Trends in Antimicrobial Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa from Bloodstream Infections: An Eight-Year Study in a Romanian Tertiary Hospital
Abstract
1. Introduction
2. Results
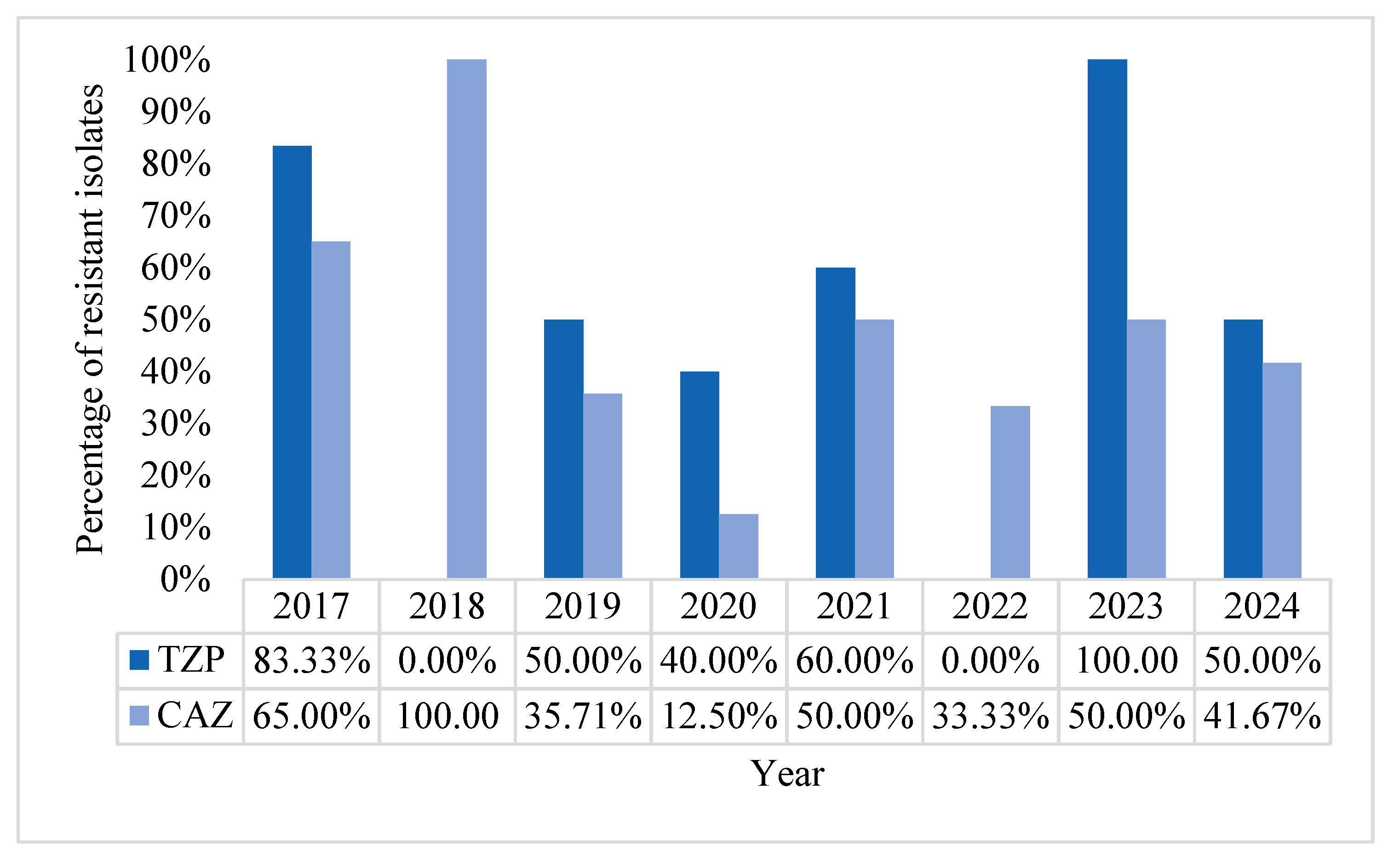
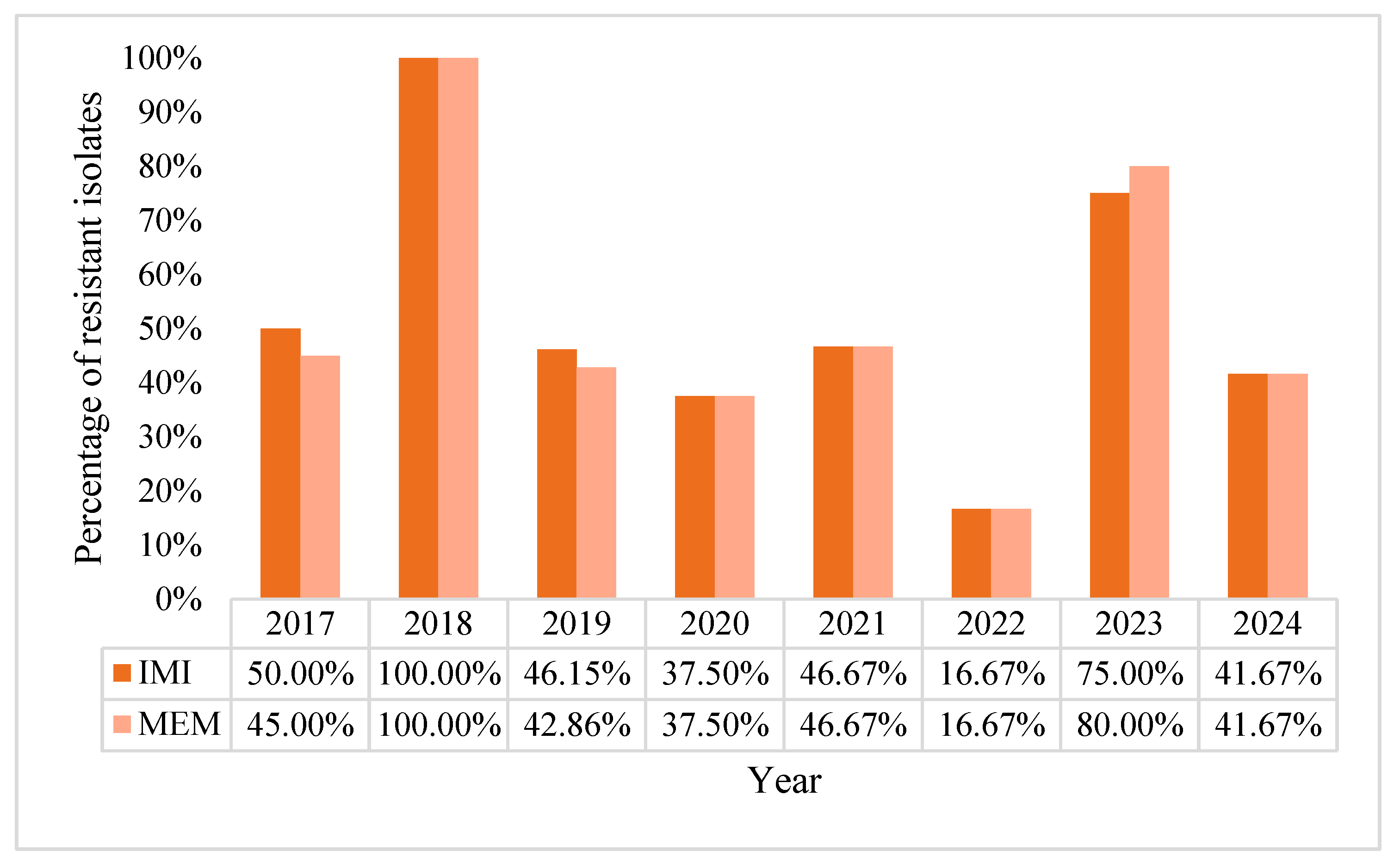
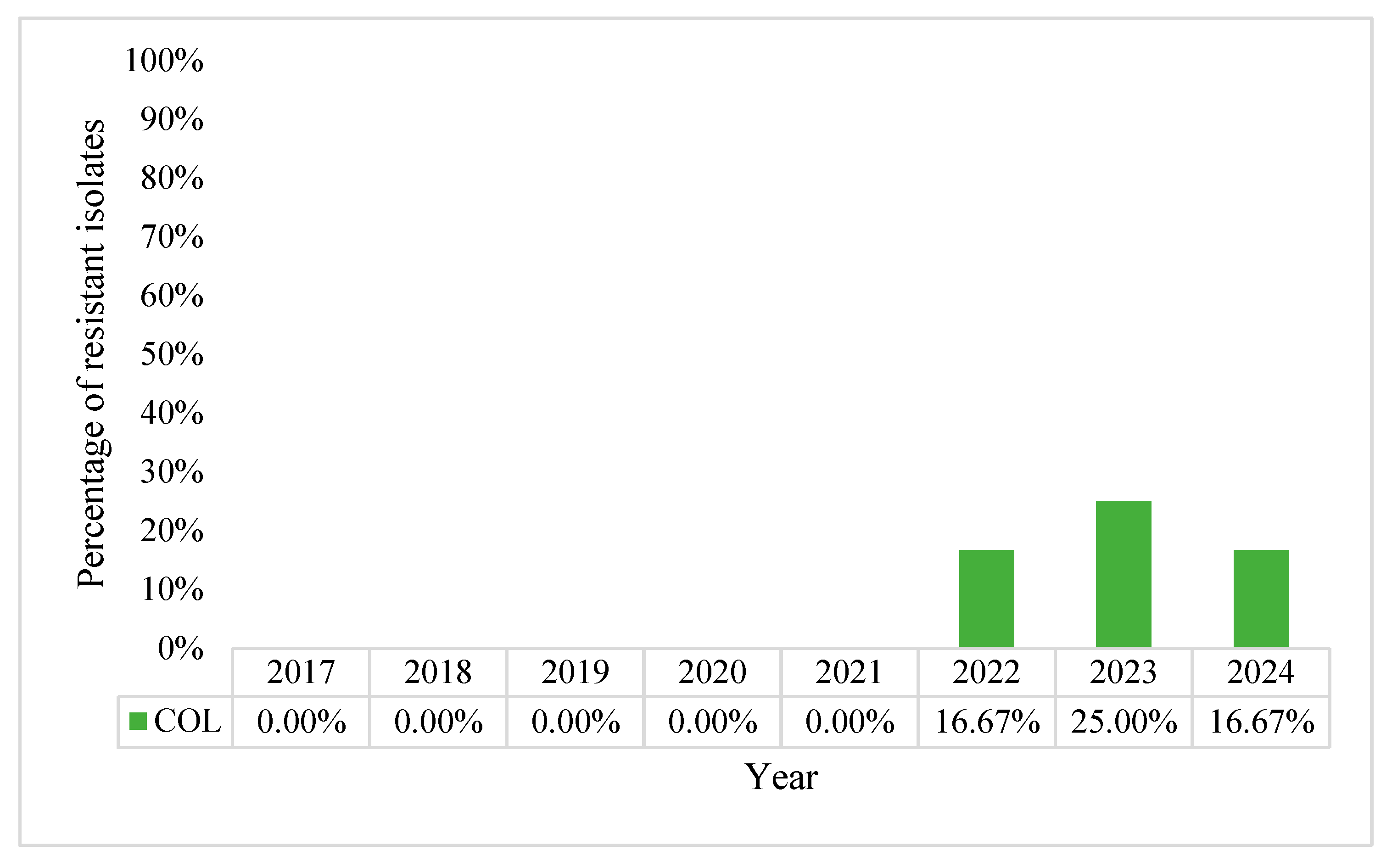
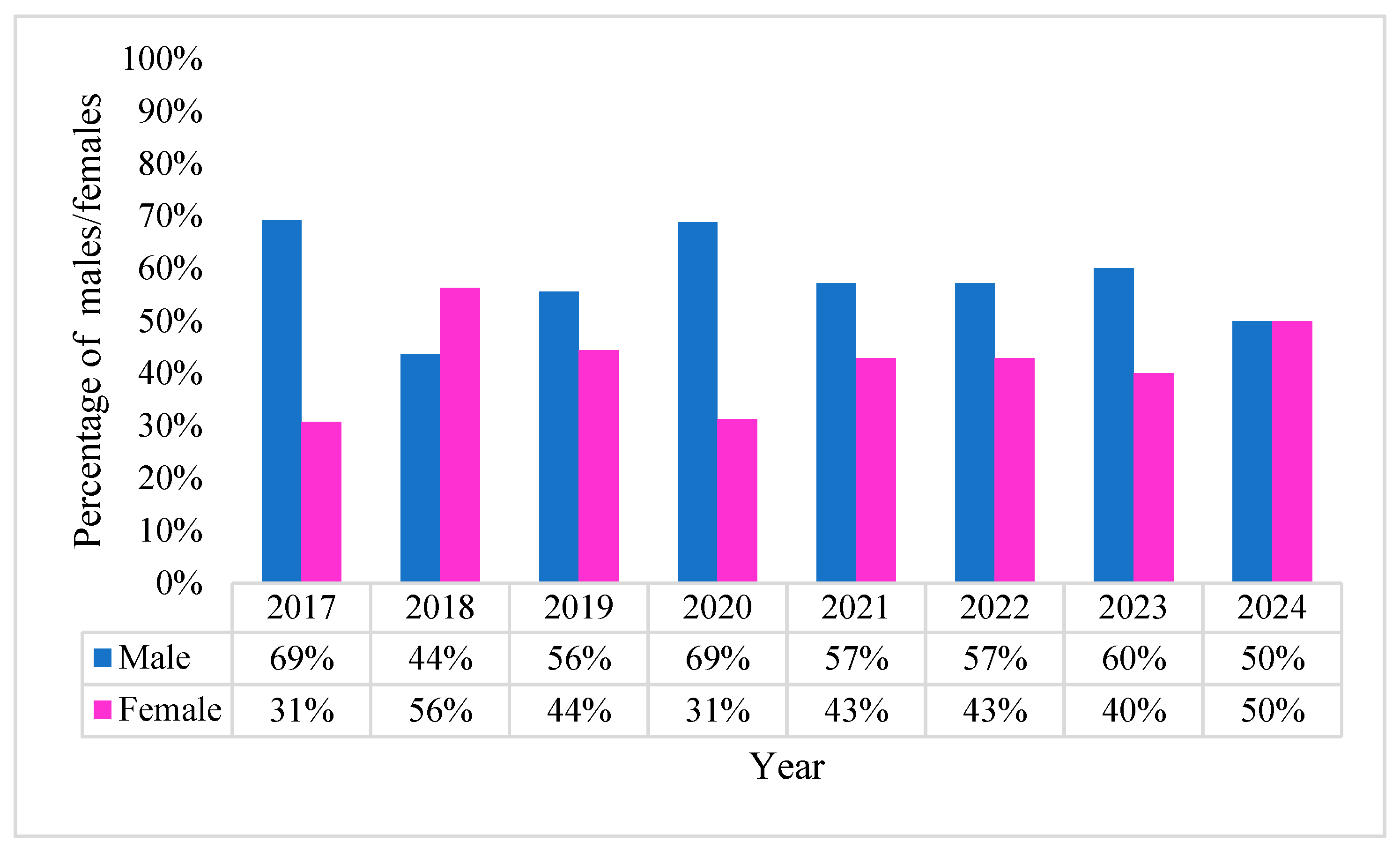
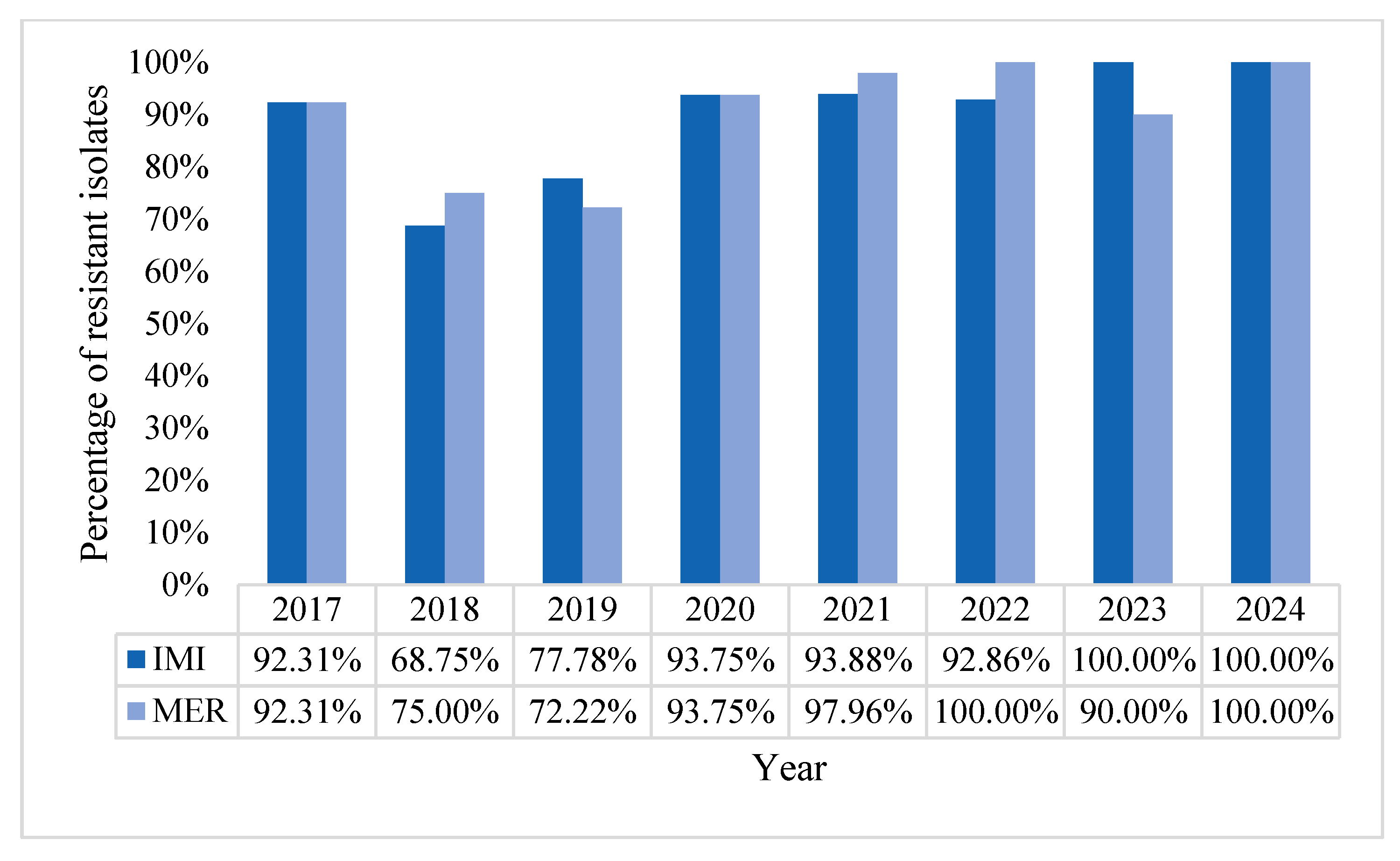
2.1. Characteristics of Pseudomonas aeruginosa Positive Blood Cultures (BC)
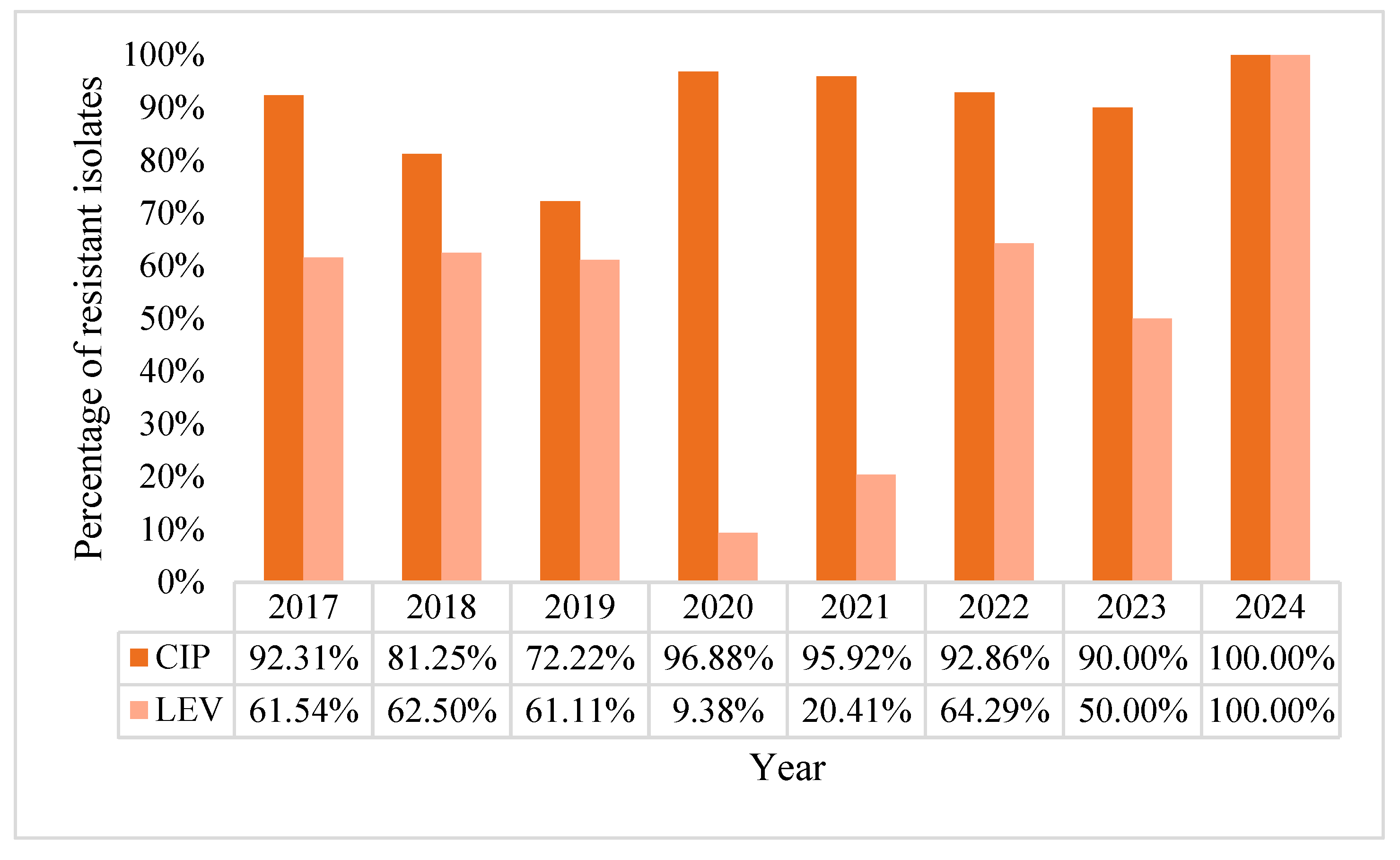
2.2. Acinetobacter baumannii Positive BCs Statistics
3. Discussions
4. Materials and Methods
4.1. Study Design and Setting
4.2. Blood Culture Processing and Bacterial Identification
4.3. Antimicrobial Susceptibility Testing
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles, A.G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; Antimicrobial Resistance Division (AMR) II and RC (IRC); WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Shoaib, M.; Tang, M.; Aqib, A.I.; Zhang, X.; Wu, Z.; Wen, Y.; Hou, X.; Xu, J.; Hao, R.; Wang, S. Dairy farm waste: A potential reservoir of diverse antibiotic resistance and virulence genes in aminoglycoside- and beta-lactam-resistant Escherichia coli in Gansu Province, China. Environ. Res. 2024, 263, 120190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Cha, C.J. Antibiotic Resistome from the One-Health Perspective: Understanding and Controlling Antimicrobial Resistance Transmission. In Experimental and Molecular Medicine; Springer Nature: Berlin, Germany, 2021; Volume 53, pp. 301–309. [Google