Role of Central Inflammatory and Oxidative Pathways in the Morphine Exacerbation of Cardiovascular Effects of Sepsis in Rats
Abstract
1. Introduction
2. Results
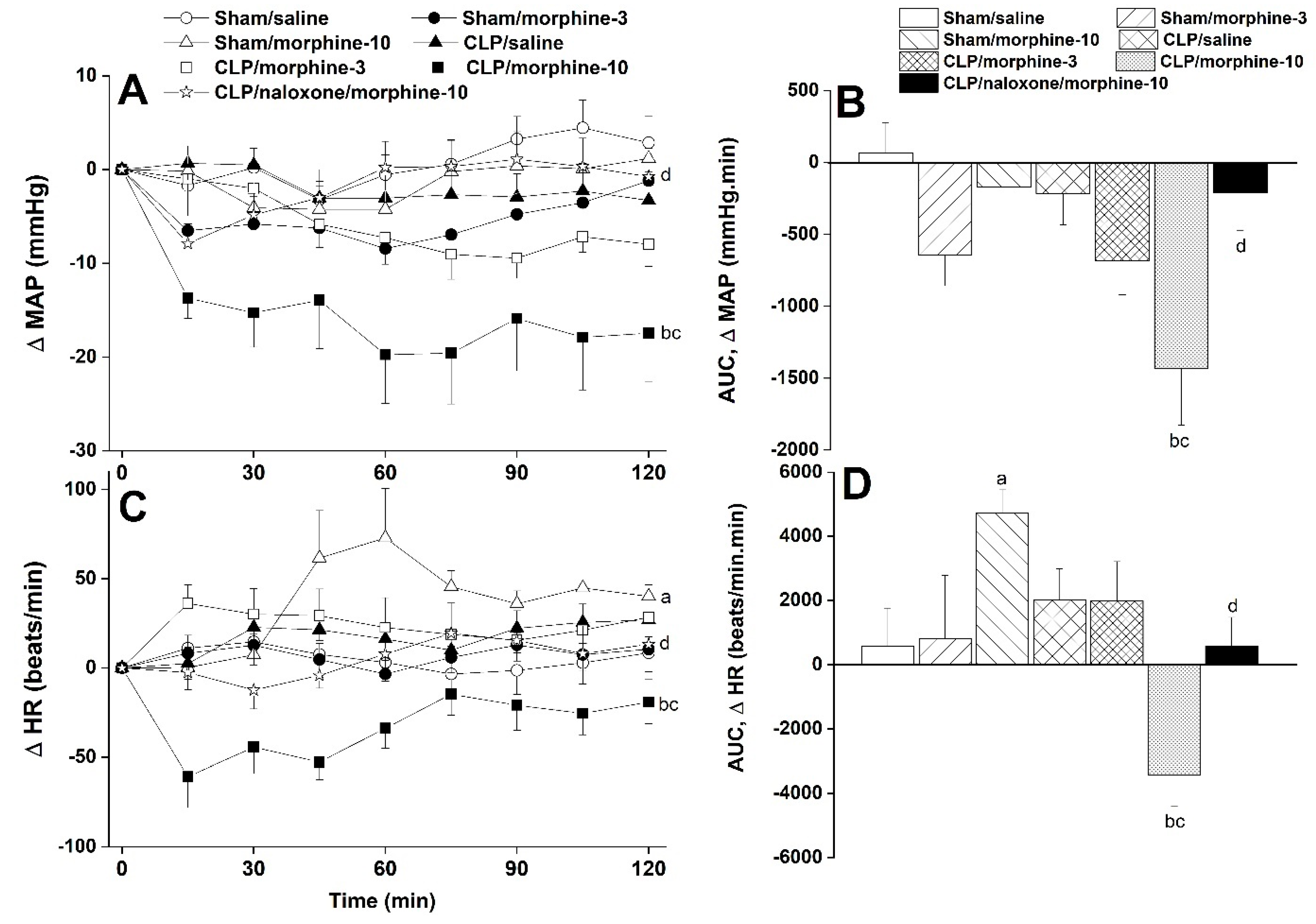
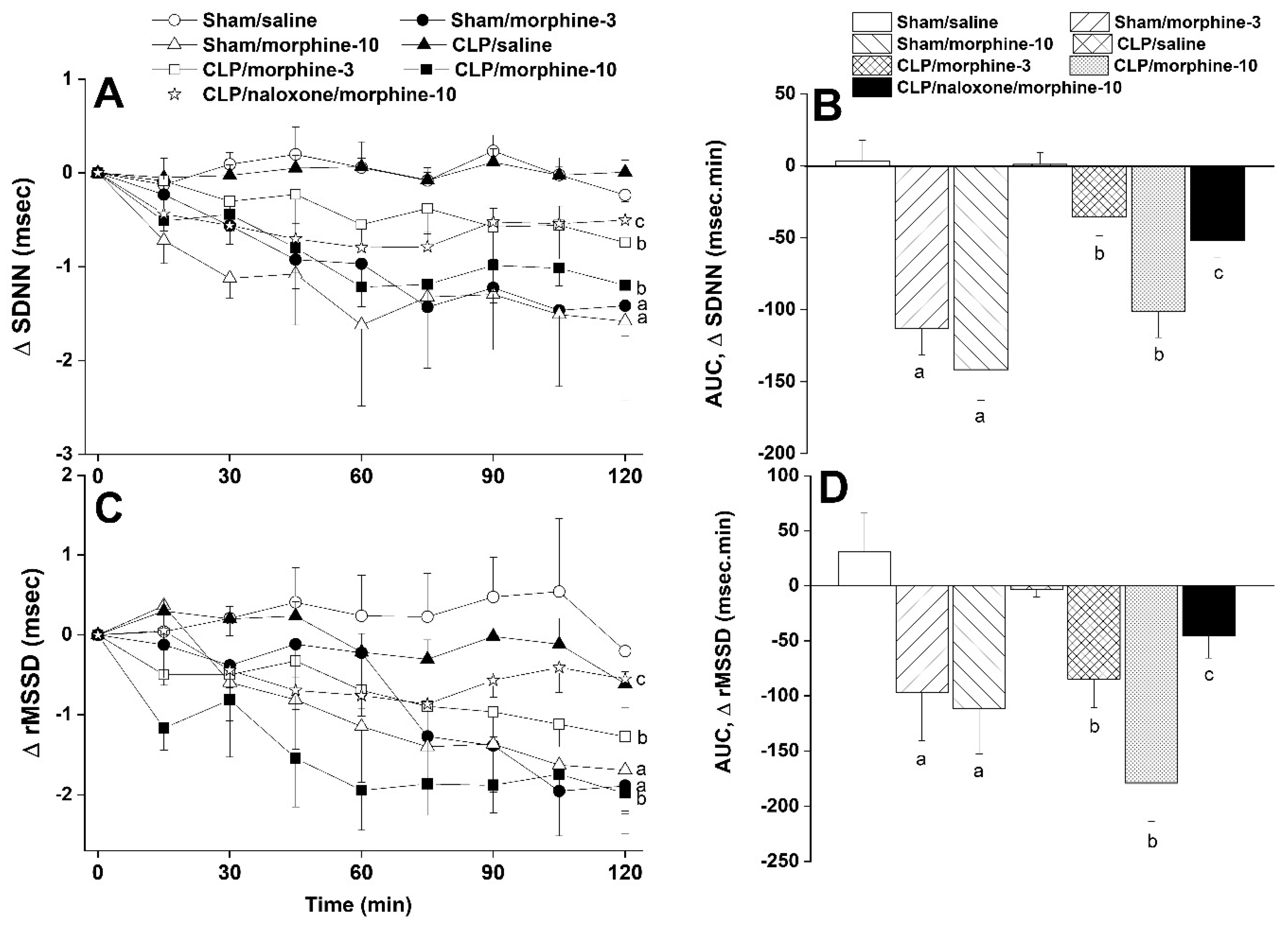
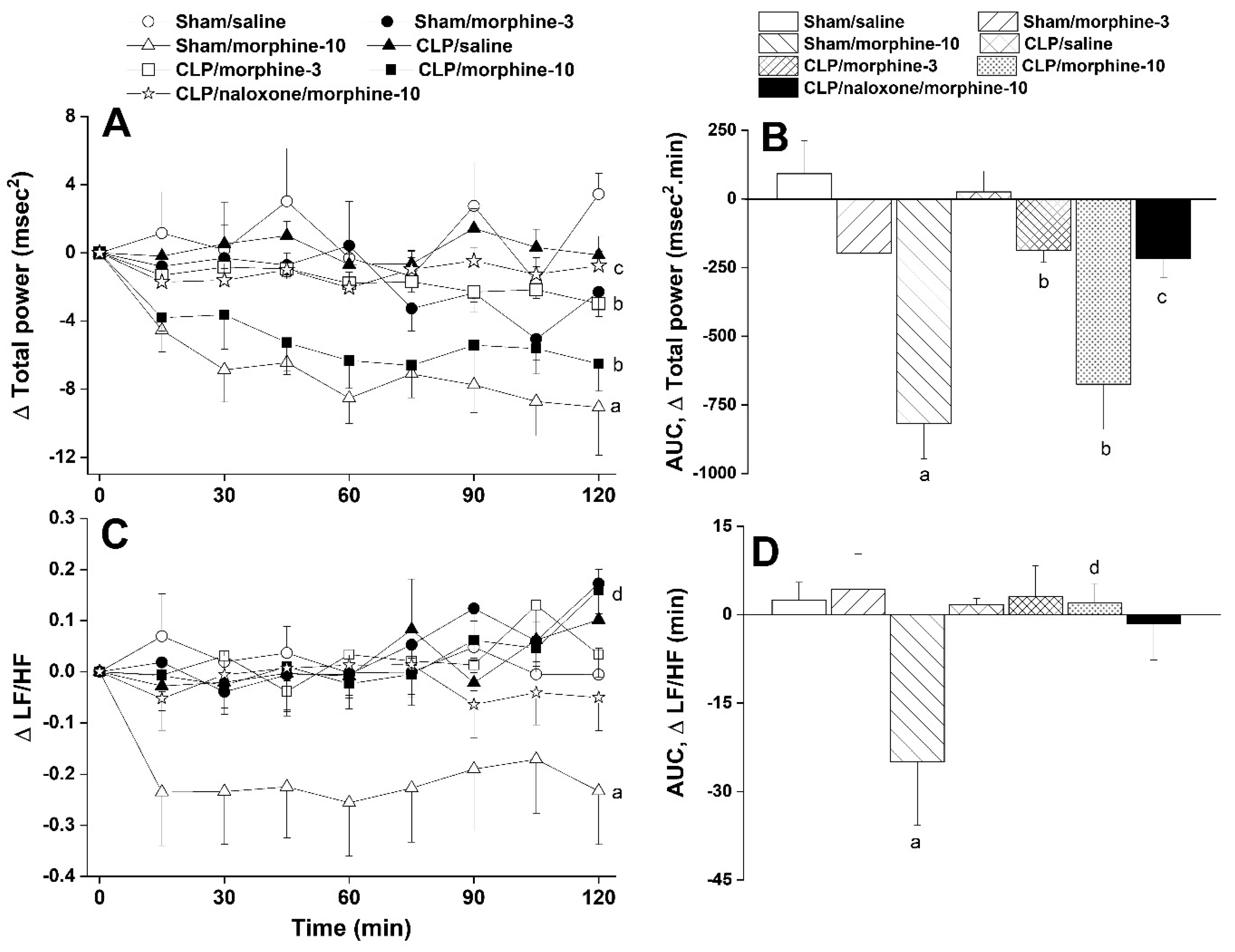
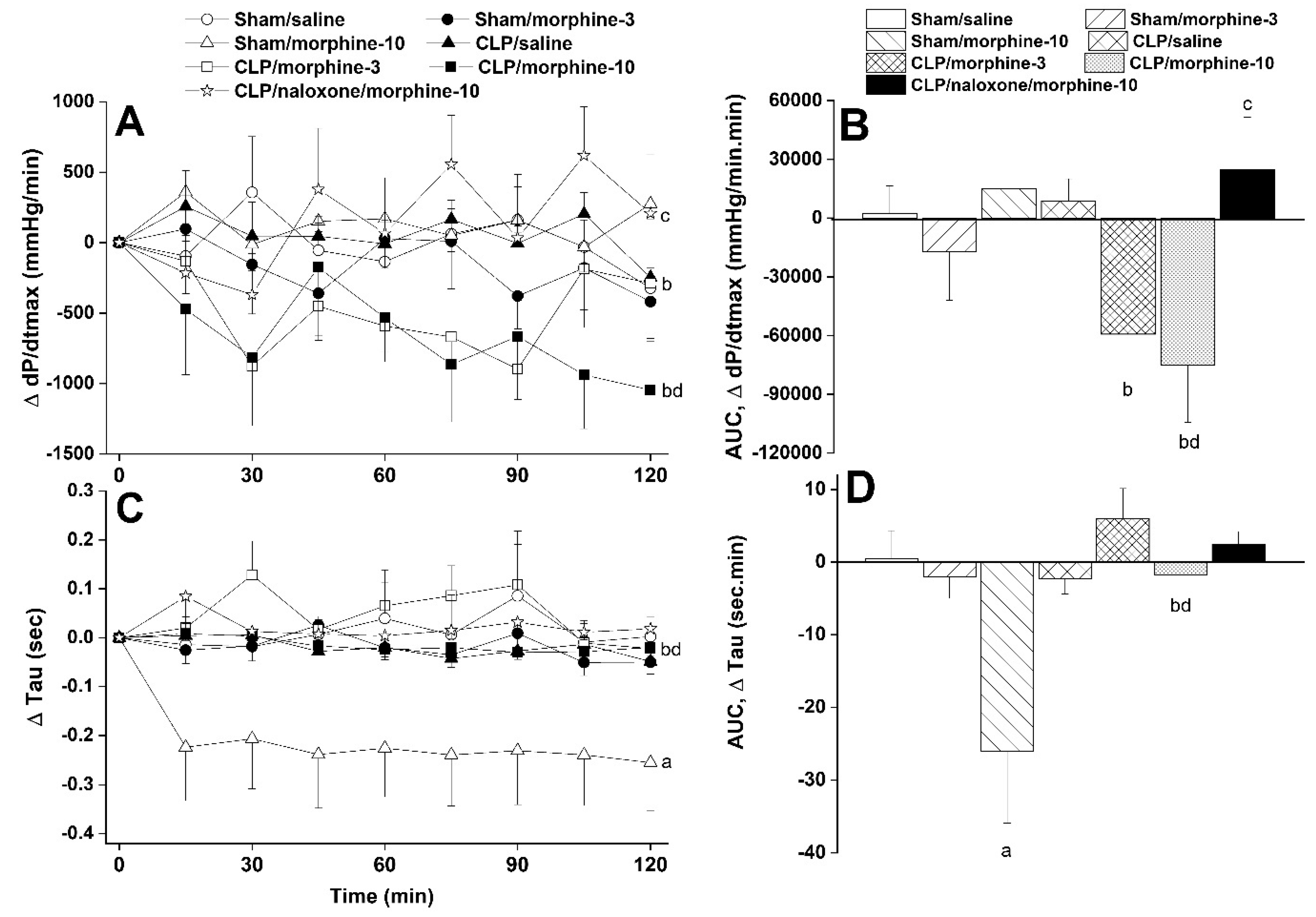
2.1. Hemodynamic and Cardiac Autonomic Effects of CLP
2.2. Morphine Aggravates Septic Manifestations of Hypotension and Autonomic Neuropathy
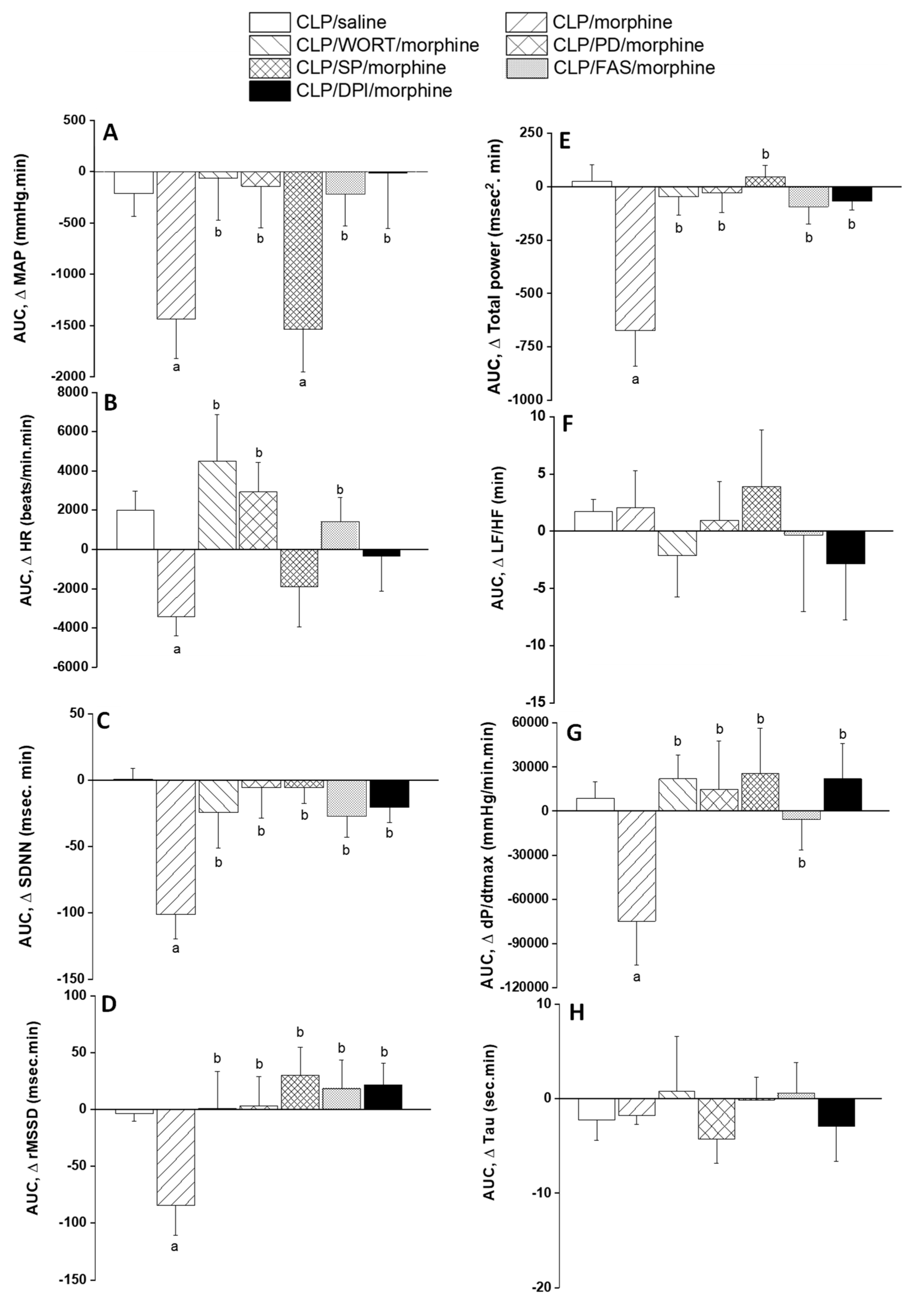
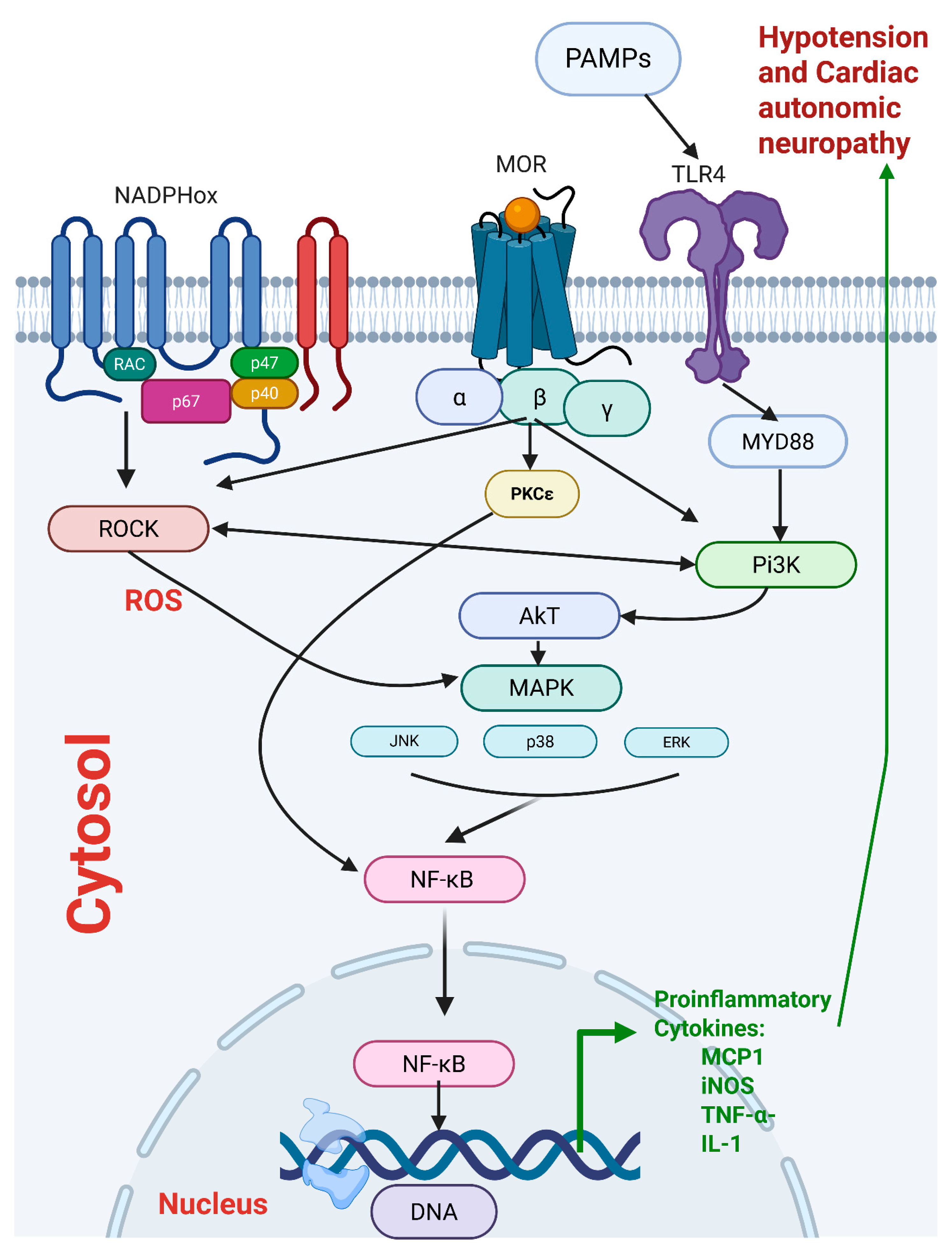
2.3. Central PI3K/MAPK/NADPHox/Rho Kinase Pathway Mediates the Sepsis/Morphine-Interaction
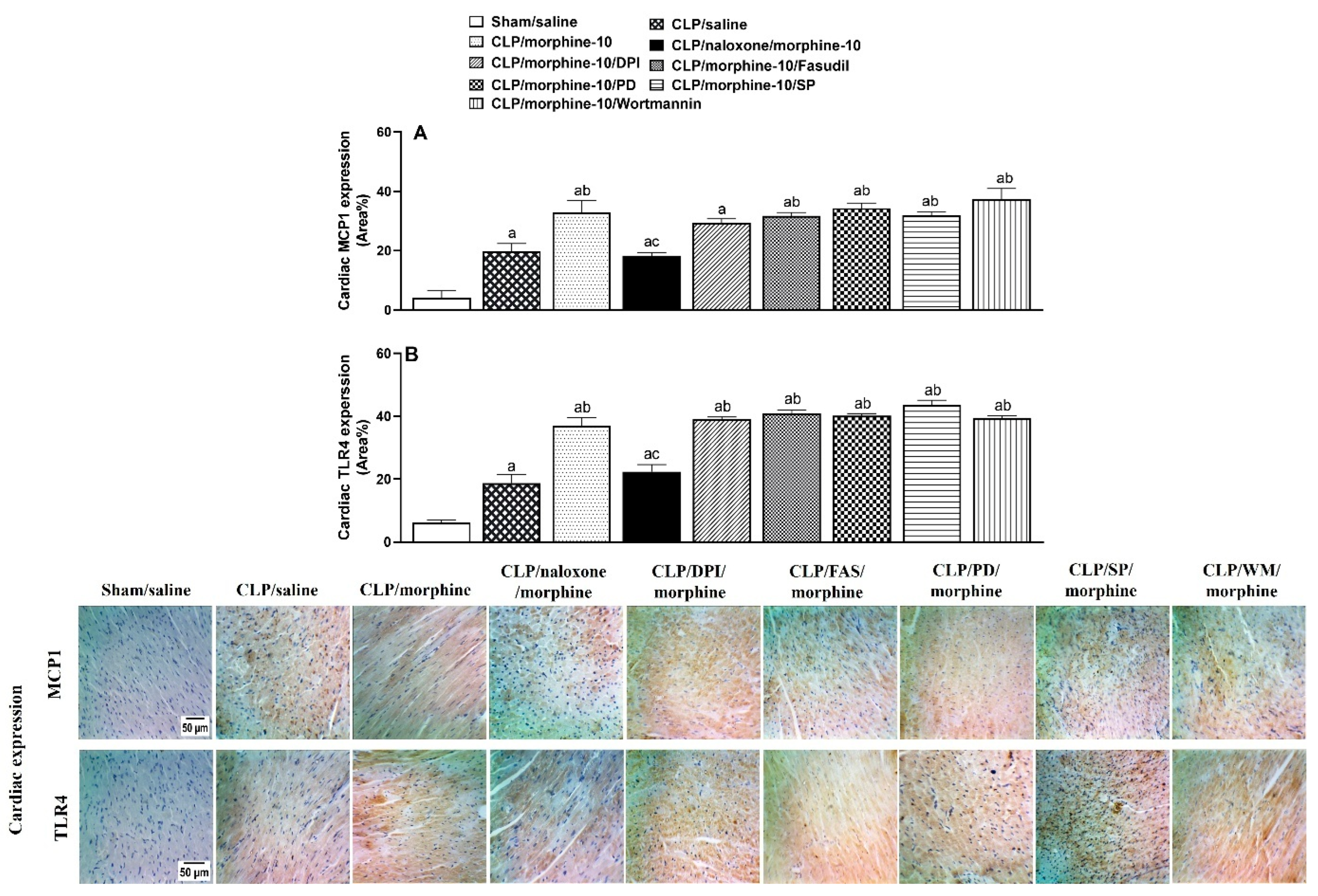
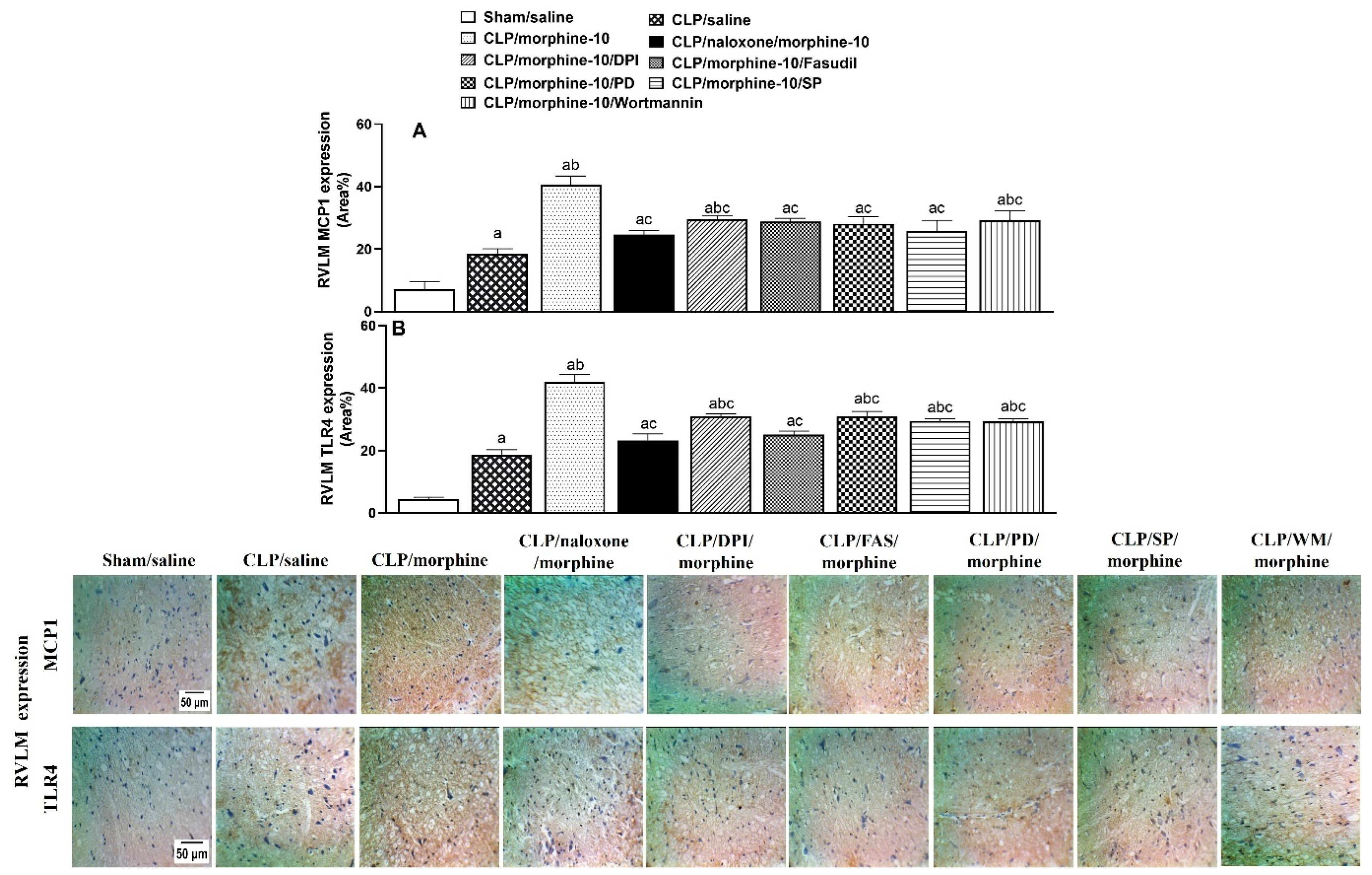
2.4. Brainstem Neuroinflammation Provokes Cardiovascular Disturbances Induced by Morphine
3. Discussion
Perspectives
4. Materials and Method
4.1. Animals
4.2. Drugs
4.3. Cecal Ligation and Puncture (CLP)
4.4. Intracisternal Cannulation (i.c.)
4.5. Intravascular Cannulation
4.6. Time-Domain Analysis of HRV
4.7. Frequency-Domain Analysis of HRV
4.8. Immunohistochemistry
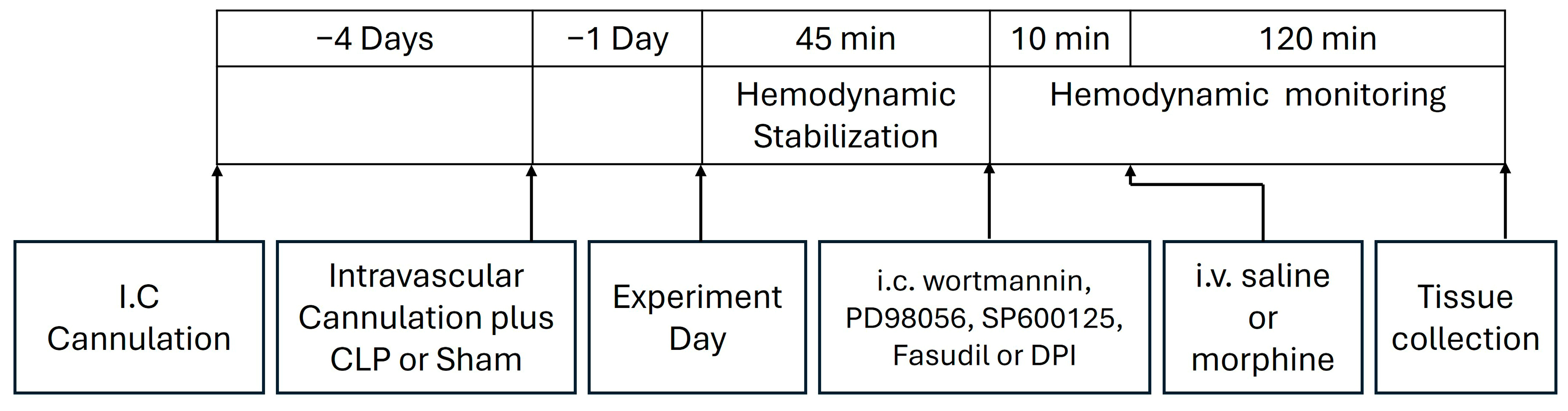
4.9. Protocols and Experimental Design
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUCs | Areas Under the Curves |
| CLP | Cecal ligation and puncture |
| dP/dtmax | maximal rate of rise of left ventricular pressure |
| ERK | Extracellular signal-regulated kinase |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| i.c. | Intracisternal cannulation |
| ICUs | Intensive Care Units |
| JNK | c-Jun N-terminal Kinase |
| LF/HF | low frequency/high frequency ratio |
| MAPK | Mitogen-activated protein kinase |
| MAP | Mean Arterial Pressure |
| MCP1 | Monocyte Chemoattractant Protein-1 |
| NADPHox | Nicotinamide adenine dinucleotide phosphate oxidase |
| PI3K | Phosphoinositide-3 kinases |
| rMSSD | the square root of the mean squared differences of successive NN intervals |
| ROCK | Rho-associated coiled-coil kinase |
| RVLM | Rostral Ventrolateral Medullary |
| SDNN | Standard Deviation of NN intervals |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Deutschman, C.S.; Tracey, K.J. Sepsis: Current dogma and new perspectives. Immunity 2014, 40, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.A.; Zaverucha-do-Valle, C.; Fleurance, R.; Sharshar, T.; Bozza, F.A.; d’Avila, J.C. Neuroinflammation in Sepsis: Molecular Pathways of Microglia Activation. Pharmaceuticals 2021, 14, 416. [Google Scholar] [CrossRef]
- Sallam, M.Y.; El-Gowilly, S.M.; Abdel-Galil, A.G.; El-Mas, M.M. Central GABAA receptors are involved in inflammatory and cardiovascular consequences of endotoxemia in conscious rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sayk, F.; Vietheer, A.; Schaaf, B.; Wellhoener, P.; Weitz, G.; Lehnert, H.; Dodt, C. Endotoxemia causes central downregulation of sympathetic vasomotor tone in healthy humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R891–R898. [Google Scholar] [CrossRef]
