The Effects of Inclisiran on the Subclinical Inflammatory Markers of Atherosclerotic Cardiovascular Disease in Patients at High Cardiovascular Risk
Abstract
1. Introduction
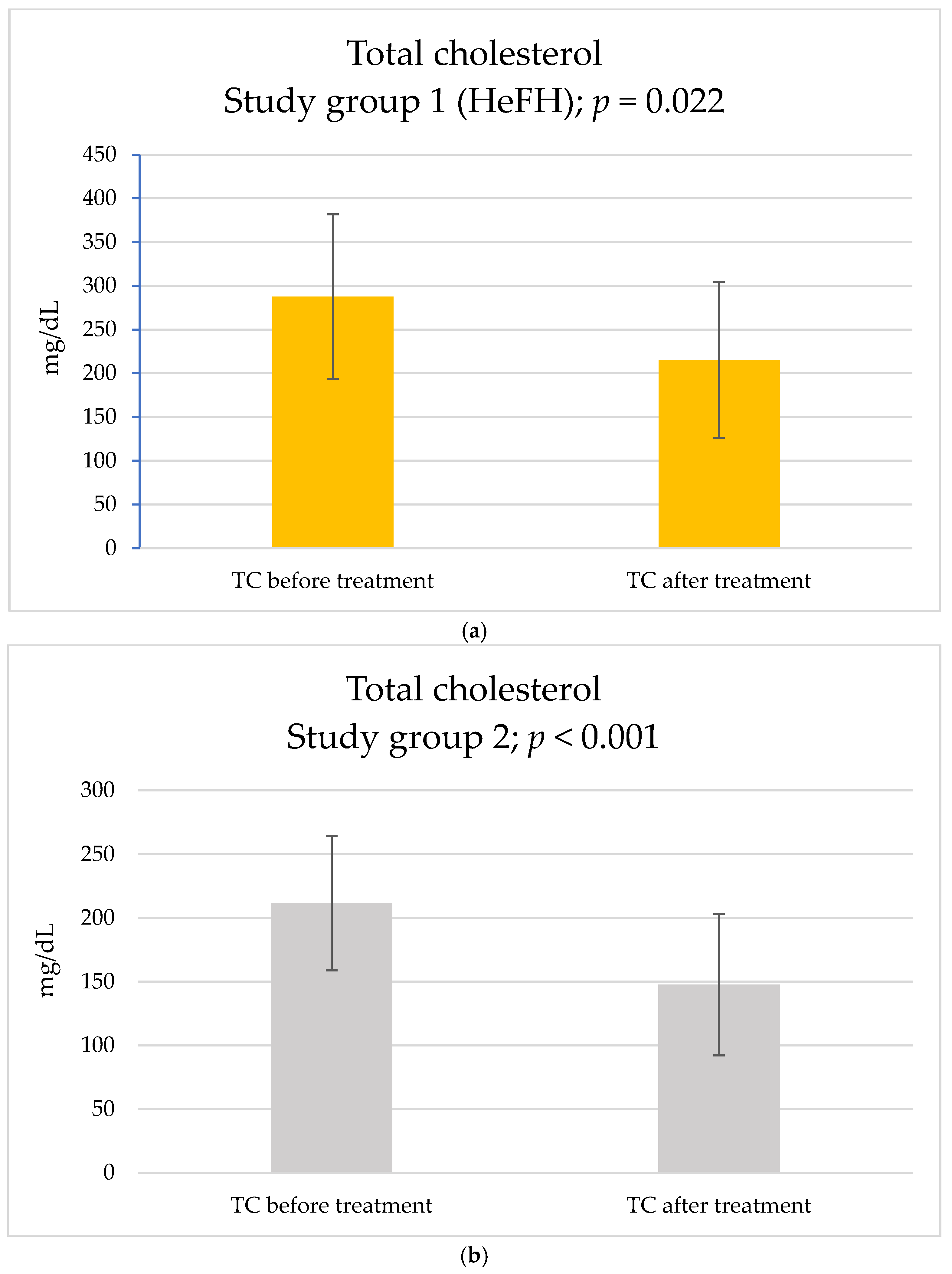
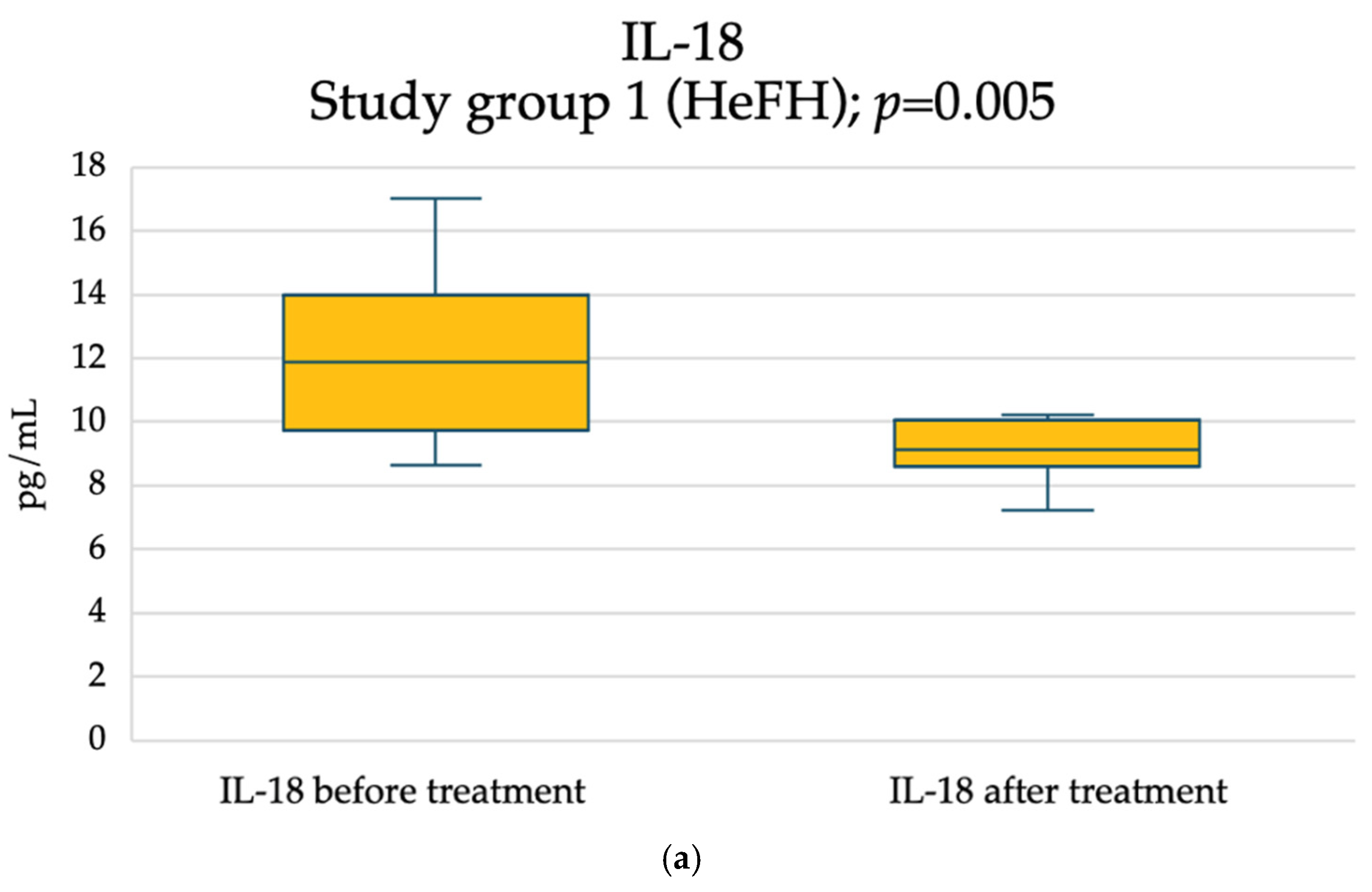
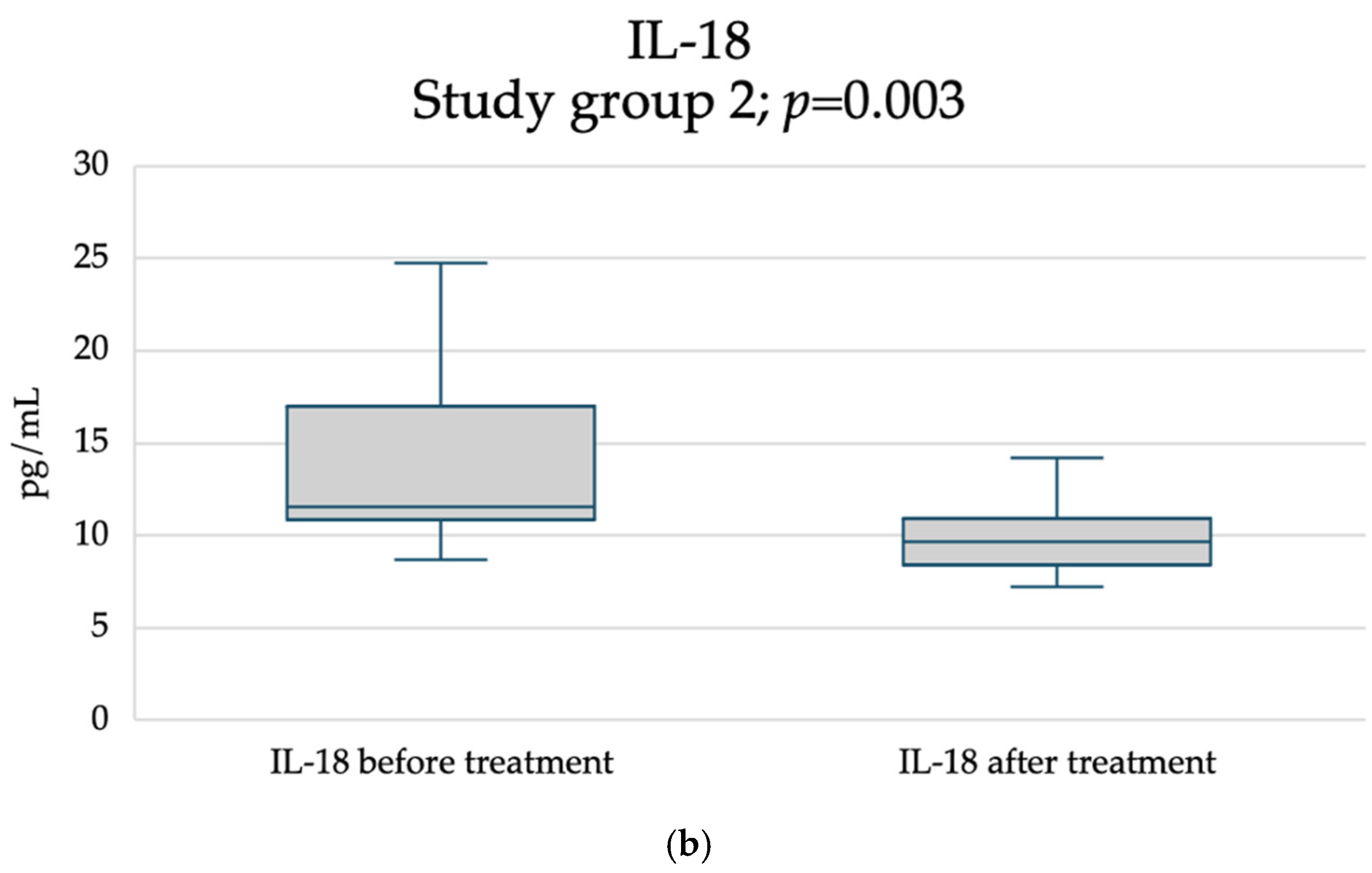
2. Results
3. Discussion
4. Materials and Methods
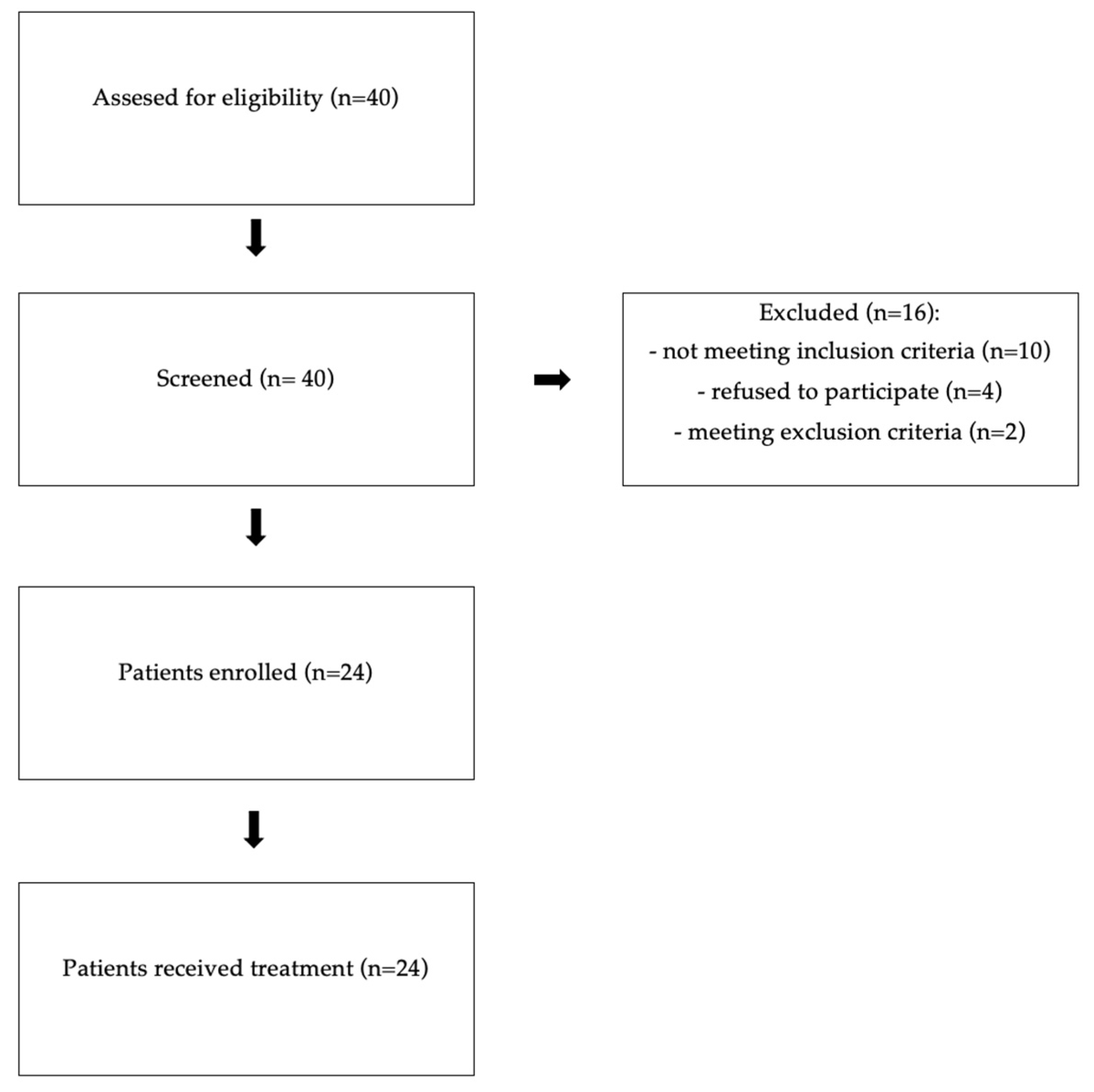
4.1. Study Design
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ApoB | Apolipoprotein B |
| ASCVD | Atherosclerotic cardiovascular disease |
| AST | Aspartate aminotransferase |
| CD40 | Cluster of differentiation 40 |
| CD40L | Cluster of differentiation 40 ligand |
| CK | Creatine kinase |
| CV | Cardiovascular |
| DLCN | Dutch Lipid Clinic Network |
| DM | Diabetes mellitus |
| eGFR | Estimated glomerular filtration rate |
| EMA | European Medicine Agency |
| ESC | European Society of Cardiology |
| FDA | American Food and Drug Administration |
| GalNAc | Triantenary N-acetylgalactosamine |
| HbA1c | Glycated hemoglobin |
| HeFH | Heterozygous familial hypercholesterolemia |
| HGB | Haemoglobin |
| HDLC | High-density lipoprotein cholesterol |
| HDL-c | High-density lipoprotein cholesterol concentration |
| hs-CRP | High sensitivity C-reactive protein |
| IFN-γ | Interferon gamma |
| IL-1 | Interleukin 1 |
| IL-18 | Interleukin 18 |
| INR | International normalized ratio |
| LDL-C | Low-density lipoprotein cholesterol |
| LDL-c | Low-density lipoprotein cholesterol concentration |
| LDLRs | Receptors for LDL-C |
| M-W | Mann–Whitney test |
| NK | Natural killer cell |
| NT-proBNP | N-terminal pro B-type natriuretic peptide |
| NYHA | New York Heart Association |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin 9 |
| PCSK9 mAbs | Monoclonal antibodies against PCSK9 |
| PLT | Platelet count |
| PTX3 | Pentraxin 3 |
| RBC | Red blood cell count |
| RCTs | Randomized controlled trials |
