] | |
| P. cubensis | 2, 5, 3, 9, 4, 10, 6, 7, 15, 16, 17, 18, 19, 20, 11, 13 and 14 | Whole mushroom (methanolic) | 2 = 0.01–1.35, 5 = 0.01–0.78, 3 = 0.05–0.11, 9 = 0.01–0.02, 10 = 0.01–0.05, 15 = 0.002, 7 = 0.02–0.8% and 6 = 0.2–3.3% | [4,18,20,21,36,41,51,52,53,54,55,56,57,58,59] |
| 21, 26 and 27. | Metabolic liquid (ethyl acetate) | 21 = 21.1%; 26 = 8.3%; 27 = 2.8% | [9,10] | |
| 2 and 5 | Cap (methanolic) | 2 = 0.102–0.76 and 5 = 0.0415–0.836 | [60] | |
| 2 and 5 | Whole mushroom (ethanolic) | 2 = 1% and 5 = 0.16% | [45] | |
| 2, 5 and 4 | Whole mushroom (hydro-methanolic) | 2 = 0.13, 5 = 0.03 and 4 = 0.0015 | [17,61] | |
| 2 and 5 | Whole mushroom (acid trichloroacetic acid 10%) | 2 <0.005% and 5 <0.005 | [62] | |
| P. cubensis | 2 and 5 | Whole mushroom (chloroform) | Not reported | [63] |
| P. cyanescens | 2, 5, 9, 3, 4, 10, 15, 16, 17, 18, 19, 11, 13 and 14 | Whole mushroom (methanolic) | 2 = 0.1–1.84, 5 = 0.06–0.76 and 3 = 0.004–0.04 | [21,31,35,36,41,46,47,64,65] |
| P. fimetaria | 2 | Whole mushroom (methanolic) | Not reported | [66] |
| P. inquilina | 2 and 5 | Whole mushroom (methanolic) | Not reported | [36] |
| P. McKennaii | Not reported | Whole mushroom (methanolic) | Not reported | [67] |
| P. medullosa | 2 and 5 | Whole mushroom (methanolic) | Not reported | [68] |
| P. merdaria | 23, 28, 24, 32, 33, 35, 25, 31, 34, 36 and 37 | Metabolic liquid (ethyl acetate) | Not reported | [8] |
| P. mexicana | 2, 5, 9, 3, 4, 10, 15, 16, 17, 18, 19, 11, 13 and 14 | Whole mushroom (methanolic) | 2 = 0.25 | [1,20,21,69] |
| P. montana | 2 and 5 | Whole mushroom (methanolic) | Not reported | [36,70] |
| P. pelliculosa | 2, 5 and 3 | Whole mushroom (methanolic) | 3 = 0.007–0.04 and 2 = 0.12–0.71 | [36,41] |
| P. pseudobullacea | 2 and 5 | Whole mushroom (methanolic) | Not reported | [70] |
| P. samauiensis | 2, 5 and 3 | Whole mushroom (methanolic) | 0.23–0.90 for all substances | [71] |
| 29 and 30 | Metabolic fluid (ethyl acetate) | Not reported | [27] | |
| P. semilanceata | 2, 5, 3, 9, 10 and 22 | Whole mushroom (methanolic) | 2 = 0.02–1.70, 22 = 0.00001–0.000145, 5 = 0.01–0.90, 3 = 0.02–1.10, 9 = 0.077 and 10 = 0.022 | [19,31,36,46,47,48,51,52,54,66,72,73,74,75,76,77,78,79] |
| 2 | Whole mushroom (methanol with ammonium nitrate) | 2 = 0.55–2.37 | [80,81] | |
| 2, 5 and 3 | Cap (methanolic) | 2 = 0.33–1.35, 3 = 0.12 and 5 = 0.04–0.68 | [48,78] | |
| 2 and 3 | Whole mushroom (hydroalcoholic acidified) | 2 = 1.09–1.19 and 3 = 0.29–0.41 | [82] | |
| 2, 5 and 3 | Whole mushroom (methanol: chloroform) | Not reported | [83] | |
| P. serbica | 2, 5, 9, 3, 4, 10, 15, 16, 17, 18, 19, 11, 13 and 14 | Whole mushroom (methanolic) | Not reported | [21] |
| P. silvatica | 2, 5 and 3 | Whole mushroom (methanolic) | 3 = 0.004–0.02 | [41,68] |
| P. strictipes | 2 | Whole mushroom (methanolic) | Not reported | [39] |
| P. stuntzii | 2 and 5 | Whole mushroom (methanolic) | 2 = 0.04–0.36, 5 = 0.006–0.012 and 3 = 0.002–0.02 | [36,41] |
| 2 and 5 | Whole mushroom (hydro -methanol) | Not reported | [84] | |
| P. subaeruginosa | 2 and 5 | Whole mushroom (methanolic) | 2 = 0.45–1.41 and 5 = 0.011–0.038 | [85,86,87] |
| P. subcubensis | 2 and 5 | Whole mushroom (methanolic) | 2 = 0.32 and 5 =0.06 | [32] |
| 2 and 5 | Whole mushroom (chloroform) | 2 = 0.80–0.86 and 5 = 0.02–0.03 | [88] | |
| P. tampanensis | 2 and 5 | Whole mushroom (methanolic) | 2 = 0.01–0.19 and 5 = 0.01–0.03 | [54] |
