Abstract
RNA interference (RNAi) was originally regarded as a mechanism of eukaryotic post-transcriptional gene regulation mediated by small interfering RNA (siRNA)-induced sequence-specific RNA degradation. It is well known to exert as an important antiviral defense mechanism in a wide range of organisms, from plants to invertebrates. The specificity, ease of design, and ability to target conserved gene regions make siRNA technology a promising approach to combat viral pathogenesis, allowing the targeting of multiple virus strains. The mechanism of sequence complementarity utilized by siRNAs against their targets presents a novel strategy to combat viral infections, as they can specifically target and degrade viral RNA. Consequently, siRNA-based therapeutics have been applied to various viral diseases. This is largely due to the design flexibility and rapid response potential of RNAi technologies, which provide advantages over traditional antiviral agents. However, the emergence of viral escape mutants poses a major barrier to the sustained antiviral activity of siRNA-based therapy. Therefore, devising strategies to overcome the emergence of escape mutants to antiviral siRNAs could enhance the efficacy of siRNA-based therapeutics in providing a rapid response to emerging viral infectious diseases. This review aims to comprehensively summarize the current knowledge on siRNA-based therapeutic approaches against viral infections and elucidate the challenges associated with implementing siRNA treatment, with a specific emphasis on antiviral resistance.
1. Introduction
RNA interference (RNAi) is a natural mechanism that defends against exogenous gene invasion [1,2], orchestrating the selective silencing of target genes through the degradation of their corresponding mRNA [3]. This process is activated by small double-stranded RNA (dsRNA) molecules, known as small interfering RNAs (siRNAs), which are typically 21–23 nucleotides long. These siRNAs bind to complementary mRNA, thereby inducing mRNA degradation. Introducing exogenous and synthetic siRNAs into cells triggers RNAi, driving RNAi-based therapies against disease-associated mRNA. This spans genetic disorders, acquired diseases, and viral infections [4,5,6,7,8,9,10]. Viral dsRNAs from infection activate RNAi [11]. These dsRNAs yield 21-nucleotide siRNA duplexes, facilitated by Dicer [12]. Within this duplex, one strand, the guide-strand, assembles with the RNA-induced silencing complex (RISC). RISC identifies viral mRNAs, leading to degradation [13,14]. Cleavage occurs around nucleotides 10–11 on the complementary antisense strand [15]. This highly orchestrated pathway underscores the therapeutic potential of siRNA therapy in combating viral infections [1,16].
RNAi offers numerous advantages as a therapeutic approach compared to small-molecule drugs, as it holds the potential to target virtually all genes using siRNA molecules. RNAi effectively hinders the replication of a wide range of viruses by using both siRNA and siRNA expression vectors [17]. Due to their ability to exhibit complete base-pairing and induce mRNA cleavage in a precise target region [18], siRNA can effectively inhibit viral infection by targeting specific genes essential for viral entry or replication [19,20]. This results in shorter research and development time and a more cost-effective therapeutic approach. Although targeting the viral genome and its sub-genomic transcripts might not prevent viral entry, this approach still provides protection to uninfected cells by introducing siRNAs that promptly degrade the viral RNA upon its release into the cytoplasm (Figure 1), thereby effectively preventing viral spread [21].
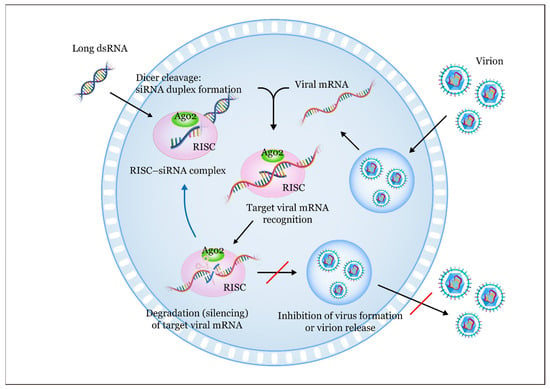
Figure 1.
The siRNA-mediated RNA interference (RNAi) pathway against a virus involves several steps: (1) Introduction of antiviral siRNAs: long double-stranded RNAs (dsRNAs) are initially introduced into the cell and subsequently cleaved by the enzyme RNase III (Dicer) in the cytoplasm. This cleavage results in the formation of small dsRNAs; (2) processing into siRNAs: these small dsRNAs are further processed by Dicer, yielding short siRNA molecules that are typically 21–30 nucleotides long [22]. These antiviral siRNAs act as the functional units in the silencing mechanism; (3) formation of siRNA duplexes: Dicer cleavage generates siRNA duplexes, consisting of two complementary strands [23]. These duplexes associate with a multi-protein complex called the RNA-induced silencing complex (RISC) [24,25,26]; (4) RISC assembly: the RNA strands within the siRNA duplexes are separated by the ATP-dependent RNA helicase domain of RISC [27]. One strand, known as the passenger (sense) strand, is degraded within the RISC; (5) guide strand function: the remaining antisense single-stranded RNA, also known as the guide RNA, assists in aligning the RISC with the target viral mRNA. This alignment allows the fragmentation of the RNA sense strand through the action of Argonaute 2 (Ago2); (6) viral mRNA cleavage: the guide strand within the RISC binds to complementary sequences in the target viral mRNA through the Watson–Crick base pairing [25]. Subsequently, the guide strand directs the RISC to cleave the target viral mRNA [28]; (7) impact on gene expression: this cleavage leads to degradation of the target viral mRNA, resulting in the inhibition of viral protein expression [29,30]. Consequently, the expression of targeted viral genes is reduced; and (8) antiviral potential: by targeting viral genes, this mechanism has the potential to inhibit viral replication and infection, making it a promising approach for antiviral therapy [31].
The advantages of siRNA, including its convenient design and production, make it a promising alternative to existing vaccines and antiviral compounds for targeting infectious diseases [32,33]. This readily adaptable approach is particularly beneficial for targeting viruses with rapidly varying dominant genotypes [34] and phenotypes. It is particularly valuable in situations where predicting vaccine efficacy is difficult, or in seasons when available vaccines inadequately match the circulating viral strains. siRNA-based therapies could potentially serve as a first line of defense against viral strains that acquire resistance to previously effective drugs and vaccines, either through natural evolution or human intervention [17]. This review is specifically focused on strategies to overcome the emergence of escape mutant viruses in the context of siRNA-based antiviral therapy. By narrowing the focus to resistance-related mechanisms, this review aims to provide a more in-depth and actionable framework for overcoming one of the most pressing barriers to siRNA-based antiviral therapies.