- Filiz, A.I.; Ozturk, A.; Kurt, Y.; Sucullu, I.; Akin, M.L.; Yildiz, M. The effects of immunosuppressive agents on inflammatory response in septic rats. Cent. Eur. J. Med. 2010, 5, 683–690. [Google Scholar] [CrossRef]
- Zhu, T.; Liao, X.; Feng, T.; Wu, Q.; Zhang, J.; Cao, X.; Li, H. Plasma Monocyte Chemoattractant Protein 1 as a Predictive Marker for Sepsis Prognosis: A Prospective Cohort Study. Tohoku J. Exp. Med. 2017, 241, 139–147. [Google Scholar] [CrossRef]
- Tunctan, B.; Korkmaz, B.; Cuez, T.; Kemal Buharalioglu, C.; Sahan-Firat, S.; Falck, J.; Malik, K.U. Contribution of vasoactive eicosanoids and nitric oxide production to the effect of selective cyclooxygenase-2 inhibitor, NS-398, on endotoxin-induced hypotension in rats. Basic Clin. Pharmacol. Toxicol. 2010, 107, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Temiz-Resitoglu, M.; Kucukkavruk, S.P.; Guden, D.S.; Cecen, P.; Sari, A.N.; Tunctan, B.; Gorur, A.; Tamer-Gumus, L.; Buharalioglu, C.K.; Malik, K.U.; et al. Activation of mTOR/IκB-α/NF-κB pathway contributes to LPS-induced hypotension and inflammation in rats. Eur. J. Pharmacol. 2017, 802, 7–19. [Google Scholar] [CrossRef]
- Anderberg, S.B.; Luther, T.; Frithiof, R. Physiological aspects of Toll-like receptor 4 activation in sepsis-induced acute kidney injury. Acta Physiol. 2017, 219, 573–588. [Google Scholar] [CrossRef]
- Cazareth, J.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: Importance of CCR2/CCL2 signaling. J. Neuroinflamm. 2014, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Wennström, M.; Janelidze, S.; Bay-Richter, C.; Minthon, L.; Brundin, L. Pro-Inflammatory Cytokines Reduce the Proliferation of NG2 Cells and Increase Shedding of NG2 In Vivo and In Vitro. PLoS ONE 2014, 9, e109387. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar] [CrossRef]
- Sessler, C.N.; Wilhelm, W. Analgesia and sedation in the intensive care unit: An overview of the issues. Crit. Care 2008, 12, S1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Meng, J.; Lian, Q.; Chen, X.; Bauman, B.; Chu, H.; Segura, B.; Roy, S. Prescription opioids are associated with higher mortality in patients diagnosed with sepsis: A retrospective cohort study using electronic health records. PLoS ONE 2018, 13, e0190362. [Google Scholar] [CrossRef]
- Hu, A.M.; Shan, Z.M.; Zhang, Z.J.; Li, H.P. Comparative Efficacy of Fentanyl and Morphine in Patients with or At Risk for Acute Respiratory Distress Syndrome: A Propensity Score-Matched Cohort Study. Drugs R D 2021, 21, 149–155. [Google Scholar] [CrossRef]
