Bevacizumab in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer: A Risk-Stratified Analysis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EOC | Epithelial ovarian cancer |
| PSROC | Platinum-sensitive recurrent ovarian cancer |
| VEGF | Vascular endothelial growth factor |
| PARP | Poly(ADP-ribose) polymerase |
| CRS | Cytoreductive surgery |
| HRD | Homologous recombination deficiency |
| FIGO | International Federation of Gynecology and Obstetrics |
| PFS | Progression-free survival |
| OS | Overall survival |
| PFI | Platinum-free interval |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Burges, A.; Schmalfeldt, B. Ovarian cancer: Diagnosis and treatment. Dtsch. Ärzteblatt Int. 2011, 108, 635–641. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, E.J.; Lee, M.; Chung, H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kim, H.S. Recurrence patterns after bevacizumab in platinum-sensitive, recurrent epithelial ovarian cancer. Int. J. Gynecol. Cancer 2020, 30, 1943–1950. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pelizzari, G.; Gerratana, L.; Bortot, L.; Lombardi, D.; Nicoloso, M.; Scalone, S.; Giorda, G.; Baldassarre, G.; Sorio, R.; et al. Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3805. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Z.; Luo, H.; Hu, X.; Zheng, L.; Zhu, X. The Benefits and Side Effects of Bevacizumab for the Treatment of Recurrent Ovarian Cancer. Curr. Drug Targets 2017, 18, 1125–1131. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Żak, K.; Satora, M.; Skrabalak, I.; Tarkowski, R.; Ostrowska-Leśko, M.; Bobiński, M. The Potential Influence of Residual or Recurrent Disease on Bevacizumab Treatment Efficacy in Ovarian Cancer: Current Evidence and Future Perspectives. Cancers 2024, 16, 1063. [Google Scholar] [CrossRef]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Java, J.J.; Cohn, D.E.; Burger, R.A. Risk factors for readmission in patients with ovarian, fallopian tube, and primary peritoneal carcinoma who are receiving front-line chemotherapy on a clinical trial (GOG 218): An NRG oncology/gynecologic oncology group study (ADS-1236). Gynecol. Oncol. 