| P. wrightii | 2 and 5 | Whole mushroom (methanolic) | Not reported | [89] |
| P. zapotecorum | 2, 5, 3, 9, 10, 8, 4, 10 and 1 | Whole mushroom (methanolic) | 2 = 1.06–3.04, 5 = 0.03–0.65, 3 = 0.0024–0.321, 9 = 0.036–0.271, 4 = 0.024–0.142, 10 = >0.01 | [90] |
Techniques for Extracting Indole Alkaloids from the Psilocybe Genus Mushrooms
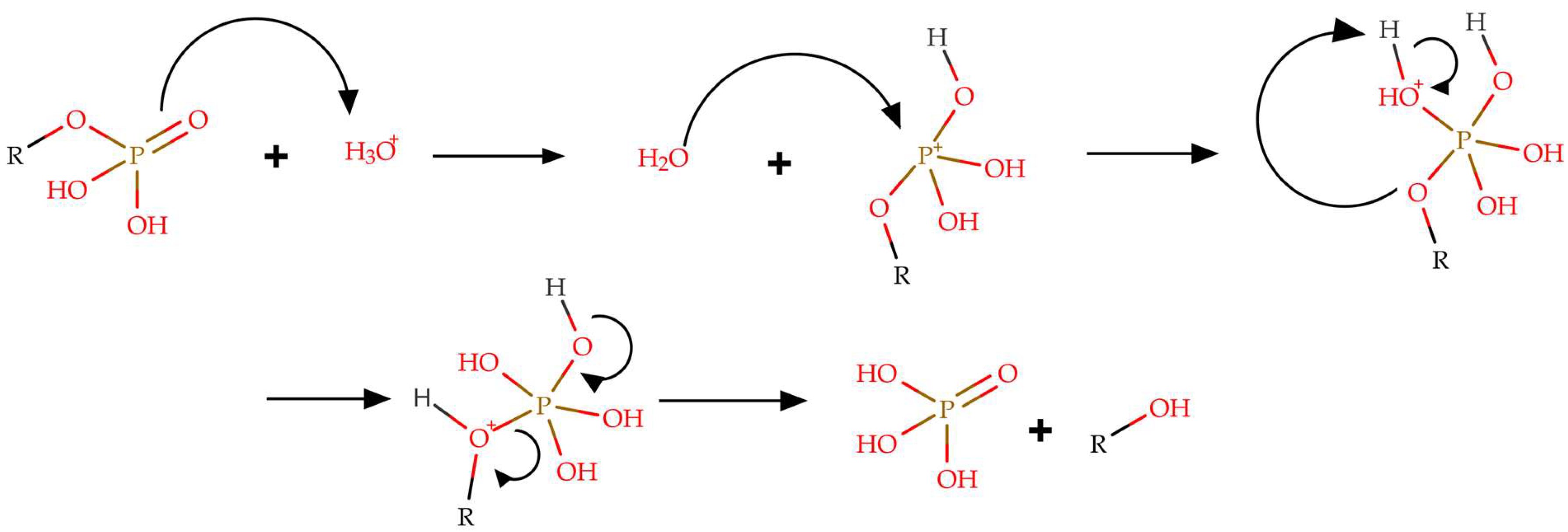
2.2. Stability of Alkaloids of the Psilocybe Genus
2.3. Biological Activities Reported for Extracts of Psilocybe Genus Mushrooms
| Species | Type of Extract | Activity | Strains/Radicals/Others Cellular Mediators | Ref. |
|---|---|---|---|---|
| P. cubensis | Ethyl Acetate (metabolic liquid) | Antimicrobial and larvicidal | Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Vibrio cholera (SA), Salmonella typhi, Staphylococcus aureus and Culex quinquefasciatus | [9] |
| P. cubensis | Aqueous | Larvicide | Artemia franciscana | [13] |
| P. cubensis | Ethanol and aqueous | Antibacterial | Escherichia coli and Bacillus cereus | [12] |
| P. merdaria | Metabolic liquid (ethyl acetate) | AChE inhibitor | Acetylthiocholine iodide | [8] |
| P. natalensis | Ethanolic and aqueous | Antioxidant, cytotoxic and anti-inflammatory | Radical ABTS, normal Vero cells, NO inhibition, PGE2 inhibition and cytokine inhibition | [14] |
Aspects of the Interaction Between Indole Alkaloids from Psilocybe Mushrooms and Serotonin Neuroreceptors
| Substance | Receivers | Positive Control | Inhibition Factor (a: Mice; b: Human; nM) | Ref. |
|---|---|---|---|---|
| Psilocybin | 5-HT1A/2A; 5-HT2B/2C | Ketanserin, LSD and serotonin | 1A: 197 a; 2A: 2096a ; 2B: 612 b; 2C: 3741 b | [104] |
| Psilocin | 5-HT1A/2A; 5-HT2B/2C | 1A: 118 a; 2A: 235 a; 2B: 8 b; 2C:34 b | ||
| Baeocystin | 5-HT1A/2A | 1A: 25 a; 2A: >5000 a | ||
| Norbaeocystin | No data | No data | No data | |
| Norpsilocin | 5-HT1A/2A; 5-HT2B/2C | Ketanserin, LSD and serotonin | 1A: 29 a; 2A: 706 a; 2B: 13 b; 2C: 32 b | |
| Aeruginascin | 5-HT1A/2A | 1A: >10.000 a; 2A: >5000 a |
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, A.; Heim, R.; Brack, A.; Kobel, H. Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz Psilocybe mexicana Heim. Experientia 1958, 14, 107–109. [Google Scholar] [CrossRef]
- Sharma, P.; Nguyen, Q.A.; Matthews, S.J.; Carpenter, E.; Mathews, D.B.; Patten, C.A.; Hammond, C.J. Psilocybin history, action and reaction: A narrative clinical review. J. Psychopharmacol. 2023, 37, 849–865. [Google Scholar] [CrossRef]
- Li, N.-X.; Hu, Y.-R.; Chen, W.-N.; Zhang, B. Dose effect of psilocybin on primary and secondary depression: A preliminary systematic review and meta-analysis. J. Affect. Disord. 2022, 296, 26–34. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; Escamilla-Orozco, R.I.; Tabal-Robles, A.R.; Martínez-Vargas, D.; Romero-Bautista, L.; Escamilla-Soto, G.; González-Romero, O.S.; Torres-Valencia, M.; González-Trujano, M.E. Antidepressant- and anxiolytic-like activities and acute toxicity evaluation of the Psilocybe cubensis mushroom in experimental models in mice. J. Ethnopharmacol. 2024, 320, 117415. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abus. 2017, 43, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Controlled and Illegal Drugs. 2005. Available online: https://www.canada.ca/en/health-canada/services/substance-use/controlled-illegal-drugs.html (accessed on 20 May 2025).
