Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Synthesis of DSPE-PEG(2000)-Maleimide-Herceptin Conjugate Was Achieved in a Three-Step Reaction
2.2. The Sizes of the Herceptin-Functionalized Liposomes Loaded with siRNA Were Lower than 200 nm in Diameter
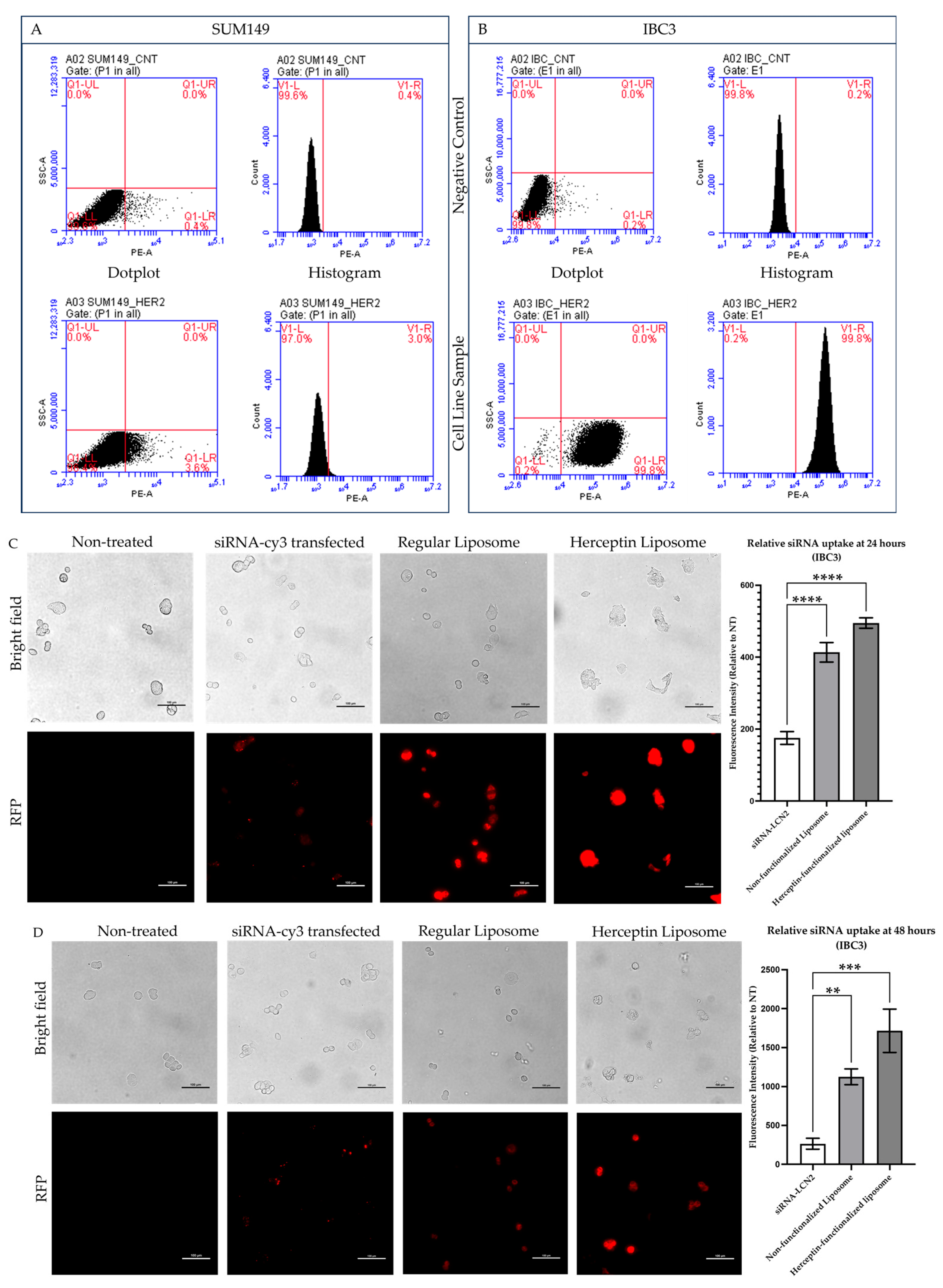
2.3. Flow Cytometry Analysis Confirmed That IBC3 Cells Have Increased HER2 Receptor Levels
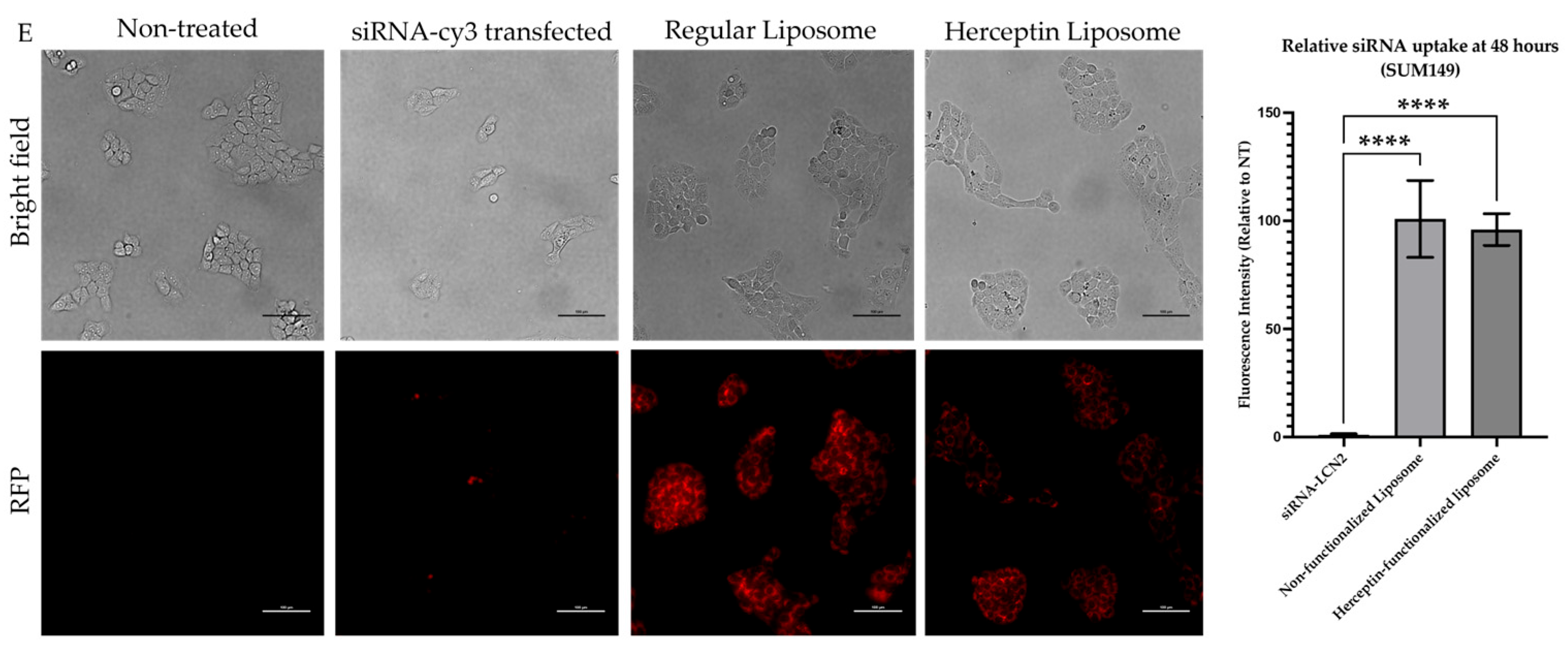
2.4. Herceptin-Conjugated Liposomes Were Efficiently Internalized by HER2+ IBC Cells
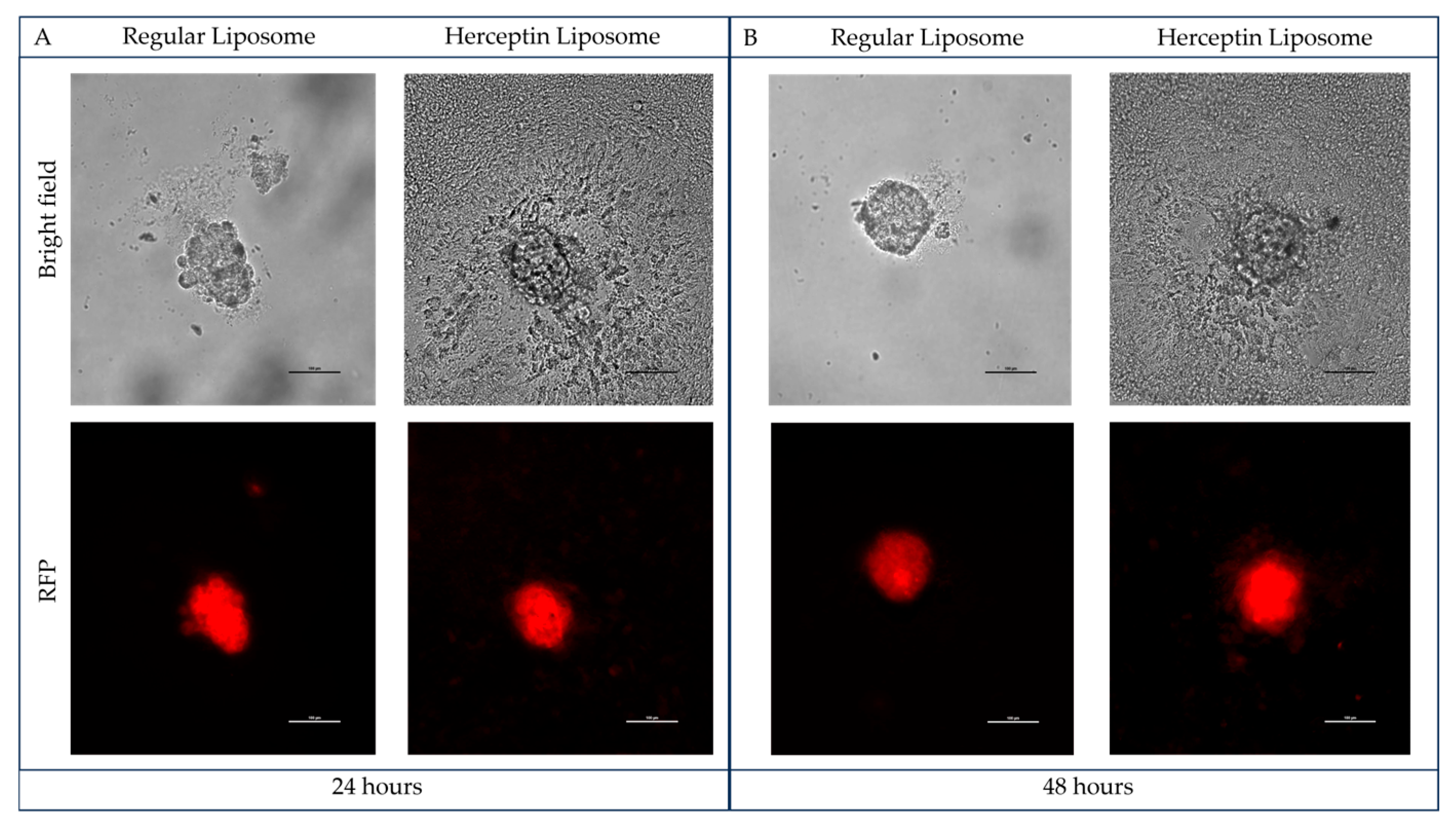
2.5. Herceptin-Conjugated Liposomes Efficiently Disrupt 3D Tumor Emboli of IBC Cells
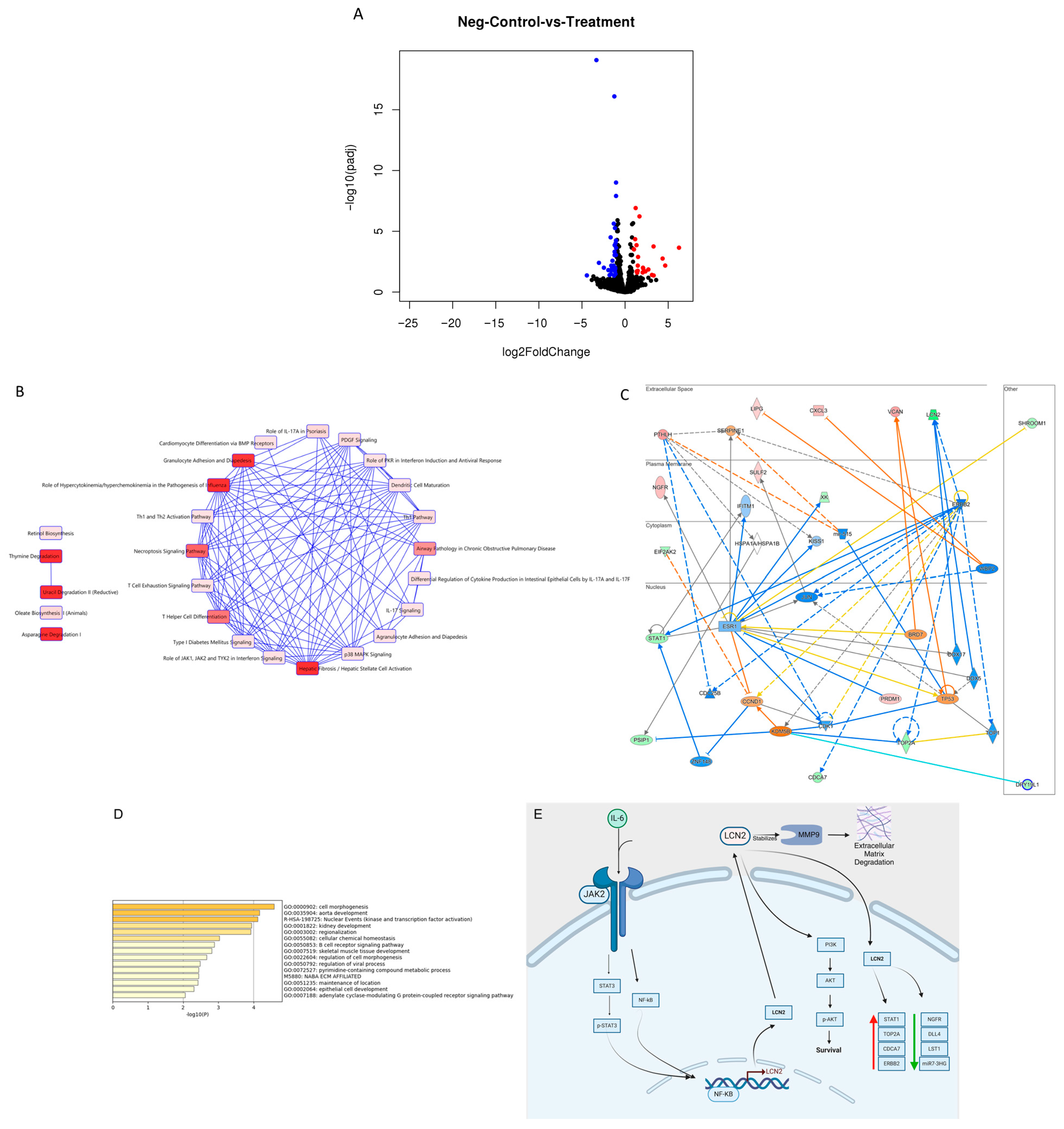
2.6. Knockdown of LCN2 in HER2+ IBC Cells Altered the Expression Levels of Transcripts Involved in Tumor Initiation and Progression
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Herceptin-Conjugated Liposomal Preparation
4.3. Liposome Internalization and LCN2 Knockdown in IBC Cells
4.4. Flow Cytometry
4.5. Tumor Emboli Formation and Treatments
4.6. SiRNA Transfection, RNA Isolation, RNA Sequencing, and Data Analysis
4.7. Bioinformatic Analysis and Network Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chippa, V.; Barazi, H. Inflammatory Breast Cancer. In StatPearls[Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564324/ (accessed on 31 January 2025).