2015, 139, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, D.M.; Fagotti, A.; Scambia, G.; Weaver, A.L.; McGree, M.; Quagliozzi, L.; Langstraat, C.; Kumar, A.; Cliby, W. Validation of a risk-based algorithm to reduce poor operative outcomes after complex surgery for ovarian cancer. Int. J. Gynecol. Cancer 2023, 33, 83–88. [Google Scholar] [CrossRef]
- Rizzuto, I.; Stavraka, C.; Chatterjee, J.; Borley, J.; Hopkins, T.G.; Gabra, H.; Ghaem-Maghami, S.; Huson, L.; Blagden, S.P. Risk of Ovarian Cancer Relapse score: A prognostic algorithm to predict relapse following treatment for advanced ovarian cancer. Int. J. Gynecol. Cancer 2015, 25, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2015, 139, 10–16. [Google Scholar] [CrossRef]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, P.; Qu, X.; Liu, Y.; Zhang, J. Phase III trials of standard chemotherapy with or without bevacizumab for ovarian cancer: A meta-analysis. PLoS ONE 2013, 8, e81858. [Google Scholar] [CrossRef]
- Liu, S.; Kasherman, L.; Fazelzad, R.; Wang, L.; Bouchard-Fortier, G.; Lheureux, S.; Krzyzanowska, M.K. The use of bevacizumab in the modern era of targeted therapy for ovarian cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2021, 161, 601–612. [Google Scholar] [CrossRef]
- Rehman, O.U.; Fatima, E.; Imran, H.; Akram, U.; Ahmad, A.B.; Nadeem, Z.A.; Fatima, L.; Hussain, A.; Mabrouk, M.A.; Farooq, M.Z. Bevacizumab Combination Therapy Versus Standard Chemotherapy for Ovarian Cancer in Shorter and Longer Follow-Up Duration: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Oncol. 2024, 47, 399–408. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, J.H.; Noh, J.J.; Kim, S.H.; Kim, T.E.; Kim, K.; Park, J.Y.; Lim, M.C.; Lee, J.W.; Kim, J.W. Impact of bevacizumab and secondary cytoreductive surgery on survival outcomes in platinum-sensitive relapsed ovarian clear cell carcinoma: A multicenter study in Korea. Gynecol. Oncol. 2022, 166, 444–452. [Google Scholar] [CrossRef]