- Nardi, G.M.; Bet, A.C.; Sordi, R.; Fernandes, D.; Assreuy, J. Opioid analgesics in experimental sepsis: Effects on physiological, biochemical, and haemodynamic parameters. Fundam. Clin. Pharmacol. 2013, 27, 347–353. [Google Scholar] [CrossRef]
- Meng, J.; Banerjee, S.; Li, D.; Sindberg, G.M.; Wang, F.; Ma, J.; Roy, S. Opioid Exacerbation of Gram-positive sepsis, induced by Gut Microbial Modulation, is Rescued by IL-17A Neutralization. Sci. Rep. 2015, 5, 10918. [Google Scholar] [CrossRef]
- Banerjee, S.; Meng, J.; Das, S.; Krishnan, A.; Haworth, J.; Charboneau, R.; Zeng, Y.; Ramakrishnan, S.; Roy, S. Morphine induced exacerbation of sepsis is mediated by tempering endotoxin tolerance through modulation of miR-146a. Sci. Rep. 2013, 3, 1977. [Google Scholar] [CrossRef]
- Brunauer, A.; Koköfer, A.; Bataar, O.; Gradwohl-Matis, I.; Dankl, D.; Dünser, M.W. The arterial blood pressure associated with terminal cardiovascular collapse in critically ill patients: A retrospective cohort study. Crit. Care 2014, 18, 719. [Google Scholar] [CrossRef]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Rudiger, A.; Singer, M. The Heart in Sepsis: From Basic Mechanisms to Clinical Management. Curr. Vasc. Pharmacol. 2013, 11, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.B.; Ozment, T.R.; Wondergem, R.; Li, C.; Williams, D.L. Impaired heart rate regulation and depression of cardiac chronotropic and dromotropic function in polymicrobial sepsis. Shock 2015, 43, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Pancoto, J.A.; Correa, P.B.; Oliveira-Pelegrin, G.R.; Rocha, M.J. Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton. Neurosci. 2008, 138, 57–63. [Google Scholar] [CrossRef]
- Ellingsrud, C.; Agewall, S. Morphine in the treatment of acute pulmonary oedema—Why? Int. J. Cardiol. 2016, 202, 870–873. [Google Scholar] [CrossRef]
- Afshari, R.; Maxwell, S.R.; Webb, D.J.; Bateman, D.N. Morphine is an arteriolar vasodilator in man. Br. J. Clin. Pharmacol. 2009, 67, 386–393. [Google Scholar] [CrossRef]
- Chen, A.; Ashburn, M.A. Cardiac Effects of Opioid Therapy. Pain Med. 2015, 16, S27–S31. [Google Scholar] [CrossRef]
- Frithiof, R.; Rundgren, M. Activation of central opioid receptors determines the timing of hypotension during acute hemorrhage-induced hypovolemia in conscious sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R987–R996. [Google Scholar] [CrossRef]
- Carrara, M.; Ferrario, M.; Bollen Pinto, B.; Herpain, A. The autonomic nervous system in septic shock and its role as a future therapeutic target: A narrative review. Ann. Intensive Care 2021, 11, 80. [Google Scholar] [CrossRef]
