Caffeic Acid and Erythromycin: Antibacterial and Synergistic Effects on Staphylococci
Abstract
1. Introduction
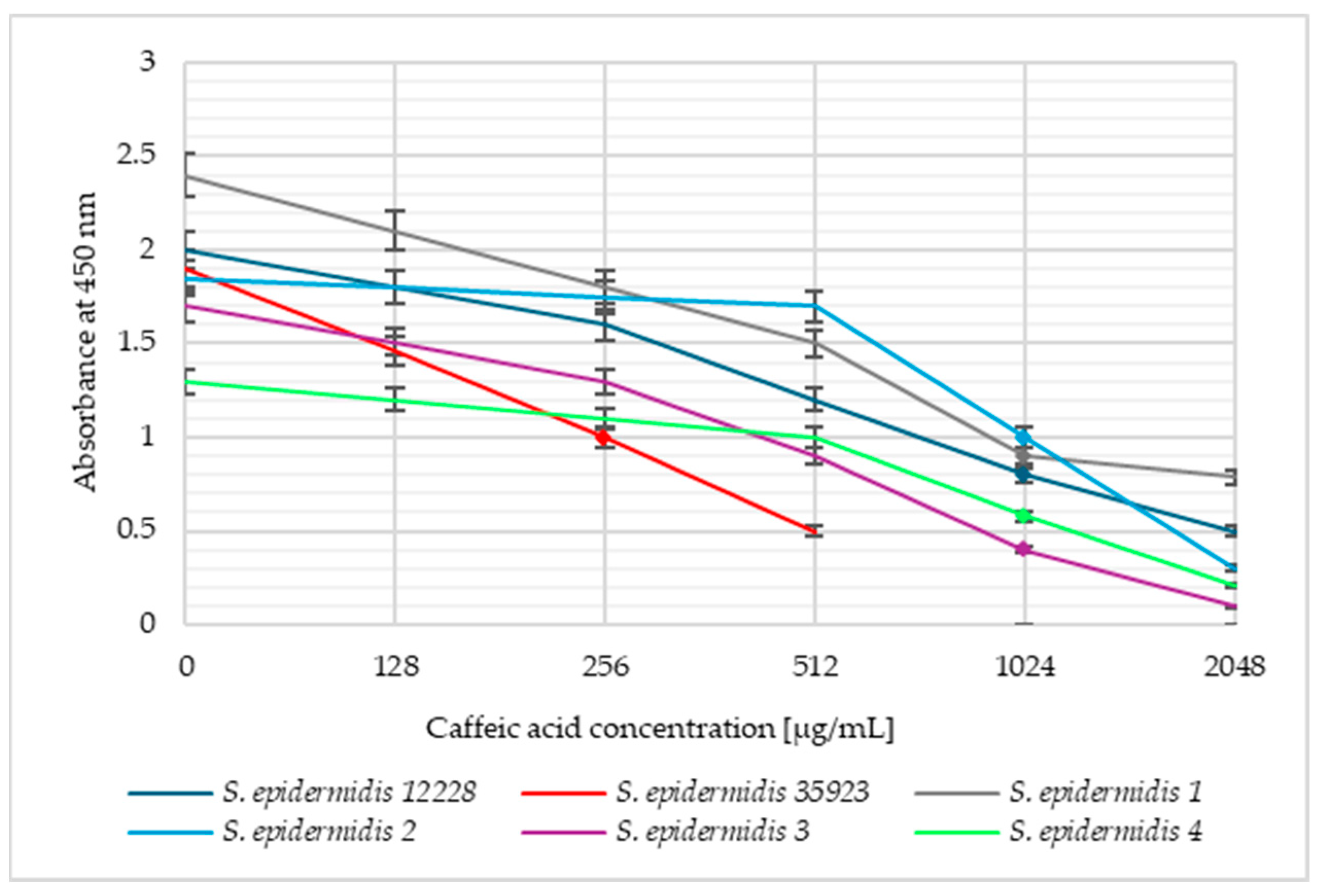
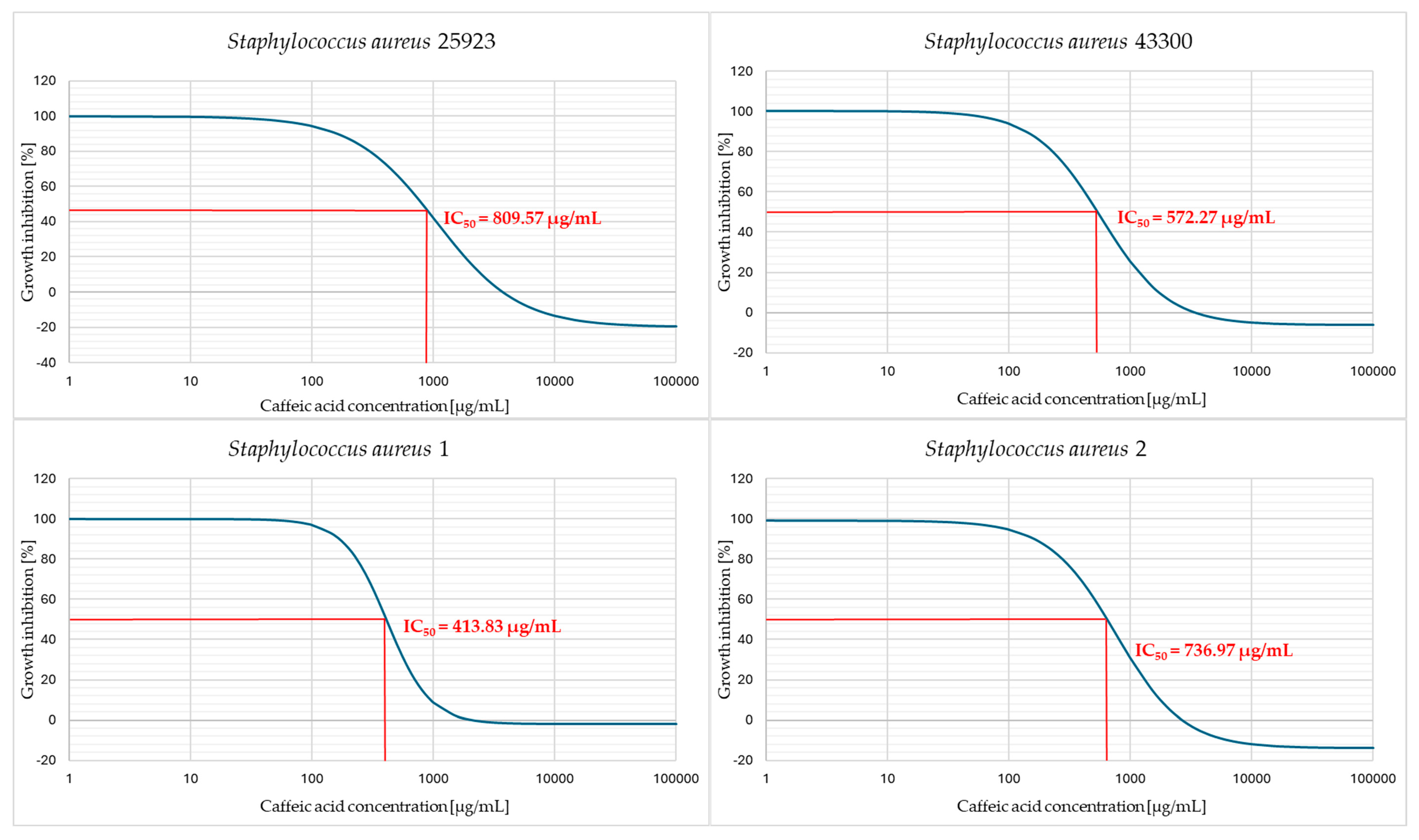
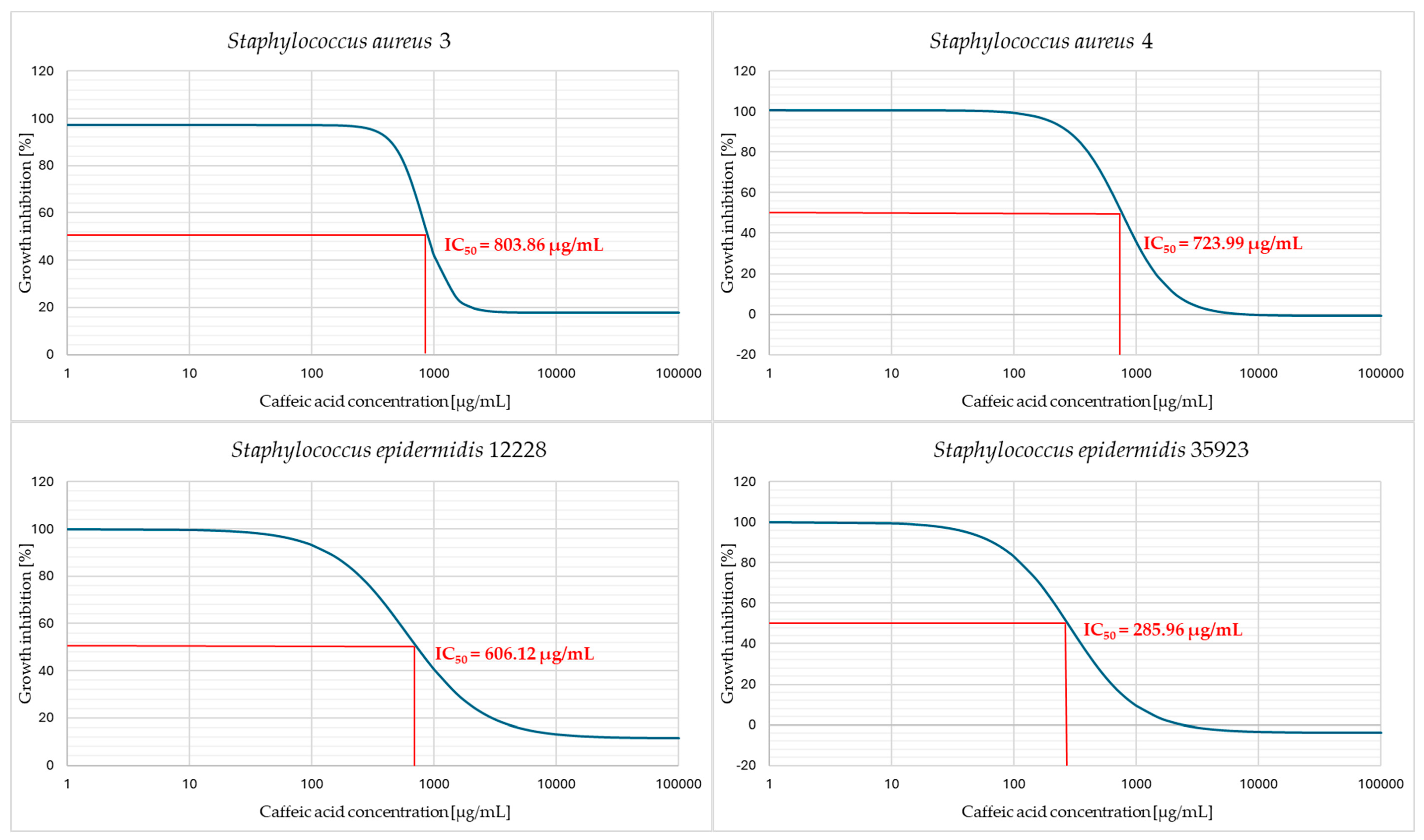
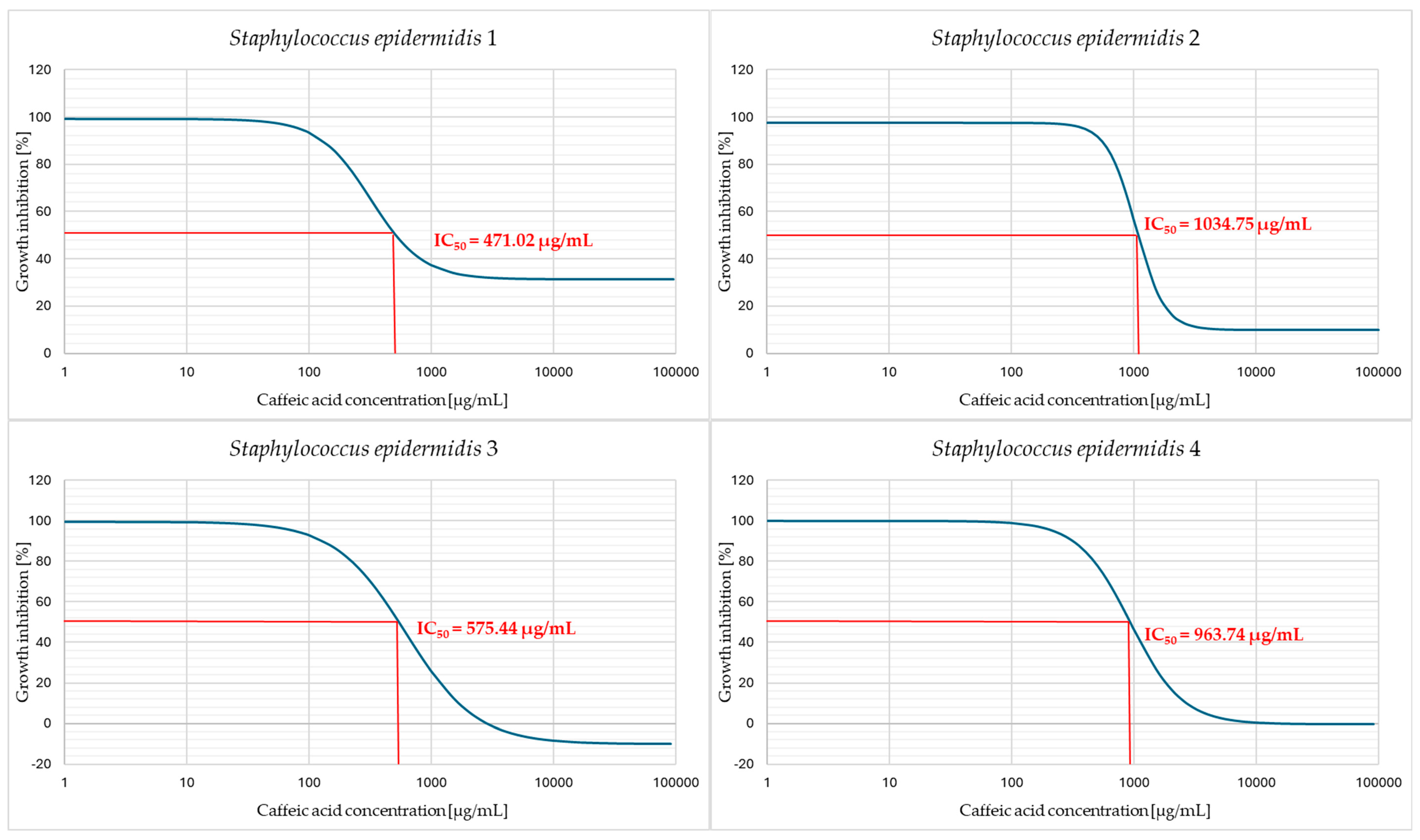
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Identification of Clinical Strains
4.3. Determination of the Resistance Profile of the Tested Clinical Strains
4.4. Determination of the Minimal Inhibitory Concentration (MIC) Value for Erythromycin and Caffeic Acid
4.5. Determination of the Bacterial Cell Viability
4.6. Determination of the Half Maximal Inhibitory Concentration (IC50)
4.7. Determination of the Fractional Inhibitory Concentration (FIC) and FIC Index
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IC50 | Half maximal inhibitory concentration |
| MIC | Minimum inhibitory concentration |
| FIC | Fractional Inhibitory Concentration |
| WHO | World Health Organization |
| PCR-RFLP | The polymerase chain reaction–restriction fragment length polymorphism |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MLSB | Macrolides, lincosamides and streptogramin B |
| cMLSB | Constitutive mechanism of resistance to macrolides, lincosamides and streptogramin B |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSE | Methicillin-sensitive Staphylococcus epidermidis |
| MRSE | Methicillin-resistant Staphylococcus epidermidis |
References
- Nguyen, T.H.; Park, M.D.; Otto, M. Host Response to Staphylococcus epidermidis Colonization and Infections. Front. Cell. Infect. Microbiol. 2017, 7, 90. [Google Scholar] [CrossRef]