Scholar]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2024, 9, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Hsueh, P.R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The microbiology of bloodstream infection: 20-year trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Marra, A.R.; Camargo, L.F.; Pignatari, A.C.; Sukiennik, T.; Behar, P.R.; Medeiros, E.A.; Ribeiro, J.; Girão, E.; Correa, L.; Guerra, C.; et al. Nosocomial bloodstream infections in Brazilian hospitals: Analysis of 2563 cases from a prospective nationwide surveillance study. J. Clin. Microbiol. 2011, 49, 1866–1871. [Google Scholar] [CrossRef]
- Tam, V.H.; Rogers, C.A.; Chang, K.T.; Weston, J.S.; Caeiro, J.P.; Garey, K.W. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob. Agents Chemother. 2010, 54, 3717–3722. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Hattemer, A.; Hauser, A.R.; Alhajhusain, A.; Vora, H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit. Care Med. 2012, 40, 1157–1163. [Google Scholar] [CrossRef]
- Gonçalves, I.R.; Dantas, R.C.C.; Ferreira, M.L.; Batistão, D.W.D.F.; Gontijo-Filho, P.P.; Ribas, R.M. Carbapenem-resistant Pseudomonas aeruginosa: Association with virulence genes and biofilm formation. Braz. J. Microbiol. 2017, 48, 211–217. [Google Scholar] [CrossRef]
- Recio, R.; Villa, J.; Viedma, E.; Orellana, M.; Lora-Tamayo, J.; Chaves, F. Bacteraemia due to extensively drug-resistant Pseudomonas aeruginosa sequence type 235 high-risk clone: Facing the perfect storm. Int. J. Antimicrob. Agents 2018, 52, 172–179. [Google Scholar] [CrossRef]