- Adam, J.; Rupprecht, S.; Künstler, E.C.S.; Hoyer, D. Heart rate variability as a marker and predictor of inflammation, nosocomial infection, and sepsis—A systematic review. Auton. Neurosci. 2023, 249, 103116. [Google Scholar] [CrossRef]
- Barnaby, D.P.; Fernando, S.M.; Ferrick, K.J.; Herry, C.L.; Seely, A.J.E.; Bijur, P.E.; Gallagher, E.J. Use of the low-frequency/high-frequency ratio of heart rate variability to predict short-term deterioration in emergency department patients with sepsis. Emerg. Med. J. 2018, 35, 96. [Google Scholar] [CrossRef] [PubMed]
- de Castilho, F.M.; Ribeiro, A.L.P.; Nobre, V.; Barros, G.; de Sousa, M.R. Heart rate variability as predictor of mortality in sepsis: A systematic review. PLoS ONE 2018, 13, e0203487. [Google Scholar] [CrossRef]
- Stein, P.K.; Bosner, M.S.; Kleiger, R.E.; Conger, B.M. Heart rate variability: A measure of cardiac autonomic tone. Am. Heart J. 1994, 127, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- El-Mas, M.M.; Abdel-Rahman, A.A. Intermittent clonidine regimen abolishes tolerance to its antihypertensive effect: A spectral study. J. Cardiovasc. Pharmacol. 2007, 49, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.H.; Rhee, S.H.; Perkins, D.J.; Medvedev, A.E.; Piao, W.; Fenton, M.J.; Vogel, S.N. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leucoc. Biol. 2009, 85, 966–977. [Google Scholar] [CrossRef]
- Pandey, S.; Kawai, T.; Akira, S. Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb. Perspect. Biol. 2014, 7, a016246. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Zhang, Y.; Zhang, Y.; Tang, H.; Yue, H.; Zhang, L.; Song, D. Macrophages in the inflammatory response to endotoxic shock. Immun. Inflamm. Dis. 2024, 12, e70027. [Google Scholar] [CrossRef]
- de Freitas, B.G.; Pereira, L.M.; Santa-Cecília, F.V.; Hösch, N.G.; Picolo, G.; Cury, Y.; Zambelli, V.O. Mitogen-Activated Protein Kinase Signaling Mediates Morphine Induced-Delayed Hyperalgesia. Front. Neurosci. 2019, 13, 1018. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, L.; Wang, J.; Chai, Z.; Xiong, W. Morphine promotes angiogenesis by activating PI3K/Akt/HIF-1α pathway and upregulating VEGF in hepatocellular carcinoma. J. Gastrointest. Oncol. 2021, 12, 1761–1772. [Google Scholar] [CrossRef]
- Malik, S.; Khalique, H.; Buch, S.; Seth, P. A Growth Factor Attenuates HIV-1 Tat and Morphine Induced Damage to Human Neurons: Implication in HIV/AIDS-Drug Abuse Cases. PLoS ONE 2011, 6, e18116. [Google Scholar] [CrossRef]