- De Schepper, M.; Nguyen, H.-L.; Richard, F.; Rosias, L.; Lerebours, F.; Vion, R.; Clatot, F.; Berghian, A.; Maetens, M.; Leduc, S.; et al. Treatment Response, Tumor Infiltrating Lymphocytes and Clinical Outcomes in Inflammatory Breast Cancer–Treated with Neoadjuvant Systemic Therapy. Cancer Res. Commun. 2024, 4, 186–199. [Google Scholar] [CrossRef]
- Wang, X.; Semba, T.; Thi, L.; Phi, H.; Chainitikun, S.; Iwase, T.; Lim, B.; Ueno, N.T. Targeting Signaling Pathways in Inflammatory Breast Cancer. Cancers 2020, 12, 2479. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 28 March 2025).
- Mamouch, F.; Berrada, N.; Aoullay, Z.; El Khanousi, B.; Errihani, H. Inflammatory Breast Cancer Disease: A Literature Review. Cancer Stud. 2019, 2, 129–135. [Google Scholar] [CrossRef]
- Santiago-Sánchez, G.S.; Noriega-Rivera, R.; Hernández-O’farrill, E.; Valiyeva, F.; Quiñones-Diaz, B.; Villodre, E.S.; Debeb, B.G.; Rosado-Albacarys, A.; Vivas-Mejía, P.E. Targeting Lipocalin-2 in Inflammatory Breast Cancer Cells with Small Interference Rna and Small Molecule Inhibitors. Int. J. Mol. Sci. 2021, 22, 8581. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Sauer, S.J.; Tarpley, M.; Vermeulen, P.; Rypens, C.; Van Laere, S.; Williams, K.P.; Devi, G.R.; Dewhirst, M.W. Inflammatory Breast Cancer Tumor Emboli Express High Levels of Anti-Apoptotic Proteins: Use of a Quantitative High Content and High-Throughput 3D IBC Spheroid Assay to Identify Targeting Strategies. Oncotarget 2017, 8, 25226–25241. [Google Scholar] [CrossRef]
- Van Uden, D.J.P.; Van Maaren, M.C.; Strobbe, L.J.A.; Bult, P.; Van Der Hoeven, J.J.; Siesling, S.; De Wilt, J.H.W.; Blanken-Peeters, C.F.J.M. Metastatic Behavior and Overall Survival According to Breast Cancer Subtypes in Stage IV Inflammatory Breast Cancer. Breast Cancer Res. 2019, 21, 113. [Google Scholar] [CrossRef]
- Fernandez, S.V.; Robertson, F.M.; Pei, J.; Aburto-Chumpitaz, L.; Mu, Z.; Chu, K.; Alpaugh, R.K.; Huang, Y.; Cao, Y.; Ye, Z.; et al. Inflammatory Breast Cancer (IBC): Clues for Targeted Therapies. Breast Cancer Res. Treat. 2013, 140, 23–33. [Google Scholar] [CrossRef]
- Chainitikun, S.; Saleem, S.; Lim, B.; Valero, V.; Ueno, N.T. Update on Systemic Treatment for Newly Diagnosed Inflammatory Breast Cancer. J. Adv. Res. 2021, 29, 1–12. [Google Scholar] [CrossRef]
- Santiago-Sánchez, G.S.; Pita-Grisanti, V.; Quiñones-Díaz, B.; Gumpper, K.; Cruz-Monserrate, Z.; Vivas-Mejía, P.E. Biological Functions and Therapeutic Potential of Lipocalin 2 in Cancer. Int. J. Mol. Sci. 2020, 21, 4365. [Google Scholar] [CrossRef]
- Du, Z.P.; Wu, B.L.; Xie, Y.M.; Zhang, Y.L.; Liao, L.D.; Zhou, F.; Xie, J.J.; Zeng, F.M.; Xu, X.E.; Fang, W.K.; et al. Lipocalin 2 Promotes the Migration and Invasion of Esophageal Squamous Cell Carcinoma Cells through a Novel Positive Feedback Loop. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 2240–2250. [Google Scholar] [CrossRef]
- Villodre, E.S.; Hu, X.; Larson, R.; Finetti, P.; Gomez, K.; Balema, W.; Stecklein, S.R.; Santiago-Sanchez, G.; Krishnamurthy, S.; Song, J.; et al. Lipocalin 2 Promotes Inflammatory Breast Cancer Tumorigenesis and Skin Invasion. Mol. Oncol. 2021, 15, 2752–2765. [Google Scholar] [CrossRef]
- Mayrovitz, H.N. Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-6453320-3-2. [Google Scholar]
- Ye, L.; Li, F.; Song, Y.; Yu, D.; Xiong, Z.; Li, Y.; Shi, T.; Yuan, Z.; Lin, C.; Wu, X.; et al. Overexpression of CDCA7 Predicts Poor Prognosis and Induces EZH2-Mediated Progression of Triple-Negative Breast Cancer. Int. J. Cancer 2018, 143, 2602–2613. [Google Scholar] [CrossRef]
- What Is Inflammatory Breast Cancer?|IBC-IC|International Consortium. Available online: https://ibcic.org/what-is-ibc/ (accessed on 29 January 2022).