- Hatlen, T.J.; Miller, L.G. Staphylococcal Skin and Soft Tissue Infections. Infect. Dis. Clin. N. Am. 2021, 35, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G. Staphylococcus aureus Infection: Pathogenesis and Antimicrobial Resistance. Int. J. Mol. Sci. 2023, 24, 8182. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Burke, Ó.; Zeden, M.S.; O’Gara, J.P. The Pathogenicity and Virulence of the Opportunistic Pathogen Staphylococcus epidermidis. Virulence 2024, 15, 2359483. [Google Scholar] [CrossRef]
- Ragheb, M.N.; Thomason, M.K.; Hsu, C.; Nugent, P.; Gage, J.; Samadpour, A.N.; Kariisa, A.; Merrikh, C.N.; Miller, S.I.; Sherman, D.R.; et al. Inhibiting the Evolution of Antibiotic Resistance. Mol. Cell 2019, 73, 157–165.e5. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Malczak, I.; Gajda, A. Interactions of Naturally Occurring Compounds with Antimicrobials. J. Pharm. Anal. 2023, 13, 1452–1470. [Google Scholar] [CrossRef]
- Al Alsheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Hamers, V.; Huguet, C.; Bourjot, M.; Urbain, A. Antibacterial Compounds from Mushrooms: A Lead to Fight ESKAPEE Pathogenic Bacteria? Planta Med. 2021, 87, 351–367. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Barinova, K.; Kudryavtseva, S.; Medvedeva, M.; Melnikova, A.; Sevostyanova, I.; Semenyuk, P.; Stroylova, Y.; Sova, M. Natural and Synthetic Derivatives of Hydroxycinnamic Acid Modulating the Pathological Transformation of Amyloidogenic Proteins. Molecules 2020, 25, 4647. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Barrino, F.; Poggetto, G.D.; Crescente, G.; Piccolella, S.; Pacifico, S.