Evaluating Guideline Alignment by Analyzing Patient Profiles of Elderly People with Type 2 Diabetes and Chronic Kidney Disease Treated or Not with SGLT2 Inhibitors
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Statistical Analysis and Ethical Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Bonner, R.; Albajrami, O.; Hudspeth, J.; Upadhyay, A. Diabetic Kidney Disease. Prim. Care 2020, 47, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Okada, K.; Yanai, M.; Takahashi, S. Aging and chronic kidney disease. Kidney Blood Press. Res. 2013, 38, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Migdalis, I.N.; Papanas, N.; Raptis, A.E.; Ioannidis, I.M.; Sotiropoulos, A.E.; Dimitriadis, G.D. Hellenic Diabetic Nephropathy Study (HDNS) Group. The prevalence of diabetic chronic kidney disease in adult Greek subjects with type 2 diabetes mellitus: A series from hospital-based diabetes clinics. Diabetes Res. Clin. Pract. 2020, 166, 108243. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Neuen, B.L.; Jun, M.; Ohkuma, T.; Neal, B.; Jardine, M.J.; Heerspink, H.L.; Wong, M.G.; Ninomiya, T.; Wada, T.; et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S181–S206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, J.I.; Xu, Y.; Chang, A.R.; Carrero, J.J.; Flaherty, C.M.; Mukhopadhyay, A.; Inker, L.A.; Blecker, S.B.; Horwitz, L.I.; Grams, M.E. Prescription Patterns for Sodium-Glucose Cotransporter 2 Inhibitors in U.S. Health Systems. J. Am. Coll. Cardiol. 2024, 84, 683–693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milder, T.Y.; Stocker, S.L.; Baysari, M.; Day, R.O.; Greenfield, J.R. Prescribing of SGLT2 inhibitors in primary care: A qualitative study of General Practitioners and Endocrinologists. Diabetes Res. Clin. Pract. 2021, 180, 109036. [Google Scholar] [CrossRef] [PubMed]
