Abstract
The neurological effects of opium were first described over 8000 years ago. Morphine was isolated in 1803 and by the mid-1800s had become both a pain-relieving blessing and an addictive curse. As part of the crusade to identify safer and more reliable alternatives to morphine, dezocine (Dalgan®) was marketed in the US in 1986. Its use was discontinued in the US in 2011 without revealing the reasons, but it remains one of the most widely used analgesic agents in China today. Dezocine’s unique pharmacology makes it an effective analgesic with limited opioid-associated side effects and little or no reported potential for dependence and addiction. In addition, dezocine’s blocking effect on serotonin and norepinephrine transporters recommends its further exploration as a potential treatment for various chronic and neuropathic pain conditions. Most recently, data suggest that dezocine might represent a viable treatment for addiction management. This report focuses on the data supporting dezocine’s non-addictive profile and its potential use to treat opioid addiction and withdrawal, as well as recent efforts to generate formulations of dezocine that support sub-chronic and chronic dosing.
1. Introduction
The first written records mentioning opium date back over 5000 years to Sumarian clay tablets [1,2] found at the site of the ancient city of Nippur in present-day Iraq (Figure 1). Its use was documented throughout the Bronze Age, the Middle Ages, and the Renaissance period and was mentioned in historic literature such as Homer’s The Odyssey, the writings of Hypocrates, and the Bible. The discovery of morphine as the active analgesic ingredient in opium is credited to the German pharmacist’s assistant Friedrich William Adam Serturner, who isolated it as a gummy substance from opium poppies in 1803 and named it after Morpheus, the Greek god of dreams [3]. Soon afterwards, morphine became a mainstay of pain treatment. The invention of the hypodermic needle in 1853 facilitated the use of morphine to treat neuralgia associated with surgical procedures, but the social use of opium was still prevalent. By the 1830s, the addictive liability of morphine was recognized, and the US government began a campaign to reduce the social use of morphine and opium and limit their uses to medicinal applications, culminating in the Opium Exclusion Act of 1909 and the Harrison Narcotics Tax Act of 1914. Drug discovery around morphine began with the synthesis of heroin in 1874 [4], but it became apparent that many of the semisynthetic analogs derived from morphine retained an addiction liability.

Figure 1.
Written records describing opium date back over 5000 years to Sumarian clay tablets from Nippur, Iraq. Used under license from Shutterstock.
Opioid use disorder (OUD) affects more than 16 million people worldwide [5]. Deaths from opioid overdose in the US alone rose to an astonishing 81,806 in 2022 [6]. That number has decreased somewhat in the past two years thanks to the use of mu opioid antagonists/partial agonists like buprenorphine, methadone, naloxone, and naltrexone (especially long-acting agents or extended release formulations), but it is a commonly held position within the medical community that not enough patients are receiving these drugs. Patients who use opioids chronically, either socially or for the treatment of pain, are at high risk for becoming addicted to those agents. Buprenorphine (Figure 2) has become the most widely used treatment for analgesic opioid addiction and withdrawal [7], but buprenorphine itself can cause addiction and withdrawal, and patients must be tapered off of it just like mu opioid full agonists. Some patients remain on buprenorphine chronically or indefinitely.
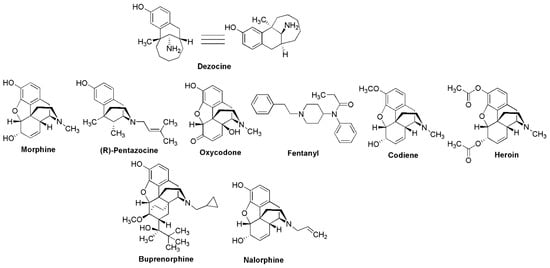
Figure 2.
Structures of dezocine and the opioid agonists, partial agonists, and antagonists discussed in this paper.
Dezocine (Dalgan®, Figure 2) is an aminotetralin derivative that shares structural similarity with the benzomorphan opioid R-pentazocine (Figure 2). It was discovered at the Wyeth division of American Home Products (WY-16,255) and first disclosed as an analgesic agent in 1973 [8] with nearly 3-fold greater analgesic potency compared to morphine. Dezocine was marketed in 1986 but was voluntarily withdrawn from the US market in 2011. No official reason for the withdrawal was given, although it is conceivable that decreasing sales and liability associated with the growing opioid crisis may have played a role in the decision [9]. However, dezocine remains one of the most frequently used opioid analgesic agents in China to this day. Until recently, its use was limited to postsurgical pain in that country. But more recently, dezocine is being used in China for other moderate to severe pain conditions such as cancer and has been examined in clinical trials for a wide range of potential applications [10]. In this review, we focus on dezocine’s potential for causing dependence/addiction, review the medicinal chemistry of dezocine, discuss possible uses for dezocine outside of acute pain, and summarize recent efforts to formulate and modify dezocine for chronic administration.
2. Medicinal Chemistry of Dezocine
Interestingly, relatively few chemistry-oriented reports around dezocine have appeared since its original disclosure. The original reported synthesis of dezocine involved seven steps (Scheme 1) and suffered from lower yields due to the need for a kinetic resolution in the last step of the synthetic scheme. In the course of large-scale syntheses of dezocine, three byproducts were produced, which were synthesized to confirm their structures (Figure 3). Two of those byproducts (Figure 3a,b) showed significantly less analgesic activity in a rat tail flick model, but the third byproduct (Figure 3c) was equipotent to morphine in that study. Since that time, a few patent applications have been filed claiming improved syntheses of dezocine, and at least four patent applications describing novel dezocine-like analogs (other than prodrugs) have appeared [11,12,13,14]. However, to our knowledge, none of these novel analogs of dezocine have advanced to clinical trials.
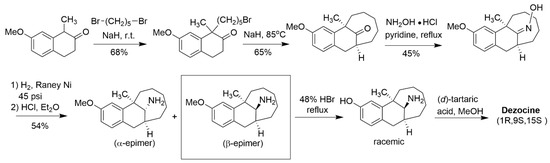
Scheme 1.