- Ruan, J.P.; Chen, L.; Ma, Z.L. Activation of spinal Extacellular Signal-Regulated Kinases and c-jun N-terminal kinase signaling pathways contributes to morphine-induced acute and chronic hyperalgesia in mice. J. Cell. Biochem. 2019, 120, 15045–15056. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pires, M.E.; Frade-Guanaes, J.O.; Quinlan, G.J. Clotting Dysfunction in Sepsis: A Role for ROS and Potential for Therapeutic Intervention. Antioxidants 2022, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Jung, J.S.; Choi, M.J.; Lee, Y.Y.; Moon, B.I.; Park, J.S.; Kim, H.S. Suppression of Lipopolysaccharide-Induced Neuroinflammation by Morin via MAPK, PI3K/Akt, and PKA/HO-1 Signaling Pathway Modulation. J. Agric. Food Chem. 2017, 65, 373–382. [Google Scholar] [CrossRef]
- Sastre, J.; Pérez, S.; Sabater, L.; Rius-Pérez, S. Redox signaling in the pancreas in health and disease. Physiol. Rev. 2025, 105, 593–650. [Google Scholar] [CrossRef]
- Akhiani, A.A.; Martner, A. Role of Phosphoinositide 3-Kinase in Regulation of NOX-Derived Reactive Oxygen Species in Cancer. Antioxidants 2022, 12, 67. [Google Scholar] [CrossRef]
- Jia, J.; Xu, G.; Zeng, X. The Biology of Morphine and Oxidative Stress. In Handbook of Substance Misuse and Addictions: From Biology to Public Health; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1955–1975. [Google Scholar] [CrossRef]
- Doyle, T.; Bryant, L.; Muscoli, C.; Cuzzocrea, S.; Esposito, E.; Chen, Z.; Salvemini, D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci. Lett. 2010, 483, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Borea, P.A.; Bencivenni, S.; Fazzi, D.; Varani, K.; Merighi, S. The activation of μ-opioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia. FEBS Lett. 2016, 590, 2813–2826. [Google Scholar] [CrossRef]
- Rodriguez, S.; Sharma, S.; Tiarks, G.; Peterson, Z.; Jackson, K.; Thedens, D.; Wong, A.; Keffala-Gerhard, D.; Mahajan, V.B.; Ferguson, P.J.; et al. Neuroprotective effects of naltrexone in a mouse model of post-traumatic seizures. Sci. Rep. 2024, 14, 13507. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Xiang, Y.; Ma, B.; Li, H.; Li, Y.; Shi, Y.; Li, S.; Bai, Y. Role of MCP-1 as an inflammatory biomarker in nephropathy. Front. Immunol. 2023, 14, 1303076. [Google Scholar] [CrossRef]
- Kishi, T. Clarification of hypertension mechanisms provided by the research of central circulatory regulation. Hypertens. Res. 2023, 46, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.E.; Helmy, M.M.; El-Gowilly, S.M.; El-Mas, M.M. Suppression by central adenosine A3 receptors of the cholinergic defense against cardiovascular aberrations of sepsis: Role of PI3K/MAPKs/NFκB signaling. Front. Pharmacol. 2024, 15, 1418981. [Google Scholar] [CrossRef]
- Ono-Saito, N.; Niki, I.; Hidaka, H. H-series protein kinase inhibitors and potential clinical applications. Pharmacol. Ther. 1999, 82, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Tibbetts, R.S.; Busby, E.C.; Kennedy, A.P.; Hill, D.E.; Abraham, R.T. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998, 58, 4375–4382. [Google Scholar]