- Reach, G.; Pechtner, V.; Gentilella, R.; Corcos, A.; Ceriello, A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017, 43, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Koufakis, T.; Doumas, M.N.; Bargiota, A.; Kotsa, K.; Maltese, G. Sodium-glucose cotransporter 2 inhibitors in frail, older people with type 2 diabetes and heart failure: Do we have enough evidence to confidently support the use? Expert. Rev. Clin. Pharmacol. 2023, 16, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.S.; Baggesen, L.M.; Lajer, M.; Nurkanovic, L.; Ustyugova, A.; Sørensen, H.T.; Thomsen, R.W. Changes in SGLT2i and GLP-1RA real-world initiator profiles following cardiovascular outcome trials: A Danish nationwide population-based study. PLoS ONE 2020, 15, e0229621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCoy, R.G.; Dykhoff, H.J.; Sangaralingham, L.; Ross, J.S.; Karaca-Mandic, P.; Montori, V.M.; Shah, N.D. Adoption of New Glucose-Lowering Medications in the U.S.-The Case of SGLT2 Inhibitors: Nationwide Cohort Study. Diabetes Technol. Ther. 2019, 21, 702–712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S239–S251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasti, E.C.; Basina, M.; Calma, J.; Maron, D.J.; Rodriguez, F.; Sandhu, A.T. Disparities in adoption of new diabetic therapies with cardiovascular benefits. Diabetes Res. Clin. Pract. 2023, 196, 110233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hösli, P.S.; Renström, F.; Laimer, M.; Cavelti-Weder, C.; Gastaldi, G.; Lehmann, R.; Brändle, M. Assessing the use of sodium-glucose cotransporter 2 inhibitor in patients with type 2 diabetes mellitus and chronic kidney disease in tertiary care: A SwissDiab Study. BMJ Open Diabetes Res. Care 2024, 12, e004108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meraz-Muñoz, A.Y.; Weinstein, J.; Wald, R. eGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney360 2021, 2, 1042–1047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, B.; Yen, C.L.; Wu, C.Y.; Tsai, C.Y.; Chen, J.J.; Hsiao, C.C.; Chen, Y.C.; Hsieh, I.C.; Yang, H.Y. SGLT2 inhibitors reduce the risk