4. Concluding Remarks and Future Perspectives
Emerging infectious diseases pose a serious threat to public health and pandemic preparedness. The COVID-19 pandemic has shown the need for effective treatments for SARS-CoV-2. RNAi, a natural process that silences gene expression, has great potential for antiviral research and development. siRNAs, synthetic molecules that target specific genes for silencing, have been explored as a promising strategy to combat viral infections. siRNAs have the advantage of being able to target almost any viral gene and being faster to develop than other types of drugs.
However, siRNA-based antiviral RNAi faces several challenges that need to be overcome. A major challenge in antiviral therapy is the continuous mutation of viral genes, which compromises treatment efficacy and narrows therapeutic options. This challenge means the adaptation of viruses to the presence of siRNAs and their evasion of the host cell’s RNA silencing machinery. This is known as the “Red Queen hypothesis,” which describes a molecular “arms race” between viruses and their hosts. Therefore, the development and efficacy of siRNA-based antiviral therapies depend on how well they can prevent viral escape mutants. To cope with the ever-changing viral genome, it is important to target therapeutics that can block viral replication at conserved regions of the genome. Using multiple siRNAs that target different regions can help prevent resistant mutants and increase the chance of maintaining efficacy against new viral strains. Moreover, the efficacy of siRNA-based antiviral therapeutics is one of the important factors in the development of antiviral-resistant escape variants.
The biotechnology industry has invested a lot in developing siRNA-based therapeutic approaches for various diseases, including viral infections, and many studies have focused on antiviral siRNA in recent years. Ongoing research into novel target discovery and siRNA optimization is essential for reducing viral escape potential and achieving durable antiviral efficacy. Despite the challenges of using siRNA as antiviral therapy, the future of RNAi therapeutics looks promising with interdisciplinary collaboration and technological advancements. The use of exogenous antiviral siRNAs to silence genes through the natural RNAi pathway has become a popular technique with great therapeutic potential that may soon be realized. Therefore, it is reasonable to expect that antiviral siRNA applications will have a huge impact on the treatment of viral infections in the future.
Looking forward, it will be crucial to integrate computational prediction, structural modeling, and high-throughput screening to proactively design siRNA combinations that can remain effective against emerging escape variants. In parallel, more research is needed to evaluate these strategies in vivo and ensure their clinical viability across different virus families. Although this review focuses on siRNA-based antiviral approaches, alternative gene-editing technologies such as CRISPR-Cas systems have also shown promise in targeting viral genomes. While CRISPR-based strategies offer certain advantages, such as permanent gene disruption, they also raise unique challenges in terms of delivery and specificity. Future comparative studies evaluating these platforms in the context of rapidly evolving viruses would be valuable.
Funding
This research was supported by an Incheon National University Research Grant in 2019.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Li, B.J.; Tang, Q.; Cheng, D.; Qin, C.; Xie, F.Y.; Wei, Q.; Xu, J.; Liu, Y.; Zheng, B.J.; Woodle, M.C.; et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005, 11, 944–951. [Google Scholar] [CrossRef]
- Zender, L.; Hutker, S.; Liedtke, C.; Tillmann, H.L.; Zender, S.; Mundt, B.; Waltemathe, M.; Gosling, T.; Flemming, P.; Malek, N.P.; et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Lee, S.K.; Wang, J.; Ince, N.; Ouyang, N.; Min, J.; Chen, J.; Shankar, P.; Lieberman, J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 2003, 9, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, L.; van der Kuip, H.; Miething, C.; Vornlocher, H.P.; Knabbe, C.; Duyster, J.; Aulitzky, W.E. Inhibition of bcr-abl gene expression by small interfering RNA sensitizes for imatinib mesylate (STI571). Blood 2003, 102, 2236–2239. [Google Scholar] [CrossRef]
- Scherr, M.; Battmer, K.; Winkler, T.; Heidenreich, O.; Ganser, A.; Eder, M. Specific inhibition of bcr-abl gene expression by small interfering RNA. Blood 2003, 101, 1566–1569. [Google Scholar] [CrossRef]
- Moore, M.D.; McGarvey, M.J.; Russell, R.A.; Cullen, B.R.; McClure, M.O. Stable inhibition of hepatitis B virus proteins by small interfering RNA expressed from viral vectors. J. Gene Med. 2005, 7, 918–925. [Google Scholar] [CrossRef]
- Konishi, M.; Wu, C.H.; Kaito, M.; Hayashi, K.; Watanabe, S.; Adachi, Y.; Wu, G.Y. siRNA-resistance in treated HCV replicon cells is correlated with the development of specific HCV mutations. J. Viral Hepat. 2006, 13, 756–761. [Google Scholar] [CrossRef]
- Wilkins, C.; Dishongh, R.; Moore, S.C.; Whitt, M.A.; Chow, M.; Machaca, K. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 2005, 436, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Galiana-Arnoux, D.; Dostert, C.; Schneemann, A.; Hoffmann, J.A.; Imler, J.L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat. Immunol. 2006, 7, 590–597. [Google Scholar] [CrossRef]