- Akers, S.N.; Riebandt, G.; Miller, A.; Groman, A.; Odunsi, K.; Lele, S. Bevacizumab for the treatment of recurrent ovarian cancer: A retrospective cohort study. Eur. J. Gynaecol. Oncol. 2013, 34, 113–119. [Google Scholar] [PubMed]
- Coleman, R.; Burger, R.; Brady, M.; Bookman, M.; Fowler, J.; Birrer, M.; Fleming, G.; Mannel, R.; Monk, B. Analysis of survivorship in high-risk patients on treated on GOG-218. Gynecol. Oncol. 2013, 130, e112–e113. [Google Scholar] [CrossRef]
- Bradley, W.; Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A. Maintenance olaparib for patients with newly diagnosed, advanced ovarian cancer and a BRCA mutation: 5-Year follow-up from SOLO1. Gynecol. Oncol. 2021, 162, S25–S26. [Google Scholar] [CrossRef]
- Liu, J.F.; Brady, M.F.; Matulonis, U.A.; Miller, A.; Kohn, E.C.; Swisher, E.M.; Tew, W.P.; Cloven, N.G.; Muller, C.; Bender, D. A phase III study comparing single-agent olaparib or the combination of cediranib and olaparib to standard platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer. J. Clin. Oncol. 2020, 38, 6003. [Google Scholar] [CrossRef]
- Pautier, P.; Harter, P.; Pisano, C.; Cropet, C.; Hernando Polo, S.; Berger, R.; Matsumoto, T.; Vergote, I.; Colombo, N.; Noettrup, T.J. Progression-free survival (PFS) and second PFS (PFS2) by disease stage in patients (pts) with homologous recombination deficiency (HRD)-positive newly diagnosed advanced ovarian cancer receiving bevacizumab (bev) with olaparib/placebo maintenance in the phase III PAOLA-1/ENGOT-ov25 trial. J. Clin. Oncol. 2021, 39, 5514. [Google Scholar]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Graybill, W.; Lorusso, D.; McCormick, C.C.; Freyer, G.; Backes, F.; Heitz, F.; Redondo, A.; et al. Progression-free survival and safety at 3.5 years of follow-up: Results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur. J. Cancer 2023, 189, 112908. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Ben-Baruch, N.E.; Werner, T.L. VELIA/GOG-3005: Integration of veliparib with front-line chemotherapy and maintenance in women with high-grade serous carcinoma of ovarian, fallopian tube, or primary peritoneal origin. Ann. Oncol. 2019, 30, v851–v934. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Chambers, L.M.; O’Malley, D.M.; Coleman, R.L.; Herzog, T.J. Is there a “low-risk” patient population in advanced epithelial ovarian cancer?: A critical analysis. Am. J. Obstet. Gynecol. 2022, 227, 728–734. [Google Scholar] [CrossRef]