- US Drug Enforcement Administration DEA. 2000. Available online: https://www.dea.gov/drug-information/drug-scheduling (accessed on 20 May 2025).
- Yang, N.-N.; Ma, Q.-Y.; Huang, S.-Z.; Kong, F.-D.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Chemical study of the fungus Psilocybe merdaria. J. Asian Nat. Prod. Res. 2017, 19, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Latha, S.; Suganya, P.; Panneerselvam, A.; Senthil Kumar, T.; Alharbi, N.S.; Arunachalam, C.; Alharbi, S.A.; Thajuddin, N. Taxonomic identification and bioactive compounds characterization of Psilocybe cubensis DPT1 to probe its antibacterial and mosquito larvicidal competency. Microb. Pathog. 2020, 143, 104138. [Google Scholar] [CrossRef]
- Pechwang, J.; Sihanonth, P.; Pornpakakul, S.; Muangsin, N.; Piapukiew, J.; Vangnai, A.; Chaichit, N.; Chuchawankul, S.; Petsom, A. Biotransformation of ent-kaur-16-en-19-oic acid by Psilocybe cubensis. Nat. Prod. Res. 2010, 24, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Pace, B.T.; Nicholas, C.R.; Raison, C.L.; Hutson, P.R. The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry Res. 2020, 284, 112749. [Google Scholar] [CrossRef]
- Sukmawati, I.K.; Dewi, F.N.O.; Nurfajri, A. Antibacterial activity of extract magic mushroom (Psilocybe cubensis (early) singer) against Staphylococcus aureus, Escherichia coli, and Bacillus cereus. J. Glob. Trends Pharm. Sci. 2020, 11, 7595–7601. [Google Scholar]
- Vega-Villasante, F.; Ruiz-González, L.E.; Guerrero-Galván, S.R.; Guzmán-Dávalos, L. Evaluación de la toxicidad de Psilocybe cubensis (Agaricales, Basidiomycota) sobre Artemia franciscana (Crustacea, Anostraca). Rev. Iberoam. Micol. 2013, 30, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.; Eloff, J.N. Phytochemical, cytotoxicity, antioxidant and anti-inflammatory effects of Psilocybe natalensis magic mushroom. Plants 2020, 9, 1127. [Google Scholar] [CrossRef]
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Vandepitte, L.; Hobern, D.; et al. Catalogue of Life. Cat. Life 2024. [Google Scholar] [CrossRef]
- Secretariat, G. GBIF Backbone Taxonomy. Checklist Dataset. 2023. Available online: https://doi.org/10.15468/39omei (accessed on 18 July 2024).