- Schott, D.H.; Cureton, D.K.; Whelan, S.P.; Hunter, C.P. An antiviral role for the RNA interference machinery in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2005, 102, 18420–18424. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Aliyari, R.; Li, W.X.; Li, H.W.; Kim, K.; Carthew, R.; Atkinson, P.; Ding, S.W. RNA interference directs innate immunity against viruses in adult Drosophila. Science 2006, 312, 452–454. [Google Scholar] [CrossRef]
- Rand, T.A.; Petersen, S.; Du, F.; Wang, X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell 2005, 123, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef]
- Leonard, J.N.; Schaffer, D.V. Antiviral RNAi therapy: Emerging approaches for hitting a moving target. Gene Ther. 2006, 13, 532–540. [Google Scholar] [CrossRef]
- Pillai, R.S.; Bhattacharyya, S.N.; Filipowicz, W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007, 17, 118–126. [Google Scholar] [CrossRef]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar] [CrossRef]
- Kalhori, M.R.; Saadatpour, F.; Arefian, E.; Soleimani, M.; Farzaei, M.H.; Aneva, I.Y.; Echeverria, J. The Potential Therapeutic Effect of RNA Interference and Natural Products on COVID-19: A Review of the Coronaviruses Infection. Front. Pharmacol. 2021, 12, 616993. [Google Scholar] [CrossRef]
- Friedrich, M.; Pfeifer, G.; Binder, S.; Aigner, A.; Vollmer Barbosa, P.; Makert, G.R.; Fertey, J.; Ulbert, S.; Bodem, J.; Konig, E.M.; et al. Selection and Validation of siRNAs Preventing Uptake and Replication of SARS-CoV-2. Front. Bioeng. Biotechnol. 2022, 10, 801870. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Parashar, D.; Rajendran, V.; Shukla, R.; Sistla, R. Lipid-based nanocarriers for delivery of small interfering RNA for therapeutic use. Eur. J. Pharm. Sci. 2020, 142, 105159. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Michler, T.; Merkel, O.M. siRNA Therapeutics against Respiratory Viral Infections-What Have We Learned for Potential COVID-19 Therapies? Adv. Heal. Mater. 2021, 10, e2001650. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Barik, S. siRNA for Influenza Therapy. Viruses 2010, 2, 1448–1457. [Google Scholar] [CrossRef]
- Zamore, P.D. RNA interference: Listening to the sound of silence. Nat. Struct. Biol. 2001, 8, 746–750. [Google Scholar] [CrossRef]
- Pantaleo, V.; Szittya, G.; Burgyán, J. Molecular bases of viral RNA targeting by viral small interfering RNA-programmed RISC. J. Virol. 2007, 81, 3797–3806. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Fu, L.; Yu, C.; Li, X.; Li, Y.; Zhang, X.; Rong, Z.; Wang, Y.; Ning, H.; et al. siRNA targeting the leader sequence of SARS-CoV inhibits virus replication. Gene Ther. 2005, 12, 751–761. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Cho, Y.; Kato, S.; Xu, A.; Way, J.J.; Lohan, S.; Tiwari, R.K. siRNA Therapeutics for the Therapy of COVID-19 and Other Coronaviruses. Mol. Pharm. 2021, 18, 2105–2121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Haasnoot, J.; ter Brake, O.; Berkhout, B.; Konstantinova, P. Inhibition of HIV-1 by multiple siRNAs expressed from a single microRNA polycistron. Nucleic Acids Res. 2008, 36, 2811–2824. [Google Scholar] [CrossRef]
- Forgham, H.; Kakinen, A.; Qiao, R.; Davis, T.P. Keeping up with the COVID’s-Could siRNA-based antivirals be a part of the answer? Exploration 2022, 2, 20220012. [Google Scholar] [CrossRef] [PubMed]
- Tolksdorf, B.; Nie, C.; Niemeyer, D.; Rohrs, V.; Berg, J.; Lauster, D.; Adler, J.M.; Haag, R.; Trimpert, J.; Kaufer, B.; et al. Inhibition of SARS-CoV-2 Replication by a Small Interfering RNA Targeting the Leader Sequence. Viruses 2021, 13, 2030. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Richardson, C.D. Hepatitis C virus replicons escape RNA interference induced by a short interfering RNA directed against the NS5b coding region. J. Virol. 2005, 79, 7050–7058. [Google Scholar] [CrossRef]
- Das, A.T.; Brummelkamp, T.R.; Westerhout, E.M.; Vink, M.; Madiredjo, M.; Bernards, R.; Berkhout, B. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J. Virol. 2004, 78, 2601–2605. [Google Scholar] [CrossRef]
- Boden, D.; Pusch, O.; Lee, F.; Tucker, L.; Ramratnam, B. Human immunodeficiency virus type 1 escape from RNA interference. J. Virol. 2003, 77, 11531–11535. [Google Scholar] [CrossRef]
- Drake, J.W.; Charlesworth, B.; Charlesworth, D.; Crow, J.F. Rates of spontaneous mutation. Genetics 1998, 148, 1667–1686. [Google Scholar] [CrossRef]
- Westerhout, E.M.; Ooms, M.; Vink, M.; Das, A.T.; Berkhout, B. HIV-1 can escape from RNA interference by evolving an alternative structure in its RNA genome. Nucleic Acids Res. 2005, 33, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Ding, H.; Liu, M.; Zhao, P.; Gao, J.; Ren, H.; Liu, Y.; Qi, Z.T. Inhibition of hepatitis C virus replication and expression by small interfering RNA targeting host cellular genes. Arch. Virol. 2007, 152, 955–962. [Google Scholar] [CrossRef]
- Gitlin, L.; Karelsky, S.; Andino, R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 2002, 418, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Pusch, O.; Boden, D.; Silbermann, R.; Lee, F.; Tucker, L.; Ramratnam, B. Nucleotide sequence homology requirements of HIV-1-specific short hairpin RNA. Nucleic Acids Res. 2003, 31, 6444–6449. [Google Scholar] [CrossRef]
- Amarzguioui, M.; Holen, T.; Babaie, E.; Prydz, H. Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Res. 2003, 31, 589–595. [Google Scholar] [CrossRef]
