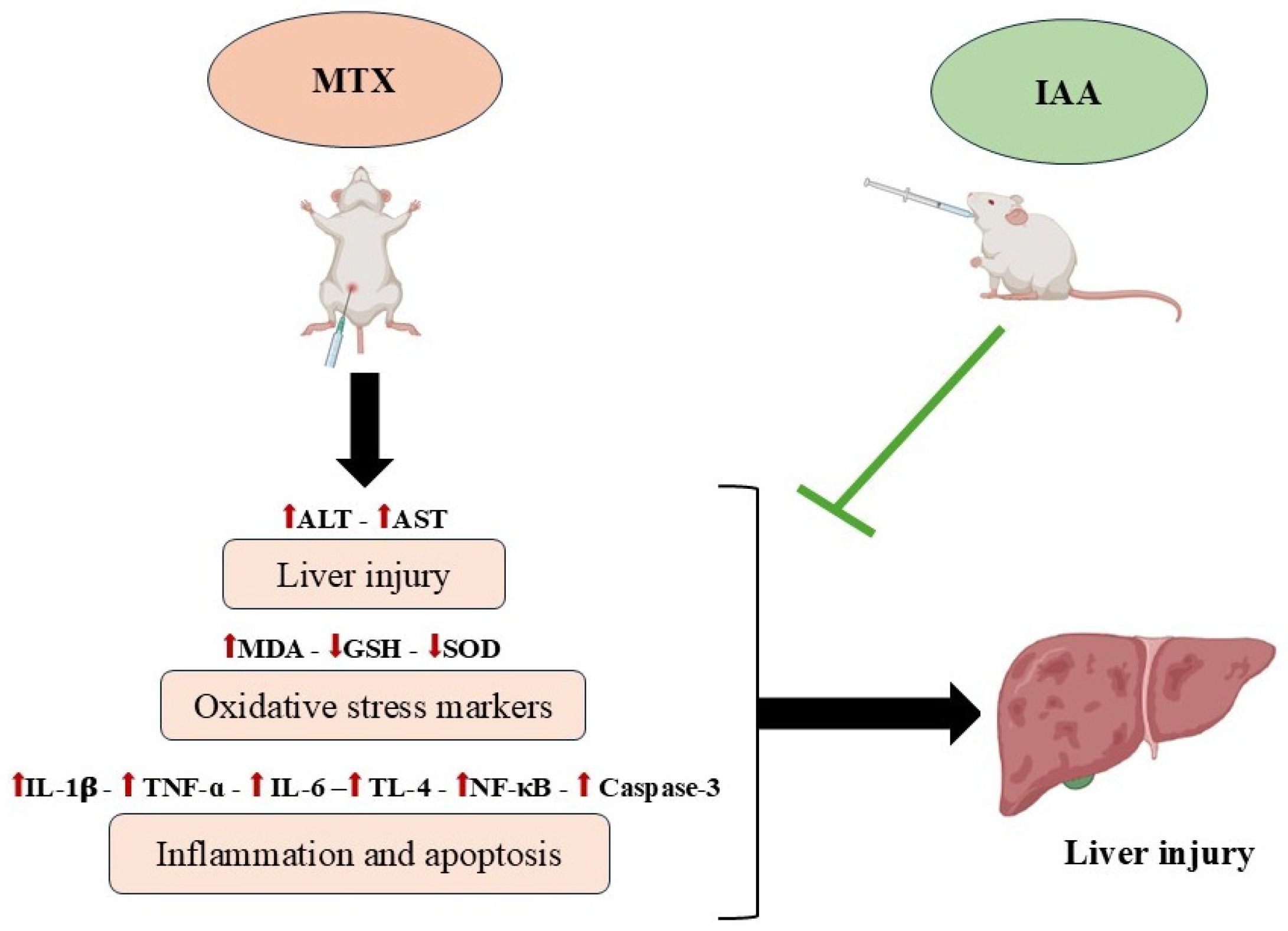
Indole-3-Acetic Acid: Promising Protective Agent Against Methotrexate-Induced Liver Injury via Modulation of TLR4/NF-κB/Caspase-3 Pathway
Abstract
1. Introduction
2. Results
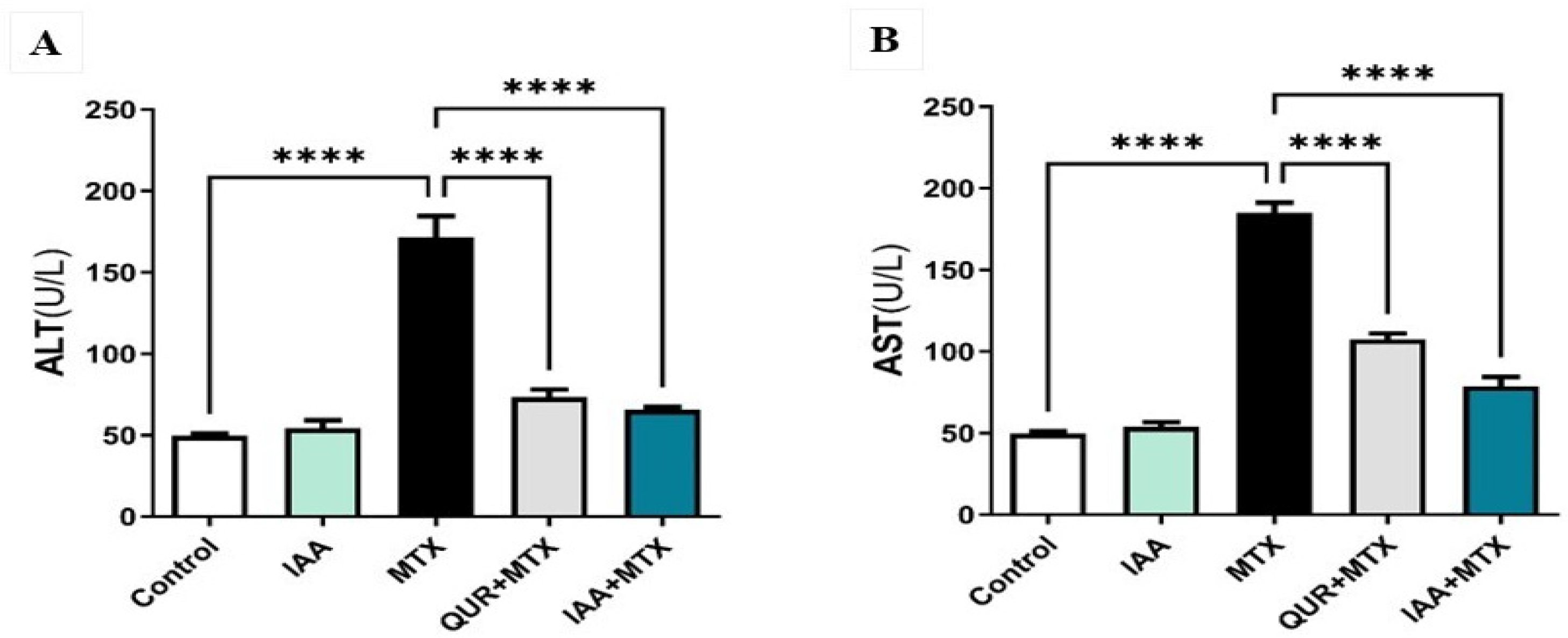
2.1. IAA Restored the Levels of Serum ALT and AST
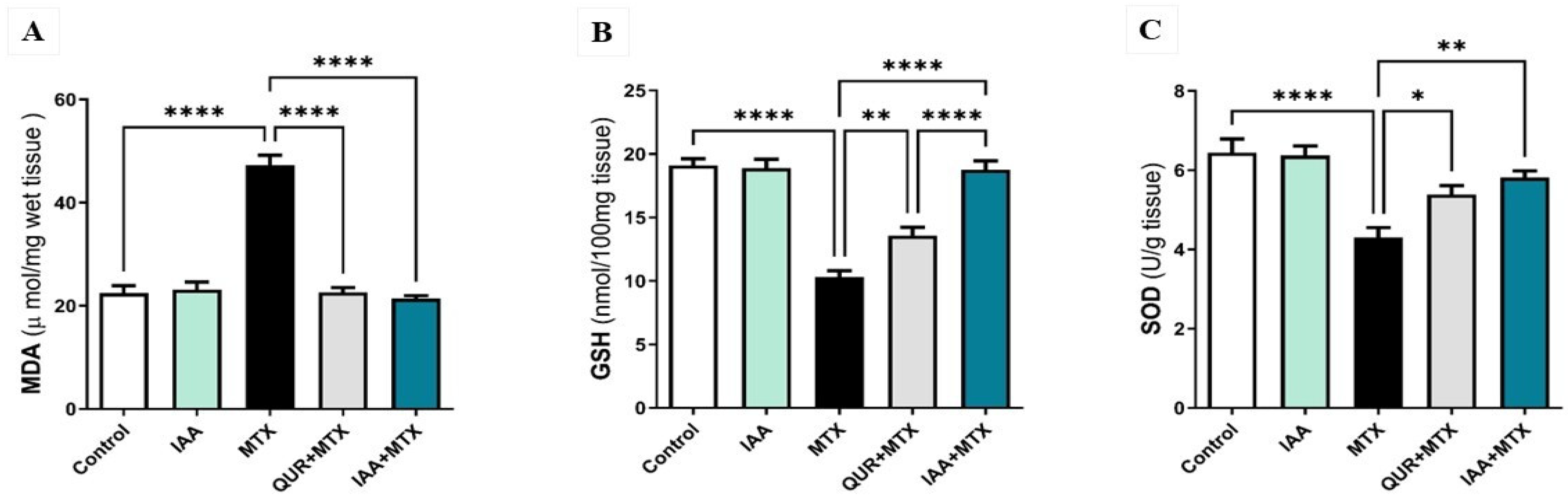
2.2. IAA Restored the Hepatic Levels of MDA, GSH, and SOD
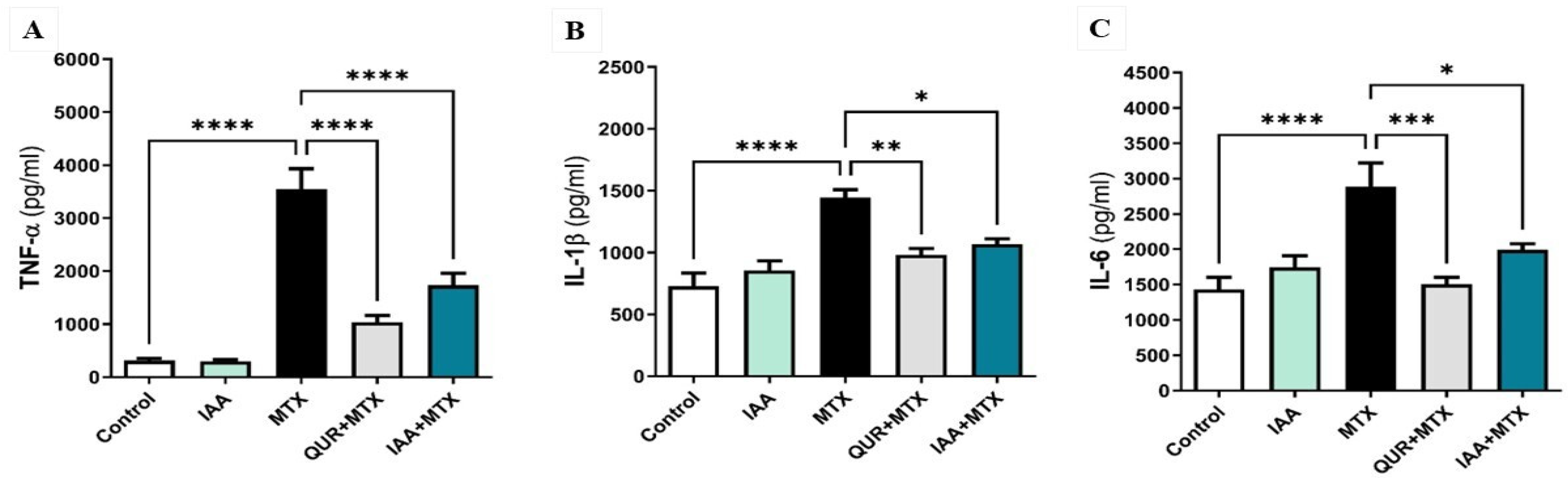
2.3. IAA Downregulated the Expression Levels of Hepatic TNF-α, IL-1β, and IL-6
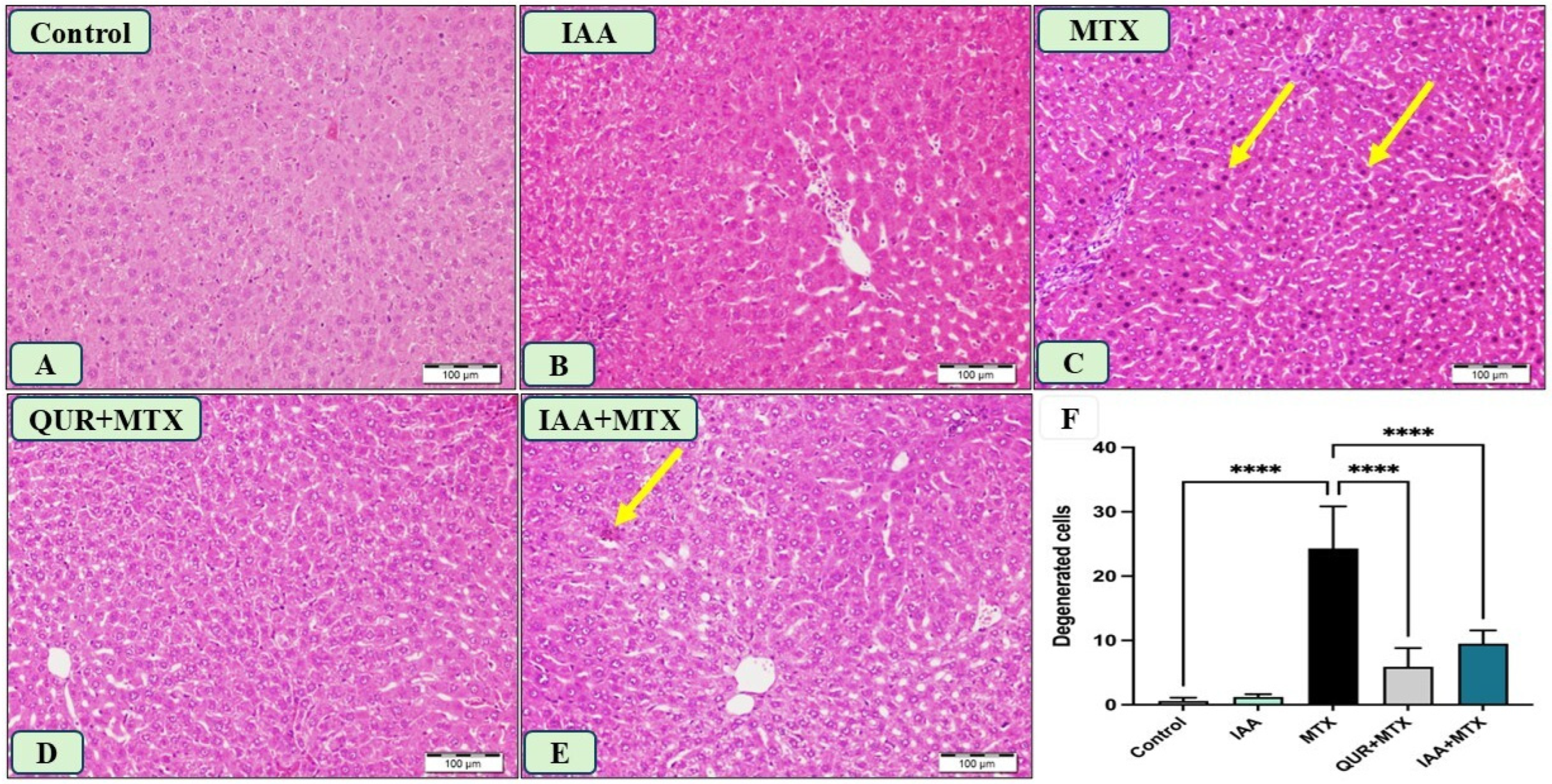
2.4. IAA Improved the Morphological Changes in Liver Sections Induced by MTX
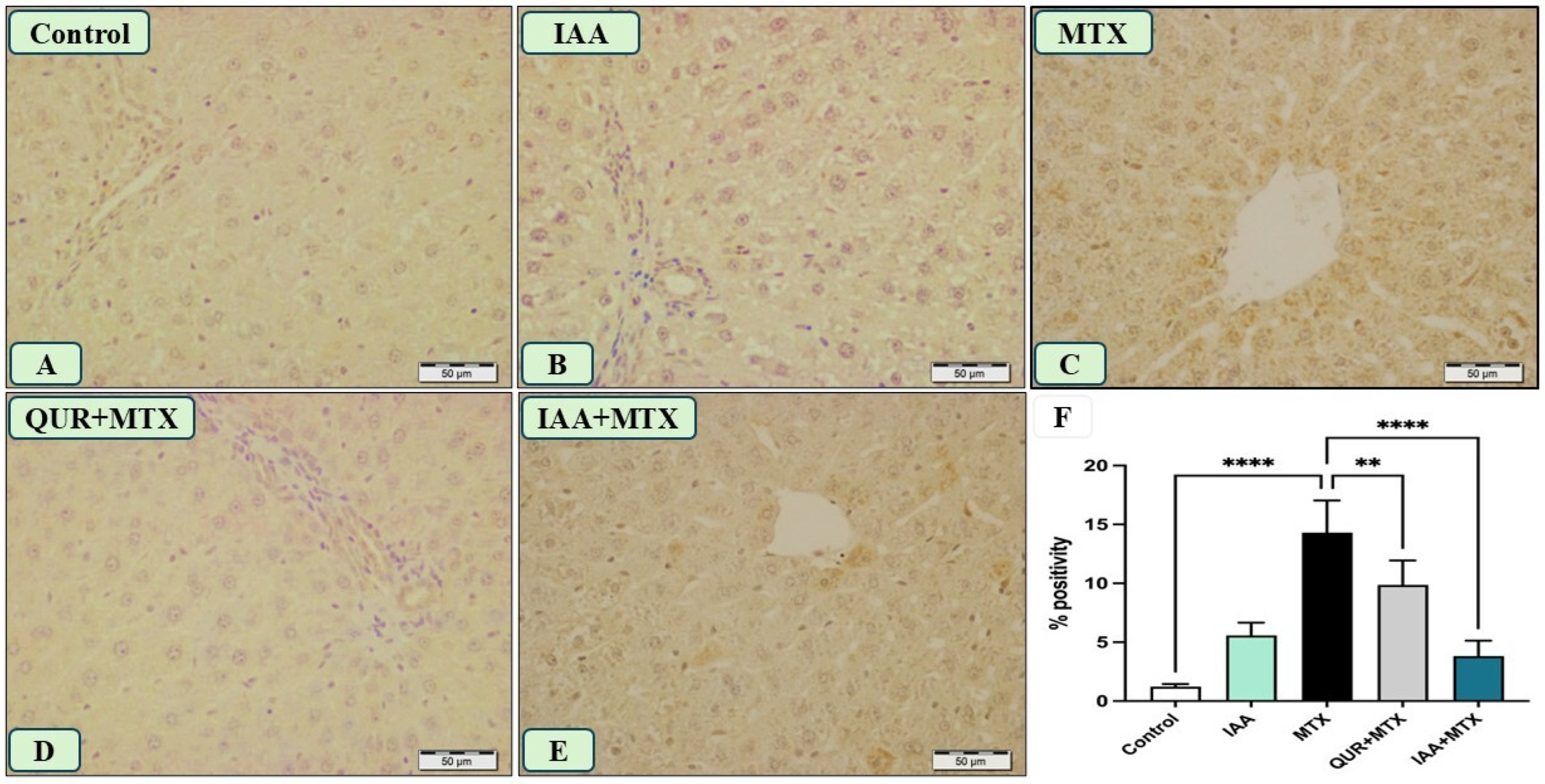