- Lenz, C.; Wick, J.; Hoffmeister, D. Identification of ω-N-Methyl-4-hydroxytryptamine (Norpsilocin) as a Psilocybe Natural Product. J. Nat. Prod. 2017, 80, 2835–2838. [Google Scholar] [CrossRef]
- Gotvaldová, K.; Hájková, K.; Borovička, J.; Jurok, R.; Cihlářová, P.; Kuchař, M. Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis. Drug Test. Anal. 2021, 13, 439–446. [Google Scholar] [CrossRef]
- Beck, O.; Helander, A.; Karlson-Stiber, C.; Stephansson, N. Presence of Phenylethylamine in Hallucinogenic Psilocybe Mushroom: Possible Role in Adverse Reactions. J. Anal. Toxicol. 1998, 22, 45–49. [Google Scholar] [CrossRef]
- Blei, F.; Dörner, S.; Fricke, J.; Baldeweg, F.; Trottmann, F.; Komor, A.; Meyer, F.; Hertweck, C.; Hoffmeister, D. Simultaneous Production of Psilocybin and a Cocktail of β-Carboline Monoamine Oxidase Inhibitors in “Magic” Mushrooms. Chem.–A Eur. J. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Dörner, S.; Rogge, K.; Fricke, J.; Schäfer, T.; Wurlitzer, J.M.; Gressler, M.; Pham, D.N.K.; Manke, D.R.; Chadeayne, A.R.; Hoffmeister, D. Genetic Survey of Psilocybe Natural Products. ChemBioChem 2022, 23, e202200249. [Google Scholar] [CrossRef] [PubMed]
- Chantarawong, W.; Kuncharoen, N.; Tanasupawat, S.; Chanvorachote, P. Lumichrome Inhibits Human Lung Cancer Cell Growth and Induces Apoptosis via a p53-Dependent Mechanism. Nutr. Cancer 2019, 71, 1390–1402. [Google Scholar] [CrossRef]
- Son, S.; Kim, E.; Kim, J.W.; Ko, S.-K.; Lee, B.; Lee, J.-S.; Hong, Y.-S.; Jang, J.-H.; Ahn, J.S. Isolation and Structure Determination of a New Lumichrome Glycoside Isolated from a Soil Streptomyces sp. KCB16C001. Nat. Prod. Commun. 2018, 13, 1934578X1801300211. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Hung, Y.-C.; Chang, F.-R.; Cosentino, M.; Wang, H.-K.; Lee, K.-H. Identification of ent-16β,17-Dihydroxykauran-19-oic Acid as an Anti-HIV Principle and Isolation of the New Diterpenoids Annosquamosins A and B from Annona squamosa. J. Nat. Prod. 1996, 59, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Zhao, Z.-Z.; Cheng, G.-G.; Zhao, K.; Han, K.-Y.; Zhou, L.; Feng, T.; Li, Z.-H.; Liu, J.-K. Immunosuppressive Nor-isopimarane Diterpenes from Cultures of the Fungicolous Fungus Xylaria longipes HFG1018. J. Nat. Prod. 2020, 83, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Stompor-Gorący, M. The Health Benefits of Emodin, a Natural Anthraquinone Derived from Rhubarb-A Summary Update. Int. J. Mol. Sci. 2021, 22, 9522. [Google Scholar] [CrossRef] [PubMed]
- Pornpakakul, S.; Suwancharoen, S.; Petsom, A.; Roengsumran, S.; Muangsin, N.; Chaichit, N.; Piapukiew, J.; Sihanonth, P.; Allen, J.W. A new sesquiterpenoid metabolite from Psilocybe samuiensis. J. Asian Nat. Prod. Res. 2009, 11, 12–17. [Google Scholar] [CrossRef]
- Bovio, E.; Garzoli, L.; Poli, A.; Luganini, A.; Villa, P.; Musumeci, R.; McCormack, G.P.; Cocuzza, C.E.; Gribaudo, G.; Mehiri, M.; et al. Marine Fungi from the Sponge Grantia compressa: Biodiversity, Chemodiversity, and Biotechnological Potential. Mar. Drugs 2019, 17, 220. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Ito, C.; Kimura, T.; Suzuki, A.; Nishida, Y.; Itoigawa, M. Isolation of Aromatic Compounds Produced by Eurotium herbariorum NU-2 from Karebushi, a Katsuobushi, and their DPPH-Radical Scavenging Activities. Food Sci. Technol. Res. 2014, 20, 139–146. [Google Scholar] [CrossRef]
- Van Court, R.C.; Wiseman, M.S.; Meyer, K.W.; Ballhorn, D.J.; Amses, K.R.; Slot, J.C.; Dentinger, B.T.M.; Garibay-Orijel, R.; Uehling, J.K. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal Biol. 2022, 126, 308–319. [Google Scholar] [CrossRef]
- Stríbrný, J.; Borovicka, J.; Sokol, M. Levels of psilocybin and psilocin in various types of mushrooms. Soud Lek 2003, 48, 45–49. [Google Scholar] [PubMed]
- Kamata, T.; Nishikawa, M.; Katagi, M.; Tsuchihashi, H. Liquid Chromatography-Mass Spectrometric and Liquid Chromatography-Tandem Mass Spectrometric Determination of Hallucinogenic Indoles Psilocin and Psilocybin in “Magic Mushroom” Samples. J. Forensic Sci. 2005, 50, JFS2004276-5. [Google Scholar] [CrossRef]
- Koike, Y.; Wada, K.; Kusano, G.; Nozoe, S.; Yokoyama, K. Isolation of psilocybin from Psilocybe argentipes and its determination in specimens of some mushrooms. J. Nat. Prod. 1981, 44, 362–365. [Google Scholar] [CrossRef]
- Benedict, R.G.; Brady, L.R.; Tyler, V.E. Occurrence of Psilocin in Psilocybe baeocystis. J. Pharm. Sci. 1962, 51, 393–394. [Google Scholar] [CrossRef]
