Valproate-Enhanced Protocols for Alcohol Withdrawal Syndrome: A Brief Review and Retrospective Study of Efficacy and the Ability to Reduce Benzodiazepine Use
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics
2.2. Valproate and Benzodiazepine Dosages
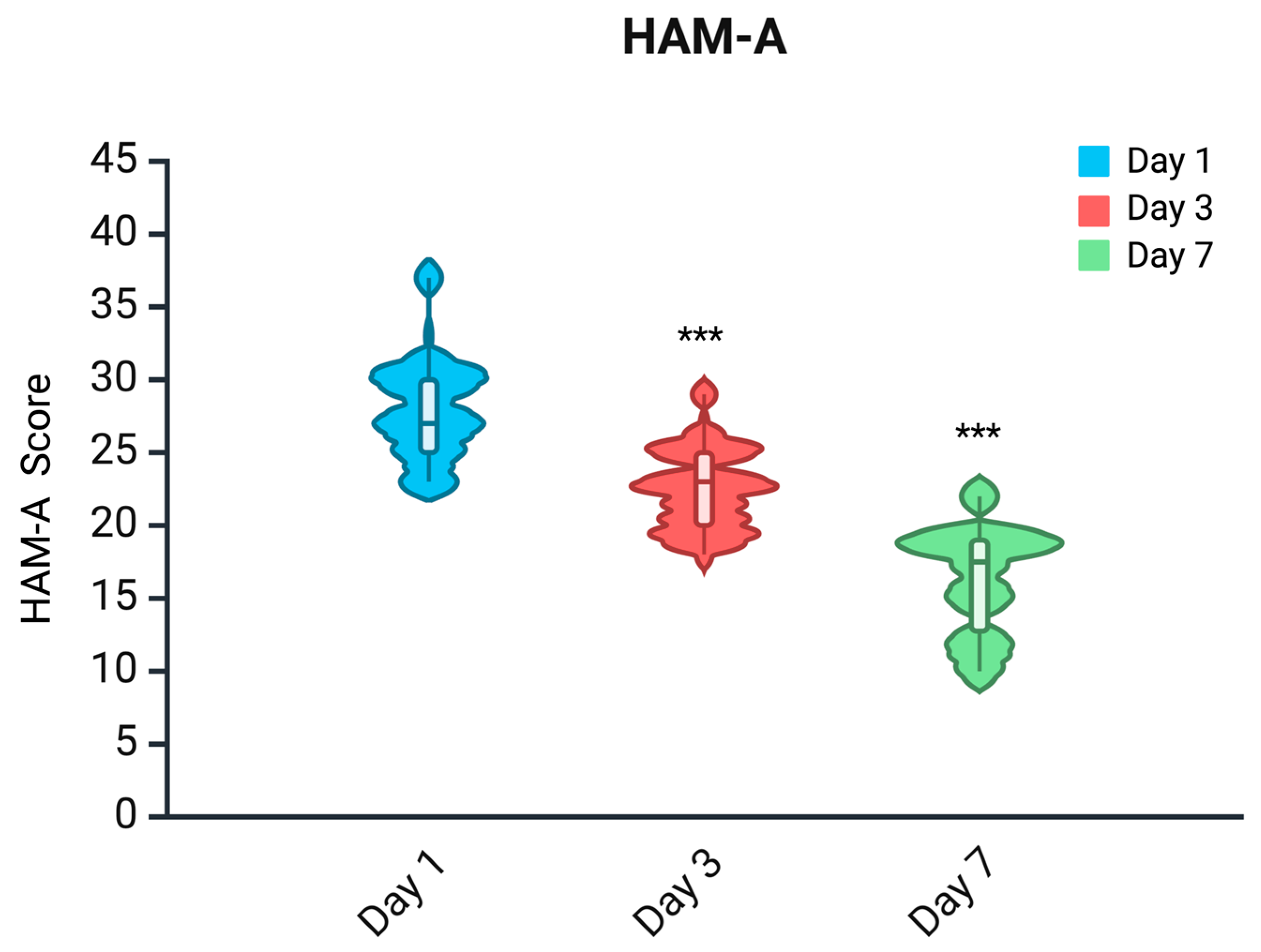
2.3. HAM-A Scale
2.4. CIWA-Ar Scale
2.5. Comparison of PO-EV
3. Discussion
4. Materials and Methods
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AWS | Alcohol Withdrawal Syndrome |
| IV | Intravenous |
| OS | Oral |
| CIWA-Ar | Clinical Institute Withdrawal Assessment for Alcohol Scale, Revised |
| HAM-A | Hamilton Anxiety Rating Scale |
| DT | Delirium Tremens |
| GABA-A | Gamma-Aminobutyric Acid Type A Receptor |
| GSK-3β | Glycogen Synthase Kinase 3 Beta |
| MSSA | Minnesota Substance Abuse Survey |
| ASQ | Alcohol Symptom Questionnaire |
| GCP | Good Clinical Practice |
References
- Canver, B.R.; Newman, R.K.; Gomez, A.E. Alcohol Withdrawal Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Attilia, F.; Perciballi, R.; Rotondo, C.; Capriglione, I.; Iannuzzi, S.; Attilia, M.L.; Coriale, G.; Vitali, M.; Cereatti, F.; Fiore, M.; et al. Alcohol withdrawal syndrome: Diagnostic and therapeutic methods. Riv. Psichiatr. 2018. [Google Scholar] [CrossRef]
- Schuckit, M.A. Recognition and Management of Withdrawal Delirium (Delirium Tremens). N. Engl. J. Med. 2014, 371, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Paul, M. Delirium Tremens(Archived). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- World Health Organization Alcohol. Available online: https://www.who.int/news-room/fact-sheets/detail/alcohol (accessed on 21 May 2025).
- Ntais, C.; Pakos, E.; Kyzas, P.; Ioannidis, J.P. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst. Rev. 2005, 2005, CD005063, Update in: Cochrane Database Syst. Rev. 2010, 2010, CD005063. https://doi.org/10.1002/14651858.CD005063.pub3. [Google Scholar] [CrossRef]
- DuPont, R.L. “Should Patients with Substance Use Disorders Be Prescribed Benzodiazepines?” No. J. Addict. Med. 2017, 11, 84–86. [Google Scholar] [CrossRef]
- Lopez, E.; Jeanne, G.; Lefort, L.; Autissier, C.; Picot, M.; Peyrière, H.; Donnadieu-Rigole, H. Characterization of Benzodiazepine Misuse and Comorbidities in Patients with Alcohol Use Disorder. Fundamemntal Clin. Pharma 2021, 35, 1133–1140. [Google Scholar] [CrossRef]
- Ghosh, A.; Mahintamani, T.; Choudhury, S.; Sharma, N.; Das, S. The Effectiveness of Non-Benzodiazepine, Non-Barbiturate Medications for Alcohol Withdrawal Syndrome: A Rapid Systematic Review. Alcohol Alcohol. 2021, 56, 513–534. [Google Scholar] [CrossRef]