- Rutherford, V.; Yom, K.; Ozer, E.A.; Pura, O.; Hughes, A.; Murphy, K.R.; Cudzilo, L.; Mitchell, D.; Hauser, A.R. Environmental reservoirs for exoS+ and exoU+ strains of Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2018, 10, 485–492. [Google Scholar] [CrossRef]
- Bertrand, X.; Thouverez, M.; Talon, D.; Boillot, A.; Capellier, G.; Floriot, C.; Hélias, J. Endemicity, molecular diversity and colonisation routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med. 2001, 27, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Resistance Threats in the United States. 2019 Antibiotic Resistance Threats Report. Antimicrobial Resistance. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 5 February 2025).
- Antimicrobial Resistance Threats in the United States. 2021–2022 Antimicrobial Resistance. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/update-2022.html (accessed on 11 April 2025).
- Gary, P.; Deirdre, C.; Geraldine, H.; Elmer, K.; Paul, S.; Gail, W. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 7th ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2017; pp. 768–774. [Google Scholar]
- Russell, D.L.; Uslan, D.Z.; Rubin, Z.A.; Grogan, T.R.; Martin, E.M. Multidrug resistant Acinetobacter baumanii: A 15-year trend analysis. Infect Control Hosp. Epidemiol. 2018, 39, 608–611. [Google Scholar] [CrossRef]
- Xu, M.; Fu, Y.; Kong, H.; Chen, X.; Chen, Y.; Li, L.; Yang, Q. Bloodstream infections caused by Klebsiella pneumoniae: Prevalence of blaKPC, virulence factors and their impacts on clinical outcome. BMC Infect. Dis. 2018, 18, 358. [Google Scholar] [CrossRef] [PubMed]