- Baert, T.; Ferrero, A.; Sehouli, J.; O’Donnell, D.M.; González-Martín, A.; Joly, F.; van der Velden, J.; Blecharz, P.; Tan, D.S.P.; Querleu, D.; et al. The systemic treatment of recurrent ovarian cancer revisited. Ann. Oncol. 2021, 32, 710–725. [Google Scholar] [CrossRef]
- Winter, W.E., III; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P. Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, Cd007565. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Manning-Geist, B.L.; Hicks-Courant, K.; Gockley, A.A.; Clark, R.M.; Del Carmen, M.G.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Muto, M.G.; Worley, M.J., Jr. A novel classification of residual disease after interval debulking surgery for advanced-stage ovarian cancer to better distinguish oncologic outcome. Am. J. Obstet. Gynecol. 2019, 221, 326.e1–326.e7. [Google Scholar] [CrossRef] [PubMed]
- Wimberger, P.; Lehmann, N.; Kimmig, R.; Burges, A.; Meier, W.; Du Bois, A. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol. Oncol. 2007, 106, 69–74. [Google Scholar] [CrossRef]
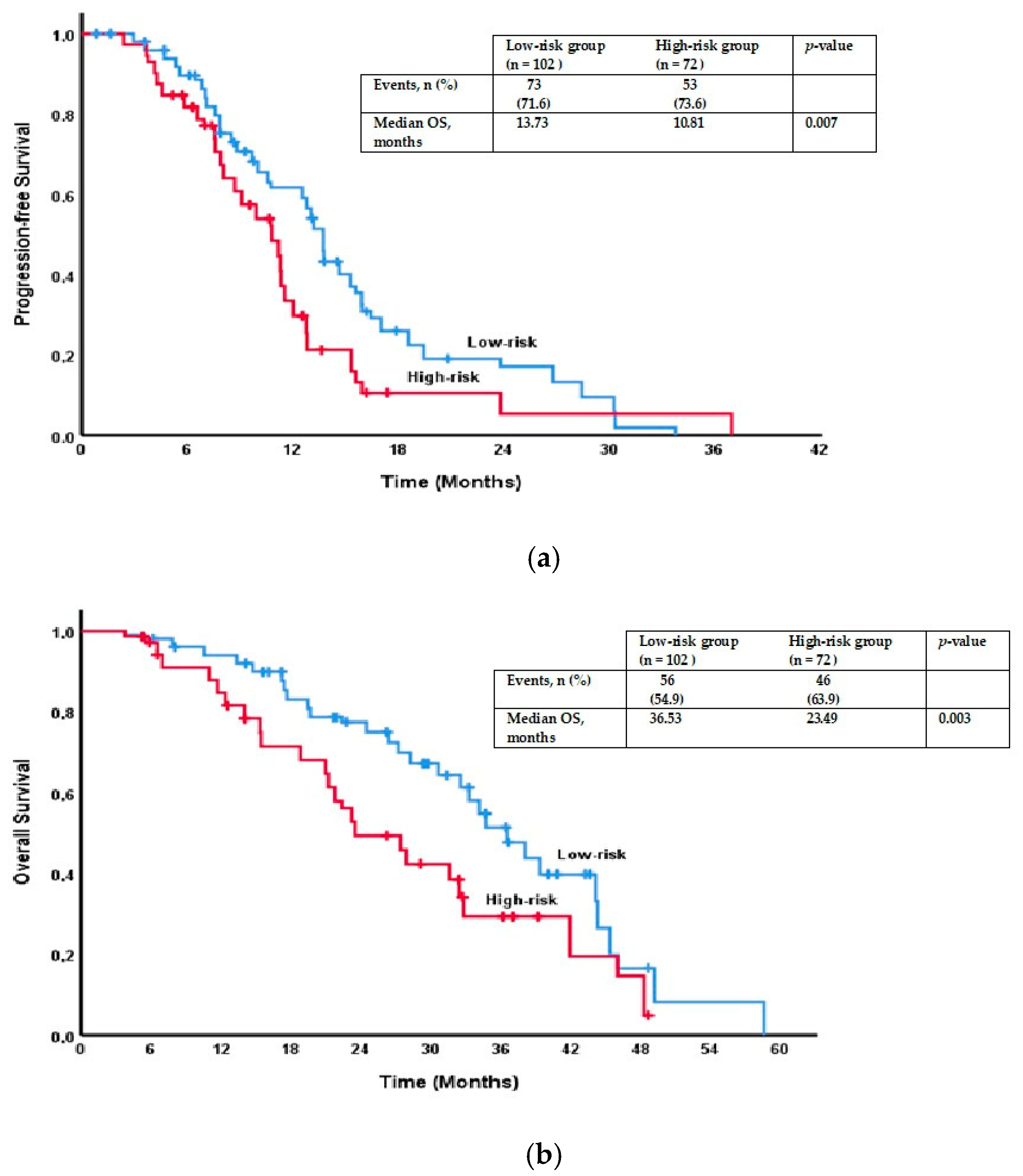
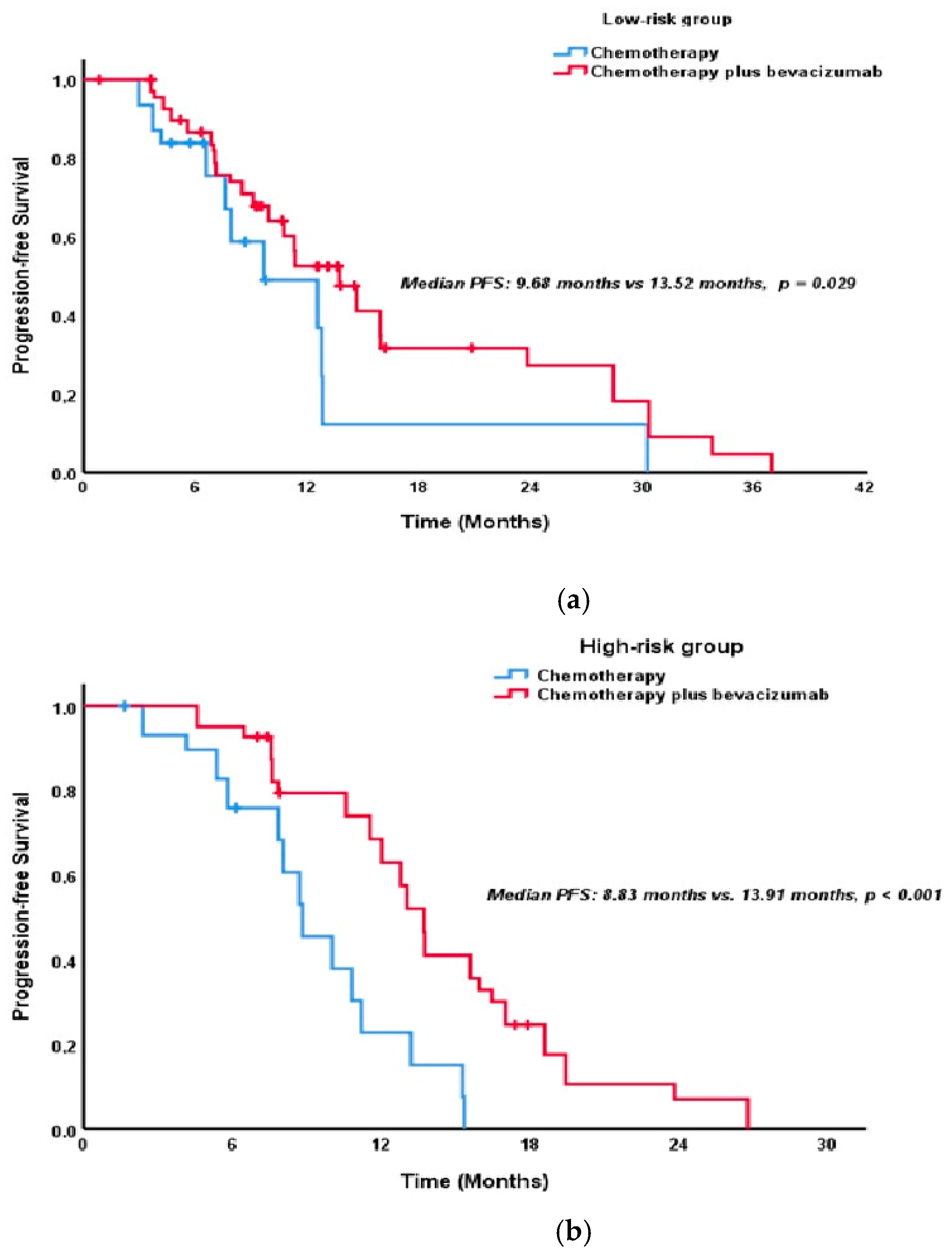
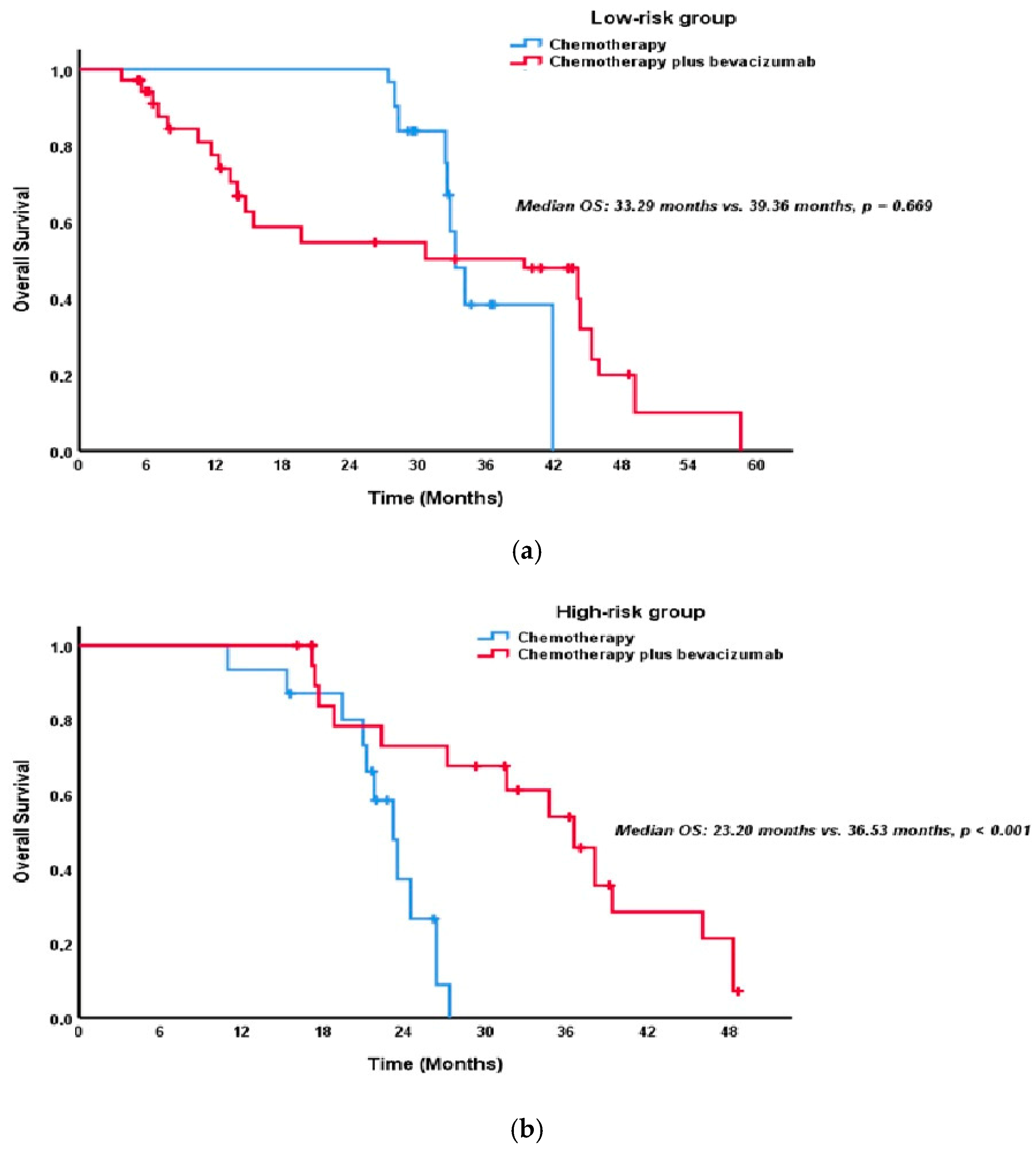
| Factor | Total n = 174 (%) | Low Risk n = 102 (58.6%) | High Risk n = 72 (41.4%) | p-Value |
|---|---|---|---|---|
| Age-year *–median | 0.130 | |||
| ≤58 | 94 (54.0%) | 60 (58.8%) | 34 (47.2%) | |
| >58 | 80 (46.0%) | 42 (41.2%) | 38 (52.8%) | |
| ECOG performance status * | <0.001 | |||
| 0–1 | 148 (85.1%) | 97 (95.1%) | 51 (70.8%) | |
| 2 | 26 (14.9%) | 5 (4.9%) | 21 (29.2%) | |
| gBRCAm | 0.946 | |||
| 1 or 2 mutants | 32 (18.4%) | 18 (17.6%) | 14 (19.4%) | |
| Wild | 45 (25.9%) | 27 (26.5%) | 18 (25.0%) | |
| Unknown | 97 (55.7%) | 57 (55.9%) | 40 (55.6%) | |
| FIGO Stage * | <0.001 | |||