| ROS | Reactive oxygen species |
| sCD40L | Soluble CD40L |
| siRNA | Small-interfering RNA |
| TC | Total cholesterol |
| TG | Triglycerides |
| TGS-14 | Tumor necrosis factor—stimulated gene 14 |
| TNF | Tumor necrosis factor |
| TSH | Thyroid-stimulating hormone |
| WBC | White blood cell count |
References
- Pirillo, A.; Norata, G.D. The Burden of Hypercholesterolemia and Ischemic Heart Disease in an Ageing World. Pharmacol. Res. 2023, 193, 106814. [Google Scholar] [CrossRef]
- Duncan, M.S.; Vasan, R.S.; Xanthakis, V. Trajectories of Blood Lipid Concentrations Over the Adult Life Course and Risk of Cardiovascular Disease and All-Cause Mortality: Observations from the Framingham Study over 35 Years. J. Am. Heart Assoc. 2019, 8, e011433. [Google Scholar] [CrossRef]
- Domanski, M.J.; Tian, X.; Wu, C.O.; Reis, J.P.; Dey, A.K.; Gu, Y.; Zhao, L.; Bae, S.; Liu, K.; Hasan, A.A.; et al. Time Course of LDL Cholesterol Exposure and Cardiovascular Disease Event Risk. J. Am. Coll. Cardiol. 2020, 76, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Bouhairie, V.E.; Goldberg, A.C. Familial Hypercholesterolemia. Cardiol. Clin. 2015, 33, 169–179. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.P.; Hosseini Dehkordi, S.H.; Moriarty, P.M.; Duell, P.B. Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia. J. Am. Heart Assoc. 2019, 8, e013225. [Google Scholar] [CrossRef]
- Bahrami, A.; Liberale, L.; Reiner, Ž.; Carbone, F.; Montecucco, F.; Sahebkar, A. Inflammatory Biomarkers for Cardiovascular Risk Stratification in Familial Hypercholesterolemia. In Reviews of Physiology, Biochemistry and Pharmacology; Pedersen, S.H.F., Ed.; Reviews of Physiology, Biochemistry and Pharmacology; Springer International Publishing: Cham, Switzerland, 2020; Volume 177, pp. 25–52. ISBN 978-3-030-61494-2. [Google Scholar]