of renal failure in CKD stage 5 patients with Type 2 DM. Sci. Rep. 2025, 15, 5872, Erratum in Sci. Rep. 2025, 15, 12440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgianos, P.I.; Vaios, V.; Koufakis, T.; Liakopoulos, V. Slowing the Progression of Chronic Kidney Disease in Patients with Type 2 Diabetes Using Four Pillars of Therapy: The Time to Act is Now. Drugs 2024, 84, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Mosenzon, O.; Raz, I.; Wiviott, S.D.; Schechter, M.; Goodrich, E.L.; Yanuv, I.; Rozenberg, A.; Murphy, S.A.; Zelniker, T.A.; Langkilde, A.M.; et al. Dapagliflozin and Prevention of Kidney Disease Among Patients With Type 2 Diabetes: Post Hoc Analyses From the DECLARE-TIMI 58 Trial. Diabetes Care 2022, 45, 2350–2359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koh, S.W.C.; Lai, M.Y.; Leong, C.K.; Lam, J.; Chew, H.S.J.; Ngoh, C.L.Y. Primary Care Physicians’ Perspective on SGLT2 Inhibitors for Chronic Kidney Disease. Kidney Med. 2025, 7, 101002. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Li, S.; Wang, Y.; Qin, X.; Deng, K.; Liu, Y.; Zou, K.; Sun, X. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2020, 22, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Butterly, E.; Wei, L.; Wightman, H.; Almazam, S.A.M.; Alsallumi, K.; Crowther, J.; McChrystal, R.; Rennison, H.; Hughes, K.; et al. Age and Sex Differences in Efficacy of Treatments for Type 2 Diabetes: A Network Meta-Analysis. JAMA 2025, 333, 1062–1073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lunati, M.E.; Cimino, V.; Gandolfi, A.; Trevisan, M.; Montefusco, L.; Pastore, I.; Pace, C.; Betella, N.; Favacchio, G.; Bulgheroni, M.; et al. SGLT2-inhibitors are effective and safe in the elderly: The SOLD study. Pharmacol. Res. 2022, 183, 106396. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.S.; Stein, D.; Matza, L.S.; Jordan, J.; Yu, R.; Norrbacka, K.; Hassan, S.W.; García-Pérez, L.E. Timing of GLP-1 Receptor Agonist Initiation for Treatment of Type 2 Diabetes in the UK. Drugs RD 2019, 19, 213–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, M.; Choi, C.Y.; Choi, Y.J.; Rhie, S.J. Clinical outcomes of renin angiotensin system inhibitor-based dual antihypertensive regimens in chronic kidney disease: A network meta-analysis. Sci. Rep. 2023, 13, 5727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawai, K.; Ishii, M.; Kokado, Y.; Horikawa, T.; Hoshino, J. Outcomes of Early Versus Delayed Anemia Treatment in Nondialysis-Dependent CKD. Kidney Int. Rep. 2024, 9, 2056–2066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cases, A.; Cigarrán, S.; Luis Górriz, J.; Nuñez, J. Effect of SGLT2 inhibitors on anemia and their possible clinical implications. Nefrologia 2024, 44, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Nangaku, M.; Kraus, B.J.; Zinman, B.; Mattheus, M.; Hantel, S.; Schumacher, M.; Ohneberg, K.; Schmoor, C.; Inzucchi, S.E. How do SGLT2 inhibitors protect the kidney? A mediation analysis of the EMPA-REG OUTCOME trial. Nephrol. Dial. Transplant. 2024, 39, 1504–1513. [Google Scholar] [CrossRef]
- Tsai, C.W.; Lin, S.Y.; Kuo, C.C.; Huang, C.C. Serum Uric Acid and Progression of Kidney Disease: A Longitudinal Analysis and Mini-Review. PLoS ONE 2017, 12, e0170393. [Google Scholar] [CrossRef]
- Packer, M. Hyperuricemia and Gout Reduction by SGLT2 Inhibitors in Diabetes and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2024, 83, 371–381. [Google Scholar] [CrossRef] [PubMed]
- La Grotta, R.; de Candia, P.; Olivieri, F.; Matacchione, G.; Giuliani, A.; Rippo, M.R.; Tagliabue, E.; Mancino, M.; Rispoli, F.; Ferroni, S.; et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell Mol. Life Sci. 2022, 79, 273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Godøy, A.; Huitfeldt, I. Regional variation in health care utilization and mortality. J. Health Econ. 2020, 71, 102254. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Bansal, N.; Cavanaugh, K.L.; Chang, A.; Crowley, S.; Delgado, C.; Estrella, M.M.; Ghossein, C.; Ikizler, T.A.; Koncicki, H.; et al. KDOQI US Commentary on the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2025, 85, 135–176. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022: A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.B.; Sarafidis, P.; Ekart, R.; Ferro, C.J.; Balafa, O.; Fernandez-Fernandez, B.; Herrington, W.G.; Rossignol, P.; Del Vecchio, L.; Valdivielso, J.M.; et al. SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: A comprehensive review. Nephrol. Dial. Transplant. 2023, 38, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Husson, N.; Watfa, G.; Laurain, M.C.; Perret-Guillaume, C.; Niemier, J.Y.; Miget, P.; Benetos, A. Characteristics of polymedicated (≥4) elderly: A survey in a community-dwelling population aged 60 years and over. J. Nutr. Health Aging 2014, 18, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Vrettos, I.; Voukelatou, P.; Panayiotou, S.; Kyvetos, A.; Kalliakmanis, A.; Makrilakis, K.; Sfikakis, P.P.; Niakas, D. Validation of the revised 9-scale clinical frailty scale (CFS) in Greek language. BMC Geriatr. 2021, 21, 393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
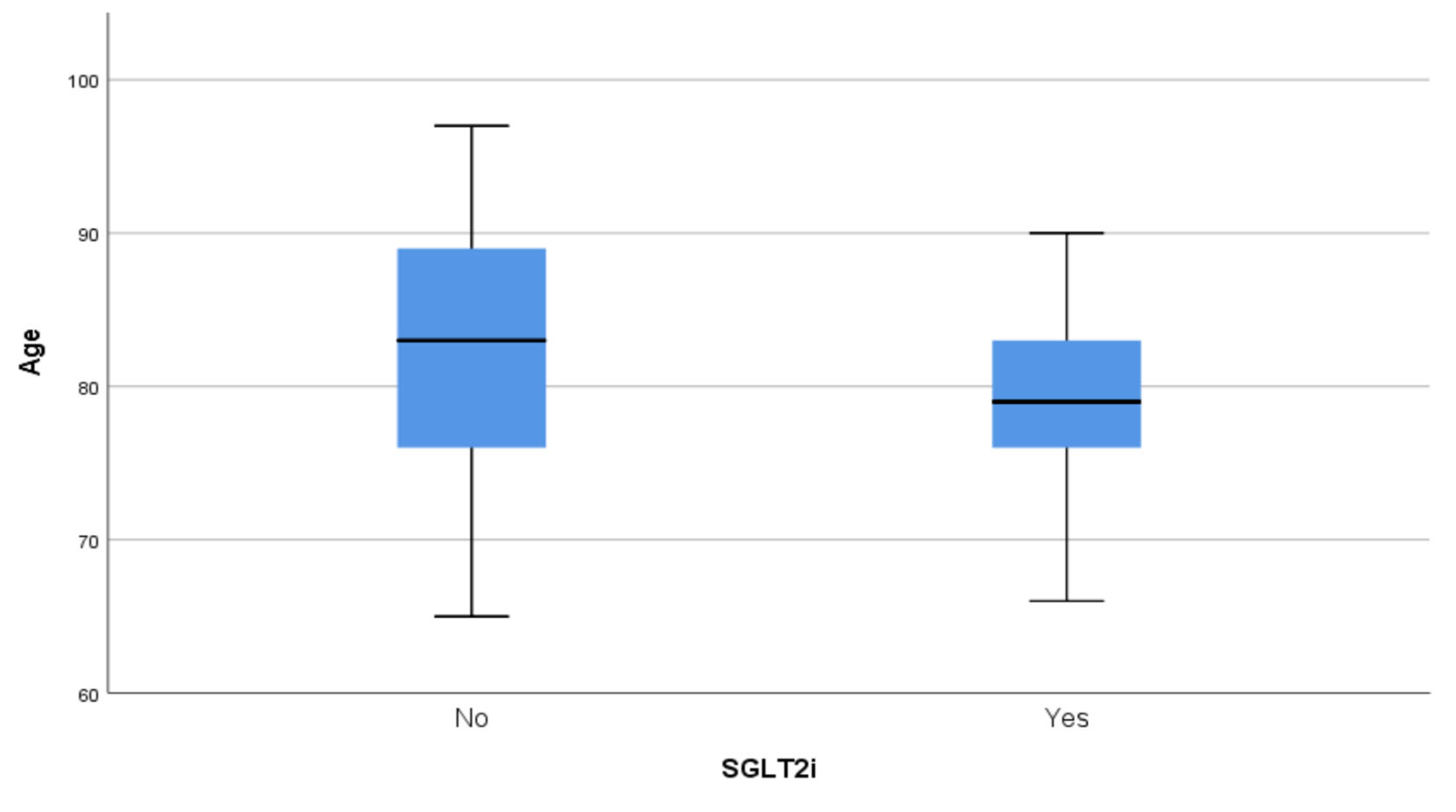

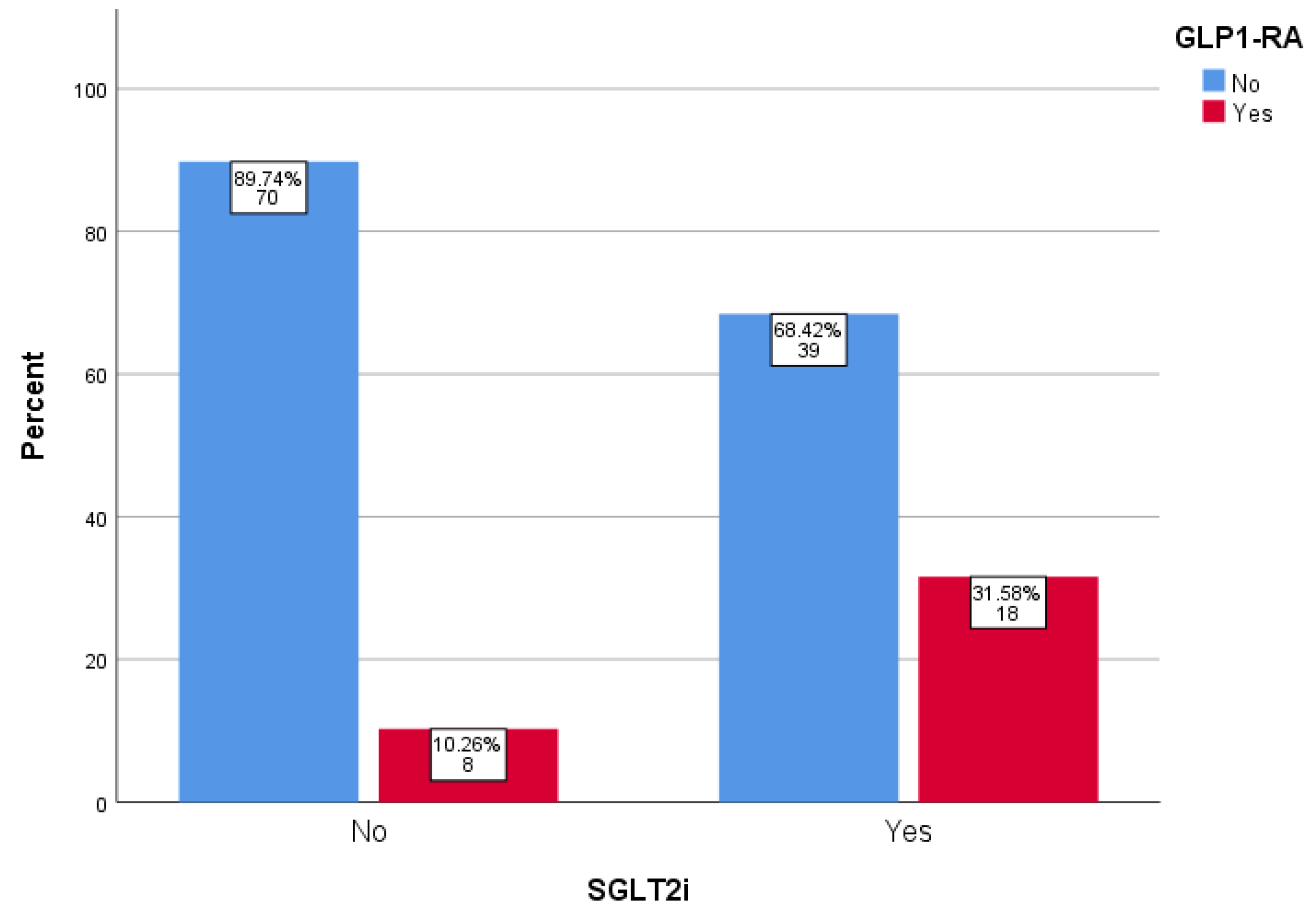
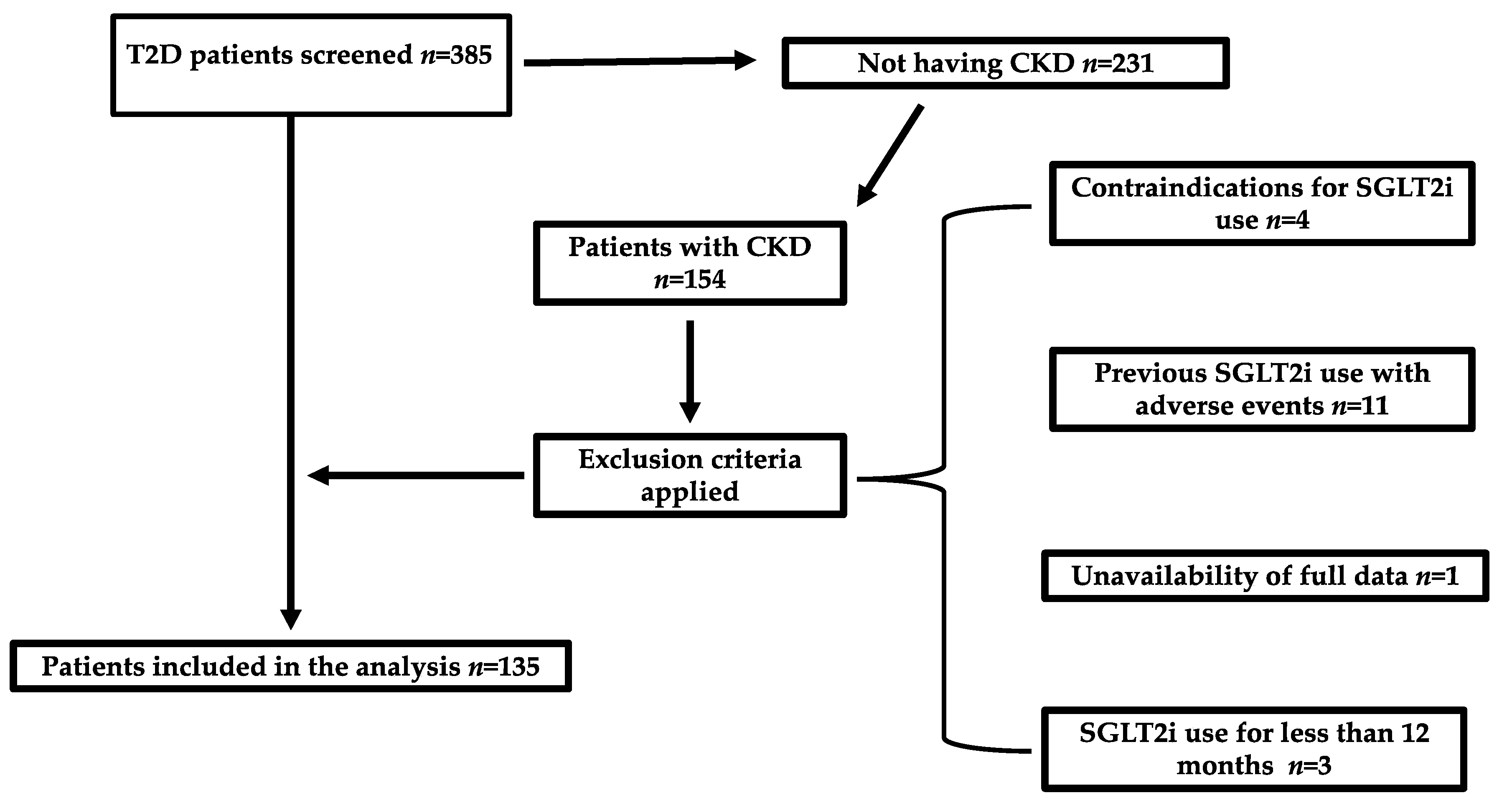
| Variable | SGLT2i Group | Non-SGLT2i Group | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 78.65 ± 5.94 (79) | 82.03 ± 7.53 (83) | 0.006 |
| Male gender (%) | 57.9 | 44.9 | 0.164 |
| Comorbidities | |||
| Heart failure (%) | 60.0 | 76.0 | 0.252 |
| Hypertension (%) | 80.7 | 75.6 | 0.535 |
| Dyslipidemia (%) | 75.4 | 46.2 | 0.001 |
| Hyperuricemia (%) | 22.8 | 26.9 | 0.586 |
| Obesity (%) | 27.8 | 25.1 | 0.138 |
| Atrial fibrillation (%) | 21.0 | 26.9 | 0.433 |
| Ischemic heart disease (%) | 29.8 | 17.9 | 0.105 |
| Stroke/TIA (%) | 17.5 | 14.1 | 0.586 |
| COPD/lung disease (%) | 12.3 | 15.4 | 0.608 |