Original synthesis of dezocine described in [7] (Freed et al., 1973).
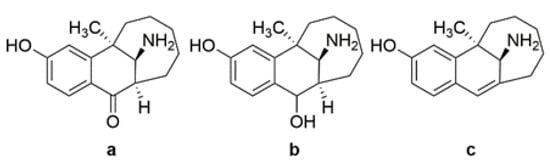
Figure 3.
Impurities from the large-scale synthesis of dezocine ((a,b) less potent; (c) more potent).
A number of patent applications specifically addressing the production of dezocine have been filed since the original synthesis was published. Table 1 summarizes those inventions, which include improved synthetic routes, improved synthesis of important intermediates, improved methods for isolating chirally pure products, and specific crystal forms. Despite the discontinuation of dezocine in the US in 2011, the continued patenting activity in China and expanded worldwide coverage for some applications (even as recently as 2022) indicate continued interest in dezocine.

Table 1.
Patent applications describing synthetic or manufacturing improvements for dezocine. Results of a Scifinder-n® search performed on 5 January 2025.
3. Biased Ligand Signaling
The pharmacology of dezocine has been extensively reviewed by us and others [10,15,16,17]. It is a partial agonist for both u- and k-opioid receptors (MOR and KOR, respectively). Dezocine’s partial agonist activity at MORs at least partly explains its ceiling effect in causing opioid-associated side effects such as respiratory depression, constipation, and sedation. It also explains how dezocine is able to antagonize both the analgesic activity and adverse effects of MOR full agonists such as morphine [18]. A similar profile is seen with other MOR partial agonists such as pentazocine. However, recent data suggest that partial agonism is not the only reason for dezocine’s superior safety profile [19,20].
Like many G-protein-coupled receptors (GPCRs), opioid receptors can signal through a second pathway involving beta-arrestins (Figure 4) [21,22]. In this pathway, the binding of a ligand to the receptor recruits kinases that phosphorylate the receptor, resulting in the recruitment of beta-arrestins that desensitize the receptor and target it for internalization. However, other kinase-associated pathways are activated by beta-arrestin, making the process more complex than just receptor regulation and blockade. MORs signal through both the GPCR and beta-arrestin pathways (Figure 3). The arrestin pathway has been implicated in a number of deleterious aspects of MOR agonists. As one might expect, opioid tolerance is caused by arrestin-associated receptor desensitization and internalization [23]. However, there is evidence that the beta2-arrestin pathway also plays a role in many of the adverse effects associated with opioid analgesics [24], including respiratory depression, constipation, and even addiction and dependence [25].
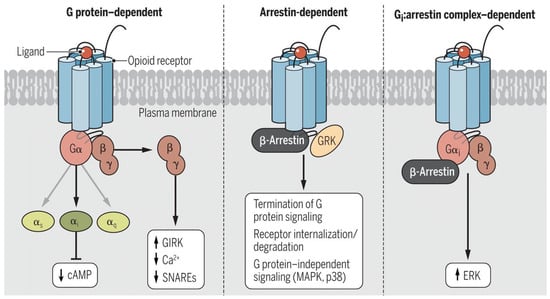
Figure 4.
Signaling pathways used by opioid receptors. Opioid receptors signal through three distinctive pathways. (Left) G-protein dependent pathway, wherein the receptor engages G-proteins to initiate intracellular signaling; (Middle) arrestin-dependent pathway, wherein the receptor becomes phosphorylated and engages beta-arrestins, resulting in receptor desensitization and internalization; (Right) Gi/arrestin complex pathway, wherein the receptor forms a complex with Gi and beta arrestins, resulting in ERK-associated downstream signaling. Reproduced with permission from Che et al., 2021 [21].
Dezocine is a biased signaling ligand at μ-opioid receptors (Figure 4). The analgesic activity of MOR agonists is mediated by G-protein signaling, while many of the undesirable side effects of MOR full agonists, such as respiratory depression, constipation, sedation, and addiction, are thought to be mediated through β-arrestin signaling [26]. Dezocine has been shown to be a biased ligand that signals primarily through G-proteins [27], which may at least partially explain its reduced propensity for typical μ-opioid side effects. In animal studies, dezocine showed a lower incidence of constipation compared to morphine, codeine, and d-propoxyphene [28]. Dezocine also produced a significantly lower incidence of respiratory depression compared to morphine in rhesus monkeys. A similar safety profile was reported for dezocine in humans [29]. Dezocine induced some respiratory depression in humans at super-clinical doses but exhibited a ceiling effect, with no further respiratory depression observed at doses exceeding 0.3 mg/kg i.v [30]. Only a limited amount of sedation is observed at clinical doses [31]. This realization has stimulated the search for MOR-biased opioids. A number of them have been identified (Figure 5), including the marketed analgesic oliceridine (Olinvyk®, TRV-130) [21,32,33,34]. However, the situation seems to be more complicated than simply a case of receptor bias. Despite its bias for MOR GPCR signaling and reduction in some opioid-associated side effects, oliceridine is a full agonist at the MOR and still generalizes to morphine. The package label comes with warnings about abuse, addiction, and withdrawal [35].
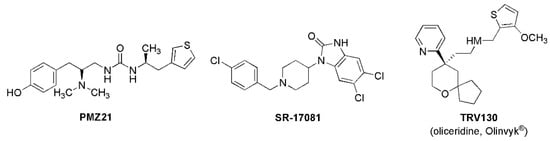
Figure 5.
Structures of selected MOR biased agonists.