- Martín-Aspas, A.; Guerrero-Sánchez, F.M.; García-Colchero, F.; Rodríguez-Roca, S.; Girón-González, J.A. Differential characteristics of Acinetobacter baumannii colonization and infection: Risk factors, clinical picture, and mortality. Infect Drug Resist. 2018, 11, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Chang, T.C.; Wu, C.J.; Chang, C.M.; Lee, H.C.; Chen, P.L.; Lee, C.-C.; Ko, N.-Y.; Ko, W.-C. Clinical manifestations, antimicrobial therapy, and prognostic factors of monomicrobial Acinetobacter baumannii complex bacteremia. J. Infect. 2010, 61, 219–227. [Google Scholar] [CrossRef]
- Shenoy, E.S.; Pierce, V.M.; Sater, M.R.A.; Pangestu, F.K.; Herriott, I.C.; Anahtar, M.N.; Bramante, J.T.; Kwon, D.S.; Hawkins, F.R.; Suslak, D.; et al. Community-acquired in name only: A cluster of carbapenem-resistant Acinetobacter baumannii in a burn intensive care unit and beyond. Infect. Control Hosp. Epidemiol. 2020, 41, 531–538. [Google Scholar] [CrossRef]
- Liou, M.L.; Chen, K.H.; Yeh, H.L.; Lai, C.Y.; Chen, C.H. Persistent nasal carriers of Acinetobacter baumannii in long-term-care facilities. Am. J. Infect. Control. 2017, 45, 723–727. [Google Scholar] [CrossRef]
- Byrne-Bailey, K.G.; Gaze, W.H.; Kay, P.; Boxall, A.B.; Hawkey, P.M.; Wellington, E.M. Prevalence of sulfonamide resistance genes in bacterial isolates from manured agricultural soils and pig slurry in the United Kingdom. Antimicrob. Agents Chemother. 2009, 53, 696–702. [Google Scholar] [CrossRef]