| III | 134 (77.0%) | 102 (100.0%) | 32 (45.2%) | |
| IV | 40 (23.0%) | 0 (0.0%) | 40 (54.8%) | |
| Histology * | 0.136 | |||
| High-grade serous | 130 (74.7%) | 72 (70.6%) | 58 (80.6%) | |
| Other or unknown | 44 (25.3%) | 30 (29.4%) | 14 (19.4%) | |
| Ascites | 0.681 | |||
| Yes | 119 (68.4%) | 71 (69.6%) | 48 (66.7%) | |
| No | 55 (31.6%) | 31 (30.4%) | 24 (33.3%) | |
| Cytoreductive surgery * | <0.001 | |||
| Suboptimal | 43 (24.7%) | 0 (0.0%) | 43 (59.7%) | |
| Optimal | 108 (62.1%) | 102 (100.0%) | 6 (8.4%) | |
| Non-operable | 23 (13.2%) | 0 (0.0%) | 23 (31.9%) | |
| Location of recurrence | 0.916 | |||
| Local | 108 (62.1%) | 62 (60.8%) | 46 (63.9%) | |
| Distant | 48 (27.6%) | 29 (28.4%) | 19 (26.4%) | |
| Lymph node | 18 (10.3%) | 11 (10.8%) | 7 (9.7%) | |
| Second-line agents | 0.451 | |||
| Chemotherapy | 62 (35.6%) | 34 (33.3%) | 28 (38.9%) | |
| Chemotherapy Plus bevacizumab | 112 (64.4%) | 68 (66.7%) | 44 (61.1%) | |
| Maintenance bevacizumab therapy | ||||
| Yes | 48 (42.9%) | 33 (48.5%) | 15 (34.1%) | 0.132 |
| No | 64 (57.1%) | 35 (51.5%) | 29 (65.9) | |
| Platinum-free interval | 0.199 | |||
| 6–12 months | 89 (51.1%) | 48 (47.1%) | 41 (56.9%) | |
| >12 months | 85 (48.9%) | 54 (52.9%) | 31 (43.1%) | |
| Median CA-125 levels at diagnosis | 0.163 | |||
| ≤500 | 81 (46.6%) | 52 (51.0%) | 29 (40.3%) | |
| >500 | 93 (53.4%) | 50 (49.0%) | 43 (59.7%) | |
| Median CA-125 levels at recurrence | 0.742 | |||
| ≤100 | 92 (52.9%) | 55 (53.9%) | 37 (51.4%) | |
| >100 | 82 (47.1%) | 47 (46.1%) | 35 (48.6%) | |
| Factor | PFS Median (95% CI) | p-Value | OS Median (95% CI) | p-Value |
|---|---|---|---|---|
| Age median (range) 1 | 0.133 | 0.433 | ||
| ≤58 | 13.21 (11.46–14.96) | 34.73 (20.22–39.24) | ||
| >58 | 11.20 (9.98–12.43) | 30.65 (26.67–34.63) | ||
| FIGO Stage 1 | 0.386 | 0.986 | ||
| III | 12.03 (10.54–13.51) | 34.14 (29.63–38.65) | ||
| IV | 11.54 (8.88–14.20) | 31.58 (26.27–36.89) | ||
| Histology 1 | 0.147 | 0.776 | ||
| High-grade serous | 12.55 (11.40–13.70) | 32.81 (27.87–37.76) | ||
| Other or unknown | 9.68 (7.83–11.53) | 32.43 (29.21–35.65) | ||
| Ascites | 0.090 | 0.912 | ||