- Park, S.E.; Song, J.D.; Kim, K.M.; Park, Y.M.; Kim, N.D.; Yoo, Y.H.; Park, Y.C. Diphenyleneiodonium induces ROS-independent p53 expression and apoptosis in human RPE cells. FEBS Lett. 2007, 581, 180–186. [Google Scholar] [CrossRef]
- Li, H.X.; Xu, K.; Chen, S.L.; Wang, S.F.; Li, W.J. Current techniques for the treatment of spasticity and their effectiveness. EFORT Open Rev. 2025, 10, 237–249. [Google Scholar] [CrossRef]
- Jiang, R.; Ai, Z.-S.; Jiang, X.; Yuan, P.; Liu, D.; Zhao, Q.-H.; He, J.; Wang, L.; Gomberg-Maitland, M.; Jing, Z.-C. Intravenous fasudil improves in-hospital mortality of patients with right heart failure in severe pulmonary hypertension. Hypertens. Res. 2015, 38, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Ohbuchi, M.; Kimura, T.; Nishikawa, T.; Horiguchi, T.; Fukuda, M.; Masaki, Y. Neuroprotective Effects of Fasudil, a Rho-Kinase Inhibitor, After Spinal Cord Ischemia and Reperfusion in Rats. Anesth. Analg. 2018, 126, 815–823. [Google Scholar] [CrossRef]
- Nagel, S.; Genius, J.; Heiland, S.; Horstmann, S.; Gardner, H.; Wagner, S. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007, 1132, 210–217. [Google Scholar] [CrossRef]
- Seok, Y.M.; Azam, M.A.; Okamoto, Y.; Sato, A.; Yoshioka, K.; Maeda, M.; Kim, I.; Takuwa, Y. Enhanced Ca2+-dependent activation of phosphoinositide 3-kinase class IIα isoform-Rho axis in blood vessels of spontaneously hypertensive rats. Hypertension 2010, 56, 934–941. [Google Scholar] [CrossRef]
- Murata, Y.; Fujiwara, N.; Seo, J.H.; Yan, F.; Liu, X.; Terasaki, Y.; Luo, Y.; Arai, K.; Ji, X.; Lo, E.H. Delayed inhibition of c-Jun N-terminal kinase worsens outcomes after focal cerebral ischemia. J. Neurosci. 2012, 32, 8112–8115. [Google Scholar] [CrossRef]
- Nguyen Thi, P.A.; Chen, M.H.; Li, N.; Zhuo, X.J.; Xie, L. PD98059 Protects Brain against Cells Death Resulting from ROS/ERK Activation in a Cardiac Arrest Rat Model. Oxidative Med. Cell. Longev. 2016, 2016, 3723762. [Google Scholar] [CrossRef] [PubMed]
- El-Lakany, M.A.; Fouda, M.A.; El-Gowelli, H.M.; El-Gowilly, S.M.; El-Mas, M.M. Gonadal hormone receptors underlie the resistance of female rats to inflammatory and cardiovascular complications of endotoxemia. Eur. J. Pharmacol. 2018, 823, 41–48. [Google Scholar] [CrossRef]
- Yap, J.Q.; Nikouee, A.; Lau, J.E.; Walsh, G.; Zang, Q.S. Mitochondria at the Heart of Sepsis: Mechanisms, Metabolism, and Sex Differences. Int. J. Mol. Sci. 2025, 26, 4211. [Google Scholar] [CrossRef]