- Qin, R.; Wang, X.; Fan, T.; Wu, T.; Lu, C.; Shao, X.; Yin, L. Bilateral Inflammatory Recurrence of HER-2 Positive Breast Cancer: A Unique Case Report and Literature Review. Front. Oncol. 2024, 14, 1276637. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Koo, M.J.; Kim, J.S.; Kim, J.S. Herceptin-Conjugated Temperature-Sensitive Immunoliposomes Encapsulating Gemcitabine for Breast Cancer. Arch. Pharm. Res. 2016, 39, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cheng, Y.; Piao, S.Z.; Xu, Y.J.; Sun, H.H.; Cui, X.; Li, X.Z.; Zhang, S.N.; Piao, L.Z.; Jin, Y.M.; et al. Correlation between HER-2/Neu(ErbB-2) Expression Level and Therapeutic Effect of Combination Treatment with HERCEPTIN and Chemotherapeutic Agents in Gastric Cancer Cell Lines. Cancer Cell Int. 2014, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yu, L.; Lu, H.; Sakthivel, T.S.; Seal, S.; Liu, B.; Zhao, J. Sensitization of Breast Cancer to Herceptin by Redox Active Nanoparticles. Am. J. Cancer Res. 2021, 11, 4884–4899. [Google Scholar]
- Reyes-gonzález, J.M.; Armaiz-peña, G.N.; Mangala, L.S.; Ivan, C.; Pradeep, S.; Echevarría-vargas, I.M.; Rivera-, A.; Sood, A.K.; Vivas-mejía, P.E. Targeting C-MYC in Platinum-Resistant Ovarian Cancer. Mol. Cancer Ther. 2016, 14, 2260–2269. [Google Scholar] [CrossRef]
- Reyes, J.; Pietri, F.; Vivas-Mejia, P.E. Nano Based Drug Delivery; IAPC Publishing: Zagreb, Croatia, 2015; Chapter 12; ISBN 978-953-56942-2-9. [Google Scholar]
- Eckhardt, B.L.; Ueno, N.T. A Target of Potential RELAvance in Inflammatory Breast Cancer. Oncotarget 2017, 8, 25226–25241. [Google Scholar] [CrossRef]
- Asada, K.; Kobayashi, K.; Joutard, S.; Tubaki, M.; Takahashi, S.; Takasawa, K.; Komatsu, M.; Kaneko, S.; Sese, J.; Hamamoto, R. Uncovering Prognosis-Related Genes and Pathways by Multi-Omics Analysis in Lung Cancer. Biomolecules 2020, 10, 524. [Google Scholar] [CrossRef]
- Watanabe, K.; Takebayashi, H.; Bepari, A.K.; Esumi, S.; Yanagawa, Y.; Watanabe, K.; Takebayashi, H.; Bepari, A.K.; Esumi, S.; Yanagawa, Y. Dpy19l1, a Multi-Transmembrane Protein, Regulates the Radial Migration of Glutamatergic Neurons in the Developing Cerebral Cortex. Development 2011, 138, 4979–4990. [Google Scholar] [CrossRef]
- DPY19L1 Dpy-19 like C-Mannosyltransferase 1 [Homo sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=23333 (accessed on 30 September 2024).
- Zhao, W.; Sun, L.; Li, X.; Wang, J.; Zhu, Y.; Jia, Y.; Tong, Z. SCD5 Expression Correlates with Prognosis and Response to Neoadjuvant Chemotherapy in Breast Cancer. Sci. Rep. 2021, 11, 8976. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, M. SSX2IP as a Novel Prognosis Biomarker Plays an Important Role in the Development of Breast Cancer. Mol. Cell. Toxicol. 2023, 19, 463–472. [Google Scholar] [CrossRef]
- Breslin, A.; Denniss, F.A.K.; Guinn, B.A. SSX2IP: An Emerging Role in Cancer. Biochem. Biophys. Res. Commun. 2007, 363, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Ikura, M. Radixin: Cytoskeletal Adopter and Signaling Protein. Int. J. Biochem. Cell Biol. 2004, 36, 2131–2136. [Google Scholar] [CrossRef]
- Yuan, J.; Xiao, C.; Lu, H.; Yu, H.; Hong, H.; Guo, C.; Wu, Z. MiR-200b Regulates Breast Cancer Cell Proliferation and Invasion by Targeting Radixin. Exp. Ther. Med. 2020, 19, 2741–2750. [Google Scholar] [CrossRef]
- Du, H.X.; Wang, H.; Ma, X.P.; Chen, H.; Dai, A.B.; Zhu, K.X. Eukaryotic Translation Initiation Factor 2α Kinase 2 in Pancreatic Cancer: An Approach towards Managing Clinical Prognosis and Molecular Immunological Characterization. Oncol. Lett. 2023, 26, 1–21. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, Y.; Zhao, X.; Wang, J.; Zhang, Y.; Wang, Q.; Yu, H.; Zhang, Y.; You, Y. EIF2AK2 Selectively Regulates the Gene Transcription in Immune Response and Histones Associated with Systemic Lupus Erythematosus. Mol. Immunol. 2021, 132, 132–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Wang, L.; Zhou, H.; Zhang, G.; Xiao, Z.; Xue, X. TOP2A Correlates with Poor Prognosis and Affects Radioresistance of Medulloblastoma. Front. Oncol. 2022, 12, 918959. [Google Scholar] [CrossRef]
- An, X.; Xu, F.; Luo, R.; Zheng, Q.; Lu, J.; Yang, Y.; Qin, T.; Yuan, Z.; Shi, Y.; Jiang, W.; et al. The Prognostic Significance of Topoisomerase II Alpha Protein in Early Stage Luminal Breast Cancer. BMC Cancer 2018, 18, 331. [Google Scholar] [CrossRef]
- Zhang, M.; Xiang, Z.; Wang, F.; Shan, R.; Li, L.; Chen, J.; Liu, B.A.; Huang, J.; Sun, L.Q.; Zhou, W.B. STARD4 Promotes Breast Cancer Cell Malignancy. Oncol. Rep. 2020, 44, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. SLF1. Available online: https://www.proteinatlas.org/ENSG00000133302-SLF1/pathology/breast+cancer (accessed on 30 September 2024).
- Räschle, M.; Smeenk, G.; Hansen, R.; Temu, T.; Oka, Y.; Hein, M.; Nagaraj, N.; Long, D.; Walter, J.; Hofmann, K.; et al. Proteomics Reveals Dynamic Assembly of Repair Complexes during Bypass of DNA Cross-Links. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Oravcová, M.; Nie, M.; Zilio, N.; Maeda, S.; Jami-Alahmadi, Y.; Lazzerini-Denchi, E.; Wohlschlegel, J.A.; Ulrich, H.D.; Otomo, T.; Boddy, M.N. The Nse5/6-like SIMC1-SLF2 Complex Localizes SMC5/6 to Viral Replication Centers. eLife 2022, 11, e79676. [Google Scholar] [CrossRef] [PubMed]
- ADGRB2 Adhesion G Protein-Coupled Receptor B2 [Homo sapiens (Human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/576 (accessed on 30 September 2024).