4. Dezocine and Biogenic Amine Reuptake
In addition to its activities at opioid receptors, dezocine displays inhibitory activity against the serotonin reuptake transporter (SERT) and the norepinephrine reuptake transporter (NET) [36]. Through binding to the site occupied by known antidepressant reuptake inhibitors (Figure 6). Dezocine possesses weaker affinity for the SERT (PKi = 6.96) compared to potent selective serotonin reuptake inhibitors (SSRI) such as paroxetine and escitalopram (PKis ~ 9.0) and weaker affinity for the NET (PKi = 6.00) compared to potent norepinephrine reuptake inhibitors (NRI) like nisoxetine (PKi = 9.15). Nevertheless, for a common clinical dose of dezocine (0,15 mg/kg, i.v.) in a 70 kg human with approximately 5.7 L blood volume, the free unbound exposure of dezocine based on its 80% protein binding and assuming a brain/plasma ration of 1.0 ((the brain to plasma ratio for dezocine has never been reported; this speculated number is compared to a B/P ratio of 0.6 for morphine and 1.9 for heroin) is around 1.6 micromolar, which covers the Ki for NET. Thus, it is anticipated that the effects of at least NET inhibition should be realized with clinically relevant doses of dezocine.
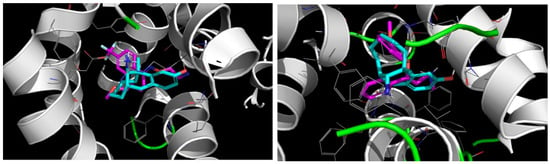
Figure 6.
Docking models of dezocine (Cyan) with the human serotonin and norepinephrine transporters. (Left) Dezocine is predicted to occupy the same binding site as the SSRI fluvoxamine (magenta, docking model using the co-crystal structure of fluvoxamine bound to the human SERT, PDB 6AWP, Coleman and Gouaux, 2017) [37]; (Right) dezocine is predicted to occupy the same binding site as the NRI reboxetine (magenta, docking model using the cryo-EM structure of reboxetine bound to the human NET, PDB 8ZP1, Song and Wu, 2024 [38]).
The NET inhibitory activity has implications for the analgesic efficacy of dezocine. Spinal norepinephrine is known to be involved in both acute and chronic/neuropathic pain perception via α2A receptors [39]. Inhibitors of NET have been shown to be effective against a number of pain conditions (summarized in Childers and Abou-Gharbia, 2021 [15]). A number of studies demonstrated that at least part of dezocine’s analgesic effects in animal models of neuropathic pain were associated with the inhibition of norepinephrine reuptake in the spinal cord [40]. Thus, this additional analgesic mechanism is another factor that distinguishes dezocine from many other opioid modulators [41]. Evidence from both animal models [42,43,44] and human conditions [19,45,46,47] support this supposition. Recently, data suggested that dezocine may exert some of its antihypersensitivity effects through the suppression of the Akt1/GSK-3β pathway, although this hypothesis needs further investigation [48].
Dezocine is reported to show antidepressant-like activity in animal models [49,50]. Reports of human antidepressant activity have also appeared [51]. KORs are involved in depression, especially withdrawal-associated depression following the discontinuation of MOR agonists [52,53]; however, α2A-noradrenergic agonists also display efficacy in reducing opioid withdrawal symptoms [54]. Since the inhibition of norepinephrine uptake enhances adrenergic tone, reuptake inhibition would be expected to show activity similar to that of α2-agonists. Thus, dezocine’s combination of KOR partial agonism and norepinephrine reuptake inhibition suggests a possible role for the drug in reducing the withdrawal symptoms associated with opioid discontinuation. Indeed, this use has recently been proposed, as discussed below.
5. Dezocine and Addiction
Opioid dependence and addiction are both complicated in their neurobiology [55]. Dezocine is not classified as a controlled substance by the World Health Organization. There are no reports of dezocine abuse or addiction in the English language literature and only a few reports of concern for dezocine abuse in the Chinese language literature [56]. That is not to say that dezocine is devoid of the euphoric effects associated with abused MOR agonists such as morphine, fentanyl, and oxycodone. Human substance abusers described dezocine as feeling “similar to dope” (a slang term for substances of abuse, specifically morphine) in a 1985 study [57]. However, the few reports of withdrawal from chronic dezocine use described the symptoms as short term and mild compared to those of traditional opioids [58,59].
Results in animal studies corroborate human experiences. Dezocine has been shown to generalize to MOR agonists in various animal models [60], but dezocine’s profile is different from that of MOR full agonists. Dependence liability studies in rhesus monkeys showed that dezocine neither acutely substituted for morphine in self-administration studies nor produced dependence after chronic administration [28]. In codeine-addicted macaque monkeys, dezocine substituted for codeine in self-administration studies but with a much shallower dose-response curve and an apparent ceiling effect compared to other opioids [61]. In drug discrimination studies performed in macaque and squirrel monkeys, dezocine generalized to morphine and fentanyl in some cases [61,62] but not in others [28]. The MOR antagonist nalorphine blocked the discriminative effects of all three drugs from saline in multiple studies. Morphine and fentanyl fully substituted for dezocine in pigeons [63]. Discrimination of all three compounds from saline was reversed by treatment with the MOR antagonist nalorphine, although much higher doses of the antagonist were required to block the effects of dezocine in pigeons compared to the two full MOR agonists. A KOR antagonist had no effect on the discriminative activity of dezocine. These data strongly suggest that dezocine displays a lower risk for addiction compared to traditional MOR agonists.
These data raise the question of why dezocine seems to be less addictive than other MOR agonists like fentanyl, morphine, and oxycodone. Dezocine is a partial agonist at MOR2 rather than a full agonist like addictive opioids. However, the MOR partial agonists that are used to treat opioid use disorder, such as buprenorphine, still cause addiction. Many patients using such agents to withdraw from, for example, fentanyl abuse, spend much of their lives dependent on those partial agonists. Dezocine is a weak partial agonist/antagonist at KORs. But drug discovery research on KOR antagonists has failed to demonstrate efficacy for such agents as anti-addiction drugs [64].