- Bate, S.T.; Clark, R.A. The Design and Statistical Analysis of Animal Experiments; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- El-Naggar, A.E.; Helmy, M.M.; El-Gowilly, S.M.; El-Mas, M.M. Adenosine A1 receptors of the medullary solitary tract arbitrate the nicotine counteraction of neuroinflammation and cardiovascular dysfunction in septic rats. Sci. Rep. 2023, 13, 17818. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wen, H. Sepsis Induced by Cecal Ligation and Puncture. Methods Mol. Biol. 2019, 1960, 249–255. [Google Scholar] [CrossRef] [PubMed]
- El-Mas, M.M.; Omar, A.G.; Helmy, M.M.; Mohy El-Din, M.M. Crosstalk between central pathways of nitric oxide and carbon monoxide in the hypertensive action of cyclosporine. Neuropharmacology 2012, 62, 1890–1896. [Google Scholar] [CrossRef]
- El-Mas, M.M.; El-Gowelli, H.M.; Ghazal, A.R.; Harraz, O.F.; Mohy El-Din, M.M. Facilitation of central imidazoline I(1)-site/extracellular signal-regulated kinase/p38 mitogen-activated protein kinase signalling mediates the hypotensive effect of ethanol in rats with acute renal failure. Br. J. Pharmacol. 2009, 158, 1629–1640. [Google Scholar] [CrossRef]
- El-Mas, M.M.; Abdel-Rahman, A.A. Role of the sympathetic control of vascular resistance in ethanol-clonidine hemodynamic interaction in SHRs. J. Cardiovasc. Pharmacol. 1999, 34, 589–596. [Google Scholar] [CrossRef]
- El-Mas, M.M.; Abdel-Rahman, A.A. Ethanol counteraction of I1-imidazoline but not alpha-2 adrenergic receptor-mediated reduction in vascular resistance in conscious spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1999, 288, 455–462. [Google Scholar] [CrossRef]
- Omar, A.G.; El-Mas, M.M. Time-Domain Evaluation of Cyclosporine Interaction with Hemodynamic Variability in Rats. Cardiovasc. Drugs Ther. 2004, 18, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. Atlas of the rat brain in stereotaxic coordinates; Academic Press: New York, NY, USA, 1986. [Google Scholar]
- Baby, S.M.; Gruber, R.B.; Young, A.P.; MacFarlane, P.M.; Teppema, L.J.; Lewis, S.J. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur. J. Pharmacol. 2018, 834, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, H.N.; Villar, V.M. Pharmacodynamics and pharmacokinetics of intravenously administered morphine in spontaneously hypertensive and normotensive Wistar-Kyoto rats. J. Pharmacol. Exp. Ther. 1992, 261, 290–296. [Google Scholar] [CrossRef]
- Kissin, I.; Kerr, C.R.; Smith, L.R. Assessment of anaesthetic action of morphine and fentanyl in rats. Can. Anaesth. Soc. J. 1983, 30, 623–628. [Google Scholar] [CrossRef]
- Dickenson, A.H.; Oliveras, J.L.; Besson, J.M. Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res. 1979, 170, 95–111. [Google Scholar] [CrossRef]