- Shi, W.; Xu, C.; Lei, P.; Sun, X.; Song, M.; Guo, Y.; Song, W.; Li, Y.; Yu, L.; Zhang, H.; et al. A Correlation Study of Adhesion G Protein-Coupled Receptors as Potential Therapeutic Targets for Breast Cancer. Breast Cancer Res. Treat. 2024, 207, 417–434. [Google Scholar] [CrossRef]
- Tan, M.-H.; De, S.; Bebek, G.; Orloff, M.S.; Wesolowski, R.; Downs-Kelly, E.; Budd, G.T.; Stark, G.R.; Eng, C. Specific Kinesin Expression Profiles Associated with Taxane Resistance in Breast Cancer. Breast Cancer Res Treat. 2012, 131, 849–858. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, L.; Lu, S.; Li, W.; Dong, W. KIFC3 Promotes Proliferation, Migration, and Invasion in Colorectal Cancer via PI3K/AKT/MTOR Signaling Pathway. Front. Genet. 2022, 13, 848926. [Google Scholar] [CrossRef]
- Ju, J.Q.; Zhang, H.L.; Wang, Y.; Hu, L.L.; Sun, S.C. Kinesin KIFC3 Is Essential for Microtubule Stability and Cytokinesis in Oocyte Meiosis. Cell Commun. Signal. 2024, 22, 199. [Google Scholar] [CrossRef]
- Li, T.F.; Zeng, H.J.; Shan, Z.; Ye, R.Y.; Cheang, T.Y.; Zhang, Y.J.; Lu, S.H.; Zhang, Q.; Shao, N.; Lin, Y. Overexpression of Kinesin Superfamily Members as Prognostic Biomarkers of Breast Cancer. Cancer Cell Int. 2020, 20, 123. [Google Scholar] [CrossRef]
- Zohny, S.F.; Zamzami, M.A.; Al-Malki, A.L.; Trabulsi, N.H. Highly Expressed DLL4 and JAG1: Their Role in Incidence of Breast Cancer Metastasis. Arch. Med. Res. 2020, 51, 145–152. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Ntounis, T.; Pergialiotis, V.; Chionis, A.; Katrachouras, A.; Palios, V.C.; Symeonidis, P.; Valsamaki, A.; Syllaios, A.; et al. The Prognostic Role and Significance of Dll4 and Toll-like Receptors in Cancer Development. Cancers 2022, 14, 1649. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M. The Role of Notch Ligand, Delta-like Ligand 4 (DLL4), in Cancer Angiogenesis—Implications for Therapy. Eur. Surg. Acta Chir. Austriaca 2021, 53, 274–280. [Google Scholar] [CrossRef]
- Hao, S.; Meng, Q.; Sun, H.; Li, Y.; Li, Y.; Gu, L.; Liu, B.; Zhang, Y.; Zhou, H.; Xu, Z.; et al. The Role of Transketolase in Human Cancer Progression and Therapy. Biomed. Pharmacother. 2022, 154, 113607. [Google Scholar] [CrossRef]
- Mao, X.; Zhou, J.; Kong, L.; Zhu, L.; Yang, D.; Zhang, Z. A Peptide Encoded by LncRNA MIR7-3 Host Gene (MIR7-3HG) Alleviates Dexamethasone-Induced Dysfunction in Pancreatic β-Cells through the PI3K/AKT Signaling Pathway. Biochem. Biophys. Res. Commun. 2023, 647, 62–71. [Google Scholar] [CrossRef]
- Wang, S.; Jin, J.; Chen, J.; Lou, W. MUC14-Related NcRNA-MRNA Network in Breast Cancer. Genes 2021, 12, 1677. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. LST1. Available online: https://www.proteinatlas.org/ENSG00000204482-LST1/pathology (accessed on 30 September 2024).
- Fabisik, M.; Tureckova, J.; Pavliuchenko, N.; Kralova, J.; Balounova, J.; Vicikova, K.; Skopcova, T.; Spoutil, F.; Pokorna, J.; Angelisova, P.; et al. Regulation of Inflammatory Response by Transmembrane Adaptor Protein LST1. Front. Immunol. 2021, 12, 618332. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Spatari, G.; Costa, C.; Giambò, F.; Fenga, C. Computational Analyses Reveal Deregulated Clock Genes Associated with Breast Cancer Development in Night Shift Workers. Int. J. Mol. Sci. 2024, 25, 8659. [Google Scholar] [CrossRef] [PubMed]
- Broad Institute of MIT and Harvard Genotype-Tissue Expression (GTEx) Portal. Available online: https://www.gtexportal.org/home/gene/ENSG00000234210.1 (accessed on 31 August 2024).
- Lu, Z.; Gao, Y. Screening Differentially Expressed Genes between Endometriosis and Ovarian Cancer to Find New Biomarkers for Endometriosis. Ann. Med. 2021, 53, 1377–1389. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Chen, C.; Jiang, Y.; Feng, X.; Liao, Y.; Yang, Z. NGFR Increases the Chemosensitivity of Colorectal Cancer Cells by Enhancing the Apoptotic and Autophagic Effects of 5-Fluorouracil via the Activation of S100A9. Front. Oncol. 2021, 11, 652081. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Steele, D.; Di Palma, S.; Jones, R.L.; Savage, K.; James, M.; Milanezi, F.; Schmitt, F.C.; Ashworth, A. Distribution and Significance of Nerve Growth Factor Receptor (NGFR/P75NTR) in Normal, Benign and Malignant Breast Tissue. Mod. Pathol. 2006, 19, 307–319. [Google Scholar] [CrossRef]
- Lee, H.; Shin, C.H.; Kim, H.R.; Choi, K.H.; Kim, H.H. MicroRNA-296-5p Promotes Invasiveness through Downregulation of Nerve Growth Factor Receptor and Caspase-8. Mol. Cells 2017, 40, 254–261. [Google Scholar] [CrossRef]
- Granulocyte Adhesion and Diapedesis. Available online: https://geneglobe.qiagen.com/us/knowledge/pathways/granulocyte-adhesion-and-diapedesis (accessed on 30 September 2024).
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A Regulated Inflammatory Mode of Cell Death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef]
- QIAGEN Th1 Pathway|GeneGlobe. Available online: https://geneglobe.qiagen.com/us/knowledge/pathways/th1-pathway (accessed on 28 March 2025).
- P38 MAPK Signaling|GeneGlobe. Available online: https://geneglobe.qiagen.com/us/knowledge/pathways/p38-mapk-signaling (accessed on 28 March 2025).
- Bertucci, F.; Finetti, P.; Rougemont, J.; Charafe-Jauffret, E.; Nasser, V.; Loriod, B.; Camerlo, J.; Tagett, R.; Tarpin, C.; Houvenaeghel, G.; et al. Gene Expression Profiling for Molecular Characterization of Inflammatory Breast Cancer and Prediction of Response to Chemotherapy. Cancer Res. 2004, 64, 8558–8565. [Google Scholar] [CrossRef] [PubMed]
- Lehman, H.L.; Dashner, E.J.; Lucey, M.; Vermeulen, P.; Dirix, L.; Van Laere, S.; Van Golen, K.L. Modeling and Characterization of Inflammatory Breast Cancer Emboli Grown in Vitro. Int. J. Cancer 2013, 132, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Scheck, M.K.; Hofheinz, R.D.; Lorenzen, S. HER2-Positive Gastric Cancer and Antibody Treatment: State of the Art and Future Developments. Cancers 2024, 16, 1336. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. FDA Amends Approval of Pembrolizumab to Treat Stomach and Esophageal Junction Cancer. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2023/fda-pembrolizumab-stomach-esophageal-her2-pdl1 (accessed on 31 August 2024).