- Rojo-Mira, J.; Pineda-Álvarez, M.; Zapata-Ospina, J.P. Efficacy and Safety of Anticonvulsants for the Inpatient Treatment of Alcohol Withdrawal Syndrome: A Systematic Review and Meta-Analysis. Alcohol Alcohol. 2022, 57, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Barrons, R.; Roberts, N. The Role of Carbamazepine and Oxcarbazepine in Alcohol Withdrawal Syndrome: Carbamazepine and Oxcarbazepine in Alcohol Withdrawal Syndrome. J. Clin. Pharm. Ther. 2010, 35, 153–167. [Google Scholar] [CrossRef]
- Bertilsson, L. Clinical Pharmacokinetics of Carbamazepine. Clin. Pharmacokinet. 1978, 3, 128–143. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, Y.; Huang, W. Gabapentinoids for Treatment of Alcohol Use Disorder: A Systematic Review and Meta-analysis. Hum. Psychopharmacol. 2020, 35, 1–11. [Google Scholar] [CrossRef]
- Rahman, M.; Awosika, A.O.; Nguyen, H. Valproic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Longo, L.P.; Campbell, T.; Hubatch, S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J. Addict. Dis. 2002, 21, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.N.; Perkel, C.; Singh, P.; Anand, O.; Miner, C.R. A Pilot Open Randomized Trial of Valproate and Phenobarbital in the Treatment of Acute Alcohol Withdrawal. Am. J. Addict. 1998, 7, 189–197. [Google Scholar] [CrossRef]
- Reoux, J.P.; Saxon, A.J.; Malte, C.A.; Baer, J.S.; Sloan, K.L. Divalproex Sodium in Alcohol Withdrawal: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Alcohol Clin. Exp. Res. 2001, 25, 1324–1329. [Google Scholar] [PubMed]
- Myrick, H.; Brady, K.T.; Malcolm, R. Divalproex in the Treatment of Alcohol Withdrawal. Am. J. Drug Alcohol Abus. 2000, 26, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Reoux, J.P.; Saxon, A.J.; Shen, D. Pharmacokinetic Profile of an Oral Loading Dose of Divalproex Sodium During Acute Alcohol Withdrawal. J. Clin. Psychopharmacol. 2006, 26, 105–107. [Google Scholar] [CrossRef]
- Hammer, B.A.; Brady, K.T. Valproate Treatment of Alcohol Withdrawal and Mania. Am. J. Psychiatry 1996, 153, 1232. [Google Scholar] [CrossRef]
- Roy-Byrne, P.P.; Ward, N.G.; Donnelly, P.J. Valproate in Anxiety and Withdrawal Syndromes. J. Clin. Psychiatry 1989, 50, 44–48. [Google Scholar]
- Foster, K.T.; Hicks, B.M.; Iacono, W.G.; McGue, M. Gender Differences in the Structure of Risk for Alcohol Use Disorder in Adolescence and Young Adulthood. Psychol. Med. 2015, 45, 3047–3058. [Google Scholar] [CrossRef]
- White, A. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. Curr. Rev. 2020, 40, 1. [Google Scholar] [CrossRef]