| No | 11.54 (9.83–13.25) | 32.43 (28.79–36.06) | ||
| Yes | 12.03 (5.42–18.63) | 34.73 (31.71–37.75) | ||
| Risk groups | 0.007 * | 0.003 * | ||
| Low risk | 13.73 (12.94–14.52) | 36.53 (32.59–40.46) | ||
| High risk | 10.81 (8.75–12.87) | 23.49 (18.41–28.58) | ||
| Cytoreductive surgery 1 | 0.056 | 0.013 * | ||
| Suboptimal | 11.34 (10.72–11.95) | 31.58 (24.43–38.72) | ||
| Optimal | 13.21 (12.31–14.10) | 36.53 (32.61–40.44) | ||
| Non-operable | 6.97 (5.43–8.51) | 18.83 (11.14–26.51) | ||
| Location of recurrence | 0.030 * | 0.011 * | ||
| Locoregional | 13.04 (12.16–13.93) | 34.73 (31.54–37.92) | ||
| Distant | 10.58 (9.34–11.82) | 31.58 (25.00–38.16) | ||
| Lymph node | 10.76 (6.68–14.84) | 21.20 (12.07–30.33) | ||
| Second-line treatment | <0.001 * | 0.178 | ||
| Chemotherapy | 9.68 (8.31–11.05) | 32.43 (27.98–36.88) | ||
| Chemotherapy Plus bevacizumab | 13.73 (12.30–15.17) | 36.53 (30.41–42.64) | ||
| Maintenance bevacizumab therapy | 0.114 | 0.914 | ||
| Yes | 15.93 (12.97–18.90) | 36.53 (26.83–46.23) | ||
| No | 12.03 (10.05–14.00) | 31.58 (16.80–46.36) | ||
| Platinum-free interval | 0.226 | 0.014 * | ||
| 6–12 months | 10.81 (9.67–11.95) | 27.92 (20.91–34.92) | ||
| >12 months | 12.81 (11.85–13.77) | 36.53 (30.22–42.83) | ||
| Median CA-125 levels at diagnosis | 0.769 | 0.867 | ||
| ≤500 | 12.77 (11.37–14.17) | 32.43 (28.00–36.85) | ||
| >500 | 11.54 (10.29–12.79) | 34.14 (29.88–38.40) | ||
| Median CA-125 levels at recurrence | 0.561 | 0.119 | ||
| ≤100 | 12.55 (9.76–15.34) | 39.26 (28.75–49.97) | ||
| >100 | 11.34 (10.11–12.56) | 32.43 (27.76–37.09) |
| PFS | OS | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Risk groups | ||||
| Low Risk | Ref | Ref | ||
| High Risk | 1.69 (1.16–2.46) | 0.007 * | 1.94 (1.15–3.26) | 0.013 * |
| Location of recurrence | ||||
| Locoregional | Ref | Ref | ||
| Distant | 1.58 (1.03–2.42) | 0.038 * | 1.50 (0.93–2.42) | 0.099 |
| Lymph node | 1.60 (0.86–2.98) | 0.138 | 2.12 (1.07–4.17) | 0.030 * |
| Second-line treatment | ||||
| Chemotherapy | Ref | - | ||
| Chemotherapy Plus bevacizumab | 0.52 (0.36–0.77) | 0.001 * | - | |