- Cheng, J.; Liang, M.; Carvalho, M.F.; Tigue, N.; Faggioni, R.; Roskos, L.K.; Vainshtein, I. Molecular Mechanism of HER2 Rapid Internalization and Redirected Trafficking Induced by Anti-HER2 Biparatopic Antibody. Antibodies 2020, 9, 49. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zheng, Z.Q.; Jia, S.; Liu, S.N.; Xiao, X.F.; Chen, G.Y.; Liang, W.Q.; Lu, X.F. Trastuzumab Resistance in HER2-Positive Breast Cancer: Mechanisms, Emerging Biomarkers and Targeting Agents. Front. Oncol. 2022, 12, 1006429. [Google Scholar] [CrossRef]
- Elamir, A.; Ajith, S.; Al Sawaftah, N.; Abuwatfa, W.; Mukhopadhyay, D.; Paul, V.; Al-Sayah, M.H.; Awad, N.; Husseini, G.A. Ultrasound-Triggered Herceptin Liposomes for Breast Cancer Therapy. Sci. Rep. 2021, 11, 7545. [Google Scholar] [CrossRef]
- Fernández, C.A.; Yan, L.; Louis, G.; Yang, J.; Kutok, J.L.; Moses, M.A. The Matrix Metalloproteinase-9/Neutrophil Gelatinase-Associated Lipocalin Complex Plays a Role in Breast Tumor Growth and Is Present in the Urine of Breast Cancer Patients. Clin. Cancer Res. 2005, 11, 5390–5395. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, X.; Lei, T.; Fu, D.; Zhang, H. Lipocalin-2 Promotes Breast Cancer Brain Metastasis by Enhancing Tumor Invasion and Modulating Brain Microenvironment. Front. Oncol. 2024, 14, 1448089. [Google Scholar] [CrossRef]
- Guo, P.; Yang, J.; Jia, D.; Moses, M.A.; Auguste, D.T. ICAM-1-Targeted, Lcn2 SiRNA-Encapsulating Liposomes Are Potent Anti-Angiogenic Agents for Triple Negative Breast Cancer. Theranostics 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Gote, V.; Pal, D. Octreotide-Targeted Lcn2 Sirna Pegylated Liposomes as a Treatment for Metastatic Breast Cancer. Bioengineering 2021, 8, 44. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. Correction to: A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoScience 2022, 12, 274–291, Erratum in Bionanoscience 2022, 12, 660. [Google Scholar] [CrossRef]
- Li, W.; Huang, Z.; MacKay, J.A.; Grube, S.; Szoka, F.C. Low-PH-Sensitive Poly(Ethylene Glycol) (PEG)-Stabilized Plasmid Nanolipoparticles: Effects of PEG Chain Length, Lipid Composition and Assembly Conditions on Gene Delivery. J. Gene Med. 2005, 7, 67–79. [Google Scholar] [CrossRef]
- Hald, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Ding, T.; Lin, H.; Wang, Y.; Hu, L.; Hu, J.; Feig, B.; Zhang, W.; Pusztai, L.; Symmans, W.F.; et al. Inhibition of Lipocalin 2 Impairs Breast Tumorigenesis and Metastasis. Cancer Res. 2009, 69, 8579–8584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Novel Function of STAT1 in Breast Cancer. Oncoimmunology 2013, 2, e25125. [Google Scholar] [CrossRef][Green Version]
- Jeon, M.; You, D.; Bae, S.Y.; Kim, S.W.; Nam, S.J.; Kim, H.H.; Kim, S.; Lee, J.E. Dimerization of EGFR and HER2 Induces Breast Cancer Cell Motility through STAT1-Dependent ACTA2 Induction. Oncotarget 2017, 8, 50570–50581. [Google Scholar] [CrossRef]
- Stevens, L.E.; Peluffo, G.; Qiu, X.; Temko, D.; Fassl, A.; Li, Z.; Trinh, A.; Seehawer, M.; Jovanović, B.; Alečković, M.; et al. JAK–STAT Signaling in Inflammatory Breast Cancer Enables Chemotherapy-Resistant Cell States. Cancer Res. 2023, 83, 264–284. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, Y.; Zhou, L.; Du, J.; Wang, J.; Liu, S.H.; Cao, Y.; Li, Y.; Yang, W.; et al. Resistance Mechanisms and Prospects of Trastuzumab. Front. Oncol. 2024, 14, 1389390. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, X.; Sun, Y.; Feng, X.; Wu, X.; Liu, W.; Gao, C.; Yan, Y.; Tian, W.; Wang, Y. TOP2A Modulates Signaling via the AKT/MTOR Pathway to Promote Ovarian Cancer Cell Proliferation. Cancer Biol. Ther. 2024, 25, 2325126. [Google Scholar] [CrossRef]
- Pei, Y.F.; Yin, X.M.; Liu, X. qiang TOP2A Induces Malignant Character of Pancreatic Cancer through Activating β-Catenin Signaling Pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 197–207. [Google Scholar] [CrossRef]
- Li, H.; Weng, Y.; Wang, S.; Wang, F.; Wang, Y.; Kong, P.; Zhang, L.; Cheng, C.; Cui, H.; Xu, E.; et al. CDCA7 Facilitates Tumor Progression by Directly Regulating CCNA2 Expression in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 734655. [Google Scholar] [CrossRef]
- Wang, H.; Ye, L.; Xing, Z.; Li, H.; Lv, T.; Liu, H.; Zhang, F.; Song, Y. CDCA7 Promotes Lung Adenocarcinoma Proliferation via Regulating the Cell Cycle. Pathol. Res. Pract. 2019, 215, 152559. [Google Scholar] [CrossRef]
- Siman, L.I.; Huang, J.; Qin, M.; Zhang, J.; Liao, C. High Expression of CDCA7 Predicts Tumor Progression and Poor Prognosis in Human Colorectal Cancer. Mol. Med. Rep. 2020, 22, 57–66. [Google Scholar] [CrossRef]
- Modi, A.P.; Nguyen, J.P.T.; Wang, J.; Ahn, J.S.; Libling, W.A.; Klein, J.M.; Mazumder, P.; Barsky, S.H. Geometric Tumor Embolic Budding Characterizes Inflammatory Breast Cancer. Breast Cancer Res. Treat. 2023, 197, 461–478. [Google Scholar] [CrossRef]