- Benedict, R.G.; Brady, L.R.; Smith, A.H.; Tyler, V.E. Occurrence of psilocybin and psilocin in certain Conocybe and Psilocybe species. Lloydia 1962, 25, 156–159. [Google Scholar]
- Beug, M.W.; Bigwood, J. Psilocybin and psilocin levels in twenty species from seven genera of wild mushrooms in the Pacific Northwest, U.S.A. J. Ethnopharmacol. 1982, 5, 271–285. [Google Scholar] [CrossRef]
- Leung, A.Y.; Paul, A.G. Baeocystin and Norbaeocystin: New Analogs of Psilocybin from Psilocybe baeocystis. J. Pharm. Sci. 1968, 57, 1667–1671. [Google Scholar] [CrossRef]
- Leung, A.Y.; Paul, A.G. Baeocystin, a mono-methyl analog of psilocybin from Psilocybe baeocystis saprophytic culture. J. Pharm. Sci. 1967, 56, 146. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.Y.; Smith, A.H.; Paul, A.G. Production of psilocybin in Psilocybe baeocystis saprophytic culture. J. Pharm. Sci. 1965, 54, 1576–1579. [Google Scholar] [CrossRef]
- Beug, M.W.; Bigwood, J. Quantitative analysis of psilocybin and psilocin and Psilocybe baecystis (singer and smith) by high-performance liquid chromatography and by thin-layer chromatography. J. Chromatogr. A 1981, 207, 379–385. [Google Scholar] [CrossRef]
- Repke, D.B.; Leslie, D.T.; Guzmán, G. Baeocystin in Psilocybe, Conocybe and Panaeolus. Lloydia 1977, 40, 566–578. [Google Scholar]
- Gartz, J.; Moller, G.K. Analysis and Cultivation of Fruit Bodies and Mycelia of Psilocybe bohemica. Biochem. Physiol. Pflanz. 1989, 184, 337–341. [Google Scholar] [CrossRef]
- Kysilka, R.; Wurst, M.; Pacáková, V.; Štulík, K.; Haškovec, L. High-performance liquid chromatographic determination of hallucinogenic indoleamines with simultaneous UV photometric and voltammetric detection. J. Chromatogr. A 1985, 320, 414–420. [Google Scholar] [CrossRef]
- Kysilka, R.; Wurst, M. High-performance liquid chromatographic determination of some psychotropic indole derivatives. J. Chromatogr. A 1991, 464, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kysilka, R.; Wurst, M. A Novel Extraction Procedure for Psilocybin and Psilocin Determination in Mushroom Samples. Planta Medica 1990, 56, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Stijve, T.; Kuyper, T.W. Occurrence of Psilocybin in Various Higher Fungi from Several European Countries. Planta Medica 1985, 51, 385–387. [Google Scholar] [CrossRef]
- Wurst, M.; Kysilka, R.; Koza, T. Analysis and isolation of indole alkaloids of fungi by high-performance liquid chromatography. J. Chromatogr. A 1992, 593, 201–208. [Google Scholar] [CrossRef]
- Wurst, M.; Semerdžieva, M.; Vokoun, J. Analysis of psychotropic compounds in fungi of the genus Psilocybe by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1984, 286, 229–235. [Google Scholar] [CrossRef]
- Ott, J.; Guzmán, G. Detection of psilocybin in species of Psilocybe, Panaeolus and Psathyrella. Lloydia 1976, 39, 258–260. [Google Scholar]
- Alarcón, J.; Foncea, L.; Águila, S.; Alderete, J.B. Biotransformation of Tryptophan by Liquid Medium Culture of Psilocybe coprophila (Basidiomycetes). Z. Für Naturforsch. C 2006, 61, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Borner, S.; Brenneisen, R. Determination of tryptamine derivatives in hallucinogenic mushrooms using high-performance liquid chromatography with photodiode array detection. J. Chromatogr. 1987, 408, 402–408. [Google Scholar] [CrossRef]
- Gartz, J. Biotransformation of tryptamine derivatives in mycelial cultures of Psilocybe. J. Basic Microbiol. 1989, 29, 347–352. [Google Scholar] [CrossRef]
- Laussmann, T.; Meier-Giebing, S. Forensic analysis of hallucinogenic mushrooms and khat (Catha edulisForsk) using cation-exchange liquid chromatography. Forensic Sci. Int. 2010, 195, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Madea, B.; Beike, J. Hallucinogenic mushrooms on the German market—Simple instructions for examination and identification. Forensic Sci. Int. 2000, 113, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Solano, J.; Anabalón, L.; Figueroa, S.; Lizama, C.; Reyes, L.C.; Gangitano, D. Psychedelic fungus (Psilocybe sp.) authentication in a case of illegal drug traffic: Sporological, molecular analysis and identification of the psychoactive substance. Sci. Justice 2019, 59, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Toyo’oka, T.; Kato, M.; Fukushima, T.; Shirota, O.; Goda, Y. Determination of psilocybin in hallucinogenic mushrooms by reversed-phase liquid chromatography with fluorescence detection. Talanta 2005, 66, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, K.; Kanamori, T.; Iwata, Y.; Ohmae, Y.; Sugita, R.; Inoue, H.; Kishi, T. Morphological and chemical analysis of magic mushrooms in Japan. Forensic Sci. Int. 2003, 138, 85–90. [Google Scholar] [CrossRef]