| Platinum-free interval | - | |||
| 6–12 months | - | Ref | ||
| >12 months | - | 0.69 (0.46–1.04) | 0.076 | |
| Cytoreductive surgery 1 | - | |||
| Suboptimal | - | Ref | ||
| Optimal | - | 1.20 (0.67–2.13) | 0.544 | |
| Non-operable | - | 1.97 (1.02–3.83) | 0.045 * | |
| Chemotherapy Plus Bevacizumab (n = 112) | Chemotherapy (n = 62) | p-Value | |
|---|---|---|---|
| Any adverse events | 104 (92.9%) | 52 (83.9%) | 0.062 |
| Adverse event of grade III or higher | 36 (32.1%) | 11 (17.7%) | 0.040 * |
| Adverse events that led to treatment discontinuation | 27 (24.1%) | 5 (8.1%) | 0.009 * |
| Neutropenia | 87 (77.7%) | 46 (74.2%) | 0.604 |
| Thrombocytopenia | 58 (51.8%) | 35 (56.5%) | 0.555 |
| Anemia | 77 (68.8%) | 48 (77.4%) | 0.223 |
| Hypertension | 34 (30.4%) | 9 (14.5%) | 0.020 * |
| Proteinuria | 22 (19.6%) | 2 (3.2%) | 0.003 * |
| Venous thromboembolic event | 12 (10.7%) | 3 (4.8%) | 0.186 |
| Decreased left ventricular ejection fraction | 9 (8.0%) | 3 (4.8%) | 0.425 |
| Fistula | 2 (1.8%) | 0 (0%) | 0.290 |
| Increased serum creatinine | 18 (16.1%) | 6 (9.7%) | 0.241 |
| Epistaxis | 40 (35.7%) | 7 (11.3%) | 0.001 * |
| Gastrointestinal perforation | 1 (0.9%) | 0 (0%) | 0.456 |
| PRES | 0 (0%) | 0 (0%) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öner, İ.; Karaçin, P. Bevacizumab in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer: A Risk-Stratified Analysis. Pharmaceuticals 2025, 18, 850. https://doi.org/10.3390/ph18060850
Öner İ, Karaçin P. Bevacizumab in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer: A Risk-Stratified Analysis. Pharmaceuticals. 2025; 18(6):850. https://doi.org/10.3390/ph18060850
Chicago/Turabian StyleÖner, İrem, and Pınar Karaçin. 2025. "Bevacizumab in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer: A Risk-Stratified Analysis" Pharmaceuticals 18, no. 6: 850. https://doi.org/10.3390/ph18060850
APA StyleÖner, İ., & Karaçin, P. (2025). Bevacizumab in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer: A Risk-Stratified Analysis. Pharmaceuticals, 18(6), 850. https://doi.org/10.3390/ph18060850