- Haley, B.; Zamore, P.D. Kinetic analysis of the RNAi enzyme complex. Nat. Struct. Mol. Biol. 2004, 11, 599–606. [Google Scholar] [CrossRef]
- Ayyagari, V.S. Design of siRNA molecules for silencing of membrane glycoprotein, nucleocapsid phosphoprotein, and surface glycoprotein genes of SARS-CoV2. J. Genet. Eng. Biotechnol. 2022, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Verma, S. An in silico analysis of effective siRNAs against COVID-19 by targeting the leader sequence of SARS-CoV-2. Adv. Cell Gene Ther. 2021, 4, e107. [Google Scholar] [CrossRef]
- Niktab, I.; Haghparast, M.; Beigi, M.H.; Megraw, T.L.; Kiani, A.; Ghaedi, K. Design of advanced siRNA therapeutics for the treatment of COVID-19. Meta Gene 2021, 29, 100910. [Google Scholar] [CrossRef]
- Chen, W.; Feng, P.; Liu, K.; Wu, M.; Lin, H. Computational Identification of Small Interfering RNA Targets in SARS-CoV-2. Virol. Sin. 2020, 35, 359–361. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hong, W.; Pan, X.; Lu, G.; Wei, X. SARS-CoV-2 Omicron variant: Characteristics and prevention. MedComm 2021, 2, 838–845. [Google Scholar] [CrossRef]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef]
- Jo, W.K.; Drosten, C.; Drexler, J.F. The evolutionary dynamics of endemic human coronaviruses. Virus Evol. 2021, 7, veab020. [Google Scholar] [CrossRef]
- Lafforgue, G.; Martínez, F.; Sardanyés, J.; de la Iglesia, F.; Niu, Q.W.; Lin, S.S.; Solé, R.V.; Chua, N.H.; Daròs, J.A.; Elena, S.F. Tempo and mode of plant RNA virus escape from RNA interference-mediated resistance. J. Virol. 2011, 85, 9686–9695. [Google Scholar] [CrossRef]
- Meister, G. Argonaute proteins: Functional insights and emerging roles. Nat. Rev. Genet. 2013, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Bolaji, A.; Roussin-Léveillée, C.; Zhao, Z.; Biga, S.; Moffett, P. Natural variation in the Arabidopsis AGO2 gene is associated with susceptibility to potato virus X. New Phytol. 2020, 226, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Holz, C.L.; Albina, E.; Minet, C.; Lancelot, R.; Kwiatek, O.; Libeau, G.; Servan de Almeida, R. RNA interference against animal viruses: How morbilliviruses generate extended diversity to escape small interfering RNA control. J. Virol. 2012, 86, 786–795. [Google Scholar] [CrossRef]
- Aguado, L.C.; Jordan, T.X.; Hsieh, E.; Blanco-Melo, D.; Heard, J.; Panis, M.; Vignuzzi, M.; tenOever, B.R. Homologous recombination is an intrinsic defense against antiviral RNA interference. Proc. Natl. Acad. Sci. USA 2018, 115, E9211–E9219. [Google Scholar] [CrossRef]
- Fopase, R.; Panda, C.; Rajendran, A.P.; Uludag, H.; Pandey, L.M. Potential of siRNA in COVID-19 therapy: Emphasis on in silico design and nanoparticles based delivery. Front. Bioeng. Biotechnol. 2023, 11, 1112755. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wernli, M.; Klimkait, T.; Erb, P. Enhanced gene silencing by the application of multiple specific small interfering RNAs. FEBS Lett. 2003, 552, 247–252. [Google Scholar] [CrossRef]
- Zheng, B.J.; Guan, Y.; Tang, Q.; Du, C.; Xie, F.Y.; He, M.L.; Chan, K.W.; Wong, K.L.; Lader, E.; Woodle, M.C.; et al. Prophylactic and therapeutic effects of small interfering RNA targeting SARS-coronavirus. Antivir. Ther. 2004, 9, 365–374. [Google Scholar] [CrossRef]
- Kahana, R.; Kuznetzova, L.; Rogel, A.; Shemesh, M.; Hai, D.; Yadin, H.; Stram, Y. Inhibition of foot-and-mouth disease virus replication by small interfering RNA. J. Gen. Virol. 2004, 85, 3213–3217. [Google Scholar] [CrossRef]
- Traube, F.R.; Stern, M.; Tolke, A.J.; Rudelius, M.; Mejias-Perez, E.; Raddaoui, N.; Kummerer, B.M.; Douat, C.; Streshnev, F.; Albanese, M.; et al. Suppression of SARS-CoV-2 Replication with Stabilized and Click-Chemistry Modified siRNAs. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204556. [Google Scholar] [CrossRef]
- Lee, Y.R.; Tsai, H.P.; Yeh, C.S.; Fang, C.Y.; Chan, M.W.Y.; Wu, T.Y.; Shen, C.H. RNA Interference Approach Is a Good Strategy against SARS-CoV-2. Viruses 2022, 15, 100. [Google Scholar] [CrossRef]
- Wu, R.; Luo, K.Q. Developing effective siRNAs to reduce the expression of key viral genes of COVID-19. Int. J. Biol. Sci. 2021, 17, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Vigne, S.; Duraffour, S.; Andrei, G.; Snoeck, R.; Garin, D.; Crance, J.M. Inhibition of vaccinia virus replication by two small interfering RNAs targeting B1R and G7L genes and their synergistic combination with cidofovir. Antimicrob. Agents Chemother. 2009, 53, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Rossi, J.J. RNAi in combination with a ribozyme and TAR decoy for treatment of HIV infection in hematopoietic cell gene therapy. Ann. N. Y. Acad. Sci. 2006, 1082, 172–179. [Google Scholar] [CrossRef]
- Flusin, O.; Vigne, S.; Peyrefitte, C.N.; Bouloy, M.; Crance, J.M.; Iseni, F. Inhibition of Hazara nairovirus replication by small interfering RNAs and their combination with ribavirin. Virol. J. 2011, 8, 249. [Google Scholar] [CrossRef]
- Li, J.; Dai, Y.; Liu, S.; Guo, H.; Wang, T.; Ouyang, H.; Tu, C. In vitro inhibition of CSFV replication by multiple siRNA expression. Antivir. Res. 2011, 91, 209–216. [Google Scholar] [CrossRef]
- Idris, A.; Davis, A.; Supramaniam, A.; Acharya, D.; Kelly, G.; Tayyar, Y.; West, N.; Zhang, P.; McMillan, C.L.D.; Soemardy, C.; et al. A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol. Ther. 2021, 29, 2219–2226. [Google Scholar] [CrossRef]