- Sarma, P.M.; Bhattacharya, D.; Krishnan, S.; Lal, B. Assessment of intra-species diversity among strains of Acinetobacter baumannii isolated from sites contaminated with petroleum hydrocarbons. Can. J. Microbiol. 2004, 50, 405–514. [Google Scholar] [CrossRef]
- Smith, A.R.; Vowles, M.; Horth, R.Z.; Smith, L.; Rider, L.; Wagner, J.M.; Sangster, A.; Young, E.L.; Schuckel, H.; Stewart, J.; et al. Infection control response to an outbreak of OXA-23 carbapenemase-producing carbapenem-resistant Acinetobacter baumannii in a skilled nursing facility in Utah. Am. J. Infect. Control. 2021, 49, 792–799. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Thom, K.A.; Johnson, J.K.; Lee, M.S.; Harris, A.D. Environmental contamination because of multidrug-resistant Acinetobacter baumannii surrounding colonized or infected patients. Am. J. Infect. Control. 2011, 39, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Thom, K.A.; Maragakis, L.L.; Richards, K.; Johnson, J.K.; Roup, B.; Lawson, P.; Harris, A.D.; Fuss, E.P.; Pass, M.A.; Blythe, D.; et al. Assessing the burden of Acinetobacter baumannii in Maryland: A statewide cross-sectional period prevalence survey. Infect. Control Hosp. Epidemiol. 2012, 33, 883–888. [Google Scholar] [CrossRef]
- Chen, C.-T.; Wang, Y.-C.; Kuo, S.-C.; Shih, F.-H.; Chen, T.-L.; How, C.-K.; Yang, Y.-S.; Lee, Y.-T. Community-acquired bloodstream infections caused by Acinetobacter baumannii: A matched case-control study. J. Microbiol. Immunol. Infect. 2018, 51, 629–635. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0, 2024. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 2 April 2025).