- Goff, R.; Smith, M.; Islam, S.; Sisley, S.; Ferguson, J.; Kuzdzal, S.; Badal, S.; Kumar, A.B.; Sreenivasan, U.; Schug, K.A. Determination of psilocybin and psilocin content in multiple Psilocybe cubensis mushroom strains using liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2024, 1288, 342161. [Google Scholar] [CrossRef]
- Katragunta, K.; Avula, B.; Chittiboyina, A.G.; Lata, H.; Khan, I.A. Quantitative LC-QToF-MS Analysis of Mycochemicals in Amanita muscaria, Psilocybe spp. (Agaricomycetes), and Consumer Products. Int. J. Med. Mushrooms 2025, 27, 29–39. [Google Scholar] [CrossRef]
- Gambaro, V.; Roda, G.; Visconti, G.L.; Arnoldi, S.; Casagni, E.; Dell’Acqua, L.; Farè, F.; Paladino, E.; Rusconi, C.; Arioli, S.; et al. DNA-based taxonomic identification of basidiospores in hallucinogenic mushrooms cultivated in “grow-kits” seized by the police: LC-UV quali-quantitative determination of psilocybin and psilocin. J. Pharm. Biomed. Anal. 2016, 125, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Polo-Castellano, C.; Álvarez, J.Á.; Palma, M.; Barbero, G.F.; Ayuso, J.; Ferreiro-González, M. Optimization through a Box–Behnken Experimental Design of the Microwave-Assisted Extraction of the Psychoactive Compounds in Hallucinogenic Fungi (Psylocibe cubensis). J. Fungi 2022, 8, 598. [Google Scholar] [CrossRef]
- Neal, J.M.; Benedict, R.G.; Brady, L.R. Interrelationship of Phosphate Nutrition, Nitrogen Metabolism, and Accumulation of Key Secondary Metabolites in Saprophytic Cultures of Psilocybe cubensis, Psilocybe cyanescens, and Panaeolus campanulatus. J. Pharm. Sci. 1968, 57, 1661–1667. [Google Scholar] [CrossRef]
- Rafati, H.; Riahi, H.; Mohammadi, A. Enhancement of Indole Alkaloids Produced by Psilocybe cubensis (Earle) Singer (Agaricomycetideae) in Controlled Harvesting Light Conditions. Int. J. Med. Mushrooms 2009, 11, 419–426. [Google Scholar] [CrossRef]
- Gross, S. Detecting Psychoactive Drugs in the Developmental Stages of Mushrooms. J. Forensic Sci. 2000, 45, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Unger, S.E.; Cooks, R.G. Application of Mass Spectrometry/Mass Spectrometry (MS/MS) to the Identification of Natural Products in Psilocybe Cyanescens. Anal. Lett. 1979, 12, 1157–1167. [Google Scholar] [CrossRef]
- Benedict, R.G.; Tyler, V.E.; Watling, R. Blueing in Conocybe, Psilocybe, and a Stropharia species and the detection of psilocybin. Lloydia 1967, 30, 150–157. [Google Scholar]
- Otvos, R.A.; Mladic, M.; Arias-Alpizar, G.; Niessen, W.M.A.; Somsen, G.W.; Smit, A.B.; Kool, J. At-Line Cellular Screening Methodology for Bioactives in Mixtures Targeting the α7-Nicotinic Acetylcholine Receptor. J. Biomol. Screen. 2016, 21, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Borovička, J.; Oborník, M.; Stříbrný, J.; Noordeloos, M.E.; Sánchez, L.A.P.; Gryndler, M. Phylogenetic and chemical studies in the potential psychotropic species complex of Psilocybe atrobrunnea with taxonomic and nomenclatural notes. Persoonia-Mol. Phylogeny Evol. Fungi 2015, 34, 1–9. [Google Scholar] [CrossRef]
- Hoffman, A. Psilocybin and Psilocin. Two Psychotropically Active Principles of Mexican Hallucinogenic Fungus. Helv. Chim. Acta 1959, 42, 1557. [Google Scholar]
- Marcano, V.; Méndez, A.M.; Castellano, F.; Salazar, F.; Martinez, L. Occurrence of psilocybin and psilocin in Psilocybe pseudobullacea (Petch) Pegler from the Venezuelan Andes. J. Ethnopharmacol. 1994, 43, 157–159. [Google Scholar] [CrossRef]
- Gartz, J.; Allen, J.W.; Merlin, M.D. Ethnomycology, biochemistry, and cultivation of Psilocybe samuiensis Guzman, Bandala and Allen, a new psychoactive fungus from Koh Samui, Thailand. J. Ethnopharmacol. 1994, 43, 73–80. [Google Scholar] [CrossRef]
- Brenneisen, R.; Borner, S. The Occurrence of Tryptamine Derivatives in Psilocybe semilanceata. Z. Für Naturforsch. C 1988, 43, 511–514. [Google Scholar] [CrossRef]
- Christiansen, A.; Rasmussen, K. Analysis of indole alkaloids in Norwegian Psilocybe semilanceata using high-performance liquid chromatography and mass spectrometry. J. Chromatogr. A 1982, 244, 357–364. [Google Scholar] [CrossRef]
- Christiansen, A.; Rasmussen, K. Screening of hallucinogenic mushrooms with high-performance liquid chromatography and multiple detection. J. Chromatogr. A 1983, 270, 293–299. [Google Scholar] [CrossRef]