- Real, J.T.; Martínez-Hervás, S.; García-García, A.B.; Civera, M.; Pallardó, F.V.; Ascaso, J.F.; Viña, J.R.; Chaves, F.J.; Carmena, R. Circulating Mononuclear Cells Nuclear Factor-kappa B Activity, Plasma Xanthine Oxidase, and Low Grade Inflammatory Markers in Adult Patients with Familial Hypercholesterolaemia. Eur. J. Clin. Investig. 2010, 40, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Carbone, F.; Liberale, L.; Sahebkar, A. Challenges in Reducing Atherosclerotic Inflammation in Patients with Familial Hypercholesterolemia. Eur. J. Prev. Cardiol. 2020, 27, 2099–2101. [Google Scholar] [CrossRef]
- Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J. Familial Hypercholesterolemias: Prevalence, Genetics, Diagnosis and Screening Recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S9–S17. [Google Scholar] [CrossRef]
- Mhaimeed, O.; Burney, Z.A.; Schott, S.L.; Kohli, P.; Marvel, F.A.; Martin, S.S. The Importance of LDL-C Lowering in Atherosclerotic Cardiovascular Disease Prevention: Lower for Longer Is Better. Am. J. Prev. Cardiol. 2024, 18, 100649. [Google Scholar] [CrossRef]
- Duell, P.B.; Gidding, S.S.; Andersen, R.L.; Knickelbine, T.; Anderson, L.; Gianos, E.; Shrader, P.; Kindt, I.; O’Brien, E.C.; McCann, D.; et al. Longitudinal Low Density Lipoprotein Cholesterol Goal Achievement and Cardiovascular Outcomes among Adult Patients with Familial Hypercholesterolemia: The CASCADE FH Registry. Atherosclerosis 2019, 289, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Pijlman, A.H.; Huijgen, R.; Verhagen, S.N.; Imholz, B.P.M.; Liem, A.H.; Kastelein, J.J.P.; Abbink, E.J.; Stalenhoef, A.F.H.; Visseren, F.L.J. Evaluation of Cholesterol Lowering Treatment of Patients with Familial Hypercholesterolemia: A Large Cross-Sectional Study in The Netherlands. Atherosclerosis 2010, 209, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Li, J.; Lei, X.; Li, Z.; Yang, X. Effectiveness and Safety of Inclisiran in Hyperlipidemia Treatment: An Overview of Systematic Reviews. Medicine 2023, 102, e32728. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Wołowiec, Ł.; Osiak, J.; Wołowiec, A.; Wijata, A.; Grześk, E.; Kozakiewicz, M.; Banach, J.; Nowaczyk, A.; Nowaczyk, J.; Grześk, G. Inclisiran-Safety and Effectiveness of Small Interfering RNA in Inhibition of PCSK-9. Pharmaceutics 2023, 15, 323. [Google Scholar] [CrossRef]
- Raschi, E.; Casula, M.; Cicero, A.F.G.; Corsini, A.; Borghi, C.; Catapano, A. Beyond Statins: New Pharmacological Targets to Decrease LDL-Cholesterol and Cardiovascular Events. Pharmacol. Ther. 2023, 250, 108507. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and Safety of Cholesterol-Lowering Treatment: Prospective Meta-Analysis of Data from 90056 Participants in 14 Randomised Trials of Statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef]
- Gunta, S.P.; O’Keefe, J.H.; O’Keefe, E.L.; Lavie, C.J. PCSK9 Inhibitor, Ezetimibe, and Bempedoic Acid: Evidence-Based Therapies for Statin-Intolerant Patients. Prog. Cardiovasc. Dis. 2023, 79, 12–18. [Google Scholar] [CrossRef]
- German, C.A.; Liao, J.K. Understanding the Molecular Mechanisms of Statin Pleiotropic Effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Roy, P.; Orecchioni, M.; Ley, K. How the Immune System Shapes Atherosclerosis: Roles of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Qiu, L.; Xu, R.; Wang, S.; Li, S.; Sheng, H.; Wu, J.; Qu, Y. Honokiol Ameliorates Endothelial Dysfunction through Suppression of PTX3 Expression, a Key Mediator of IKK/IκB/NF-κB, in Atherosclerotic Cell Model. Exp. Mol. Med. 2015, 47, e171. [Google Scholar] [CrossRef] [PubMed]
- Zlibut, A.; Bocsan, I.C.; Agoston-Coldea, L. Pentraxin-3 and Endothelial Dysfunction. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 91, pp. 163–179. ISBN 978-0-12-817471-5. [Google Scholar]