Part of the answer may lie in the fact that dezocine is a biased agonist that signals only through G-proteins. Research into the role of opioid signaling in addiction supports the hypothesis that beta2-arrestin plays a significant role in the neurochemical changes that lead to addiction. Beta2-arrestin engagement leads to the internalization of MORs and opioid tolerance, but tolerance is not the same as dependence. However, both animal and human studies suggest that β2-arrestin engagement plays a role in the behavioral effects of multiple classes of abused drugs, including opioids, cannabinoids, stimulants, and possibly even nicotine and alcohol [65]. These pro-addictive effects are mediated through dopamine receptors, which are well known to play a role in reward and dependence [66]. However, as discussed above, the recently approved G-protein-biased MOR agonist oliceridine still possesses a risk of dependence and withdrawal. Oliceridine is a full agonist MOR. So, it may be dezocine’s unique combination of pharmacological properties that bestow it with a lower risk of becoming addicted.
6. Dezocine and Lack of Withdrawal Symptoms
Recently, there has been interest in exploring the use of dezocine to help people with an addiction taper off opioids [56,67]. But evidence suggests that dezocine may not just act as a less efficacious opioid; it may prevent the withdrawal symptoms. Like the opioid antagonist nalophine, dezocine reversed morphine-induced narcosis and loss of righting reflex in rats. In morphine-dependent rats, dezocine alleviated withdrawal symptoms, inhibited the reinstatement of conditional place preference, and prevented morphine-stimulated astrocyte activation and KOR internalization [68]. Depression is well described as a symptom associated with opioid withdrawal [69]. As an NRI and a weak KOR partial agonist, dezocine would be expected to demonstrate antidepressant activity, and indeed, a few reports support that assertion [52,70,71]. However, agonists of α2A adrenergic receptors, such as lofexidine (Lucemyra®), have shown effectiveness in treating both opioid withdrawal symptoms and relapse [72]. As an NRI, dezocine would be expected to increase adrenergic tone. Thus, dezocine’s complex pharmacology might make a good candidate to help people with an addiction withdraw from opioid use.
7. Dezocine and Addressing Physicochemical Properties
At present, dezocine is administered in the clinical setting as an intravenous (i.v.) or intramuscular (i.m.) injection. This is due to its physicochemical and ADME properties. The molecule displays an in vivo half-life of around 2–2.5 h in humans due to rapid metabolism, which involves both CYP-450 mediated oxidative metabolism and glucuronidation of the phenolic hydroxyl group. If dezocine is ever to be used for indications that require sub-chronic administration, then other modes of administration will have to be developed, and the rapid metabolism will have to be overcome.
To date, the development of oral formulations for dezocine has met with limited success, primarily due to the high first-pass metabolism that rapidly reduces plasma levels of the drug following oral administration. Recently, patent applications have been published describing alternatives to i.v. administration, including transdermal patches and intramuscular/subcutaneous depots (Table 2).

Table 2.
Recent patent applications describing improvements to dezocine’s route of administration and pharmacokinetic profile. Results of a Scifinder-n® search performed on 5 January 2025. Only patent applications specifically claiming dezocine or presenting data for dezocine are included.
A plethora of such inventions have appeared that generically claim dezocine as one of a number of drugs suitable for the technology described, but several inventions specifically target dezocine or provide data with dezocine. Most of these patent applications come from Chinese organizations. One recent paper from the University of Pennsylvania [73] describes a neutral formulation of dezocine employing cyclodextrin, a biodegradable cyclic oligosaccharide that serves as both a neutral solubilizing agent and a controlled release agent [74]. Dezocine in this formulation was able to reduce self-administration of oxycodone in rodents when administered intraperitoneally at doses previously shown to be effective in traditional formulations (0.5–10 mg/kg) in a volume of 30 μg/μL.
A number of patent applications have also appeared addressing dezocine’s limited in vivo half-life (Table 2). These efforts include sustained-release formulations and prodrug analogs designed to interact with hydrolytic enzymes to slowly release dezocine in a controlled manner. Examples of such prodrugs, which involve ester analogs of the phenolic hydroxyl group, are provided in Figure 7.
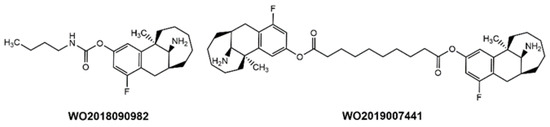
Figure 7.
Examples of dezocine prodrugs in the recent patent literature.
One intriguing approach is the development of an intranasal formulation of dezocine. Until recently, dezocine’s limited aqueous solubility and requirement for acidic formulations hindered the ability to produce formulations with suitable pH and high enough drug concentration to be suitable for intranasal administration. However, in 2022, the University of Pennsylvania group published a paper describing a neutral nano-formulation of dezocine delivered intranasally that provided higher plasma and brain concentrations of the drug than an identical dose delivered intraperitoneally [27]. The cyclodextrin-based formulation described above was also shown to reduce oxycodone self-administration in rodents [73]. Thus, intranasal administration could open the way for using dezocine chronically in an out-patient setting.
Several patent applications have also appeared targeting dezocine’s limited in vivo half-life (Table 2). These efforts include sustained-release formulations and product analogs designed to interact with hydrolytic enzymes to slow the release of dezocine in a controlled manner. Examples of such prodrugs, which involve ester, amide, and carbamate analogs of the phenolic hydroxyl group, are provided in Figure 7.
8. Opinion: Uses of Dezocine
The use of dezocine as a non-addictive analgesic has been recently reviewed by us and others [10,15]. Dezocine is widely used as a short-acting alternative to MOR full agonists for the treatment of pain in China. As discussed above, the G-protein biased MOR partial agonist activity may be part of the reason for dezocine’s lower propensity to induce dependence, but other MOR partial agonists and other G-protein biased MOR agonists currently used to treat opioid use disorder still possess a risk of addiction. Dezocine’s NRI activity likely contributes to its anti-nociceptive activity but may play a role in the apparent lack of dependence due to the indirect increase in adrenergic tone at α2A receptors associated with inhibiting norepinephrine reuptake. Dezocine is currently off the market in the US. But recently, an international panel of policy experts, society leaders, anesthesiologists, pain experts, scientists, pharmaceutical industry leaders, entrepreneurs, and educators met to discuss issues related to perioperative pain management. One subject of the meeting was the reintroduction of dezocine to the US market [75].