| MASLD (%) | 53.5 | 57.8 | 0.176 |
| Liver cirrhosis (%) | 0.0 | 7.7 | 0.039 |
| Malignancy (%) | 19.3 | 19.2 | 1.000 |
| Dementia (%) | 5.3 | 14.1 | 0.096 |
| Autoimmune disease/arthritis (%) | 5.3 | 3.8 | 0.697 |
| Mental health disorder (%) | 7.0 | 2.6 | 0.240 |
| FRAIL scale | 3.7 ± 1.2 | 3.3 ± 0.6 | 0.341 |
| Variable | SGLT2i Group | Non-SGLT2i Group | p-Value |
|---|---|---|---|
| Pharmacological treatment | |||
| Metformin (%) | 47.4 | 57.7 | 0.235 |
| Insulin (%) | 38.6 | 32.1 | 0.468 |
| GLP-1 RA (%) | 31.6 | 10.3 | 0.002 |
| DPP4i (%) | 40.4 | 55.1 | 0.090 |
| Sulphonylureas (%) | 21.1 | 20.5 | 0.939 |
| Thiazolidinediones (%) | 12.3 | 6.4 | 0.236 |
| Meglitinides (%) | 0.0 | 1.3 | 1.000 |
| RAASi (%) | 75.4 | 65.4 | 0.210 |
| Polypharmacy ≥ 4 drugs (%) | 69.2 | 73.4 | 0.451 |
| Laboratory parameters | |||
| HbA1c (%) | 7.69 ± 1.56 (7.25) | 8.29 ± 10.61 (6.9) | 0.010 |
| Fasting glucose (mg/dL) | 150.51 ± 62.33 (131) | 135.20 ± 52.33 (123) | 0.156 |
| Urea (mg/dL) | 86.53 ± 45.18 (76.8) | 71.25 ± 30.13 (65) | 0.038 |
| Creatinine (mg/dL) | 1.74 ± 0.44 (1.74) | 1.52 ± 0.39 (1.46) | 0.001 |
| eGFR (mL/min/1.73 m2) | 37.02 ± 10.14 (34) | 40.23 ± 10.01 (38.5) | 0.059 |
| UACR (mg/g) | 101.00 ± 133.56 (31) | 103.50 ± 139.30 (103.5) | 0.769 |
| Total cholesterol (mg/dL) | 135.33 ± 36.64 (135) | 138.20 ± 54.21 (131) | 0.849 |
| Triglycerides (mg/dL) | 160.45 ± 103.20 (128.5) | 132.77 ± 84.37 (110) | 0.164 |
| LDL-C (mg/dL) | 65.39 ± 29.79 (69) | 73.49 ± 45.83 (67) | 0.530 |
| HDL-C (mg/dL) | 40.28 ± 12.13 (41.65) | 38.93 ± 13.64 (38.8) | 0.306 |
| Uric acid (mg/dL) | 6.33 ± 1.85 (6.3) | 7.43 ± 2.50 (6.91) | 0.025 |
| Hemoglobin (g/dL) | 12.32 ± 2.01 (12.1) | 11.14 ± 3.43 (11.1) | 0.001 |
| Hematocrit (%) | 38.06 ± 5.76 (37.7) | 33.42 ± 7.29 (33.6) | <0.001 |
| Sodium (mmol/L) | 138.06 ± 5.26 (138) | 138.99 ± 4.71 (139) | 0.160 |
| Potassium (mmol/L) | 4.54 ± 0.69 (4.5) | 4.17 ± 0.67 (4.09) | 0.005 |
| Calcium (mg/dL) | 9.43 ± 0.60 (9.4) | 9.15 ± 0.68 (9.1) | 0.036 |