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S.; et al. New SiO2/Caffeic Acid Hybrid Materials: Synthesis. Materials 2020, 13, 394. [Google Scholar] [CrossRef]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. Biomed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Merlani, M.; Barbakadze, V.; Amiranashvili, L.; Gogilashvili, L.; Poroikov, V.; Petrou, A.; Geronikaki, A.; Ciric, A.; Glamoclija, J.; Sokovic, M. New Caffeic Acid Derivatives as Antimicrobial Agents: Design, Synthesis, Evaluation and Docking. Curr. Top. Med. Chem. 2019, 19, 292–304. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and Antioxidant Activity of Caffeic Acid Derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [PubMed]
- Lukáč, M.; Slobodníková, L.; Mrva, M.; Dušeková, A.; Garajová, M.; Kello, M.; Šebová, D.; Pisárčik, M.; Kojnok, M.; Vrták, A.; et al. Caffeic Acid Phosphanium Derivatives: Potential Selective Antitumor, Antimicrobial and Antiprotozoal Agents. Int. J. Mol. Sci. 2024, 25, 1200. [Google Scholar] [CrossRef] [PubMed]
- Ble-González, E.A.; Gómez-Rivera, A.; Zamilpa, A.; López-Rodríguez, R.; Lobato-García, C.E.; Álvarez-Fitz, P.; Gutierrez-Roman, A.S.; Perez-García, M.D.; Bugarin, A.; González-Cortazar, M. Ellagitannin, Phenols, and Flavonoids as Antibacterials from Acalypha arvensis (Euphorbiaceae). Plants 2022, 11, 300. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Bouchmaa, N.; Kabach, I.; Zouaoui, Z.; Chtibi, H.; El Maadoudi, M.; Kounnoun, A.; Cacciola, F.; El Majdoub, Y.O.; Mondello, L.; et al. Leaves: A Valuable Source of Bioactive Compounds with Multiple Pharmacological Effects. Molecules 2022, 27, 2108. [Google Scholar] [CrossRef]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- Mude, H.; Maroju, P.A.; Balapure, A.; Ganesan, R.; Ray Dutta, J. Water-Soluble Caffeic Acid-Dopamine Acid-Base Complex Exhibits Enhanced Bactericidal, Antioxidant, and Anticancer Properties. Food Chem. 2022, 374, 131830. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Liu, H.; Guo, J.; Long, S. Hybrid Molecules Based on Caffeic Acid as Potential Therapeutics: A Focused Review. Eur. J. Med. Chem. 2022, 243, 114745. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.F.S.; Tintino, S.R.; de Freitas, T.S.; Campina, F.F.; Irwin, I.R.; Siqueira-Júnior, J.P.; Coutinho, H.D.M.; Cunha, F.A.B. In Vitro e in Silico Evaluation of the Inhibition of Staphylococcus aureus Efflux Pumps by Caffeic and Gallic Acid. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 22–28. [Google Scholar] [CrossRef]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial Potential of Northeastern Portugal Wild Plant Extracts and Respective Phenolic Compounds. Biomed Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Válega, M.; Silva, A.M.S.; Cardoso, S.M. Metabolites and Biological Activities of Thymus Zygis, Thymus Pulegioides, and Thymus Fragrantissimus Grown under Organic Cultivation. Molecules 2018, 23, 1514. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evidence-based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef]
- Rodrigues, A.B.; De Almeida-Apolonio, A.A.; Alfredo, T.M.; Da Silva Dantas, F.G.; Campos, J.F.; Cardoso, C.A.L.; De Picoli Souza, K.; De Oliveira, K.M.P. Chemical Composition, Antimicrobial Activity, and Antioxidant Activity of Ocotea minarum (Nees & Mart.) Mez. Oxid. Med. Cell. Longev. 2019, 2019, 5736919. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of Antioxidant, Antimicrobial Activities and Chemical Profiles of Three Coffee (Coffea arabica L.) Pulp Aqueous Extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Xu, P.; Xu, X.B.; Khan, A.; Fotina, T.; Wang, S.H. Antibiofilm Activity against Staphylococcus aureus and Content Analysis of Taraxacum Officinale Phenolic Extract. Pol. J. Vet. Sci. 2021, 24, 243–251. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, B.M.; Arora, S.; Lim, C.S. Bactericidal Antibiotic-Phytochemical Combinations against Methicillin Resistant Staphylococcus aureus. Brazilian J. Microbiol. 2012, 43, 938–945. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, W.; Cui, X.; Qiao, R.; Li, C. Caffeic Acid and Cyclen Based Hydrogel for Synergistic Antibacterial Therapy. ACS Appl. Mater. Interfaces 2024, 16, 44493–44503. [Google Scholar] [CrossRef]
- Chiu, P.-H.; Wu, Z.-Y.; Hsu, C.-C.; Chang, Y.-C.; Huang, C.-M.; Hu, C.-T.; Chang, S.-C.; Dai, C.-A. Enhancement of antibacterial activity in electrospun fibrous membranes based on quaternized chitosan with caffeic acid and berberine chloride for wound dressing applications. RSC Adv. 2024, 14, 34756–34768. [Google Scholar] [CrossRef]
- Jokubaite, M.; Ramanauskiene, K. Potential Unlocking of Biological Activity of Caffeic Acid by Incorporation into Hydrophilic Gels. Gels 2024, 10, 794. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Khan, M.S.; Iqbal, J. Fluoroquinolones as Imaging Agents for Bacterial Infection. Dalton Trans. 2017, 46, 13220–13232. [Google Scholar] [CrossRef]
- Shah, M.M.; Iihara, H.; Noda, M.; Song, S.X.; Nhung, P.H.; Ohkusu, K.; Kawamaura, Y.; Ezaki, T. dnaJ gene sequence-based assay for species identification and phylogenetic grouping in the genus Staphylococcus. Int. J. Syst. Evol. Microbiol. 2007, 57, 25–30. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0, 2024. Available online: http://www.eucast.org (accessed on 9 November 2024).