- Veerbeek, M.A.; Ten Have, M.; Van Dorsselaer, S.A.; Oude Voshaar, R.C.; Rhebergen, D.; Willemse, B.M. Differences in Alcohol Use Between Younger and Older People: Results from a General Population Study. Drug Alcohol Depend. 2019, 202, 18–23. [Google Scholar] [CrossRef]
- Kaplan, E.M.; DuPont, R.L. Benzodiazepines and Anxiety Disorders: A Review for the Practicing Physician. Curr. Med. Res. Opin. 2005, 21, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.R.; Korn, C.W.; Vunder, J.; Bantel, A. Effect of Valproate and Pregabalin on Human Anxiety-like Behaviour in a Randomised Controlled Trial. Transl. Psychiatry 2018, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, N.A.; Aliyev, Z.N. Valproate (Depakine-Chrono) in the Acute Treatment of Outpatients with Generalized Anxiety Disorder without Psychiatric Comorbidity: Randomized, Double-Blind Placebo-Controlled Study. Eur. Psychiatry 2008, 23, 109–114. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Sykora, K.; Schneiderman, J.; Naranjo, C.A.; Sellers, E.M. Assessment of Alcohol Withdrawal: The Revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar). Br. J. Addict. 1989, 84, 1353–1357. [Google Scholar] [CrossRef]
- Ashton, H. The Diagnosis and Management of Benzodiazepine Dependence. Curr. Opin. Psychiatry 2005, 18, 249–255. [Google Scholar] [CrossRef]
- Kan, C.C.; Hilberink, S.R.; Breteler, M.H.M. Determination of the Main Risk Factors for Benzodiazepine Dependence Using a Multivariate and Multidimensional Approach. Compr. Psychiatry 2004, 45, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.C.; McCurdy, L. A Double-Blind Comparison of the Efficacy and Safety of Lorazepam and Diazepam in the Treatment of the Acute Alcohol Withdrawal Syndrome. Clin. Ther. 1984, 6, 364–371. [Google Scholar]
- Gulati, P.; Chavan, B.S.; Sidana, A. Comparative Efficacy of Baclofen and Lorazepam in the Treatment of Alcohol Withdrawal Syndrome. Indian J. Psychiatry 2019, 61, 60–64. [Google Scholar] [PubMed] [PubMed Central]
- Malcolm, R.; Myrick, H.; Roberts, J.; Wang, W.; Anton, R.F.; Ballenger, J.C. The Effects of Carbamazepine and Lorazepam on Single versus Multiple Previous Alcohol Withdrawals in an Outpatient Randomized Trial. J. Gen. Intern. Med. 2002, 17, 349–355. [Google Scholar] [CrossRef]
- Collins-Yoder, A.; Lowell, J. Valproic Acid: Special Considerations and Targeted Monitoring. J. Neurosci. Nurs. 2017, 49, 56–61. [Google Scholar] [CrossRef]