- Vermeulen, P.B.; Van Golen, K.L.; Dirix, L.Y. Angiogenesis, Lymphangiogenesis, Growth Pattern, and Tumor Emboli in Inflammatory Breast Cancer: A Review of the Current Knowledge. Cancer 2010, 116, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.M.; Shammas, R.L.; Fales, A.M.; Brown, D.A.; Hollenbeck, S.T.; Vo-Dinh, T.; Devi, G.R. Photothermal Ablation of Inflammatory Breast Cancer Tumor Emboli Using Plasmonic Gold Nanostars. Int. J. Nanomed. 2017, 12, 6259–6272. [Google Scholar] [CrossRef]
- Dobiasova, B.; Mego, M. Biomarkers for Inflammatory Breast Cancer: Diagnostic and Therapeutic Utility. Breast Cancer Targets Ther. 2020, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody–Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, Y.; Ma, L.; Jing, X.; Ke, X.; Lian, J.; Zhao, Q.; Yan, B.; Zhang, J.; Yao, J.; et al. Comparison of Anti-EGFR-Fab’ Conjugated Immunoliposomes Modified with Two Different Conjugation Linkers for SiRNA Delivery in SMMC-7721 Cells. Int. J. Nanomed. 2013, 8, 3271–3283. [Google Scholar] [CrossRef]

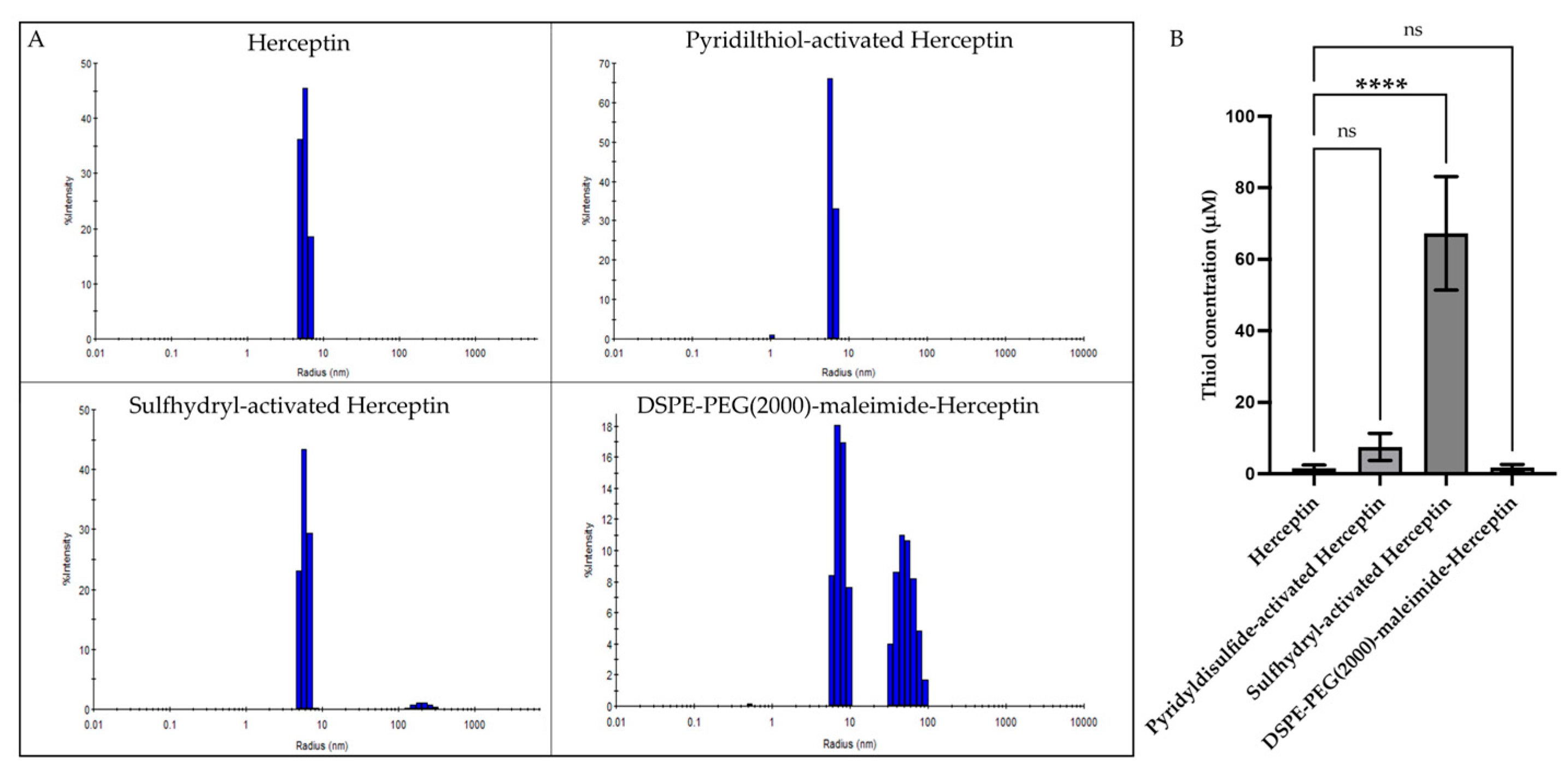


| Particle | Radius (nm) | Polydispersity Index (PDI: %) | Thiol Concentration (μM) | Reaction Percent Yield (%) |
|---|---|---|---|---|
| Herceptin | 5.6 ± 0.2 | 9.3± 3.0 | 1.6 ± 0.88 | - |
| Pyridilthiol-activated Herceptin | 6.2 ± 0.1 | 9.1 ± 2.8 | 7.4 ± 3.8 | 63.1 ± 8.9 |
| Sulfhydryl-activated Herceptin | 5.8 ± 0.3 | 14.4 ± 2.7 | 67.2 ± 15.9 | 85.5 ± 2.2 |
| DSPE-PEG(2000)-maleimide-Herceptin | 8.4 ± 2.2 40.5 ± 12.1 | 11.1 ± 4.5 24.2 ± 4.1 | 1.69 ± 0.90 | 81.6 ± 5.7 |
| Particle | Radius (nm) | Polydispersity Index (PDI: %) | Zeta-Potential (mV) |
|---|---|---|---|
| DOPC-PEG(2000)-PE liposome | 15.4 ± 6.8 43.0 ± 2.9 | 13.6 ± 4.3 28.9 ± 9.0 | −0.009 |
| DOPC-PEG(2000)-maleimide-Herceptin liposome | 22.3 ± 5.7 66.7 ± 10.5 | 17.8 ± 6.9 27.9 ± 3.5 | −0.161 |
| Gene | Gene Name | log2fold Change | p-Value | Biological Role |
|---|---|---|---|---|
| Downregulated genes | ||||
| LCN2 | Lipocalin-2 | −3.33 | 5.88 × 10−24 | Glycoproteins are associated with the transport of hydrophobic ligands, homeostasis, and epithelial cell differentiation. Associated with aggressive cancers [6] |
| DPY19L1 | Dpy-19-like C-mannosyltransferase 1 | −1.68 | 3.82 × 10−8 | Part of the DPY19 family, predicted to enable mannosyltransferase activity. A multi-transmembrane protein known to regulate the migration of neuroblasts in C. elegans. Has been reported as a pro-survival gene in lung adenocarcinoma [25,26,27] |
| SCD5 | Stearoyl-CoA Desaturase 5 | −1.51 | 5.58 × 10−5 | Involved in lipid metabolism. Overexpression is linked to increased survival in lung adenocarcinoma but shorter survival in acute myeloid carcinoma. Downregulated in breast cancer tumors [28] |
| SSX2IP | Synovial Sarcoma X breaking point 2 Interacting Protein | −1.94 | 0.000106 | Found in normal tissues, with the highest expression in the brain. Overexpressed in various cancers, including breast and acute myeloid leukemia. Enhances tumor progression, proliferation, migration, and invasion in breast cancer [29,30] |
| RDX | Radixin | −1.60 | 0.000110 | Membrane-cytoskeletal crosslinker in actin-rich cell surface structures. mRNA expression is higher in breast cancer tissues than in normal tissues, increasing with tumor-node-metastasis stage [31,32] |
| EIF2AK2 | Eukaryotic translation initiation factor 2 alpha kinase 2 | −1.64 | 0.000283 | Regulates protein synthesis through the phosphorylation of translation initiation factor eIF2α. Expression is higher in invasive ductal carcinoma compared to normal tissue. In pancreatic cancer, high expression is related to poorer survival [33,34] |
| TOP2A | DNA topoisomerase II alpha | −1.80 | 0.000380 | Catalytic enzyme that initiates DNA replication. In breast cancer, high levels correlate to higher tumor grade, increased incidence of distant metastasis, and shorter distant metastasis-free survival [35,36] |
| STARD4 | Steroidogenic acute regulatory protein-related lipid transfer 4 | −1.77 | 0.00118 | Cholesterol transporter. Elevated levels have been associated with reduced distant metastasis-free survival in breast cancer. Has been identified as an oncogene by promoting cell proliferation and inhibiting apoptosis [37] |
| SLF1 | SMC5-SMC6 complex localization factor 1 | −1.61 | 0.00199 | Has a role in DNA damage response; it is recruited together with SLF2 to DNA lesions. While it has low cancer specificity, high expression in breast cancer patients is associated with decreased survival probability [38,39,40] |
| ADGRB2 | Adhesion G protein-coupled receptor B2 | −1.50 | 0.00516 | Encodes a transmembrane protein that is a brain-specific inhibitor of angiogenesis. High transcription is found in breast cancer primary tumors [41,42] |
| Upregulated Genes | ||||