- Zirlik, A.; Abdullah, S.M.; Gerdes, N.; MacFarlane, L.; Schönbeck, U.; Khera, A.; McGuire, D.K.; Vega, G.L.; Grundy, S.; Libby, P.; et al. Interleukin-18, the Metabolic Syndrome, and Subclinical Atherosclerosis: Results from the Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2043–2049. [Google Scholar] [CrossRef]
- Gissler, M.C.; Scherrer, P.; Anto-Michel, N.; Pennig, J.; Hoppe, N.; Füner, L.; Härdtner, C.; Stachon, P.; Li, X.; Mitre, L.S.; et al. Deficiency of Endothelial CD40 Induces a Stable Plaque Phenotype and Limits Inflammatory Cell Recruitment to Atherosclerotic Lesions in Mice. Thromb. Haemost. 2021, 121, 1530–1540. [Google Scholar] [CrossRef]
- Thim, T.; Hagensen, M.K.; Bentzon, J.F.; Falk, E. From Vulnerable Plaque to Atherothrombosis. J. Intern. Med. 2008, 263, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Libby, P. CD40 Signaling and Plaque Instability. Circ. Res. 2001, 89, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ji, W.; Zhang, Y.; Zhu, W.; Sun, H.; Sun, Y. Risk Factors for Progression to Type 2 Diabetes in Prediabetes: A Systematic Review and Meta-Analysis. BMC Public Health 2025, 25, 1220. [Google Scholar] [CrossRef]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Scott Wright, R.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-Term Efficacy and Safety of Inclisiran in Patients with High Cardiovascular Risk and Elevated LDL Cholesterol (ORION-3): Results from the 4-Year Open-Label Extension of the ORION-1 Trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Lei, W.; Shen, M.; Tang, J.; Xu, X.; Yang, Y.; Zhang, H. Pentraxin 3: A Promising Therapeutic Target for Cardiovascular Diseases. Ageing Res. Rev. 2024, 93, 102163. [Google Scholar] [CrossRef]
- Rolph, M.S.; Zimmer, S.; Bottazzi, B.; Garlanda, C.; Mantovani, A.; Hansson, G.K. Production of the Long Pentraxin PTX3 in Advanced Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 5. [Google Scholar] [CrossRef]