| Phosphorus (mg/dL) | 3.93 ± 1.23 (3.64) | 3.78 ± 0.94 (3.61) | 0.553 |
| SGOT (IU/L) | 31.24 ± 54.37 (20.8) | 37.01 ± 80.94 (21.1) | 0.637 |
| SGPT (IU/L) | 24.09 ± 22.90 (17.65) | 22.81 ± 47.24 (12.5) | 0.027 |
| CPK (IU/L) | 80.33 ± 86.65 (45.6) | 148.76 ± 330.56 (63.1) | 0.216 |
| CRP (mg/dL) | 8.17 ± 10.26 (4.76) | 6.09 ± 10.26 (3.27) | 0.754 |
| Ferritin (ng/mL) | 182.26 ± 210.77 (113) | 246.68 ± 530.41 (117) | 0.607 |
| ESR (mm/h) | 61.48 ± 35.46 (61) | 60.36 ± 35.17 (50) | 0.821 |
| Guidelines | Recommendations on SGLT2is | eGFR Threshold | Elderly-Specific Considerations |
|---|---|---|---|
| KDIGO 2024 | SGLT2i use for all patients with T2D and CKD with eGFR ≥20 mL/min/1.73 m2 | Initiate if eGFR ≥ 20 mL/min/1.73 m2 and continue use until initiation of dialysis or transplantation | No specific recommendations for elderly patients; individualized treatment is emphasized |
| KDOQI 2025 | SGLT2is as first-line therapy for renal and cardiovascular protection in patients with eGFR ≥ 20 mL/min/1.73 m2 and UACR ≥200 mg/g | Initiate if eGFR ≥ 20 mL/min/1.73 m2 and continue use until initiation of dialysis or transplantation | No specific recommendations for elderly patients; individualized treatment is emphasized |
| ADA 2024 | SGLT2i use to reduce CKD progression and cardiovascular events in patients with T2D and CKD with eGFR ≥ 20 mL/min/1.73 m2, regardless of albuminuria level | Initiate if eGFR ≥ 20 mL/min/1.73 m2 and continue use until initiation of dialysis or transplantation | No specific recommendations for elderly patients; individualized treatment is emphasized |
| EASD 2022 | SGLT2i use in patients with CKD and eGFR ≥ 20 mL/min/1.73 m2 and UACR > 30 mg/g to reduce MACEs and HF and improve kidney outcomes | Initiate if eGFR ≥ 20 mL/min/1.73 m2 and continue use until initiation of dialysis or transplantation | No specific recommendations for elderly patients; individualized treatment is emphasized |
| ERA 2023 | SGLT2i use for cardiorenal protection in patients with T2D and CKD with eGFR ≥ 20–60 mL/min/1.73 m2 or UACR > 30 mg/g | Initiate if eGFR ≥ 20 mL/min/1.73 m2 and continue use until initiation of dialysis or transplantation | No specific recommendations for elderly patients; individualized treatment is emphasized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafeidou, K.; Psoma, O.; Dimakopoulos, G.; Apostolidis, E.; Sarvani, A.; Gavriilaki, E.; Doumas, M.; Tsimihodimos, V.; Kotsa, K.; Koufakis, T. Evaluating Guideline Alignment by Analyzing Patient Profiles of Elderly People with Type 2 Diabetes and Chronic Kidney Disease Treated or Not with SGLT2 Inhibitors. Pharmaceuticals 2025, 18, 807. https://doi.org/10.3390/ph18060807
Vafeidou K, Psoma O, Dimakopoulos G, Apostolidis E, Sarvani A, Gavriilaki E, Doumas M, Tsimihodimos V, Kotsa K, Koufakis T. Evaluating Guideline Alignment by Analyzing Patient Profiles of Elderly People with Type 2 Diabetes and Chronic Kidney Disease Treated or Not with SGLT2 Inhibitors. Pharmaceuticals. 2025; 18(6):807. https://doi.org/10.3390/ph18060807
Chicago/Turabian StyleVafeidou, Kyriaki, Ourania Psoma, Georgios Dimakopoulos, Evangelos Apostolidis, Anastasia Sarvani, Eleni Gavriilaki, Michael Doumas, Vassilios Tsimihodimos, Kalliopi Kotsa, and Theocharis Koufakis. 2025. "Evaluating Guideline Alignment by Analyzing Patient Profiles of Elderly People with Type 2 Diabetes and Chronic Kidney Disease Treated or Not with SGLT2 Inhibitors" Pharmaceuticals 18, no. 6: 807. https://doi.org/10.3390/ph18060807
APA StyleVafeidou, K., Psoma, O., Dimakopoulos, G., Apostolidis, E., Sarvani, A., Gavriilaki, E., Doumas, M., Tsimihodimos, V., Kotsa, K., & Koufakis, T. (2025). Evaluating Guideline Alignment by Analyzing Patient Profiles of Elderly People with Type 2 Diabetes and Chronic Kidney Disease Treated or Not with SGLT2 Inhibitors. Pharmaceuticals, 18(6), 807. https://doi.org/10.3390/ph18060807