- He, M.L.; Zheng, B.J.; Chen, Y.; Wong, K.L.; Huang, J.D.; Lin, M.C.; Peng, Y.; Yuen, K.Y.; Sung, J.J.; Kung, H.F. Kinetics and synergistic effects of siRNAs targeting structural and replicase genes of SARS-associated coronavirus. FEBS Lett. 2006, 580, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Zheng, B.J.; Chen, Y.; Wong, K.L.; Huang, J.D.; Lin, M.C.; Yuen, K.Y.; Sung, J.J.; Kung, H.F. Development of interfering RNA agents to inhibit SARS-associated coronavirus infection and replication. Hong Kong Med. J. 2009, 15, 28–31. [Google Scholar] [PubMed]
- Xing, X.K.; Li, S.J.; He, J.L.; Chen, Z. Inhibition of hepatitis C virus replication by single and dual small interfering RNA using an HCV-infected cell model. Biotechnol. Lett. 2012, 34, 295–301. [Google Scholar] [CrossRef]
- Leonard, J.N.; Schaffer, D.V. Computational design of antiviral RNA interference strategies that resist human immunodeficiency virus escape. J. Virol. 2005, 79, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, L.; Stone, J.K.; Andino, R. Poliovirus escape from RNA interference: Short interfering RNA-target recognition and implications for therapeutic approaches. J. Virol. 2005, 79, 1027–1035. [Google Scholar] [CrossRef]
- Ghosh, S.; Firdous, S.M.; Nath, A. siRNA could be a potential therapy for COVID-19. Excli J. 2020, 19, 528–531. [Google Scholar] [CrossRef]
- Chowdhury, U.F.; Sharif Shohan, M.U.; Hoque, K.I.; Beg, M.A.; Sharif Siam, M.K.; Moni, M.A. A computational approach to design potential siRNA molecules as a prospective tool for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2. Genomics 2021, 113, 331–343. [Google Scholar] [CrossRef]
- Hasan, M.; Ashik, A.I.; Chowdhury, M.B.; Tasnim, A.T.; Nishat, Z.S.; Hossain, T.; Ahmed, S. Computational prediction of potential siRNA and human miRNA sequences to silence orf1ab associated genes for future therapeutics against SARS-CoV-2. Inf. Med. Unlocked 2021, 24, 100569. [Google Scholar] [CrossRef]
- Randall, G.; Rice, C.M. Interfering with hepatitis C virus RNA replication. Virus Res. 2004, 102, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, S.; Jahan, S.; Pervaiz, A.; Ali Ashfaq, U.; Hassan, S. Down-regulation of IRES containing 5’UTR of HCV genotype 3a using siRNAs. Virol. J. 2011, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Small interfering RNA targeted to hepatitis C virus 5’ nontranslated region exerts potent antiviral effect. J. Virol. 2007, 81, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.A.; Perry, S.T.; Buck, M.D.; Oehmen, C.S.; Fischer, M.A.; Poore, E.; Smith, J.L.; Lancaster, A.M.; Hirsch, A.J.; Slifka, M.K.; et al. Inhibition of dengue virus infections in cell cultures and in AG129 mice by a small interfering RNA targeting a highly conserved sequence. J. Virol. 2011, 85, 10154–10166. [Google Scholar] [CrossRef]
- Kumar, P.; Lee, S.K.; Shankar, P.; Manjunath, N. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 2006, 3, e96. [Google Scholar] [CrossRef]
- Baldassarre, A.; Paolini, A.; Bruno, S.P.; Felli, C.; Tozzi, A.E.; Masotti, A. Potential use of noncoding RNAs and innovative therapeutic strategies to target the 5’UTR of SARS-CoV-2. Epigenomics 2020, 12, 1349–1361. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Grunert, H.P.; Zeichhardt, H.; Lena, S.W.; Wengel, J.; Kurreck, J. Design of LNA-modified siRNAs against the highly structured 5’ UTR of coxsackievirus B3. FEBS Lett. 2008, 582, 3061–3066. [Google Scholar] [CrossRef]
- Wu, C.J.; Huang, H.W.; Liu, C.Y.; Hong, C.F.; Chan, Y.L. Inhibition of SARS-CoV replication by siRNA. Antivir. Res. 2005, 65, 45–48. [Google Scholar] [CrossRef]
- Wu, H.L.; Huang, L.R.; Huang, C.C.; Lai, H.L.; Liu, C.J.; Huang, Y.T.; Hsu, Y.W.; Lu, C.Y.; Chen, D.S.; Chen, P.J. RNA interference-mediated control of hepatitis B virus and emergence of resistant mutant. Gastroenterology 2005, 128, 708–716. [Google Scholar] [CrossRef]
- Chang, Y.C.; Yang, C.F.; Chen, Y.F.; Yang, C.C.; Chou, Y.L.; Chou, H.W.; Chang, T.Y.; Chao, T.L.; Hsu, S.C.; Ieong, S.M.; et al. A siRNA targets and inhibits a broad range of SARS-CoV-2 infections including Delta variant. EMBO Mol. Med. 2022, 14, e15298. [Google Scholar] [CrossRef]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. Chembiochem 2020, 21, 730–738. [Google Scholar] [CrossRef]
- von Eije, K.J.; ter Brake, O.; Berkhout, B. Human immunodeficiency virus type 1 escape is restricted when conserved genome sequences are targeted by RNA interference. J. Virol. 2008, 82, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Thonberg, H.; Wang, J.; Wahlestedt, C.; Liang, Z. A systematic analysis of the silencing effects of an active siRNA at all single-nucleotide mismatched target sites. Nucleic Acids Res. 2005, 33, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L. Inhibition of multiple strains of Venezuelan equine encephalitis virus by a pool of four short interfering RNAs. Antivir. Res. 2007, 75, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, J. In Silico Design of siRNAs Targeting Existing and Future Respiratory Viruses with VirusSi. bioRxiv 2020. [Google Scholar] [CrossRef]
- Nur, S.M.; Hasan, M.A.; Amin, M.A.; Hossain, M.; Sharmin, T. Design of Potential RNAi (miRNA and siRNA) Molecules for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Gene Silencing by Computational Method. Interdiscip. Sci. 2015, 7, 257–265. [Google Scholar] [CrossRef]
- Qureshi, A.; Tantray, V.G.; Kirmani, A.R.; Ahangar, A.G. A review on current status of antiviral siRNA. Rev. Med. Virol. 2018, 28, e1976. [Google Scholar] [CrossRef]