- Dejanović, V.V.; Stevuljević, J.K.; Vukašinović, A.; Miljković, M.; Kafedzic, S.; Zdravković, M.; Ilić, I.; Hinić, S.; Cerović, M.; Stefanović, M.; et al. Oxidative Stress and Inflammatory Markers PTX3, CypA, and HB-EGF: How Are They Linked in Patients with STEMI? Angiology 2020, 71, 713–720. [Google Scholar] [CrossRef]
- Kamarullah, W.; Nurcahyani; Multazam, R.B.; Josephine, C.M. Pentraxin 3 Concentration Is Associated with Poor Outcomes in Patients with Coronary Artery Disease: A Systematic Review and Dose–Response Meta-Analysis. Acta Cardiol. 2022, 77, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, S.; Winkels, H.; Kelm, M.; Gerdes, N. IL-1 Family Cytokines in Cardiovascular Disease. Cytokine 2019, 122, 154215. [Google Scholar] [CrossRef]
- Bahrami, A.; Sathyapalan, T.; Sahebkar, A. The Role of Interleukin-18 in the Development and Progression of Atherosclerosis. Curr. Med. Chem. 2021, 28, 1757–1774. [Google Scholar] [CrossRef]
- Haybar, H.; Bandar, B.; Torfi, E.; Mohebbi, A.; Saki, N. Cytokines and Their Role in Cardiovascular Diseases. Cytokine 2023, 169, 156261. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Tiret, L.; Bickel, C.; Peetz, D.; Cambien, F.; Meyer, J.; Rupprecht, H.J. Interleukin-18 Is a Strong Predictor of Cardiovascular Death in Stable and Unstable Angina. Circulation 2002, 106, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Kosowski, M.; Hachula, M.; Okopien, B. Impact of PCSK9 Inhibition on Proinflammatory Cytokines and Matrix Metalloproteinases Release in Patients with Mixed Hyperlipidemia and Vulnerable Atherosclerotic Plaque. Pharmaceuticals 2022, 15, 802. [Google Scholar] [CrossRef]
- Tian, S.; Wang, Y.; Wan, J.; Yang, M.; Fu, Z. Co-Stimulators CD40-CD40L, a Potential Immune-Therapy Target for Atherosclerosis: A Review. Medicine 2024, 103, e37718. [Google Scholar] [CrossRef]
- Weber, C.; Habenicht, A.J.R.; Von Hundelshausen, P. Novel Mechanisms and Therapeutic Targets in Atherosclerosis: Inflammation and Beyond. Eur. Heart J. 2023, 44, 2672–2681. [Google Scholar] [CrossRef]