| KIFC3 | Kinesin family member C3 | 1.51 | 4.60 × 10−6 | Has roles associated with centrosome cohesion, cytokinesis, vesicle transportation, and cell proliferation in mitosis. Overexpression in breast cancer is correlated to paclitaxel and docetaxel resistance; however, reports exist of decreased expression in breast cancer tumor samples [43,44,45,46] |
| DLL4 | Delta-like canonical Notch ligand 4 | 3.30 | 3.40 × 10−7 | Component of the Notch signaling pathway, involved in endothelial tip cells, related to pro-angiogenic factors. mRNA overexpression correlated to poor prognosis in various cancers, including breast and endometrial cancer, with roles in metastasis, angiogenesis, and stem cell activation [47,48,49] |
| TKTL1 | Transketolase like 1 | 6.26 | 5.11 × 10−7 | Critical for glucose metabolism and the pentose phosphate pathway. Elevated levels correlate with chemoresistance, cell proliferation, invasion, and metastasis [50] |
| MIR7-3HG | MIR7-3 host gene | 4.35 | 6.61 × 10−6 | Long non-coding RNA that encodes a peptide that has a protective role in pancreatic β-cells through the activation PI3K/AKT pathway [51] |
| MUC22 | Mucin 22 | 4.65 | 3.39 × 10−5 | Encodes a membrane-bound glycoprotein. mRNA expression in breast cancer showed no significant difference compared to normal controls [52] |
| LST1 | Leukocyte-specific transcript 1 | 4.60 | 5.27 × 10−5 | Encodes a small adaptor protein involved in cytoskeleton regulation. Unfavorable prognostic marker in renal and testis cancer; favorable prognostic marker in cervical cancer [53,54] |
| CIART | Circadian-associated repressor of transcription | 2.08 | 5.87 × 10−5 | The clock gene plays a crucial role in the synchronization of homeostatic processes. Implicated in breast cancer initiation and progression. It is overexpressed in Luminal A, grade 2, and stage 1 breast cancer [55] |
| AC006372.4 | Long intergenic non-coding RNA | 5.91 | 5.92 × 10−5 | Annotated uncharacterized gene [56] |
| IGHM | Immunoglobulin heavy constant mu | 6.62 | 6.23 × 10−5 | Encodes the C region of the mu heavy chain, which defines the IgM isotype. Elevated IGHM levels are associated with increased overall survival and disease-free survival in ovarian cancer [57] |
| NGFR | Nerve growth factor receptor | 2.10 | 7.13 × 10−5 | Cell surface receptor with roles in cell death and survival that belongs to the tumor necrosis factor receptor superfamily. Identified as a tumor suppressor, influencing proliferation and metastasis in various breast cancer, colorectal cancer, and glioblastoma studies [58,59,60] |
| Pathway | p-Value | Number of Genes | Genes | Biological Role |
|---|---|---|---|---|
| Granulocyte Adhesion and Diapedesis | 0.00534 | 3 | CXCL3, NGFR, RDX | An immune defense process wherein white blood cells adhere to blood vessel walls and migrate into tissues to fight infection and inflammation [61]. |
| Necroptosis Signaling Pathway | 0.00865 | 3 | EIF2AK2, NGFR, STAT1 | Programmed form of necrosis characterized by cell swelling and membrane rupture, typically triggered by death receptors. It functions as a defense mechanism against pathogens occurring when caspase-8 is inhibited [62] |
| Th1 Pathway | 0.0326 | 2 | DLL4, STAT1 | A T cell differentiation pathway in which naive CD4+ T cells become Th1 cells in response to antigen and cytokine stimulation. Th1 cells produce IFN-γ and activate macrophages and cytotoxic T cells to defend against intracellular pathogens such as viruses and certain bacteria [63] |
| p38 MAPK | 0.0443 | 2 | MEF2C, STAT1 | A stress-activated signaling pathway is triggered by inflammatory cytokines and environmental factors as UV and oxidative stress. It leads to transcription of genes involved in inflammation, cytokine production, and apoptosis [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Colón, M.; Rivera-Serrano, M.; Peterson-Peguero, E.A.; Vivas-Rivera, P.E.; Valiyeva, F.; Vivas-Mejía, P.E. Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer. Pharmaceuticals 2025, 18, 1053. https://doi.org/10.3390/ph18071053
Flores-Colón M, Rivera-Serrano M, Peterson-Peguero EA, Vivas-Rivera PE, Valiyeva F, Vivas-Mejía PE. Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer. Pharmaceuticals. 2025; 18(7):1053. https://doi.org/10.3390/ph18071053
Chicago/Turabian StyleFlores-Colón, Marienid, Mariela Rivera-Serrano, Esther A. Peterson-Peguero, Pablo E. Vivas-Rivera, Fatima Valiyeva, and Pablo E. Vivas-Mejía. 2025. "Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer" Pharmaceuticals 18, no. 7: 1053. https://doi.org/10.3390/ph18071053
APA StyleFlores-Colón, M., Rivera-Serrano, M., Peterson-Peguero, E. A., Vivas-Rivera, P. E., Valiyeva, F., & Vivas-Mejía, P. E. (2025). Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer. Pharmaceuticals, 18(7), 1053. https://doi.org/10.3390/ph18071053