- Gartz, J. Quantitative Bestimmung der Indolderivate von Psilocybe semilanceata (Fr.) Kumm. Biochem. Physiol. Pflanz. 1986, 181, 117–124. [Google Scholar] [CrossRef]
- Mantle, P.; Waight, E. Occurrence of psilocybin in the sporophores of Psilocybe semilanceata. Trans. Br. Mycol. Soc. 1969, 53, 302–304. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Sannes, E.; Rasmussen, K.E.; Tønnesen, F. Determination of psilocybin in Psilocybe semilanceata by capillary zone electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1997, 694, 375–381. [Google Scholar] [CrossRef]
- Repke, D.B.; Leslie, D.T. Baeocystin in Psilocybe semilanceata. J. Pharm. Sci. 1977, 66, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Zhuk, O.; Jasicka-Misiak, I.; Poliwoda, A.; Kazakova, A.; Godovan, V.V.; Halama, M.; Wieczorek, P.P. Research on acute toxicity and the behavioral effects of methanolic extract from psilocybin mushrooms and psilocin in mice. Toxins 2015, 7, 1018–1029. [Google Scholar] [CrossRef]
- Christiansen, A.; Rasmussen, K.; Tønnesen, F. Determination of psilocybin in Psilocybe semilanceata using high-performance liquid chromatography on a silica column. J. Chromatogr. A 1981, 210, 163–167. [Google Scholar] [CrossRef]
- Jokiranta, J.; Mustola, S.; Ohenoja, E.; Airaksinen, M.M. Psilocybin in Finnish Psilocybe semilanceata. Planta Medica 1984, 50, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Vanhaelen-Fastré, R.; Vanhaelen, M. Qualitative and quantitative determinations of hallucinogenic components of Psilocybe mushrooms by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1984, 312, 467–472. [Google Scholar] [CrossRef]
- White, P. Analysis of extracts from Psilocybe semilanceata mushrooms by high-pressure liquid chromatography. J. Chromatogr. A 1979, 169, 453–456. [Google Scholar] [CrossRef]
- Guzmán, G.; Ott, J. Description and chemical analysis of a new species of hallucinogenic Psilocybe from the Pacific Northwest. Mycologia 1976, 68, 1261–1267. [Google Scholar] [CrossRef]
- Anastos, N.; Barnett, N.W.; Lewis, S.W.; Gathergood, N.; Scammells, P.J.; Sims, D.N. Determination of psilocin and psilocybin using flow injection analysis with acidic potassium permanganate and tris (2,2′-bipyridyl) ruthenium (II) chemiluminescence detection respectively. Talanta 2005, 67, 354–359. [Google Scholar] [CrossRef]
- Anastos, N.; Lewis, S.W.; Barnett, N.W.; Sims, D.N. The Determination of Psilocin and Psilocybin in Hallucinogenic Mushrooms by HPLC Utilizing a Dual Reagent Acidic Potassium Permanganate and Tris (2,2′-bipyridyl) ruthenium (II) Chemiluminescence Detection System. J. Forensic Sci. 2006, 51, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Picker, J.; Rickards, R. The occurrence of the psychotomimetic agent psilocybin in an Australian agaric, Psilocybe subaeruginosa. Aust. J. Chem. 1970, 23, 853–855. [Google Scholar] [CrossRef]
- Keller, T.; Schneider, A.; Regenscheit, P.; Dirnhofer, R.; Rücker, T.; Jaspers, J.; Kisser, W. Analysis of psilocybin and psilocin in Psilocybe subcubensis GUZMAN by ion mobility spectrometry and gas chromatography–mass spectrometry. Forensic Sci. Int. 1999, 99, 93–105. [Google Scholar] [CrossRef]
- Rossato, L.G.; Cortez, V.G.; Limberger, R.P.; Guzmán, G. Taxonomy and chemical aspects of Psilocybe wrightii from southern Brazil. Mycotaxon 2009, 108, 223–229. [Google Scholar] [CrossRef]
- Miller, D.R.; Jacobs, J.T.; Rockefeller, A.; Singer, H.; Bollinger, I.M.; Conway, J.; Slot, J.C.; Cliffel, D.E. Cultivation, chemistry, and genome of Psilocybe zapotecorum. J. Psychedelic Stud. 2023, 8, 63–81. [Google Scholar] [CrossRef]
- Galdino, T.P.; Oliveira, L.C.; Luz, M.A.; Jesus, R.A.; Lima, E.P.N.; Torres, M.C.M.; Sivieri, K.; Afonso, V.I.; Delgado, J.M.P.Q.; Lima, A.G.B.; et al. Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications. Pharmaceuticals 2025, 18, 380. [Google Scholar] [CrossRef]
- Casale, J. An Aqueous-Organic Extraction Method for the Isolation and Identification of Psilocin from Hallucinogenic Mushrooms. J. Forensic Sci. 1985, 30, 247–250. [Google Scholar] [CrossRef]
- Hu, Q.; Jayasinghe-Arachchige, V.M.; Sharma, G.; Serafim, L.F.; Paul, T.J.; Prabhakar, R. Mechanisms of peptide and phosphoester hydrolysis catalyzed by two promiscuous metalloenzymes (insulin degrading enzyme and glycerophosphodiesterase) and their synthetic analogues. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1466. [Google Scholar] [CrossRef]
- Lenz, C.; Wick, J.; Braga, D.; García-Altares, M.; Lackner, G.; Hertweck, C.; Gressler, M.; Hoffmeister, D. Injury-triggered blueing reactions of Psilocybe “magic” mushrooms. Angew. Chem. 2020, 132, 1466–1470. [Google Scholar] [CrossRef]