- Lampka, M.; Olszewska-Słonina, D.; Hołyńska-Iwan, I.; Grąbczewska, Z.; Obońska, K.; Cwynar, A.; Stępowska, J.; Szewczyk-Golec, K. Effect of Low High-Density Lipoprotein Level on Endothelial Activation and Prothrombotic Processes in Coronary Artery Disease—A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 8637. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.-Q.; Zhao, S.-P.; Li, Y.-F.; Li, J.; Zhou, H.-N. Elevated Soluble CD40 Ligand Is Related to the Endothelial Adhesion Molecules in Patients with Acute Coronary Syndrome. Clin. Chim. Acta 2002, 319, 19–26. [Google Scholar] [CrossRef]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 Ligand System. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef]
- Ohbayashi, H.; Miyazawa, C.; Miyamoto, K.; Sagara, M.; Yamashita, T.; Onda, R. Pitavastatin Improves Plasma Pentraxin 3 and Arterial Stiffness in Atherosclerotic Patients with Hypercholesterolemia. J. Atheroscler. Thromb. 2009, 16, 490–500. [Google Scholar] [CrossRef]
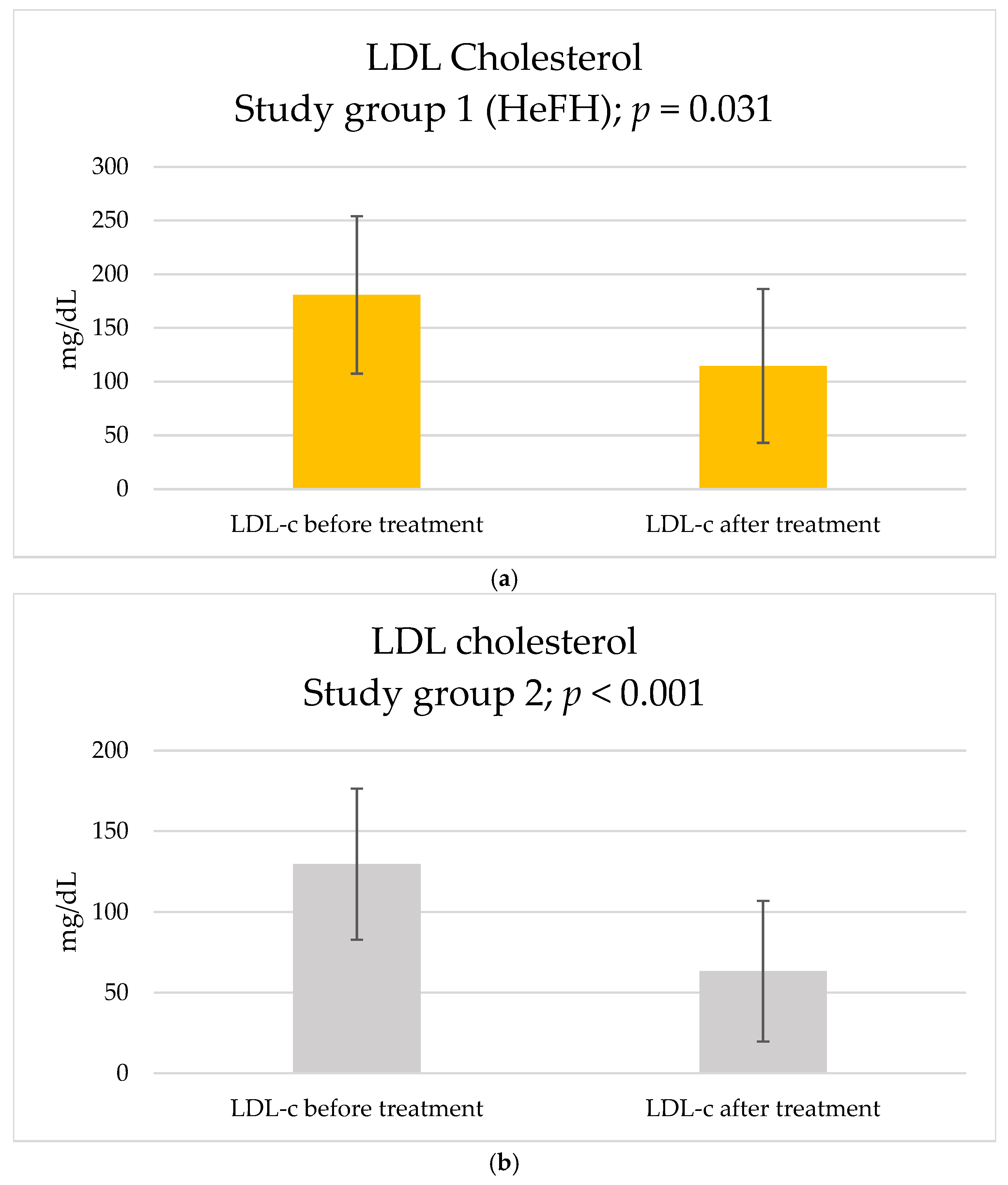
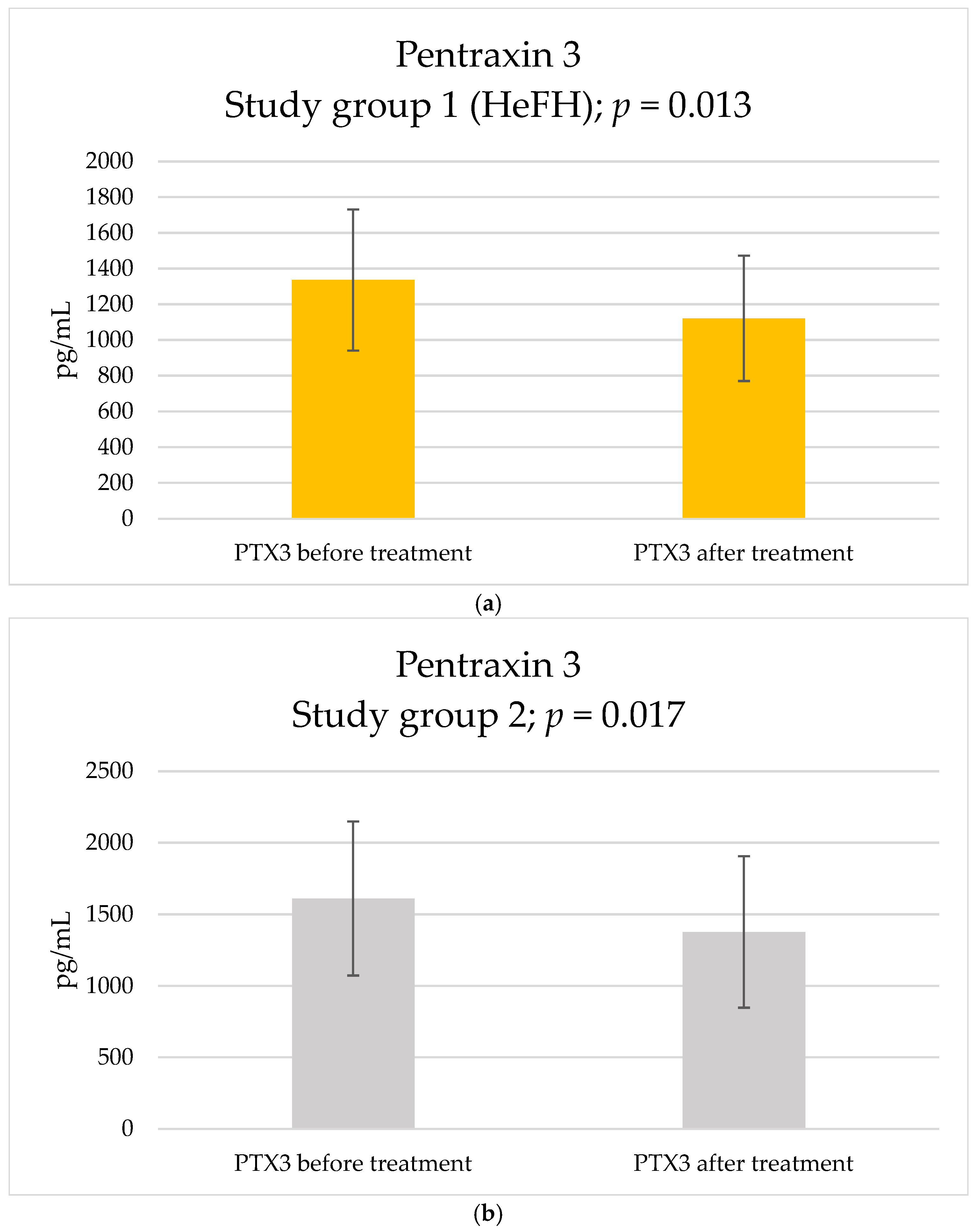
| Study Group 1 (HeFH); n = 10 | Study Group 2; n = 14 | Statistical Test | p Value | |
|---|---|---|---|---|
| Age, years | 54 ± 10 | 63 ± 10 | Welch’s t-test | 0.032 |
| WBC, K/μL | 5.87 ± 1.39 | 6.4 ± 1.06 | 0.333 | |
| RBC, M/μL | 4.57 ± 0.43 | 4.78 ± 0.56 | 0.333 | |
| HGB, g/dL | 13.73 ± 1.48 | 14.69 ± 1.37 | 0.126 | |
| PLT, K/μL | 247.6 ± 68.89 | 247.92 ± 42.38 | 0.99 | |
| TC, mg/dL | 287.6 ± 94.1 | 211.7 ± 52.7 | 0.038 | |
| LDL-c, mg/dL | 180.8 ± 73.3 | 129.6 ± 46.8 | 0.072 | |
| HDL-c, mg/dL | 57.5 (51.2–121.75) | 47.6 (40.95–59.63) | M-W | 0.064 |
| TG, mg/dL | 117.3 ± 50.8 | 144.9 ± 50.8 | Welch’s t-test | 0.206 |
| Total bilirubin, mg/dL | 0.46 ± 0.18 | 0.71 ± 0.3 | 0.043 | |
| ALT, U/L | 26 ± 13.8 | 33 ± 16.9 | 0.28 | |