- Su, L.-X.; Meng, K.; Zhang, X.; Wang, H.-J.; Yan, P.; Jia, Y.-H.; Feng, D.; Xie, L.-X. Diagnosing ventilator-associated pneumonia in critically ill patients with sepsis. Am. J. Crit. Care 2012, 21, e110–e119. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Baudet, A.; Regad, M.; Gibot, S.; Conrath, É.; Lizon, J.; Demoré, B.; Florentin, A. Pseudomonas aeruginosa Infections in Patients with Severe COVID-19 in Intensive Care Units: A Retrospective Study. Antibiotics 2024, 13, 390. [Google Scholar] [CrossRef]
- Magrini, E.; Rando, E.; Liguoro, B.; Salvati, F.; del Vecchio, P.; Fantoni, M.; Torti, C.; Murri, R. Risk factors associated with bloodstream infections caused by Acinetobacter baumannii in hospital settings: A systematic review and meta-analysis. CMI Commun. 2025, 2, 105060. [Google Scholar] [CrossRef]
- Chotiprasitsakul, D.; Ao-udomsuk, K.; Santinirand, P. Impact of COVID-19 on epidemiology and mortality risk factors in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections in a tertiary care hospital in Thailand. J. Glob. Antimicrob. Resist. 2025, 43, 155–161. [Google Scholar] [CrossRef]
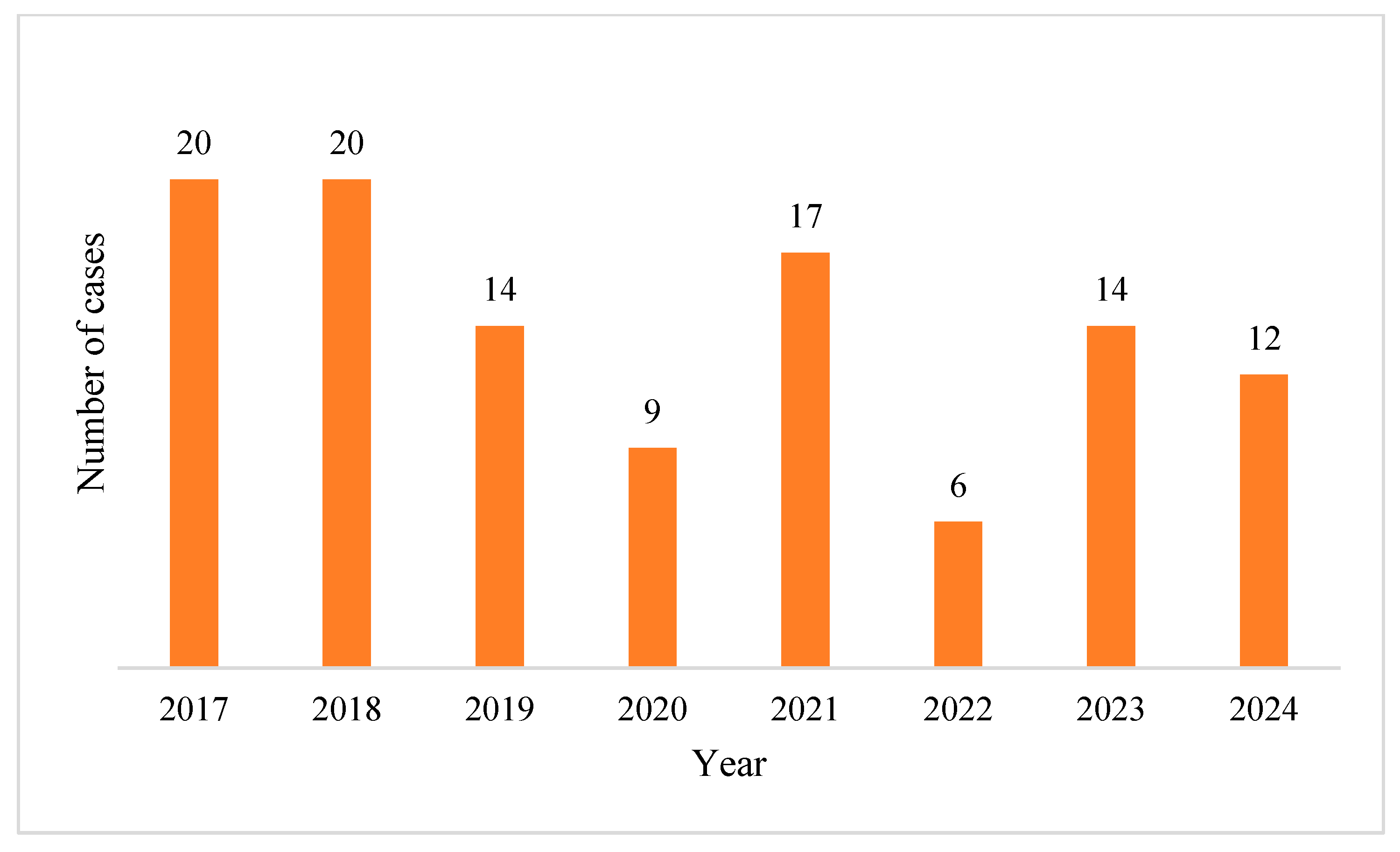



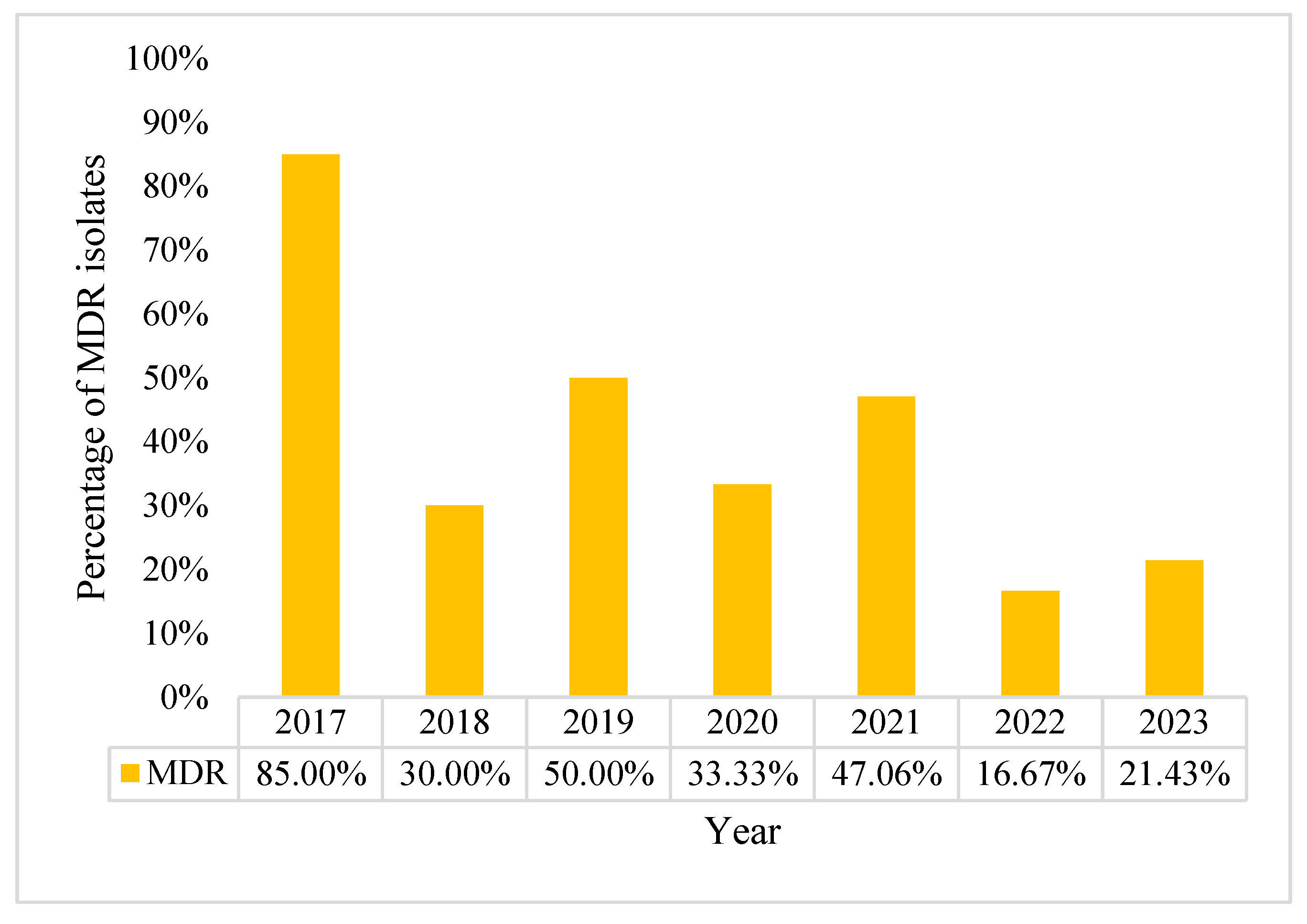
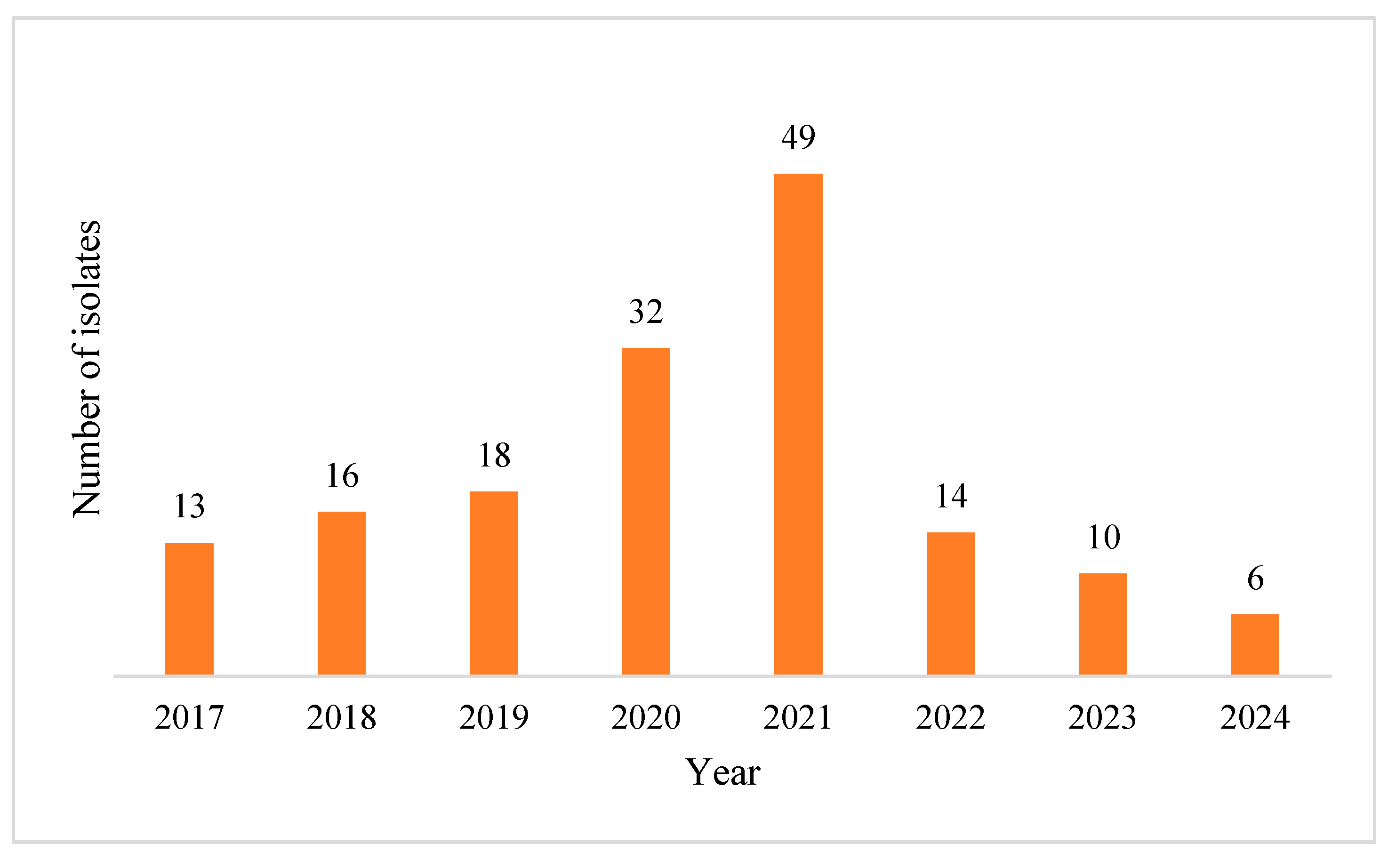
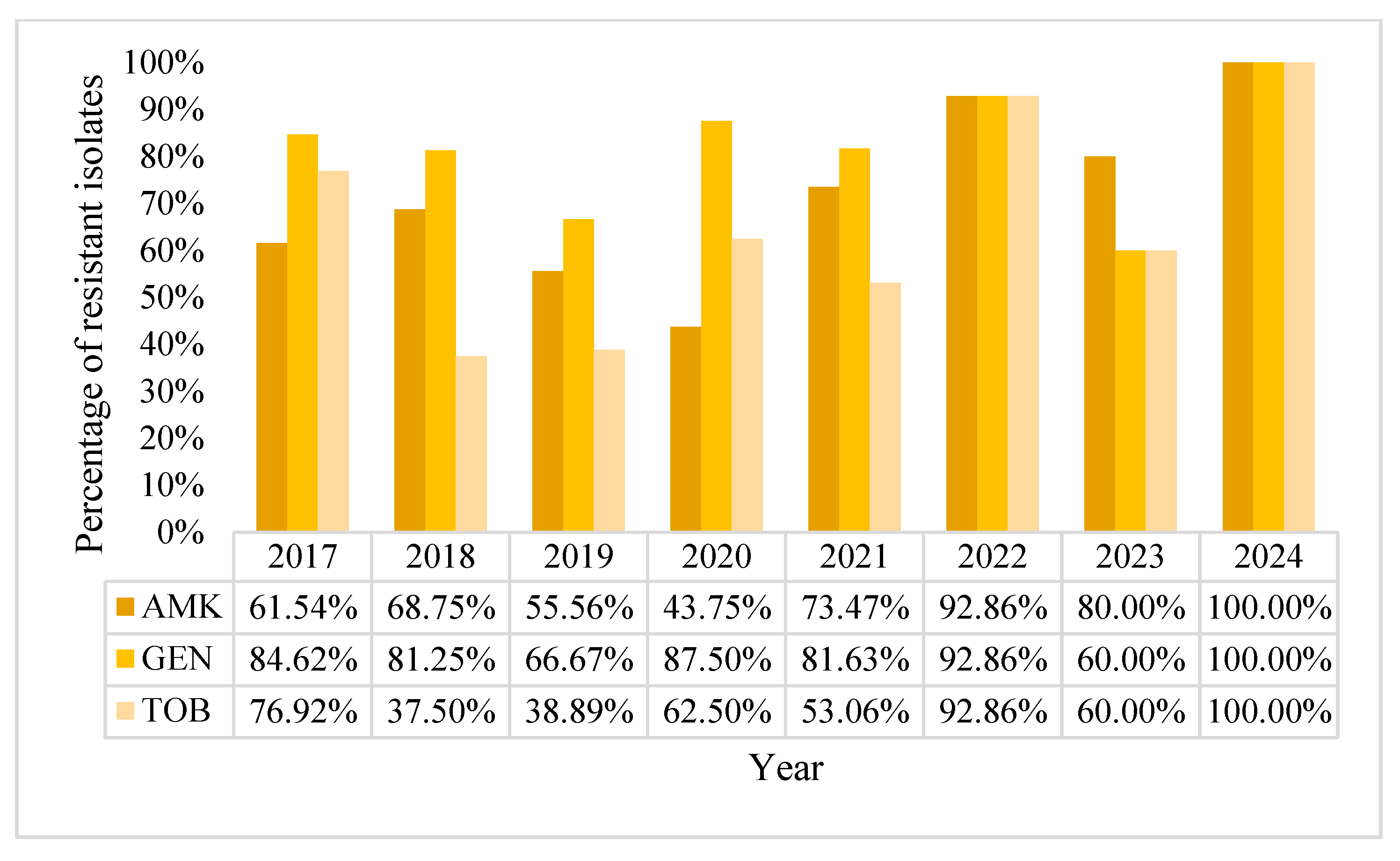

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borcan, A.M.; Rotaru, E.; Caravia, L.G.; Filipescu, M.-C.; Simoiu, M. Trends in Antimicrobial Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa from Bloodstream Infections: An Eight-Year Study in a Romanian Tertiary Hospital. Pharmaceuticals 2025, 18, 948. https://doi.org/10.3390/ph18070948
Borcan AM, Rotaru E, Caravia LG, Filipescu M-C, Simoiu M. Trends in Antimicrobial Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa from Bloodstream Infections: An Eight-Year Study in a Romanian Tertiary Hospital. Pharmaceuticals. 2025; 18(7):948. https://doi.org/10.3390/ph18070948
Chicago/Turabian StyleBorcan, Alina Maria, Elena Rotaru, Laura Georgiana Caravia, Mihai-Cezar Filipescu, and Mădălina Simoiu. 2025. "Trends in Antimicrobial Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa from Bloodstream Infections: An Eight-Year Study in a Romanian Tertiary Hospital" Pharmaceuticals 18, no. 7: 948. https://doi.org/10.3390/ph18070948
APA StyleBorcan, A. M., Rotaru, E., Caravia, L. G., Filipescu, M.-C., & Simoiu, M. (2025). Trends in Antimicrobial Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa from Bloodstream Infections: An Eight-Year Study in a Romanian Tertiary Hospital. Pharmaceuticals, 18(7), 948. https://doi.org/10.3390/ph18070948