- Amsterdam, D. Susceptibility Testing of Antimicrobials in Liquid Media. In Antibiotics in Laboratory Medicine, 5th ed.; Loman, V., Ed.; Williams and Wilkins: Philadelphia, PA, USA, 2005; pp. 61–143. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. dis 5.1. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Cudic, M.; Condie, B.A.; Weiner, D.J.; Lysenko, E.S.; Xiang, Z.Q.; Insug, O.; Bulet, P.; Otvos, L. Development of Novel Antibacterial Peptides That Kill Resistant Isolates. Peptides 2002, 23, 2071–2083. [Google Scholar] [CrossRef]
- Devienne, K.F.; Raddi, M.S.G. Screening for Antimicrobial Activity of Natural Products Using a Microplate Photometer. Braz. J. Microbiol. 2002, 33, 166–168. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. EUCAST definitive document E. Def 1.2. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, X.; Zhang, D.; Wu, M.; Xue, Z.; Liu, Z.; Yang, S.; Li, H.; Gong, G. Evaluation of the membrane damage mechanism of protocatechualdehyde against Yersinia enterocolitica and simulation of growth inhibition in pork. Food Chem. 2021, 363, 130340. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sorgi, C.A.; de Campos Chaves Lamarque, G.; Verri, M.P.; Nelson-Filho, P.; Faccioli, L.H.; Paula-Silva, F.W.G. Multifaceted effect of caffeic acid against Streptococcus mutans infection: Microbicidal and immunomodulatory agent in macrophages. Arch Microbiol. 2021, 203, 2979–2987. [Google Scholar] [CrossRef]
- “Quest Graph™ IC50 Calculator.” AAT Bioquest, Inc. 29 November 2024. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 9 November 2024).