- Tyagi, A.; Ahmed, F.; Thakur, N.; Sharma, A.; Raghava, G.P.; Kumar, M. HIVsirDB: A database of HIV inhibiting siRNAs. PLoS ONE 2011, 6, e25917. [Google Scholar] [CrossRef]
- Thakur, N.; Qureshi, A.; Kumar, M. VIRsiRNAdb: A curated database of experimentally validated viral siRNA/shRNA. Nucleic Acids Res. 2012, 40, D230–D236. [Google Scholar] [CrossRef]
- Naito, Y.; Ui-Tei, K.; Nishikawa, T.; Takebe, Y.; Saigo, K. siVirus: Web-based antiviral siRNA design software for highly divergent viral sequences. Nucleic Acids Res. 2006, 34, W448–W450. [Google Scholar] [CrossRef]
- Yogev, O.; Weissbrod, O.; Battistoni, G.; Bressan, D.; Naamti, A.; Falciatori, I.; Berkyurek, A.C.; Rasnic, R.; Hosmillo, M.; Ilan, S.; et al. Genome wide screen of RNAi molecules against SARS-CoV-2 creates a broadly potent prophylaxis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tan, X.; Lu, Z.J.; Gao, G.; Xu, Q.; Hu, L.; Fellmann, C.; Li, M.Z.; Qu, H.; Lowe, S.W.; Hannon, G.J.; et al. Tiling genomes of pathogenic viruses identifies potent antiviral shRNAs and reveals a role for secondary structure in shRNA efficacy. Proc. Natl. Acad. Sci. USA 2012, 109, 869–874. [Google Scholar] [CrossRef]
- Fellmann, C.; Zuber, J.; McJunkin, K.; Chang, K.; Malone, C.D.; Dickins, R.A.; Xu, Q.; Hengartner, M.O.; Elledge, S.J.; Hannon, G.J.; et al. Functional identification of optimized RNAi triggers using a massively parallel sensor assay. Mol. Cell 2011, 41, 733–746. [Google Scholar] [CrossRef] [PubMed]
- El-Kafrawy, S.A.; Sohrab, S.S.; Mirza, Z.; Hassan, A.M.; Alsaqaf, F.; Azhar, E.I. In Vitro Inhibitory Analysis of Rationally Designed siRNAs against MERS-CoV Replication in Huh7 Cells. Molecules 2021, 26, 2610. [Google Scholar] [CrossRef] [PubMed]
- ElHefnawi, M.; Kim, T.; Kamar, M.A.; Min, S.; Hassan, N.M.; El-Ahwany, E.; Kim, H.; Zada, S.; Amer, M.; Windisch, M.P. In Silico Design and Experimental Validation of siRNAs Targeting Conserved Regions of Multiple Hepatitis C Virus Genotypes. PLoS ONE 2016, 11, e0159211. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Thakur, N.; Kumar, M. VIRsiRNApred: A web server for predicting inhibition efficacy of siRNAs targeting human viruses. J. Transl. Med. 2013, 11, 305. [Google Scholar] [CrossRef]
- Beerenwinkel, N.; Daumer, M.; Oette, M.; Korn, K.; Hoffmann, D.; Kaiser, R.; Lengauer, T.; Selbig, J.; Walter, H. Geno2pheno: Estimating phenotypic drug resistance from HIV-1 genotypes. Nucleic Acids Res. 2003, 31, 3850–3855. [Google Scholar] [CrossRef]
- Pozzuto, T.; Roger, C.; Kurreck, J.; Fechner, H. Enhanced suppression of adenovirus replication by triple combination of anti-adenoviral siRNAs, soluble adenovirus receptor trap sCAR-Fc and cidofovir. Antivir. Res. 2015, 120, 72–78. [Google Scholar] [CrossRef]
- Yuen, M.F.; Locarnini, S.; Lim, T.H.; Strasser, S.I.; Sievert, W.; Cheng, W.; Thompson, A.J.; Given, B.D.; Schluep, T.; Hamilton, J.; et al. Combination treatments including the small-interfering RNA JNJ-3989 induce rapid and sometimes prolonged viral responses in patients with CHB. J. Hepatol. 2022, 77, 1287–1298. [Google Scholar] [CrossRef]
- Bramsen, J.B.; Laursen, M.B.; Nielsen, A.F.; Hansen, T.B.; Bus, C.; Langkjaer, N.; Babu, B.R.; Hojland, T.; Abramov, M.; Van Aerschot, A.; et al. A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity. Nucleic Acids Res. 2009, 37, 2867–2881. [Google Scholar] [CrossRef]
- Robbins, M.; Judge, A.; Liang, L.; McClintock, K.; Yaworski, E.; MacLachlan, I. 2’-O-methyl-modified RNAs act as TLR7 antagonists. Mol. Ther. 2007, 15, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Swiderski, P.; Li, H.; Zhang, J.; Neff, C.P.; Akkina, R.; Rossi, J.J. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009, 37, 3094–3109. [Google Scholar] [CrossRef]
- Gish, R.G.; Yuen, M.F.; Chan, H.L.; Given, B.D.; Lai, C.L.; Locarnini, S.A.; Lau, J.Y.; Wooddell, C.I.; Schluep, T.; Lewis, D.L. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antivir. Res. 2015, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Almazi, J.G.; Ong, H.X.; Johansen, M.D.; Ledger, S.; Traini, D.; Hansbro, P.M.; Kelleher, A.D.; Ahlenstiel, C.L. Nanoparticle Delivery Platforms for RNAi Therapeutics Targeting COVID-19 Disease in the Respiratory Tract. Int. J. Mol. Sci. 2022, 23, 2408. [Google Scholar] [CrossRef] [PubMed]
- Snead, N.M.; Escamilla-Powers, J.R.; Rossi, J.J.; McCaffrey, A.P. 5’ Unlocked Nucleic Acid Modification Improves siRNA Targeting. Mol. Ther. Nucleic Acids 2013, 2, e103. [Google Scholar] [CrossRef]
- Janas, M.M.; Schlegel, M.K.; Harbison, C.E.; Yilmaz, V.O.; Jiang, Y.; Parmar, R.; Zlatev, I.; Castoreno, A.; Xu, H.; Shulga-Morskaya, S.; et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018, 9, 723. [Google Scholar] [CrossRef]
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E.; et al. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021, 76, 2840–2854. [Google Scholar] [CrossRef]
- Tai, W. Current Aspects of siRNA Bioconjugate for In Vitro and In Vivo Delivery. Molecules 2019, 24, 2211. [Google Scholar] [CrossRef]
- Terada, T.; Kulkarni, J.A.; Huynh, A.; Chen, S.; van der Meel, R.; Tam, Y.Y.C.; Cullis, P.R. Characterization of Lipid Nanoparticles Containing Ionizable Cationic Lipids Using Design-of-Experiments Approach. Langmuir 2021, 37, 1120–1128. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef]