- Lenz, C.; Dörner, S.; Sherwood, A.; Hoffmeister, D. Structure elucidation and spectroscopic analysis of chromophores produced by oxidative psilocin dimerization. Chem.–A Eur. J. 2021, 27, 12166–12171. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.J.; Ramírez-Cruz, V.; Awan, A.R.; Furci, G.; Guzmán-Dávalos, L.; Dentinger, B.T.M. Phylogenomics of the psychoactive mushroom genus Psilocybe and evolution of the psilocybin biosynthetic gene cluster. Proc. Natl. Acad. Sci. USA 2024, 121, e2311245121. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Siegel, J.; Gold, J.A. Psilocybin-assisted psychotherapy for depression: Emerging research on a psychedelic compound with a rich history. J. Neurol. Sci. 2022, 434, 120096. [Google Scholar] [CrossRef]
- Bergh, V.J.; Bruzell, E.; Hegge, A.B.; Tønnesen, H.H. Influence of formulation on photoinactivation of bacteria by lumichrome. Die Pharm. 2015, 70, 574–580. [Google Scholar] [CrossRef]
- Jasemi, E.; Razmi, A.; Vaseghi, S.; Amiri, S.; Najafi, S.M.A. The effect of Psilocybe cubensis alkaloids on depressive-like behavior in mice exposed to maternal separation with respect to hippocampal gene expression and DNA methylation of Slc6a4 and Nr3c1. Behav. Pharmacol. 2025, 36, 115–126. [Google Scholar] [CrossRef]
- Ghofrani-Jahromi, Z.; Nouri-Darehno, S.; Rahimi-Danesh, M.; Talaee, N.; Jasemi, E.; Razmi, A.; Vaseghi, S. Psilocybe cubensis extract potently prevents fear memory recall and freezing behavior in short-but not long-term in a rat model of posttraumatic stress disorder. Behav. Neurosci. 2024, 138, 73. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The therapeutic potential of psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Halberstadt, A.L.; Klein, A.K.; McCorvy, J.D.; Kaylo, K.W.; Kargbo, R.B.; Meisenheimer, P. Synthesis and biological evaluation of tryptamines found in hallucinogenic mushrooms: Norbaeocystin, baeocystin, norpsilocin, and aeruginascin. J. Nat. Prod. 2020, 83, 461–467. [Google Scholar] [CrossRef]
- Glatfelter, G.C.; Pottie, E.; Partilla, J.S.; Sherwood, A.M.; Kaylo, K.; Pham, D.N.; Naeem, M.; Sammeta, V.R.; DeBoer, S.; Golen, J.A.; et al. Structure–activity relationships for psilocybin, baeocystin, aeruginascin, and related analogues to produce pharmacological effects in mice. ACS Pharmacol. Transl. Sci. 2022, 5, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Chadeayne, A.R.; Pham, D.N.; Reid, B.G.; Golen, J.A.; Manke, D.R. Active metabolite of aeruginascin (4-Hydroxy-N, N, N-trimethyltryptamine): Synthesis, structure, and serotonergic binding affinity. ACS Omega 2020, 5, 16940–16943. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Experimental works | Articles with score 0 |
| Articles presenting the isolation of compounds from species of the Psilocybe genus | Articles that are not experimental |
| Articles presenting biological activities for species of the Psilocybe genus | Articles lacking the keywords in the text |
| Forensic analysis of human biological samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luz, M.A.; Guedes, H.V.S.; Bisneto, A.B.M.; Jesus, R.A.d.; Galdino, T.P.; Oliveira, L.C.; Afonso, V.I.; Fook, M.V.L.; Lima, A.G.B.; Silva, S.M.d.L.; et al. Chemical Composition and Biological Activities of Psilocybe Mushrooms: Gaps and Perspectives. Pharmaceuticals 2025, 18, 989. https://doi.org/10.3390/ph18070989
Luz MA, Guedes HVS, Bisneto ABM, Jesus RAd, Galdino TP, Oliveira LC, Afonso VI, Fook MVL, Lima AGB, Silva SMdL, et al. Chemical Composition and Biological Activities of Psilocybe Mushrooms: Gaps and Perspectives. Pharmaceuticals. 2025; 18(7):989. https://doi.org/10.3390/ph18070989
Chicago/Turabian StyleLuz, Mateus A., Hellen V. S. Guedes, Antônio B. M. Bisneto, Raquel A. de Jesus, Taynah P. Galdino, Lucas C. Oliveira, Victor Ignacio Afonso, Marcus Vinícius L. Fook, Antônio G. B. Lima, Suedina M. de L. Silva, and et al. 2025. "Chemical Composition and Biological Activities of Psilocybe Mushrooms: Gaps and Perspectives" Pharmaceuticals 18, no. 7: 989. https://doi.org/10.3390/ph18070989
APA StyleLuz, M. A., Guedes, H. V. S., Bisneto, A. B. M., Jesus, R. A. d., Galdino, T. P., Oliveira, L. C., Afonso, V. I., Fook, M. V. L., Lima, A. G. B., Silva, S. M. d. L., & Torres, M. C. M. (2025). Chemical Composition and Biological Activities of Psilocybe Mushrooms: Gaps and Perspectives. Pharmaceuticals, 18(7), 989. https://doi.org/10.3390/ph18070989