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin Against Staphylococcus epidermidis. IJN 2020, 15, 3551–3562. [Google Scholar] [CrossRef]
- Chai, B.; Jiang, W.; Hu, M.; Wu, Y.; Si, H. In Vitro Synergistic Interactions of Protocatechuic Acid and Chlorogenic Acid in Combination with Antibiotics against Animal Pathogens. Synergy 2019, 9, 100055. [Google Scholar] [CrossRef]


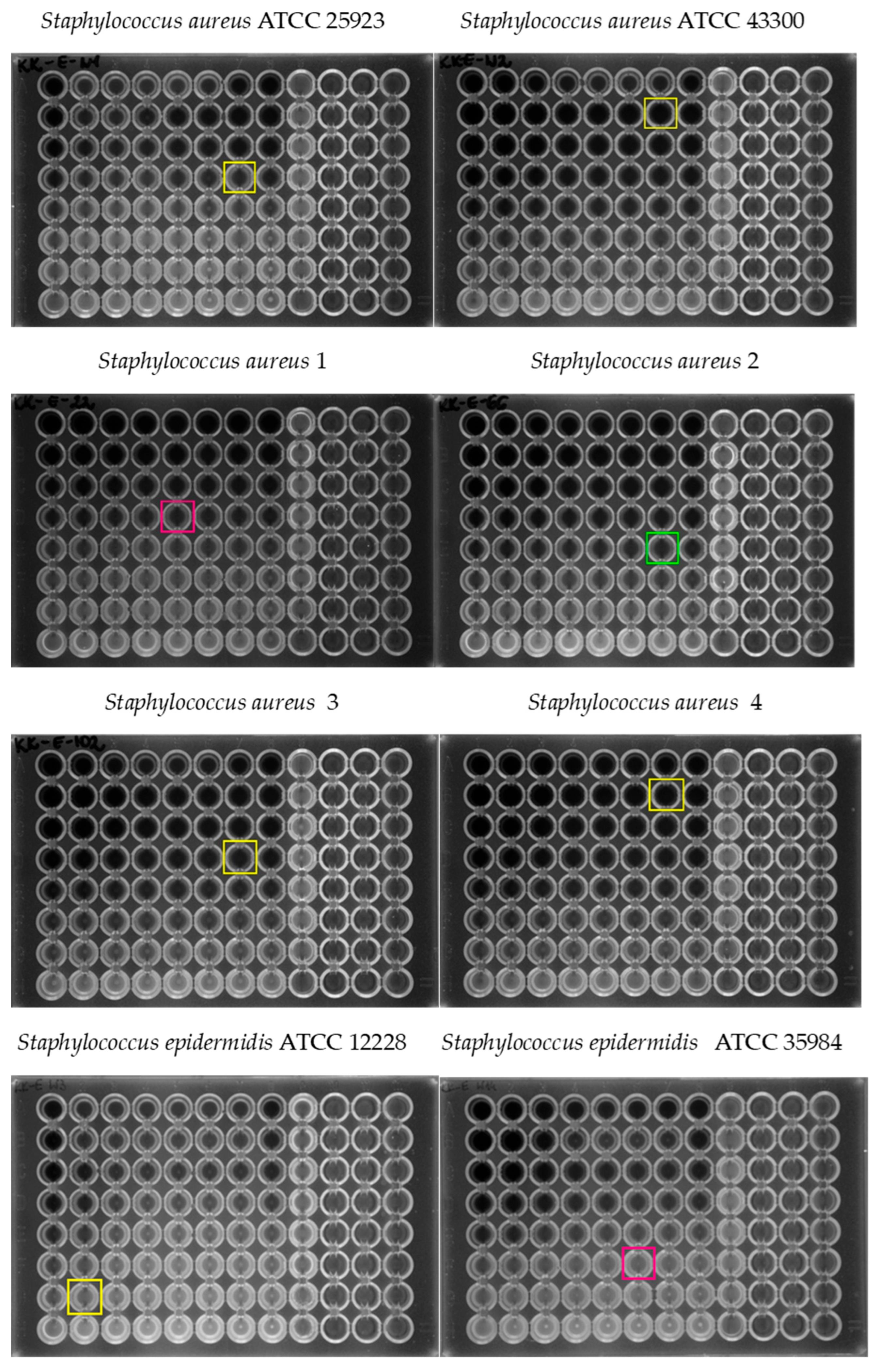
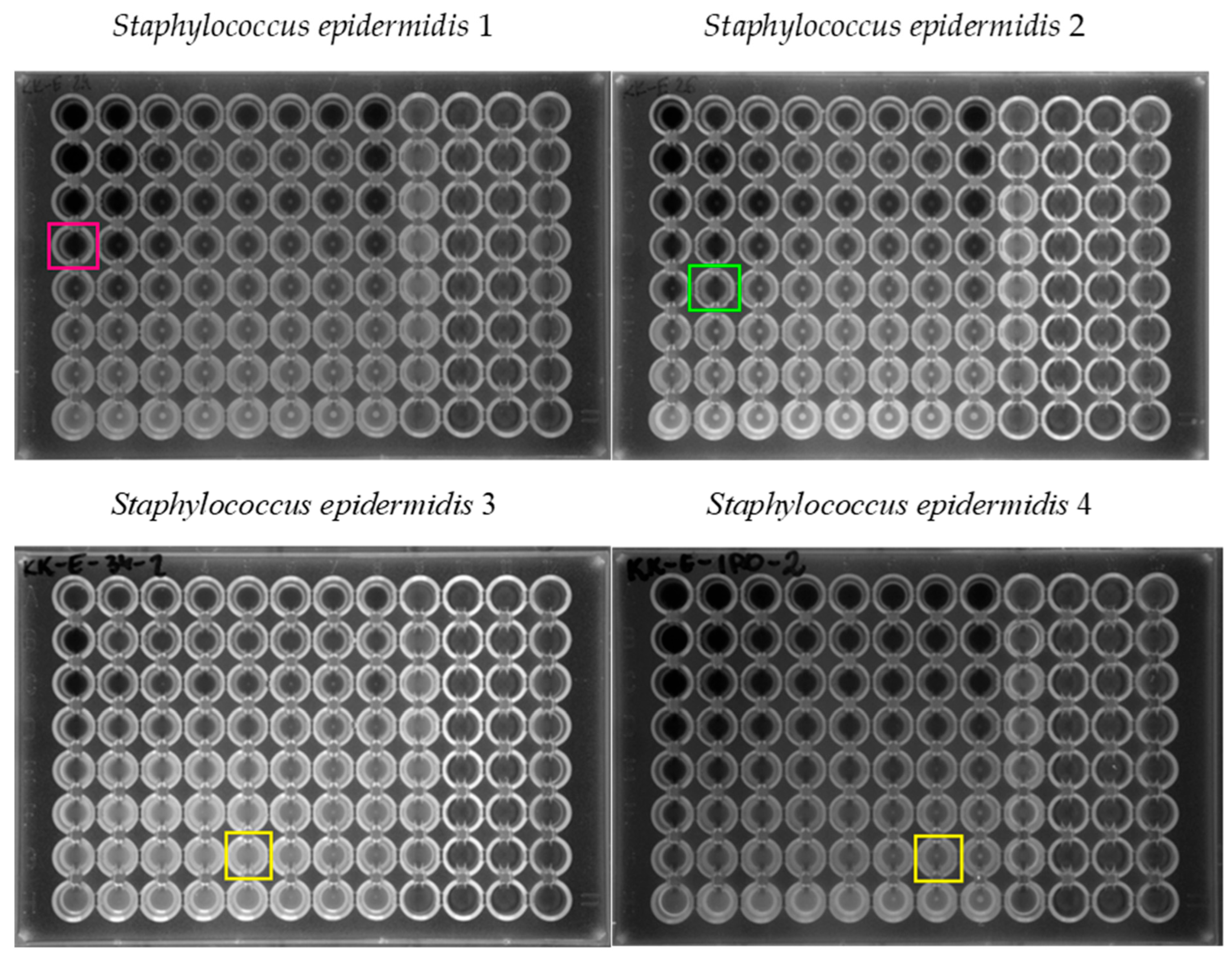
| Tested Strain | MIC0 of Erythromycin (µg/mL) | MIC0 of Caffeic Acid (µg/mL) | Mechanism of Resistance to MLSB Antibiotics | Mechanism of Resistance to Methicillin |
|---|---|---|---|---|
| S. aureus ATCC 25923 | 8 | 1024 | - | MSSA |
| S. aureus ATCC 43300 | 1024 | 1024 | cMLSB | MRSA |
| S. aureus 1 | 1 | 512 | - | MSSA |
| S. aureus 2 | 1 | 1024 | - | MSSA |
| S. aureus 3 | 1024 | 1024 | cMLSB | MRSA |
| S. aureus 4 | 1024 | 1024 | cMLSB | MRSA |
| S. epidermidis ATCC 12228 | 0.25 | 1024 | - | MSSE |
| S. epidermidis ATCC 35984 | 1024 | 256 | cMLSB | MRSE |
| S. epidermidis 1 | 1024 | 1024 | cMLSB | MRSE |
| S. epidermidis 2 | 1024 | 1024 | cMLSB | MRSE |
| S. epidermidis 3 | 1 | 1024 | - | MSSE |
| S. epidermidis 4 | 1 | 1024 | - | MSSE |
| Tested Strain | MIC of Erythromycin (µg/mL) | MIC of Caffeic Acid (µg/mL) | MIC of Erythromycin with Caffeic Acid (µg/mL) | FIC Index | Interpretation |
|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 0.25 | 1024 | 0.25 | 1.031 | indifferent |
| S. aureus ATCC 43300 | 2048 | 1024 | 32 | 1.016 | indifferent |
| S. aureus 1 | 0.25 | 1024 | 0.125 | 0.516 | additive |