| AST, U/L | 24.7 (23.1–26.7) | 26 (19–31.1) | M-W | 0.886 |
| INR | 0.92 (0.87–1) | 0.92 (0.88–1.02) | 0.761 | |
| Creatinine, mg/dL | 0.67 (0.64–0.8) | 0.86 (0.76–0.97) | 0.053 | |
| CK, U/L | 152.5 (128.5–183.3) | 122.5 (66.5–166.3) | 0.187 | |
| Glucose, mg/dL | 93.9 (89–95.3) | 102 (98.5–106) | 0.009 | |
| HbA1c, % | 5.58 (5.18–5.7) | 5.95 (5.61–6.06) | 0.053 | |
| TSH, uIU/mL | 1.76 (1.41–2.88) | 1.54 (0.81–2.88) | 0.656 | |
| NT-proBNP, pg/mL | 95.35 (35.15–154.75) | 81 (39.48–183) | 0.721 | |
| PTX3, pg/mL | 1336.33 ± 395.15 | 1610.76 ± 537.78 | Welch’s t-test | 0.164 |
| IL-18, pg/mL | 11.89 (9.72–13.98) | 11.58 (10.87–16.97) | M-W | 0.558 |
| sCD40L, ng/mL | 4.37 ± 2.56 | 3.79 ± 2.08 | Welch’s t-test | 0.563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maligłówka, M.; Dec, A.; Bułdak, Ł.; Okopień, B. The Effects of Inclisiran on the Subclinical Inflammatory Markers of Atherosclerotic Cardiovascular Disease in Patients at High Cardiovascular Risk. Pharmaceuticals 2025, 18, 832. https://doi.org/10.3390/ph18060832
Maligłówka M, Dec A, Bułdak Ł, Okopień B. The Effects of Inclisiran on the Subclinical Inflammatory Markers of Atherosclerotic Cardiovascular Disease in Patients at High Cardiovascular Risk. Pharmaceuticals. 2025; 18(6):832. https://doi.org/10.3390/ph18060832
Chicago/Turabian StyleMaligłówka, Mateusz, Adrianna Dec, Łukasz Bułdak, and Bogusław Okopień. 2025. "The Effects of Inclisiran on the Subclinical Inflammatory Markers of Atherosclerotic Cardiovascular Disease in Patients at High Cardiovascular Risk" Pharmaceuticals 18, no. 6: 832. https://doi.org/10.3390/ph18060832
APA StyleMaligłówka, M., Dec, A., Bułdak, Ł., & Okopień, B. (2025). The Effects of Inclisiran on the Subclinical Inflammatory Markers of Atherosclerotic Cardiovascular Disease in Patients at High Cardiovascular Risk. Pharmaceuticals, 18(6), 832. https://doi.org/10.3390/ph18060832