- Ge, X.; Chen, L.; Zhao, B.; Yuan, W. Rationale and Application of PEGylated Lipid-Based System for Advanced Target Delivery of siRNA. Front. Pharmacol. 2020, 11, 598175. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Tobaiqy, M.; Al Faraj, A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics 2020, 10, 5932–5942. [Google Scholar] [CrossRef]
- Mehta, M.; Paudel, K.R.; Shukla, S.D.; Shastri, M.D.; Satija, S.; Singh, S.K.; Gulati, M.; Dureja, H.; Zacconi, F.C.; Hansbro, P.M.; et al. Rutin-loaded liquid crystalline nanoparticles attenuate oxidative stress in bronchial epithelial cells: A PCR validation. Future Med. Chem. 2021, 13, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Mehta, M.; Chellappan, D.K.; Gupta, G.; Hansbro, N.G.; Tambuwala, M.M.; Aa Aljabali, A.; Paudel, K.R.; Liu, G.; Satija, S.; et al. Antiproliferative effects of boswellic acid-loaded chitosan nanoparticles on human lung cancer cell line A549. Future Med. Chem. 2020, 12, 2019–2034. [Google Scholar] [CrossRef]
- Khatak, S.; Mehta, M.; Awasthi, R.; Paudel, K.R.; Singh, S.K.; Gulati, M.; Hansbro, N.G.; Hansbro, P.M.; Dua, K.; Dureja, H. Solid lipid nanoparticles containing anti-tubercular drugs attenuate the Mycobacterium marinum infection. Tuberculosis 2020, 125, 102008. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Mehta, M.; Paudel, K.R.; Madheswaran, T.; Panneerselvam, J.; Gupta, G.; Su, Q.P.; Hansbro, P.M.; MacLoughlin, R.; Dua, K.; et al. Versatility of liquid crystalline nanoparticles in inflammatory lung diseases. Nanomedicine 2021, 16, 1545–1548. [Google Scholar] [CrossRef]
- Aigner, A.; Kogel, D. Nanoparticle/siRNA-based therapy strategies in glioma: Which nanoparticles, which siRNAs? Nanomedicine 2018, 13, 89–103. [Google Scholar] [CrossRef]
- ter Brake, O.; Konstantinova, P.; Ceylan, M.; Berkhout, B. Silencing of HIV-1 with RNA interference: A multiple shRNA approach. Mol. Ther. 2006, 14, 883–892. [Google Scholar] [CrossRef]
- ter Brake, O.; Hooft, K.; Liu, Y.P.; Centlivre, M.; von Eije, K.J.; Berkhout, B. Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 557–564. [Google Scholar] [CrossRef]
- Kronke, J.; Kittler, R.; Buchholz, F.; Windisch, M.P.; Pietschmann, T.; Bartenschlager, R.; Frese, M. Alternative approaches for efficient inhibition of hepatitis C virus RNA replication by small interfering RNAs. J. Virol. 2004, 78, 3436–3446. [Google Scholar] [CrossRef]
- Chen, Y.; Mahato, R.I. siRNA pool targeting different sites of human hepatitis B surface antigen efficiently inhibits HBV infection. J. Drug Target. 2008, 16, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Mendez, C.; Ledger, S.; Petoumenos, K.; Ahlenstiel, C.; Kelleher, A.D. RNA-induced epigenetic silencing inhibits HIV-1 reactivation from latency. Retrovirology 2018, 15, 67. [Google Scholar] [CrossRef]
- Li, M.J.; Bauer, G.; Michienzi, A.; Yee, J.K.; Lee, N.S.; Kim, J.; Li, S.; Castanotto, D.; Zaia, J.; Rossi, J.J. Inhibition of HIV-1 infection by lentiviral vectors expressing Pol III-promoted anti-HIV RNAs. Mol. Ther. 2003, 8, 196–206. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, G.J.; Groneman, J.L.; Yu, Y.H.; Tran, A.; Applegate, T.L. Multiple shRNA combinations for near-complete coverage of all HIV-1 strains. AIDS Res. Ther. 2011, 8, 1. [Google Scholar] [CrossRef]
- Ketzinel-Gilad, M.; Shaul, Y.; Galun, E. RNA interference for antiviral therapy. J. Gene Med. 2006, 8, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Parashar, D.; Paingankar, M.S.; Kumar, S.; Gokhale, M.D.; Sudeep, A.B.; Shinde, S.B.; Arankalle, V.A. Administration of E2 and NS1 siRNAs inhibit chikungunya virus replication in vitro and protects mice infected with the virus. PLoS Negl. Trop. Dis. 2013, 7, e2405. [Google Scholar] [CrossRef]
- Jeengar, M.K.; Kurakula, M.; Patil, P.; More, A.; Sistla, R.; Parashar, D. Effect of Cationic Lipid Nanoparticle Loaded siRNA with Stearylamine against Chikungunya Virus. Molecules 2022, 27, 1170. [Google Scholar] [CrossRef]
- Merl, S.; Michaelis, C.; Jaschke, B.; Vorpahl, M.; Seidl, S.; Wessely, R. Targeting 2A protease by RNA interference attenuates coxsackieviral cytopathogenicity and promotes survival in highly susceptible mice. Circulation 2005, 111, 1583–1592. [Google Scholar] [CrossRef]
- Lee, H.S.; Ahn, J.; Jee, Y.; Seo, I.S.; Jeon, E.J.; Jeon, E.S.; Joo, C.H.; Kim, Y.K.; Lee, H. Universal and mutation-resistant anti-enteroviral activity: Potency of small interfering RNA complementary to the conserved cis-acting replication element within the enterovirus coding region. J. Gen. Virol. 2007, 88, 2003–2012. [Google Scholar] [CrossRef]
- Tompkins, S.M.; Lo, C.Y.; Tumpey, T.M.; Epstein, S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 8682–8686. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Günther, S. Broad-spectrum antiviral activity of small interfering RNA targeting the conserved RNA termini of Lassa virus. Antimicrob. Agents Chemother. 2007, 51, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Elbashir, S.; Borland, T.; Toudjarska, I.; Hadwiger, P.; John, M.; Roehl, I.; Morskaya, S.S.; Martinello, R.; Kahn, J.; et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob. Agents Chemother. 2009, 53, 3952–3962. [Google Scholar] [CrossRef]
- Ong, S.P.; Choo, B.G.; Chu, J.J.; Ng, M.L. Expression of vector-based small interfering RNA against West Nile virus effectively inhibits virus replication. Antivir. Res. 2006, 72, 216–223. [Google Scholar] [CrossRef]