| S. aureus 2 | 0.25 | 2048 | 0.03125 | 0.141 | synergistic |
| S. aureus 3 | 2048 | 256 | 32 | 1.016 | indifferent |
| S. aureus 4 | 2048 | 1024 | 32 | 1.016 | indifferent |
| S. epidermidis ATCC 12228 | 0.25 | 2048 | 0.25 | 1.016 | indifferent |
| S. epidermidis ATCC 35984 | 2048 | 128 | 64 | 0.281 | synergistic |
| S. epidermidis 1 | 2048 | 1024 | 2048 | 0.750 | additive |
| S. epidermidis 2 | 2048 | 2048 | 1024 | 0.313 | synergistic |
| S. epidermidis 3 | 0.125 | 1024 | 0.125 | 1.031 | indifferent |
| S. epidermidis 4 | 0.0313 | 512 | 0.0313 | 1.063 | indifferent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kępa, M.; Miklasińska-Majdanik, M.; Haczyk, A.; Matuła, A.; Wojtyczka, R.D. Caffeic Acid and Erythromycin: Antibacterial and Synergistic Effects on Staphylococci. Pharmaceuticals 2025, 18, 964. https://doi.org/10.3390/ph18070964
Kępa M, Miklasińska-Majdanik M, Haczyk A, Matuła A, Wojtyczka RD. Caffeic Acid and Erythromycin: Antibacterial and Synergistic Effects on Staphylococci. Pharmaceuticals. 2025; 18(7):964. https://doi.org/10.3390/ph18070964
Chicago/Turabian StyleKępa, Małgorzata, Maria Miklasińska-Majdanik, Aleksandra Haczyk, Arkadiusz Matuła, and Robert D. Wojtyczka. 2025. "Caffeic Acid and Erythromycin: Antibacterial and Synergistic Effects on Staphylococci" Pharmaceuticals 18, no. 7: 964. https://doi.org/10.3390/ph18070964
APA StyleKępa, M., Miklasińska-Majdanik, M., Haczyk, A., Matuła, A., & Wojtyczka, R. D. (2025). Caffeic Acid and Erythromycin: Antibacterial and Synergistic Effects on Staphylococci. Pharmaceuticals, 18(7), 964. https://doi.org/10.3390/ph18070964