- Olivola, M.; Civardi, S.; Damiani, S.; Cipriani, N.; Silva, A.; Donadeo, A.; Politi, P.; Brondino, N. Effectiveness and Safety of Intravenous Valproate in Agitation: A Systematic Review. Psychopharmacology 2022, 239, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Fontana, E.; Mandolini, G.M.; Delvecchio, G.; Bressi, C.; Soares, J.C.; Brambilla, P. Intravenous Valproate in the Treatment of Acute Manic Episode in Bipolar Disorder: A Review. J. Affect. Disord. 2020, 260, 738–743. [Google Scholar] [CrossRef] [PubMed]

| Study | Groups | Scale | Result |
|---|---|---|---|
| Lambie et al., 1980 [15] | Placebo (n = 27) vs. Valproate (n = 22) | 3 grades of symptoms:
| Faster amelioration of withdrawal symptoms with valproate |
| Longo et al., 2002 [15] | Benzodiazepine n = 7) vs. Divalproex Sodium (n = 5) 5 Days vs. Divalproex Sodium 5 Days + 6-Week Maintenance (n = 5) | CIWA-Ar | Greater reduction in CIWA-Ar in valproate groups; Abstinence better maintained in the 6-week valproate maintenance group |
| Reoux et al., 2001 [17] | Placebo (n = 18) vs. Valproate (n = 18) | CIWA-Ar | Greater reduction in withdrawal symptoms observed in the valproate group |
| Rosenthal et al., 1998 [16] | Phenobarbital (n = NR) vs. Valproate (n = NR) | MSSA; ASQ | No significant difference between the two groups in the improvement in withdrawal symptoms |
| Myrick et al., 2000 [18] | Divalproex Sodium + Lorazepam (n = 6) vs. Lorazepam (n = 5) | CIWA-Ar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardossi, S.; Cuomo, A.; Gualtieri, G.; Pinzi, M.; Piumini, G.; Fagiolini, A. Valproate-Enhanced Protocols for Alcohol Withdrawal Syndrome: A Brief Review and Retrospective Study of Efficacy and the Ability to Reduce Benzodiazepine Use. Pharmaceuticals 2025, 18, 855. https://doi.org/10.3390/ph18060855
Pardossi S, Cuomo A, Gualtieri G, Pinzi M, Piumini G, Fagiolini A. Valproate-Enhanced Protocols for Alcohol Withdrawal Syndrome: A Brief Review and Retrospective Study of Efficacy and the Ability to Reduce Benzodiazepine Use. Pharmaceuticals. 2025; 18(6):855. https://doi.org/10.3390/ph18060855
Chicago/Turabian StylePardossi, Simone, Alessandro Cuomo, Giacomo Gualtieri, Mario Pinzi, Giuditta Piumini, and Andrea Fagiolini. 2025. "Valproate-Enhanced Protocols for Alcohol Withdrawal Syndrome: A Brief Review and Retrospective Study of Efficacy and the Ability to Reduce Benzodiazepine Use" Pharmaceuticals 18, no. 6: 855. https://doi.org/10.3390/ph18060855
APA StylePardossi, S., Cuomo, A., Gualtieri, G., Pinzi, M., Piumini, G., & Fagiolini, A. (2025). Valproate-Enhanced Protocols for Alcohol Withdrawal Syndrome: A Brief Review and Retrospective Study of Efficacy and the Ability to Reduce Benzodiazepine Use. Pharmaceuticals, 18(6), 855. https://doi.org/10.3390/ph18060855