- Ibrahim, K.S.; El-Yazbi, A.F.; El-Gowelli, H.M.; El-Mas, M.M. Heme oxygenase byproducts variably influences myocardial and autonomic dysfunctions induced by the cyclosporine/diclofenac regimen in female rats. Biomed Pharmacother. 2018, 101, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.Y.; El-Gowilly, S.M.; Abdel-Galil, A.G.; El-Mas, M.M. Modulation by Central MAPKs/PI3K/sGc of the TNF-α/iNOS-dependent Hypotension and Compromised Cardiac Autonomic Control in Endotoxic Rats. J. Cardiovasc. Pharmacol. 2016, 68, 171–181. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, N. Fasudil hydrochloride ameliorates memory deficits in rat model of streptozotocin-induced Alzheimer’s disease: Involvement of PI3-kinase, eNOS and NFkappaB. Behav. Brain Res. 2018, 351, 4–16. [Google Scholar] [CrossRef]
- Fujita, M.; Ando, K.; Nagae, A.; Fujita, T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 2007, 50, 360–367. [Google Scholar] [CrossRef]
- Nassar, N.; Abdel-Rahman, A.A. Brainstem adenosine A1 receptor signaling masks phosphorylated extracellular signal-regulated kinase 1/2-dependent hypotensive action of clonidine in conscious normotensive rats. J. Pharmacol. Exp. Ther. 2009, 328, 83–89. [Google Scholar] [CrossRef]
| Parameter | Sham | CLP |
|---|---|---|
| MAP, mmHg | 105.5 ± 1.8 | 90.5 ± 1.9 * |
| HR, beats/min | 383.2 ± 9.6 | 427.2 ± 8.1 * |
| SDNN, msec | 3.98 ± 0.21 | 2.59 ± 0.11 * |
| rMSSD, msec | 4.23 ± 0.31 | 2.99 ± 0.21 * |
| Total power, msec2 | 14.22 ± 1.54 | 6.33 ± 0.48 * |
| LF/HF | 0.23 ± 0.03 | 0.12 ± 0.02 * |
| +dP/dtmax, mmHg/min | 2998.0 ± 256.6 | 3242.6 ± 245.7 |
| Tau, sec | 0.246 ± 0.06 | 0.094 ± 0.010 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnaby, M.; Sallam, M.Y.; Helmy, M.M.; El-Gowelli, H.M.; El-Mas, M.M. Role of Central Inflammatory and Oxidative Pathways in the Morphine Exacerbation of Cardiovascular Effects of Sepsis in Rats. Pharmaceuticals 2025, 18, 882. https://doi.org/10.3390/ph18060882
Abdelnaby M, Sallam MY, Helmy MM, El-Gowelli HM, El-Mas MM. Role of Central Inflammatory and Oxidative Pathways in the Morphine Exacerbation of Cardiovascular Effects of Sepsis in Rats. Pharmaceuticals. 2025; 18(6):882. https://doi.org/10.3390/ph18060882
Chicago/Turabian StyleAbdelnaby, Mohamed, Marwa Y. Sallam, Mai M. Helmy, Hanan M. El-Gowelli, and Mahmoud M. El-Mas. 2025. "Role of Central Inflammatory and Oxidative Pathways in the Morphine Exacerbation of Cardiovascular Effects of Sepsis in Rats" Pharmaceuticals 18, no. 6: 882. https://doi.org/10.3390/ph18060882
APA StyleAbdelnaby, M., Sallam, M. Y., Helmy, M. M., El-Gowelli, H. M., & El-Mas, M. M. (2025). Role of Central Inflammatory and Oxidative Pathways in the Morphine Exacerbation of Cardiovascular Effects of Sepsis in Rats. Pharmaceuticals, 18(6), 882. https://doi.org/10.3390/ph18060882