As discussed above, dezocine has demonstrated efficacy in the management of neuropathic pain in both animals and the clinical setting. Data suggest that it does so by suppressing spinal hypersensitivity through both MOR and NRI pathways [40]. In fact, there is evidence that the NRI activity inherent in dezocine may actually prevent opioid tolerance that occurs with chronic use of MOR full agonist analgesics [76]. The ceiling effect seen for side effects that occur with dezocine should make it a safer alternative to full agonists like morphine. The opioid-like euphoria that some patients report after taking dezocine, albeit less intense than that of MOR full agonists, is still a concern for chronic use such as treating neuropathic. However, gabapentin, a widely used drug for treating neuropathic pain, is also reported to produce psychological effects and, unlike dezocine, can also be addictive if misused [77]. In its present formulations (i.v., i.m.), dezocine’s use as a practical anti-neuropathic agent is limited. However, the scientific and patent literature indicate that research into novel, easier-to-use administration routes is occurring. Clearly, formulations that patients can administer themselves in an out-patient setting are needed, and additional clinical trials are warranted to study the overall efficacy and safety of dezocine for treating neuropathic pain conditions. Still, the possibilities are intriguing.
As discussed above (Section 6), there is recent interest in exploring dezocine as an agent for treating opioid use disorder. Based on data from both animal and human studies, its activity as a G-protein-biased partial MOR agonist may facilitate weaning off of MOR full agonists without withdrawal-associated side effects and without inducing dependence or addiction in its own right. The NRI activity displayed by dezocine may further eliminate withdrawal side effects by increasing adrenergic tone at α2A receptors. Of course, clinical trials will be needed to validate that hypothesis, but the ability to perform those trials may be closer to becoming a reality due to the recent development of formulations that support convenient administration in an out-patient setting, such as intranasal.
9. Conclusions
The preclinical and clinical data accumulated to date indicate that dezocine is an effective analgesic with efficacy similar to or better than that of morphine. Its unique pharmacology makes it a safer agent for treating acute pain compared to traditional opioids. Dezocine also displays a significantly lower risk of developing dependence and addiction compared to other opioids, thanks to its G-protein-biased partial agonist activity at MORs and possibly due to its NRI activity.
There is currently a discussion among international experts on the possibility of reintroducing dezocine to the US market [75]. However, that effort would meet some competition (see Table 1 in Kingwell, 2025 [78]). At the time of this writing, the US Food and Drug Administration had just recently approved Vertex’s suzetrigine (VX-549, Journavx®), a selective, peripherally restricted, allosteric inhibitor of the voltage-gated sodium channel Nav1.8 [78,79]. Suzetrigine was devoid of the typical side effects associated with opioids. In phase 3 clinical trials, suzetrigine performed comparably to an opioid/acetaminophen combination in relieving pain from abdominoplasty and bunionectomy but did not differentiate itself from the placebo in a Phase 2 clinical trial for lumbosacral radiculopathy (a form of nerve damage-induced neuropathic pain). Thus, the extent of its efficacy in different pain conditions remains to be seen. Another consideration will be the willingness of insurers to cover Journavx® at an estimated cost of $31/day compared to oxycodone, which costs around $2/day. It remains to be seen how broadly effective Journavx® is in the general patient population and whether or not physicians worldwide are willing to move away from their traditional use of opioids for pain. But for now, the approval of Journavx®, the first US approval of a new analgesic in 27 years, has generated excitement as the world still suffers from the opioid crisis. Dezocine will need to differentiate itself from suzetrigine. There is some evidence that dezocine may be effective against at least some forms of neuropathic pain. Further clinical trials are needed to confirm these observations and better understand the extent of dezocine’s usefulness in this complicated therapeutic area.
The use of dezocine as an agent to support the withdrawal of addicted patients from full agonist opioids is intriguing. Unlike some of the agents used for this indication, dezocine’s lack of dependence and addiction would be a significant improvement. In addition, there is evidence that dezocine reduces or prevents opioid withdrawal symptoms. Dezocine is not devoid of the risk of respiratory depression, but other partial MOR agonists like buprenorphine also cause respiratory depression. Like those agents that are used today, dezocine displays a “ceiling effect” that limits the degree of respiratory depression that it can cause. This does not seem to be a hurdle to its use for opioid use disorder. Until recently, the need to administer dezocine i.v. or i.m. has prevented its consideration as a drug for sub-chronic use. However, recent advances such as intranasal formulations may resolve that weakness.
Dezocine remains something of a “double-edged sword”. While it does not cause dependence and addiction, it still causes euphoric effects, which could lead to misuse. This may prevent its use in conditions that require chronic administration, such as depression. However, that antidepressant activity may be part of why dezocine is effective in preventing opioid withdrawal and relapse. Thus, the data to date indicate that dezocine is an effective, non-addictive analgesic with a superior safety profile compared to other opioids. Its usefulness in other indications like neuropathic pain and opioid use disorder will need to be assessed in clinical trials before we truly know the full extent of dezocine’s benefits. But for now, it is safe to say that dezocine seems to be more friend than foe.
Author Contributions
Conceptualization, W.C. and M.A.-G.; writing—reviewing and editing, W.C., K.E. and M.A.-G.; computational chemistry, K.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the journal Science Signaling for their kind permission to reproduce the graphic provided in Figure 4.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schiff, P.L., Jr. Opium and its alkaloids. Am. J. Pharma. Ed. 2002, 66, 186–194. [Google Scholar]
- Bandyopadhyay, S. An 8000-year history of use and abuse of opium and opioids: How that matters for a successful control of the epidemic? Neurology 2019, 92 (Suppl. S15), 49–55. [Google Scholar]
- Klockgether-Radke, A.P. FW Serturner and the discovery of morphine. 200 years of pain therapy with opioids. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2002, 37, 244–249. [Google Scholar] [CrossRef]
- Sneader, W. The discovery of heroin. Lancet 1998, 352, 1697–1699. [Google Scholar] [CrossRef]
- Dydyk, A.M.; Jain, N.K. Opioid Use Disorder. StatPearls. 2024. Available online: https://www.statpearls.com/point-of-care/42233#:~:text=Opioid%20Use%20Disorder,dependence%20on%20opi (accessed on 2 January 2025).
- Drug Overdose Deaths: Facts and Figures, National Institute on Drug Abuse. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates#Fig3 (accessed on 2 January 2025).
- Ling, W.; Mooney, L.; Torrington, M. Buprenorphine for opioid addiction. Pain Manag. 2012, 2, 345–350. [Google Scholar] [CrossRef]
- Freed, M.E.; Potoski, J.R.; Freed, E.H.; Conklin, G.L.; Malis, J.M. Bridged aminotetralins as novel potent analgesic substances. J. Med. Chem. 1973, 16, 595–599. [Google Scholar] [CrossRef]
- Haffajee, R.; Mello, M.M. Drug companies’ liability for the opioid epidemic. N. Engl. J. Med. 2017, 377, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.-R.; Jiang, S.; Xu, X.; Lu, Y.; Wang, Y.-J.; Liu, J.-G. Dezocine as a potent analgesic: Overview of its pharmacological characterization. Acta. Pharmacol. Sin. 2022, 43, 1646–1657. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Li, Z.; Yang, G.; Fang, Y.; Zhang, T.; Gu, W.; Chen, S.; Wang, F.; Li, J. Dezocine Analog. WO2017118375A1, 13 July 2017. [Google Scholar]
- Rice, K.C.; Jacobson, A.E.; Li, F.; Gutman, E.S.; Bow, E.W. Biased Potent Opioid-Like Agonists as Improved Medications to Treat Chronic and Acute Pain and Methods of Using the Same. WO2019183950A1, 26 September 2019. [Google Scholar]
- Shao, L.; Hu, T.; Xu, H.; Chen, L.; Zou, Y.; Cai, W.; Yang, X.; Li, H.; Xue, D. Dezocine Derivative and Medical Use Thereof. WO2020165522A1, 6 August 2020. [Google Scholar]
- Yao, S.; Zhang, L.; An, R.; Sun, C.; Lu, S.; Lyu, H.; Li, H.; Li, B.; Luo, H.; Zhu, X. Opioid Compound as Well as Preparation Method and Application Thereof. CN115124435A, 30 September 2022. [Google Scholar]
- Childers, W.E.; Abou-Gharbia, M.A. “I’ll be back”: The resurrection of dezocine. ACS Med. Chem. Lett. 2021, 12, 961–969. [Google Scholar] [CrossRef]
- Grothusen, J. Dezocine: A totally different opioid. Transl. Perioper. Pain Med. 2022, 9, 430–433. [Google Scholar]
- Want, Y.-H.; Chai, J.-R.; Xu, X.-J.; Ye, R.-F.; Zan, G.-Y.; Liu, G.Y.-K.; Long, J.-D.; Ma, Y.; Huang, X.; Xaio, Z.-C.; et al. Pharmacological characterization of dezocine, a potent analgesic acting as a κ partial agonist and μ partial agonist. Sci. Rep. 2018, 8, 14087. [Google Scholar]
- Li, N.N.; Huang, Y.Q.; Huang, L.E.; Guo, S.H.; Shen, M.R.; Guo, C.L.; Zhu, S.M.; Yao, Y.X. Dezocine antagonizes morphine analgesia upon simultaneous administration in rodent models of acute nociception. Pain Physician 2017, 20, E401–E409. [Google Scholar] [PubMed]
- Hoskin, P.J.; Hanks, G.W. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 1991, 41, 326–344. [Google Scholar] [CrossRef]
- Stahl, E.L.; Bohn, L.M. Low intrinsic efficacy alone cannot explain the improved side effect profiles of new opioid agonists. Biochem. 2022, 61, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Dwivedi-Agnihotri, H.; Shukla, A.K.; Roth, B.L. Biased ligands at opioid receptors: Current status and future directions. Sci. Signal. 2021, 14, eaac0320. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Kaur, S.; Shenoy, S.K. GPCR signaing via β-arrestin-dependent mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Auo, A. The role of opioid receptor internalization and β-arrestins in the development of opioid tolerance. Anesth. Analg. 2005, 101, 728–734. [Google Scholar]
- Raehal, K.M.; Bohn, L.M. β-Arrestins: Regulatory role and therapeutic potential in opioid and cannabinoid receptor-mediated analgesia. Handb. Exp. Pharmacol. 2014, 219, 427–443. [Google Scholar]
- Gillis, A.; Kliewer, A.; Kelly, E.; Henderson, G.; Christie, M.J.; Schulz, S.; Canals, M. Critical assessment of G-protein-biased agonism at the μ-opioid receptor. Trends Pharmacol. Sci. 2020, 12, 947–959. [Google Scholar] [CrossRef]
- Madariaga-Mazon, A.; Marmolejo-Valencia, A.F.; Li, Y.; Toll, L.; Houghten, R.A.; Martinez-Mayorga, K. Mu-opioid receptor biased ligands: A safer and painless discovery of analgesics? Drug Disc. Today 2017, 22, 1719–1729. [Google Scholar] [CrossRef]
- Grothusen, J.; Lin, W.; Xi, J.; Zanni, G.; Rarr, G.A.; Liu, R. Dezocine is a biased ligand without significant beta-arrestin activation of the mu opioid receptor. Transl. Perioper. Pain Med. 2022, 9, 424–439. [Google Scholar]
- Malis, J.L.; Rosenthale, M.E.; Gluckman, M.I. Animal pharmacology of WY-16,225, a new analgesic agent. J. Pharmacol. Exp. Ther. 1975, 194, 488–498. [Google Scholar] [CrossRef] [PubMed]
- O’Brian, J.J.; Benfield, P. Dezocine: A preliminary review of its pharmacodynamics and pharmacokinetic properties, and therapeutic efficacy. Drugs 1989, 38, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Gal, T.J.; DiFazio, C.A. Ventilatory and analgesic effects of dezocine in humans. Anesthesiology 1984, 61, 716–722. [Google Scholar] [CrossRef]
- Yang, H.; Shen, R.; Zhan, F.-F.; Shao, J.-L.; Lu, Y.-J.; Wang, L. Effects of dezocine combined with dexmedetomidine on adverse reactions and inflammatory factors in patients undergoing HIPEC after intestinal surgery and its protective effect on the heart in the perioperative period. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3437–3443. [Google Scholar]
- Faouzi, A.; Varga, B.R.; Majumdar, S. Biased opioid ligands. Molecules 2020, 25, 4257. [Google Scholar] [CrossRef] [PubMed]
- Kise, R.; Inoue, A. GPCR signaling bias: An emerging framework for opioid drug development. J. Biochem. 2024, 175, 367–376. [Google Scholar] [CrossRef]
- Conibear, A.E.; Kelly, E. A biased view of μ-opioid receptors? Mol. Pharmacol. 2019, 96, 542–549. [Google Scholar] [CrossRef]
- Olinvyk Package Insert. Drugs.com. Available online: https://www.drugs.com/pro/olinvyk.html#LINK_21f3751b-1f8d-42ff-94bb-b723545069b7 (accessed on 2 January 2025).
- Liu, R.; Huang, X.P.; Yeliseer, A.; Xi, J. Novel molecular targets of dezocine and their clinical implications. Anesthesiology 2014, 120, 714–723. [Google Scholar] [CrossRef]
- Coleman, J.A.; Gureaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 2018, 25, 170–175. [Google Scholar] [CrossRef]
- Song, A.L.; Wu, X.D. Mechanistic insights of substrate transport and inhibitor binding revealed by high-resolution structures of human epinephrine transporter. Cell Res. 2024, 34, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Pertovaarda, A. Noradrenergic pain modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Mao, X.F.; Li, F.F.; Gong, N.; Zhang, M.A. Dexocine exhibits antihypersensitivity activities in neuropathy through spinal mu-opioid receptor activation and norepinephrine reuptake inhibition. Sci. Rep. 2017, 7, 43137. [Google Scholar]
- Mao, X.F.; Ahsan, M.Z.; Apryani, E.; Tang, X.Q.; Zhao, M.J.; Li, X.Y. Dual mu-opioid receptor and norepinephrine reuptake mechanisms contribute to dezocine- and tapenfadol-induced mechanical antiallodynia in cancer pain. Eur. J. Pharmacol. 2020, 876, 173062. [Google Scholar] [CrossRef]
- Fu, B.; Jiang, J.; Huang, Y. The analgesic effects of dezocine in rats with chronic constriction injuries. Exp. Anim. 2023, 72, 496–504. [Google Scholar] [CrossRef]
- Wu, F.X.; Pan, R.R.; Yu, W.F.; Liu, R. The anti-nociception effect of dezocine in a rat neuropathic pain model. Transl. Perioper. Pain Med. 2014, 1, 5–8. [Google Scholar]
- Ahzan, M.Z.; Zhao, M.J.; Shoaib, R.M.; Zhang, Y.; Wang, Y.X. Comparative study of dezocine, pentazocine and tapentadol on antinociception and physical dependence. Life Sci. 2021, 285, 119996. [Google Scholar] [CrossRef]
- Ding, Y.; White, P.F. Comparative effects of ketodolac, dezocine and fentanyl as adjuvants during outpatient anesthesia. Anesth. Analg. 1992, 75, 566–571. [Google Scholar] [CrossRef]
- Huang, R.; Jiang, L.; Cao, Y.; Liu, H.; Ping, M.; Li, W.; Xu, Y.; Ning, J.; Chen, Y.; Wang, X. Comparative Efficacy of therapeutics for chronic cancer pain: A Bayesian network meta-analysis. J. Clin. Oncol. 2019, 37, 1742–1752. [Google Scholar] [CrossRef]
- Stambaugh, J.E., Jr.; McAdams, J. Comparison in intramuscular dezocine with butorphanol and placebo in chronic cancer pain: A method to evaluate analgesia after both single and repeated doses. Clin. Parmacol. Ther. 1987, 42, 210–219. [Google Scholar] [CrossRef]
- Gong, W.Y.; Xu, B.; Liu, L.; Li, S.T. Dezocine relieves the postoperative hyperalgesia in rats through suppressing the hyper-action of Akt1/GSK-3β pathway. Exp. Brain Res. 2022, 240, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Leon, P.; Miranda-Paez, A.; Valencia-Flores, K.; Sanchez-Cstillo, H. Defensive and emotional behavior modulation by serotonin in the eriaqueductal gray. Cell Mol. Neurobiol. 2023, 43, 1453–1468. [Google Scholar] [CrossRef]
- Shang, L.; Duan, C.; Chang, S.; Chang, N.; Jia, S. Antidepressant-like effects of dezocine in mice: Involvement of 5-HT1A and κ-opioid receptors. Behav. Pharmacol. 2021, 32, 472–478. [Google Scholar] [CrossRef]
- Wang, H.; Lyu, N.; Zhao, Q. Case report: Dezocine’s rapid and sustained antidepressant effects. Front. Pharmacol. 2024, 15, 1411119. [Google Scholar] [CrossRef]
- Wong, S.; Le, G.H.; Vasudeva, S.; Teopiz, K.M.; Phan, L.; Meshkat, S.; Kwan, A.T.H.; Rhee, T.G.; Ho, R.; Choi, H.; et al. Preclinical and clinical efficacy of kappa opioid receptor antagonists for depression: A systematic review. J. Affect. Disord. 2024, 362, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y.; Jia, M.; Bai, Y.; Sun, L.; Dong, Z.; Tian, W.; Yin, F.; Sei, S.; Wang, Y. Kappa opioid receptor in nucleus accumbens regulates depressive-like behaviors following prolonged morphine withdrawal in mice. iScience 2023, 26, 107536. [Google Scholar] [CrossRef]
- Gowing, L.; Farrell, M.; Ali, R.; White, J.M. Alphs2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2016, 5, CD002024. [Google Scholar]
- Grothusen, J.R.; Blendy, J.A.; Barr, G.A. A brief overview of the neuropharmacology of opioid addiction. Transl. Perioper. Pain Med. 2022, 9, 491–496. [Google Scholar] [PubMed]
- Barr, G.A.; Schmidt, H.D.; Thakrar, A.P.; Kranzler, H.R.; Liu, R. Revisiting dezocine for opioid use disorder: A narrative review of its potential abuse liability. CNS Neurisci. Ther. 2024, 30, e70034. [Google Scholar] [CrossRef]
- Jasinski, D.R.; Preston, K.L. Assessment of dezocine for morphine-like subjective effects and miosis. Clin. Pharmacol. Ther. 1985, 38, 544–548. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Zeng, J.; Fei, Y. Clinical analysis of 8 drugs dependence with dezocine. Clin. J. Drug Depend. 2020, 29, 474–475. [Google Scholar]
- Shen, Q.; Zhang, L. Analysis of a case of addiction caused by dezocine injection in a patient with human immunodeficiency virus infection. Chin. Pharmacovigil. 2023, 20, 1181–1183. [Google Scholar]
- Holtzman, S.G. Drug discrimination studies. Drug Alcohol Depend. 1985, 14, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Stephens, K.R.; Hein, D.W.; Woods, J.H. Reinforcing and discriminative stimulus properties of mixed agonist-antagonist opioids. J. Pharmacol. Exp. Ther. 1984, 229, 118–126. [Google Scholar] [CrossRef]
- Schaefer, G.J.; Holtzman, S.G. Morphine-like stimulus effects in the monkey: Opioids with antagonist properties. Pharmacol. Biochem. Behav. 1981, 14, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Picker, M.J. Discriminative stimulus effects of the mixed-opioid agonist/antagonist dezocine: Cross-substitution by mu and delta opioid agonists. J. Pharmacol. Exp. Ther. 1997, 283, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.L. The rise and fall of kappa-opioid receptors in drug abuse research. In Substance Use Disorders, From Etology to Treatment. Handbook of Experimental Pharmacology; Nader, M., Hurd, Y., Eds.; Springer Nature: Bern, Switzerland, 2019; Volume 258, pp. 147–166. [Google Scholar]
- Porter-Stransky, K.A.; Weinshenker, D. Arresting the development of addiction: The role of β-arrestin 2 in drug abuse. J. Pharmacol. Exp. Ther. 2017, 361, 341–348. [Google Scholar] [CrossRef]
- Wise, R.A.; Robbie, M.A. Dopamine and addiction. Ann. Rev. Psychol. 2020, 71, 79–106. [Google Scholar] [CrossRef]
- Liu, R.; Milas, B.; Grothusen, J. Non-scheduled short-acting opioid to taper off opioids? CNS Neurosci. Ther. 2024, 30, e14705. [Google Scholar] [CrossRef]
- Wu, F.X.; Babazada, H.; Gao, H.; Huang, X.P.; Xi, C.H.; Chen, C.H.; Xi, J.; Yu, W.F.; Liu, R. Dezocine alleviates morphine-induced dependence in rats. Anesth. Analg. 2019, 128, 1328–1335. [Google Scholar] [CrossRef]
- Pdergolizzi, J.V., Jr.; Raffa, R.B.; Rosenblatt, M.H. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J. Clin. Pharm. Ther. 2020, 45, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wu, Z.; Li, C.; Yang, G.; Ding, J.; Wang, K.; Wang, M.; Feng, L.; Duan, G.; Li, H. Postoperative analgesia using dezocine alleviates depressive symptoms after colorectal cancer surgery: A randomized controlled, double-blind trial. PLoS ONE 2020, 15, e0233412. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.D.; Yang, K.; Wen, X. Dezocine can alleviate depression of breast cancer. Int. J. Clin. Stud. Med. Case Rep. 2023, 33, 003. [Google Scholar] [CrossRef]
- Foster, S.L.; Weinshanker, D. The role of norepinephrine in drug addiction: Past, present, and future. In Neural Mechanisms of Addiction; Torregrossa, M., Ed.; Elsevier: Amsterdam, The Netherland, 2018; pp. 221–236. [Google Scholar]
- Schmidt, H.D.; Zhang, Y.; Xi, J.; Zanni, G.; Liu, R.; Barr, G.A. A new formulation of dezocine, Cyc-dezocine, reduces oxycodone self-administration in female and male rats. Neurosci. Lett. 2023, 815, 137479. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: London, UK, 2019; Chapter 4; pp. 113–172. [Google Scholar]
- Liu, R.; Abou-Gharbia, M.; Cao, H.; Chen, X.; Fleisher, L.A.; Hasan, A.; Huang, Y.; Li, J.; Liu, H.; Ma, H.; et al. 2024 US-China round table consensus discussion on perioperative opioid management. Transl. Perioper Pain Med. 2024, 11, 683–686. [Google Scholar]
- Ozdemir, E.; Gursoy, S.; Bagcivan, I. The effects of serotonin/norepinephrine reuptake inhibitors and serotonin receptor agonist on morphine analgesia and tolerance in rats. J. Physio. Sci. 2012, 62, 317–323. [Google Scholar] [CrossRef]
- Smith, B.H.; Higgins, C.; Baldacchino, A.; Kidd, B.; Bannister, J. Substance misuse of gabapentin. Br. J. Gen. Pract. 2012, 62, 406–407. [Google Scholar] [CrossRef]
- Kingwell, K. Nav1.8 inhibitor poised to provide opioid-free pain relief. Nat. Rev. Drug Disc. 2025, 24, 3–5. [Google Scholar] [CrossRef]
- Osteen, J.D.; Immani, S.; Tapley, T.L.; Indersmitten, T.; Hurst, N.W.; Healey, T.; Aertgeerts, K.; Negulescu, P.A.; Lechner, S.M. Pharmacology and mechanism of action of suzetrigine, a potent and selective Nav1.8 pain signal inhibitor for the treatment of moderate to severe pain. Pain Ther. 2025. published online ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).