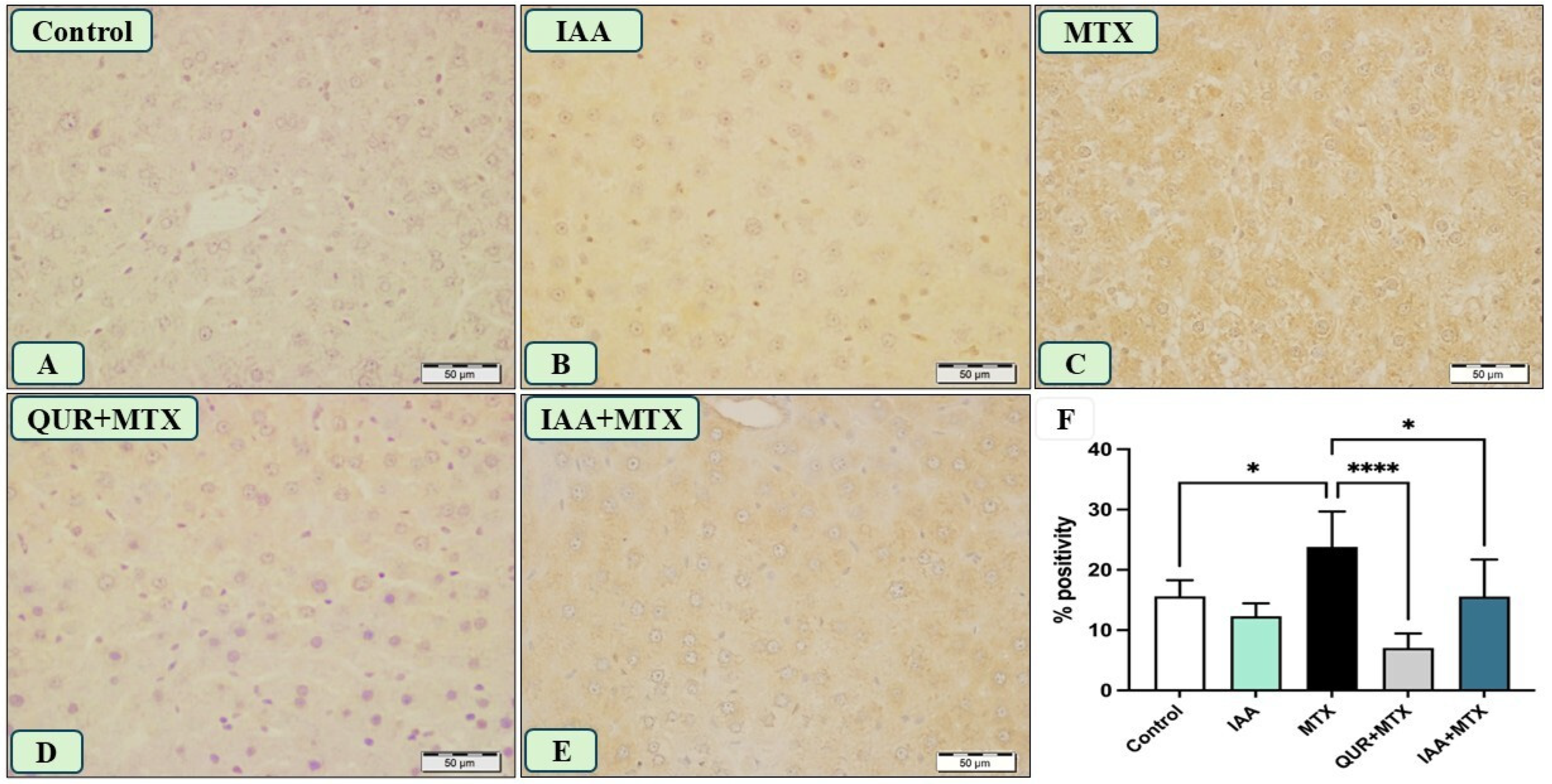
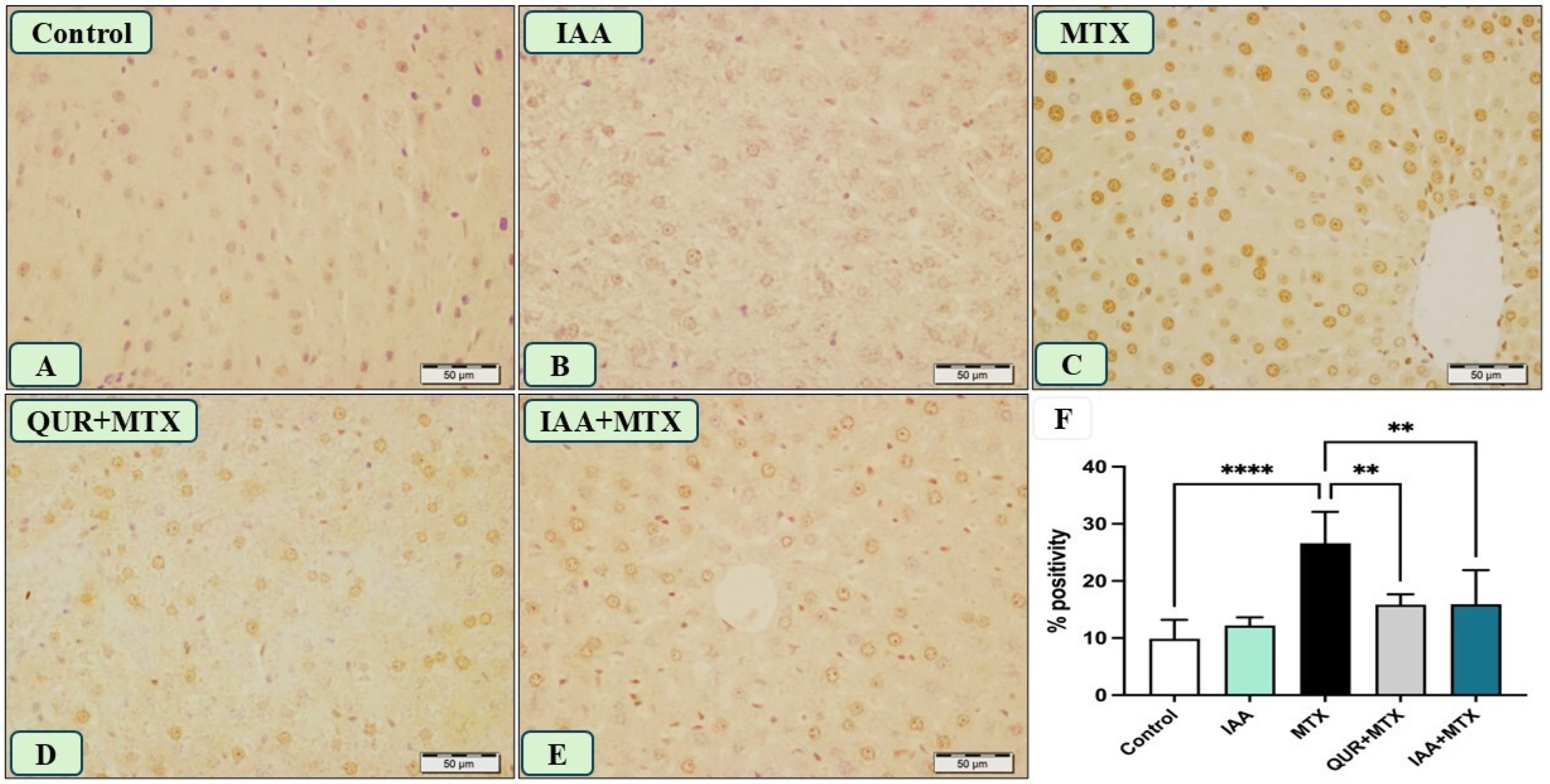
2.5. IAA Reduced the Positive Immunoreactivity of TLR4, NF-κB, and Caspase-3 in the Hepatocytes Induced by MTX
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
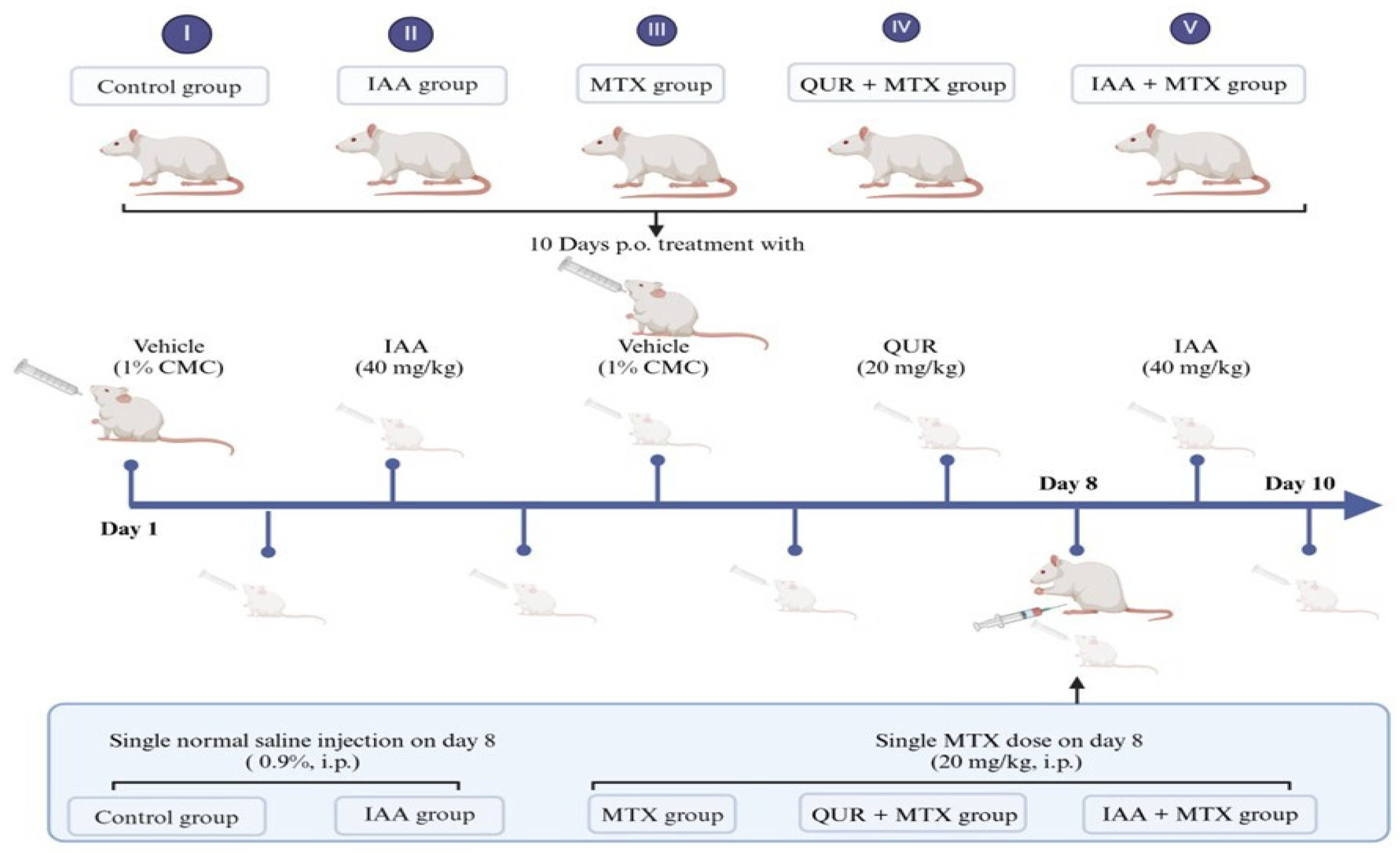
4.2. Experimental Animals and Treatment
4.3. Liver Function Tests in Serum
4.4. Examination of Oxidative Stress in Liver Tissue
4.5. Determination of Inflammatory Responses in Liver Tissue
4.6. Histopathological and Immunohistochemical Studies
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; van Haandel, L.; Kiptoo, P.; Becker, M.L.; Siahaan, T.J.; Funk, R.S. Methotrexate disposition, anti-folate activity and efficacy in the collagen-induced arthritis mouse model. Eur. J. Pharmacol. 2019, 853, 264–274. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an Old Drug with New Tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Cheng, H.; Rademaker, M. Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: Utility of transient elastography. Psoriasis Targets Ther. 2018, 8, 21–29. [Google Scholar] [CrossRef]
- Letertre, M.P.; Munjoma, N.; Wolfer, K.; Pechlivanis, A.; McDonald, J.A.; Hardwick, R.N.; Cherrington, N.J.; Coen, M.; Nicholson, J.K.; Hoyles, L.; et al. A Two-Way Interaction between Methotrexate and the Gut Microbiota of Male Sprague-Dawley Rats. J. Proteome Res. 2020, 19, 3326–3339. [Google Scholar] [CrossRef]
- Vardi, N.; Parlakpinar, H.; Cetin, A.; Erdogan, A.; Ozturk, I.C. Protective effect of β-carotene on methotrexate-induced oxidative liver damage. Toxicol. Pathol. 2010, 38, 592–597. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Hepatotoxic potentials of methotrexate: Understanding the possible toxicological molecular mechanisms. Toxicology 2021, 458, 152840. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, H.; Kumar, U.; Mayachari, A.; Sangli, G.; Singh, S. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: An experimental approach. J. Ethnopharmacol. 2020, 263, 113209. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Gaiotte, L.B.; Cesário, R.C.; Seiva, F.R.F.; de Almeida Chuffa, L.G. The role of Toll-like receptor 4 signaling pathway in ovarian, cervical, and endometrial cancers. Life Sci. 2020, 247, 117435. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O.; Al-Shaalan, N.H. Hepatoprotective effect of Ginkgo biloba extract against methotrexate-induced hepatotoxicity via targeting STAT3/miRNA-21 axis. Drug Chem. Toxicol. 2022, 45, 1723–1731. [Google Scholar] [CrossRef]
- García-González, C.M.; Baker, J. Treatment of early rheumatoid arthritis: Methotrexate and beyond. Curr. Opin. Pharmacol. 2022, 64, 102227. [Google Scholar] [CrossRef]
- Dalaklioglu, S.; Genc, G.E.; Aksoy, N.H.; Akcit, F.; Gumuslu, S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum. Exp. Toxicol. 2013, 32, 662–671. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Hodgson, K.; Miranda-Hernandez, S.; Hughes, S.; Kulur, A.B.; Ketheesan, N. Flavonoid quercetin-methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 2018, 26, 1219–1232. [Google Scholar] [CrossRef]
- Solanki, K.; Patel, J.; Desai, D.; Chaudhari, M. Protective effect Quercetin on Methotrexate induced toxicity in Wistar Rats. Int. J. Pharm. Sci. Res. 2024, 15, 2709–2718. [Google Scholar] [CrossRef]
- Sax, S.L.; Centomo, M.L.; Centofanti, F.; Rizzacasa, B.; Cox, S.; Cox, C.; Latini, A.; D’Apice, M.R.; Mannucci, L.; Novelli, G.; et al. The Senolytic Effect of Indole-3-Carbinol (I3C) on Mouse Embryonic (MEF) and Human Fibroblast Cell Lines. Int. J. Mol. Sci. 2024, 25, 11652. [Google Scholar] [CrossRef]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A systematic review on hepatoprotective activity of quercetin against various drugs and toxic agents: Evidence from preclinical studies. Phytother. Res. 2020, 34, 5–32. [Google Scholar] [CrossRef]
- Moghadam, A.R.; Tutunchi, S.; Namvaran-Abbas-Abad, A.; Yazdi, M.; Bonyadi, F.; Mohajeri, D.; Mazani, M.; Marzban, H.; Łos, M.J.; Ghavami, S. Pre-administration of turmeric prevents methotrexate-induced liver toxicity and oxidative stress. BMC Complement. Altern. Med. 2015, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Cure, E.; Kirbas, A.; Tumkaya, L.; Cure, M.C.; Kalkan, Y.; Yilmaz, A.; Yuce, S. Protective effect of infliximab on methotrexate-induced liver injury in rats: Unexpected drug interaction. J. Cancer Res. Ther. 2015, 11, 164–169. [Google Scholar] [CrossRef]
- Moodi, H.; Hosseini, M.; Abedini, M.R.; Hassanzadeh-Taheri, M.; Hassanzadeh-Taheri, M. Ethanolic Extract of Iris Songarica Rhizome Attenuates Methotrexate-Induced Liver and Kidney Damages in Rats. Avicenna J. Phytomed. 2019, 10, 372. [Google Scholar]
- Owumi, S.E.; Ajijola, I.J.; Agbeti, O.M. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum. Exp. Toxicol. 2019, 38, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.M.; Alanazi, A.M.; