1. Introduction
Melanoma is a malignant tumor arising from melanocytes and represents the most aggressive form of skin cancer. Its global incidence is increasing, and advanced-stage melanoma remains difficult to treat due to high metastatic potential, drug resistance mechanisms, and low response rates [
1,
2]. Despite advances in targeted and immunotherapies, the median survival in widely metastatic melanoma remains between 6 and 11 months, depending on the site and burden of metastases [
3].
Cisplatin is a conventional chemotherapeutic agent that exerts its antitumor effect by inducing DNA damage, leading to cell cycle arrest and apoptosis. However, its clinical use is limited by severe systemic side effects, including nephrotoxicity and chemotherapy-induced resistance [
4]. To address these challenges, combining chemotherapeutic agents with natural products has gained increasing attention as a strategy to enhance efficacy and reduce toxicity.
Flavonoids, a class of plant-derived polyphenolic compounds, are known for their diverse biological activities, including antioxidant, anti-inflammatory, and anticancer effects [
5,
6]. Hesperidin, a flavanone glycoside predominantly found in citrus peels, has shown antiproliferative and pro-apoptotic effects in malignant cell lines derived from various cancer types, including colon, breast, liver, prostate, and multiple myeloma [
7,
8]. These effects are often mediated through activation of the intrinsic apoptotic pathway, involving Bax upregulation, caspase-3/7 activation, and downregulation of the anti-apoptotic protein survivin [
9,
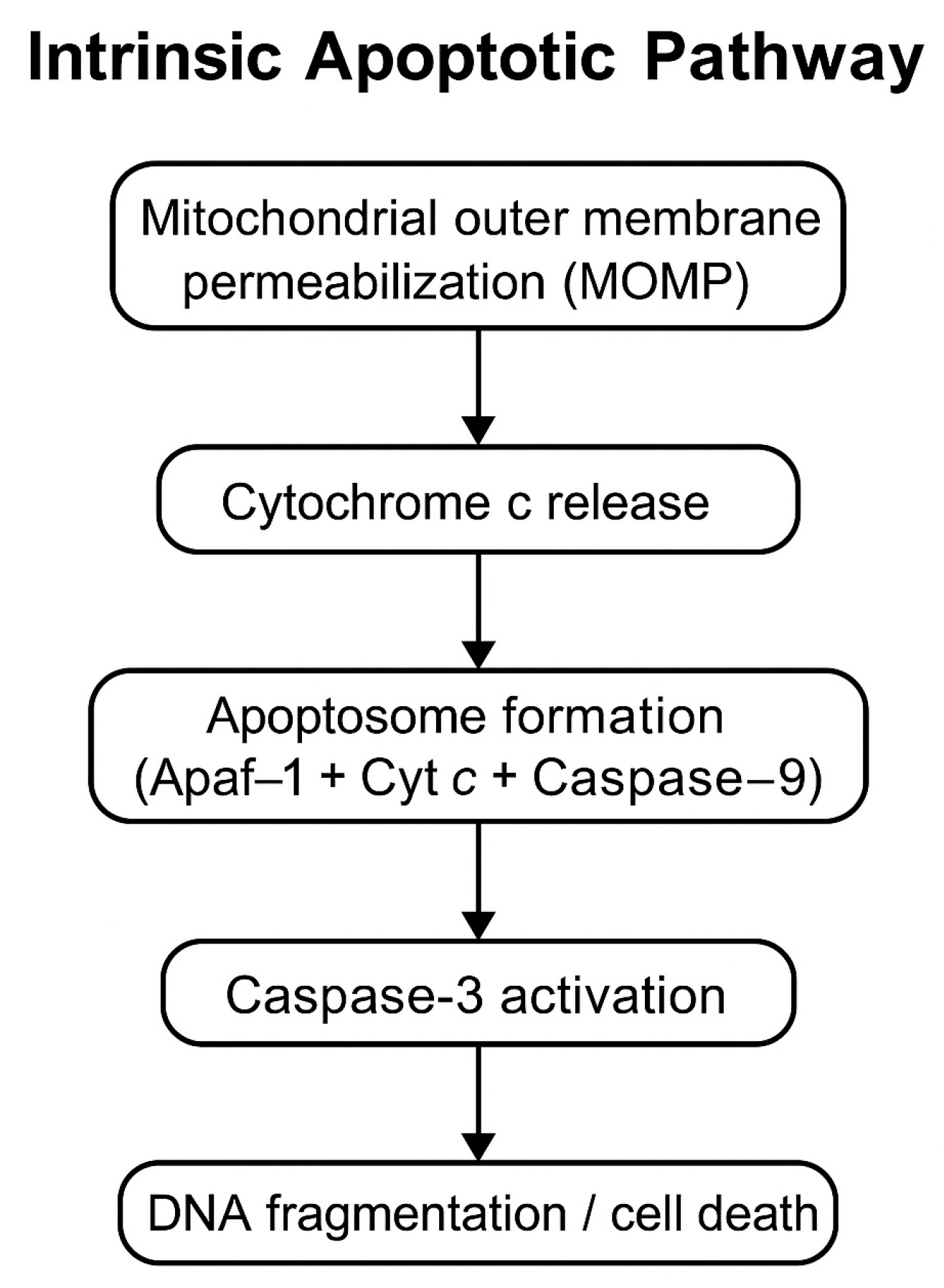
10]. Caspase-3 is a key executioner caspase that acts as a mediator in the intrinsic apoptotic pathway. Its activation reflects not only the presence of apoptosis but also the molecular mechanism by which it is executed (
Figure 1).
In addition to its direct anticancer activity, Hesperidin influences several signaling pathways implicated in tumor progression, including PI3K/Akt and NF-κB [
11]. Furthermore, its combination with chemotherapeutics like Cisplatin has been shown to augment cytotoxicity while mitigating drug-induced toxicity. For instance, in hepatocellular carcinoma models, Hesperidin co-administration with Cisplatin enhanced treatment efficacy and reduced oxidative damage in liver tissues [
12]. Moreover, Hesperidin has demonstrated selective cytotoxicity, preferentially targeting cancer cells while sparing healthy ones, thereby improving the safety profile of chemotherapy [
13].
Despite the promising findings regarding Hesperidin’s anticancer effects, studies evaluating its combination with Cisplatin in epidermoid carcinoma or melanoma models remain limited. Given the aggressive and treatment-resistant nature of such cancers, exploring the potential synergy between Hesperidin and Cisplatin is of clinical significance.
In this study, we hypothesize that Hesperidin, alone or in combination with Cisplatin, exerts a synergistic cytotoxic effect in A431 cells via activation of intrinsic apoptotic mechanisms. To test this, we assessed cell viability using MTT assays, evaluated apoptosis using Annexin V/PI staining and caspase-3/7 activity, and analyzed the expression of apoptosis-related genes (Bax, caspase-3/7, survivin) by RT-qPCR. The findings of this study may support the development of Hesperidin as a potential adjunct to chemotherapy for epidermoid carcinoma.
3. Discussion
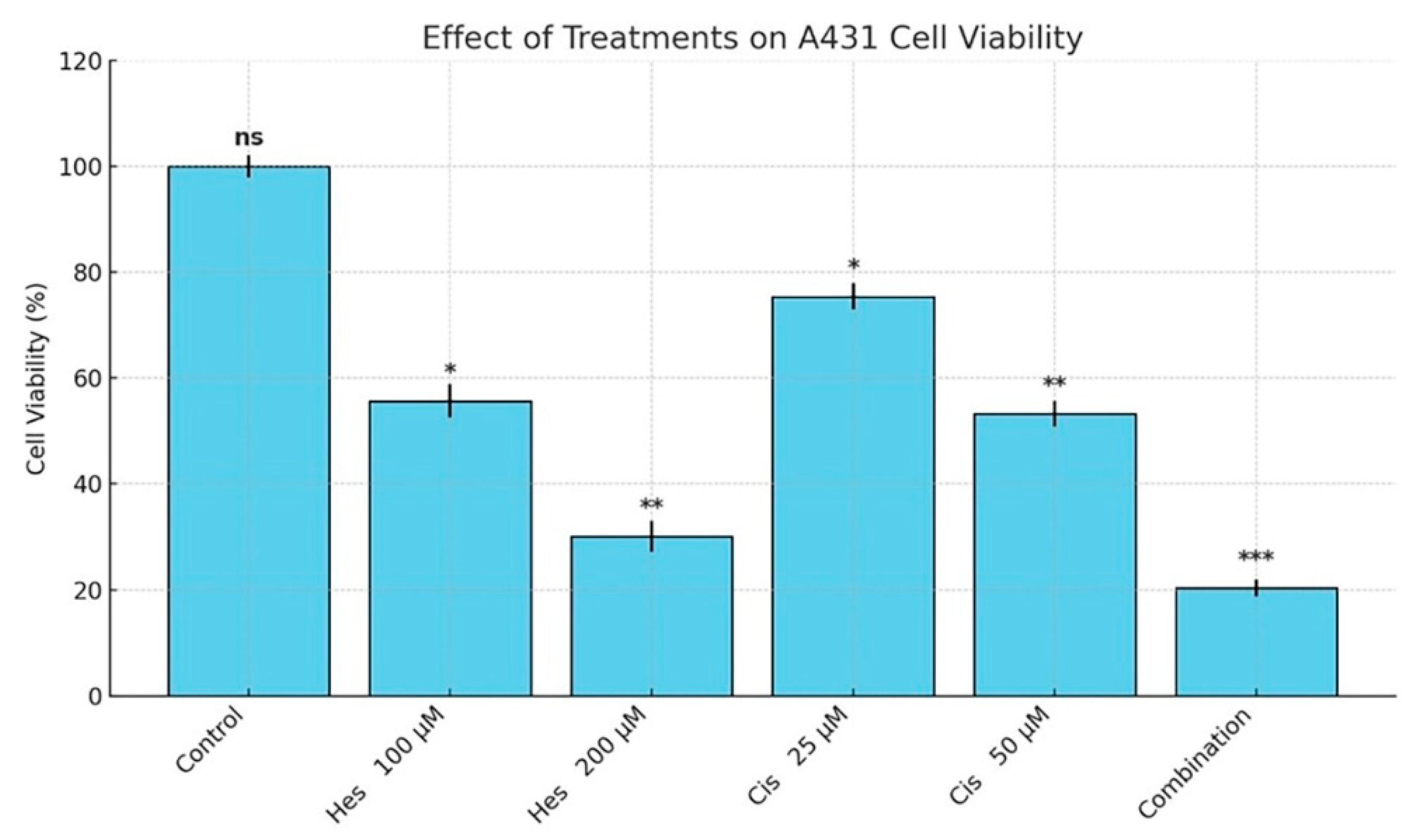
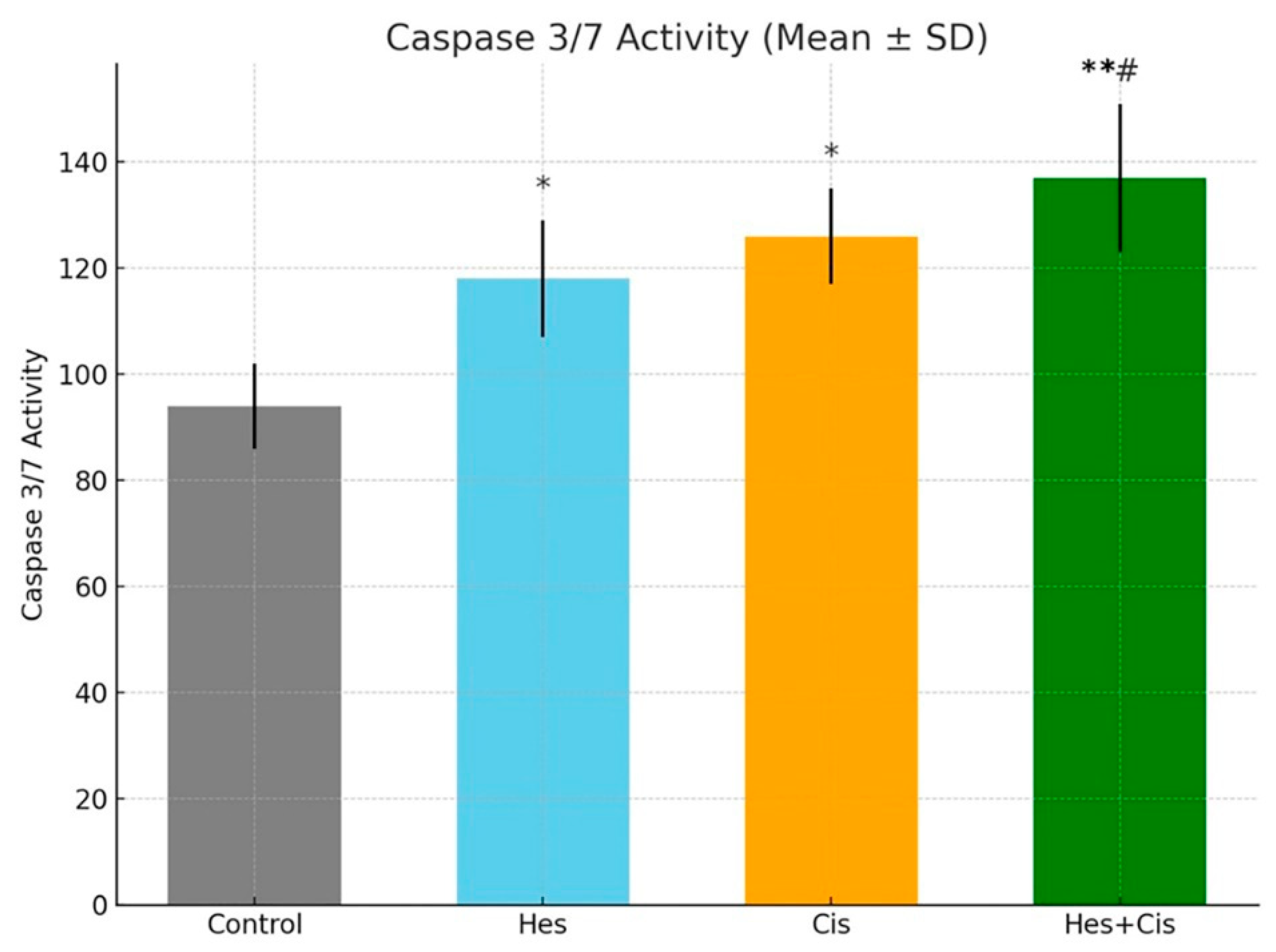
In this study, we demonstrated that Hesperidin exerts significant cytotoxic and pro-apoptotic effects on A431 human melanoma cells and that its combination with Cisplatin results in a synergistic enhancement of these effects. MTT assay results revealed a dose-dependent reduction in cell viability following Hesperidin treatment, with 200 µM causing a 65.2% decrease, while Cisplatin also reduced viability, and their combination yielded the most pronounced inhibitory effect (p < 0.001). These findings were supported by Annexin V/PI staining, which showed marked increases in both early and late apoptotic populations, especially in the combination group. Furthermore, caspase-3/7 activity was significantly elevated in all treatment groups, with the highest activity observed in the Hesperidin + Cisplatin group, suggesting enhanced execution of apoptosis. Gene expression analyses via RT-qPCR confirmed the activation of the intrinsic apoptotic pathway, as evidenced by the upregulation of pro-apoptotic Bax, caspase-3, and caspase-7 and the downregulation of anti-apoptotic survivin. The synergy observed in combination therapy was further validated by the Bliss independence model, indicating a mild but consistent synergistic interaction, particularly at moderate to high dose levels. Collectively, these data highlight the potential of Hesperidin to augment Cisplatin-induced cytotoxicity through mitochondrial-mediated apoptosis, suggesting a promising combinatorial strategy for melanoma treatment.
Traditional methods used in cancer treatment can often cause various side effects during the treatment process. Therefore, it is of great importance to develop more targeted, effective, and safe treatment options. In this context, this study, which aimed to evaluate the effects of two different compounds, Hesperidin and Cisplatin, on melanoma, provided important findings for treatment. In this study, the cytotoxic and apoptotic effects of Hesperidin on A431 melanoma cells and its synergistic interactions with Cisplatin were investigated. The findings revealed that Hesperidin dose-dependently decreased cell viability, activated apoptotic pathways, and showed a stronger anticancer effect in combination with Cisplatin.
MTT assay results showed that Hesperidin showed a dose-dependent cytotoxic effect in A431 melanoma cells, and the IC
50 value was 108.4 µM. This result is consistent with other studies in the literature. For example, Febriansah et al. reported that Hesperidin showed a similar apoptotic effect in MCF-7 breast cancer cells with an IC
50 value of 120 µM [
14]. Here, the 200 µM Hesperidin treatment decreased cell viability by 65.2%, indicating that this flavonoid has a strong cytotoxic potential against melanoma cells. Annexin V/PI staining results revealed that Hesperidin induced apoptotic cell death at both early and late stages. These findings are in agreement with the study by Li et al., which found that Hesperidin induced apoptosis in colon cancer cells [
15]. It also overlaps with the mechanism published in 2023, showing that Hesperidin triggers apoptosis by inhibiting the PI3K/Akt pathway [
16]. Our RT-qPCR analysis showed that Hesperidin treatment significantly increased pro-apoptotic Bax gene expression (
p < 0.01) and suppressed anti-apoptotic survivin expression (
p < 0.01). This suggests that Hesperidin activates the intrinsic (mitochondrial) apoptotic pathway. The increase in Bax may trigger the caspase cascade by disruption of mitochondrial membrane permeability and cytochrome c release [
17].
Hesperidin, as a flavonoid, promotes the process of apoptosis (programmed cell death) in cancer cells by increasing intracellular reactive oxygen species (ROS) levels. The increase in ROS can lead to cell membrane disruption and cell death. In studies, it has been observed that Hesperidin increases intracellular ROS levels in human melanoma cell lines, and this triggers cell death. This effect of Hesperidin may constitute a strategy for the elimination of treatment-resistant cells in cancer therapy. However, the anticancer effects of Hesperidin are not limited to ROS metabolism; they may also be related to many other mechanisms, such as reducing oxidative stress and controlling inflammation in the cell [
18]. Cisplatin is a common chemotherapeutic agent that induces apoptotic cell death through DNA damage. In this study, administration of Cisplatin alone decreased cell viability and increased apoptotic markers as expected. However, the most striking finding was that the combination of Hesperidin and Cisplatin showed a synergistic effect. Bliss analysis confirmed synergy, especially at moderate and high doses. The mechanism underlying this synergistic effect is probably that Hesperidin potentiates the intrinsic apoptotic pathway in addition to the DNA damage caused by Cisplatin. RT-qPCR data showed that the combination treatment increased the expression of Bax, caspase-3, and caspase-7 to a greater extent compared to the treatments alone. This suggests that both components potentiate apoptotic signaling by different mechanisms. Moreover, the effect of Hesperidin in balancing the oxidative stress induced by Cisplatin and repairing cellular damages may have positive results in the treatment process. However, optimization of dosage and treatment protocols is necessary for this combination therapy to achieve clinical success. Hesperidin, due to its antioxidant properties, may play a role in reducing the side effects of Cisplatin treatment, leading to better patient tolerance to the treatment. Hesperidin has also been shown to offer a mechanism of action that prevents cancer cells from becoming desensitized to Cisplatin treatment, making the treatment process more effective [
19,
20]. The combination of Hesperidin and Cisplatin is a therapeutic strategy that promises to specifically target treatment-resistant melanoma cells. However, further studies are required to confirm the efficacy of this treatment modality at the clinical level. Although the results obtained in preclinical models indicate that this treatment approach is promising, larger and long-term clinical trials are needed to determine the safety and efficacy of both compounds. Furthermore, optimizing the pharmacokinetic properties, dosages, and combinations of both compounds could improve the efficacy of the treatment process. For example, the side effects of Cisplatin treatment can be minimized with Hesperidin, but the dosage needs to be carefully adjusted. The low toxic effect of Hesperidin may help to reduce side effects and improve the treatment process [
19]. The apoptotic effects of Cisplatin through DNA damage are described in detail in a recent study published in 2021 [
21]. Current strategies for nanoformulation studies published in 2025 may be instructive [
22]. This study revealed that Hesperidin exhibited dose-dependent cytotoxic and apoptotic effects in A431 melanoma cells. Importantly, the combination of Hesperidin and Cisplatin exhibited a synergistic effect and markedly increased apoptotic cell death. These findings suggest that Hesperidin is a potential adjuvant agent that can be used in combination with Cisplatin in melanoma treatment. However, these results need to be confirmed by preclinical and clinical studies.
Although the current study demonstrates promising in vitro synergistic cytotoxicity between Hesperidin and Cisplatin, the clinical translation of Hesperidin remains challenged by its limited bioavailability. Human pharmacokinetic studies have shown that the glycosidic form of Hesperidin, particularly Hesperidin -7-O-rutinoside, exhibits poor absorption in the gastrointestinal tract, resulting in low systemic exposure. However, enzymatic conversion to its glucoside form markedly enhances absorption, shifting the uptake site from the colon to the small intestine, as evidenced by a fourfold increase in plasma levels and higher urinary excretion following consumption of α-rhamnosidase-treated orange juice [
23]. Furthermore, recent investigations revealed that both the diastereoisomeric composition and micronization process substantially affect Hesperidin’s bioavailability. Specifically, micronized 2S- Hesperidin showed the highest urinary excretion and systemic availability, underscoring the significance of formulation in enhancing therapeutic potential [
24]. Finally, literature reviews emphasize that poor aqueous solubility and limited permeability are central to Hesperidin’s low oral bioavailability, and improving these parameters through technological approaches is essential for clinical translation in chronic diseases, including cancer [
25]. Collectively, these findings suggest that future studies exploring Hesperidin and Cisplatin combinations should integrate bioavailability-enhancing strategies to ensure efficacy and reproducibility in vivo.
Despite the promising results of this study, several limitations should be acknowledged. First, the in vitro nature of the study restricts the generalizability of the findings to in vivo systems. Although the A431 human melanoma cell line provides a valuable model for evaluating the effects of Hesperidin and Cisplatin, it may not fully recapitulate the biological complexity of primary melanoma tumors in patients. Additionally, although the combination of Hesperidin and Cisplatin demonstrated significant synergistic effects in vitro, their pharmacokinetics, biodistribution, and potential systemic toxicity remain uncharacterized and require further investigation in preclinical animal models and clinical settings. Another limitation lies in the reliance on four synergy models (Chou–Talalay, Bliss, Loewe, and HSA), each with distinct assumptions and sensitivity parameters. While these models are widely employed to assess drug interactions, the variability in synergy scores observed in this study underscores the need for additional validation using alternative computational and experimental synergy frameworks. Furthermore, the long-term effects of Hesperidin and Cisplatin co-administration—particularly concerning the development of drug resistance—were not addressed and should be explored in future longitudinal studies. Importantly, we focused primarily on a limited set of apoptosis-related genes (Bax, caspase-3/7, and survivin). Other apoptotic, anti-apoptotic, or survival-related molecular pathways may also contribute to the observed cytotoxic effects and should be incorporated into future mechanistic investigations. Moreover, the potential impact of cellular dormancy and quiescence must be considered. Since non-dividing tumor cells are often less sensitive to cytotoxic agents like Cisplatin that primarily target proliferating cells, future studies should evaluate the efficacy of Hesperidin and Cisplatin in models enriched with quiescent cell populations. This approach would better reflect the tumor heterogeneity encountered in clinical scenarios. Another key limitation is the absence of non-malignant cell lines in the current study. Evaluating IC50 values in both malignant and non-malignant cells is crucial to assess the selectivity and translational safety of the combination treatment. Additionally, analyses of the culture supernatants—such as measuring cytokine levels, ROS generation, or other cellular stress markers—could provide deeper insights into the selective cytotoxic mechanisms of Hesperidin. Lastly, validation of the present findings in additional melanoma and non-melanoma cell lines is warranted to ensure reproducibility and broader applicability across diverse tumor models.
Moreover, previous studies have indicated that Hesperidin exhibits limited pro-apoptotic or antiproliferative activity in certain cancer types. For example, it showed negligible modulation of cell viability and apoptosis in human colorectal cancer cells [
26], and it was found to be ineffective both alone and in combination with Cisplatin in ovarian cancer cells [
27]. These tumor-specific differences in Hesperidin’s efficacy highlight the need for validation across a broader range of tumor models. Lastly, validation of the present findings in additional melanoma and non-melanoma cell lines is warranted to ensure reproducibility and broader applicability across diverse tumor models.
While this study presents promising in vitro results regarding the synergistic effects of Hesperidin and Cisplatin in human melanoma cells, further research is needed to translate these findings into clinical applications. Future studies should focus on optimizing the dosages and treatment regimens to assess the therapeutic potential and minimize possible side effects in vivo. Additionally, exploring the long-term effects of this combination therapy on melanoma progression and drug resistance is crucial. Investigating the pharmacokinetics and biodistribution of Hesperidin, in combination with Cisplatin, through animal models will be essential for determining its clinical feasibility. Moreover, expanding this research to include a broader range of melanoma cell lines and other cancer types would provide more comprehensive insights into the generalizability of the observed effects. The development of novel delivery systems, such as nanoparticle-based formulations, could further enhance the bioavailability and effectiveness of this combination therapy. Ultimately, large-scale clinical trials will be required to evaluate the safety, efficacy, and potential for personalized treatment strategies based on patient-specific tumor characteristics.
4. Material and Methods
4.1. Cell Culture
A431 (CRL-1555™) cells, a human epidermoid carcinoma cell line commonly utilized in melanoma-related research due to its aggressive proliferative characteristics, were used in this study. A431 melanoma cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured following established guidelines. The cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) enriched with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin–streptomycin solution (Gibco) under standard incubation conditions. Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Upon reaching 80–90% confluence, cells were passaged using 0.25% trypsin-EDTA solution (Gibco).
4.2. Hesperidin and Cisplatin Administration
Hesperidin (≥95% purity; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was dissolved in dimethyl sulfoxide (DMSO) to prepare a 100 mM stock solution and subsequently diluted in DMEM to the desired working concentrations. Cisplatin (Sigma-Aldrich) was dissolved in sterile distilled water to obtain a stock solution at a concentration of 1 mg/mL. A431 cells were seeded in 96-well and 6-well plates and treated with varying concentrations of Hesperidin (100 and 200 µM) and Cisplatin (25 and 50 µM) for 48 h under standard incubation conditions.
4.3. Cytotoxicity Analysis (MTT Assay)
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was employed to determine the viability of A431 cells. Cells were plated in 96-well culture plates at a density of 5 × 103 cells per well. After drug treatment, each well received 10 µL of 5 mg/mL MTT solution and was incubated at 37 °C for 4 h. Following incubation, the supernatant was discarded, and the insoluble formazan crystals were solubilized using DMSO. The optical density was then measured at 570 nm using a BioTek ELx800 microplate spectrophotometer. The results were expressed as a percentage of the absorbance values relative to the untreated control group.
4.4. Determination of Effective Dose (IC50)
Based on the MTT assay results, the half-maximal inhibitory concentration (IC
50) values for Hesperidin and Cisplatin were calculated using probit analysis with the SPSS 20.0 statistical software. These values were determined separately for each treatment group and used to identify effective concentrations. The calculated IC
50 doses were subsequently applied in downstream experiments, including RT-qPCR analysis. Although only two concentrations were shown in
Figure 2, additional intermediate concentrations (e.g., 25, 50, 75, 150 µM for Hesperidin and 10, 25, 40 µM for Cisplatin) were tested in preliminary experiments. These values were included in the IC
50 calculations using probit regression in SPSS 20.0. The resulting dose–response curves are shown in
Supplementary Figure S1.
4.5. Caspase-3/7 Activity Findings
Caspase-3/7 activity was evaluated using the Caspase-Glo® 3/7 Assay Kit (Promega, Madison, WI, USA) to confirm activation of the apoptotic pathway. Following treatment, cells cultured in 96-well plates were incubated with the caspase reagent according to the manufacturer’s instructions. Plates were then kept at room temperature in the dark for 1 h to allow luminescence development. Luminescence was measured using a microplate reader, and the results were compared to the untreated control group to assess relative caspase activity.
4.6. Apoptosis Analysis (Annexin V/PI Cell Staining Method)
Apoptosis rates were evaluated using the Annexin V-FITC/propidium iodide (PI) double staining method. Apoptosis analysis was performed using IC50 concentrations: Hesperidin at 108.4 µM and Cisplatin at 32.8 µM, determined from the MTT assay. Following treatment, A431 cells were harvested by trypsinization, washed twice with phosphate-buffered saline (PBS), and resuspended in PBS at a density of 1 × 10⁶ cells/mL. Subsequently, 5 µL of Annexin V-FITC and 5 µL of PI were added to each sample, and the cells were incubated in the dark at room temperature for 15 min. After staining, samples were analyzed under a fluorescence microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan).
Apoptotic status was determined based on fluorescence signals as follows:
Viable cells were identified as negative for both Annexin V-FITC and propidium iodide (PI) staining.
Cells in early-stage apoptosis exhibited Annexin V-FITC positivity while remaining negative for PI staining.
Late-stage apoptotic cells showed positivity for both Annexin V-FITC and PI.
Necrotic cells were characterized by the absence of Annexin V-FITC staining but were PI-positive.
Cells were examined in at least five random fields per condition, and the number of cells in each category was recorded to determine the distribution of cell populations across experimental groups.
4.7. Gene Expression Analysis
Quantitative reverse transcription PCR (RT-qPCR) was employed to evaluate the mRNA expression levels of key apoptosis-associated genes, including Bax, caspase-3, caspase-7, and survivin (BIRC5). Total RNA was isolated using TRIzol™ reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) following the protocol provided by the manufacturer. The RNA’s concentration and purity were verified using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). All RT-qPCR assays were carried out using Hesperidin at 108.4 µM and Cisplatin at 32.8 µM, corresponding to the IC50 concentrations calculated in cytotoxicity assays.
Complementary DNA (cDNA) synthesis was carried out using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) with 1 µg of total RNA as a template. The synthesized cDNA was then used for RT-qPCR analysis, which was performed on an Applied Biosystems 7500 Real-Time PCR System using SYBR™ Green PCR Master Mix (Thermo Fisher Scientific). The primer sequences of the genes used in the study are as follows (
Table 3):
The PCR cycling protocol was performed as follows: Initial denaturation at 95 °C for 10 min, followed by denaturation at 95 °C for 15 s, and elongation at 60 °C for 60 s for 40 cycles. Each sample was analyzed in three technical replicates. Gene expression levels were normalized to GAPDH, which was used as a reference gene. Relative gene expression was calculated using the 2−ΔΔCt method and compared to the control group.
4.8. Synergy Evaluation Using Multiple Models
To assess the nature of the pharmacological interaction between Hesperidin and Cisplatin, CI and synergy analyses were performed using four standard models: Chou–Talalay CI method, Bliss independence model, Loewe Additivity model, and HSA model.
For the Chou–Talalay method, the CI values were calculated using CompuSyn software version 1.0 (ComboSyn, Inc., Paramus, NJ, USA) based on the median-effect equation, which determines drug interactions as synergistic (CI < 1), additive (CI = 1), or antagonistic (CI > 1).
Bliss independence and Loewe Additivity analyses were performed with SynergyFinder v2.0 (
https://synergyfinder.fimm.fi, accessed on 1 May 2025), a web-based platform that uses dose–response matrices to calculate synergy scores. The Bliss model evaluates the expected non-interacting effect as EBliss = EA + EB – EA × EB, while Loewe’s model is based on the principle that a drug cannot be more effective than itself. Scores above +10 suggest synergism, between −10 and +10 suggest additive effects, and below −10 indicate antagonism.
The HSA model, which compares the combination effect with the most effective single agent, was also used to provide a robust overview of potential interactions. All synergy assays were conducted using 4 × 4 dose matrices and analyzed in triplicates.
4.9. Statistical Analysis
All experiments were independently conducted in three replicates, and the outcomes are presented as mean values with their corresponding standard deviations (SD). Statistical evaluations were performed using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was applied to examine the normality of the data distributions, while Levene’s test was utilized to verify the equality of variances across groups. Differences among the four experimental groups (Control, Hesperidin, Cisplatin, and Hesperidin + Cisplatin) were evaluated using ANOVA, followed by Tukey’s post hoc test for multiple comparisons. A
p-value of less than 0.05 was considered statistically significant. Graphical visualizations of the data, including bar charts with error bars, were generated using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). In this study, drug interactions were interpreted using four standard models: Bliss independence, Loewe Additivity, HSA, and CI. These models differ in their assumptions and definitions of synergy. Bliss Independence assumes probabilistic independence of drug actions; Loewe Additivity is based on the idea that a drug cannot interact with itself and models dose equivalence; the HSA model compares the combination effect with the more effective single agent; and the Chou–Talalay CI model is based on the median-effect principle. In general, synergy is defined as an interaction where the combination effect is greater than the sum of individual effects (CI < 1), additivity is when effects are equal (CI = 1), and antagonism is when the combination is less effective (CI > 1). For this analysis, Combenefit v2.02 software was used [
28,
29].