- Kachko, A.V.; Ivanova, A.V.; Protopopova, E.V.; Netesov, V.; Loktev, V.B. Inhibition of West Nile virus replication by short interfering RNAs. Dokl. Biochem. Biophys. 2006, 410, 260–262. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; Wu, J.; Nerurkar, V.R.; Yanagihara, R.; Lu, Y. Inhibition of West Nile Virus replication by retrovirus-delivered small interfering RNA in human neuroblastoma cells. J. Med. Virol. 2008, 80, 930–936. [Google Scholar] [CrossRef]
- Anthony, K.G.; Bai, F.; Krishnan, M.N.; Fikrig, E.; Koski, R.A. Effective siRNA targeting of the 3’ untranslated region of the West Nile virus genome. Antivir. Res. 2009, 82, 166–168. [Google Scholar] [CrossRef]
- Karothia, D.; Dash, P.K.; Parida, M.; Bhagyawant, S.; Kumar, J.S. Inhibition of West Nile virus Replication by Bifunctional siRNA Targeting the NS2A and NS5 Conserved Region. Curr. Gene Ther. 2018, 18, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Ursic-Bedoya, R.; Mire, C.E.; Robbins, M.; Geisbert, J.B.; Judge, A.; MacLachlan, I.; Geisbert, T.W. Protection against lethal Marburg virus infection mediated by lipid encapsulated small interfering RNA. J. Infect. Dis. 2014, 209, 562–570. [Google Scholar] [CrossRef]
- Thi, E.P.; Mire, C.E.; Lee, A.C.; Geisbert, J.B.; Ursic-Bedoya, R.; Agans, K.N.; Robbins, M.; Deer, D.J.; Cross, R.W.; Kondratowicz, A.S.; et al. siRNA rescues nonhuman primates from advanced Marburg and Ravn virus disease. J. Clin. Investig. 2017, 127, 4437–4448. [Google Scholar] [CrossRef]
- Weinberger, L.S.; Burnett, J.C.; Toettcher, J.E.; Arkin, A.P.; Schaffer, D.V. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell 2005, 122, 169–182. [Google Scholar] [CrossRef]
- Uludag, H.; Parent, K.; Aliabadi, H.M.; Haddadi, A. Prospects for RNAi Therapy of COVID-19. Front. Bioeng. Biotechnol. 2020, 8, 916. [Google Scholar] [CrossRef] [PubMed]
- Amarzguioui, M.; Prydz, H. An algorithm for selection of functional siRNA sequences. Biochem. Biophys. Res. Commun. 2004, 316, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- ElHefnawi, M.; Hassan, N.; Kamar, M.; Siam, R.; Remoli, A.L.; El-Azab, I.; AlAidy, O.; Marsili, G.; Sgarbanti, M. The design of optimal therapeutic small interfering RNA molecules targeting diverse strains of influenza A virus. Bioinformatics 2011, 27, 3364–3370. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-based therapeutics: An overview and prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Krueger, U.; Bergauer, T.; Kaufmann, B.; Wolter, I.; Pilk, S.; Heider-Fabian, M.; Kirch, S.; Artz-Oppitz, C.; Isselhorst, M.; Konrad, J. Insights into effective RNAi gained from large-scale siRNA validation screening. Oligonucleotides 2007, 17, 237–250. [Google Scholar] [CrossRef]
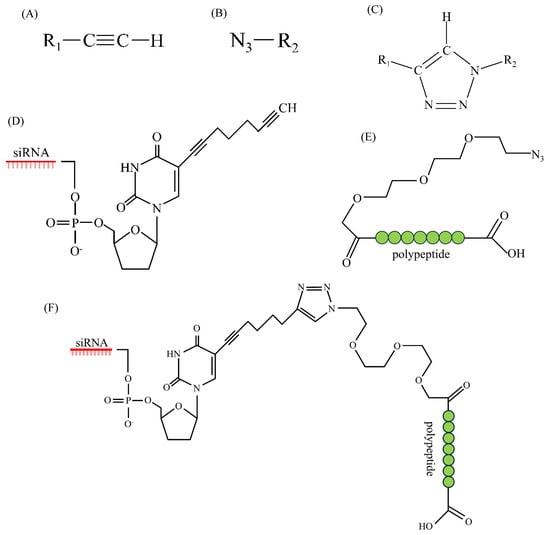
- Bauer, D.; Sarrett, S.M.; Lewis, J.S.; Zeglis, B.M. Click chemistry: A transformative technology in nuclear medicine. Nat. Protoc. 2023, 18, 1659–1668. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Sakla, A.P.; Laxmikeshav, K.; Tokala, R. Reliability of Click Chemistry on Drug Discovery: A Personal Account. Chem. Rec. 2020, 20, 253–272. [Google Scholar] [CrossRef]
- Mercier, F.; Paris, J.; Kaisin, G.; Thonon, D.; Flagothier, J.; Teller, N.; Lemaire, C.; Luxen, A. General Method for Labeling siRNA by Click Chemistry with Fluorine-18 for the Purpose of PET Imaging. Bioconjug. Chem. 2011, 22, 108–114. [Google Scholar] [CrossRef]
- Sajid, M.I.; Moazzam, M.; Kato, S.; Yeseom Cho, K.; Tiwari, R.K. Overcoming barriers for siRNA therapeutics: From bench to bedside. Pharmaceuticals 2020, 13, 294. [Google Scholar] [CrossRef]
- Choung, S.; Kim, Y.J.; Kim, S.; Park, H.-O.; Choi, Y.-C. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006, 342, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. A New Access to 2′-O-Alkylated Ribonucleosides and Properties of 2′-O-Alkylated Oligoribonucleotides. ChemInform 1995, 78, 486–504. [Google Scholar] [CrossRef]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Haussecker, D. Current issues of RNAi therapeutics delivery and development. J. Control. Release 2014, 195, 49–54. [Google Scholar] [CrossRef]
- Shah, P.S.; Schaffer, D.V. Antiviral RNAi: Translating science towards therapeutic success. Pharm. Res. 2011, 28, 2966–2982. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L.-S. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: Principles, challenges, and strategies. Yale J. Biol. Med. 2012, 85, 187–200. [Google Scholar]
- Salehirozveh, M.; Dehghani, P.; Mijakovic, I. Synthesis, Functionalization, and Biomedical Applications of Iron Oxide Nanoparticles (IONPs). J. Funct. Biomater. 2024, 15, 340. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv. Drug Deliv. Rev. 2023, 197, 114822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal nanoparticle hybrid hydrogels: The state-of-the-art of combining hard and soft materials to promote wound healing. Theranostics 2024, 14, 1534–1560. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Chen, X.; Zhao, X.; Gu, L.; Huang, H.; Yan, J.; Xu, C.; Li, G.; Wu, J.; et al. Plasmonic twinned silver nanoparticles with molecular precision. Nat. Commun. 2016, 7, 12809. [Google Scholar] [CrossRef]
- Yoosefian, M.; Sabaghian, H. Silver nanoparticle-based drug delivery systems in the fight against COVID-19: Enhancing efficacy, reducing toxicity and improving drug bioavailability. J. Drug Target. 2024, 32, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Lan, X. Quantum Dot-Based Simultaneous Multicolor Imaging. Mol. Imaging Biol. 2020, 22, 820–831. [Google Scholar] [CrossRef]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).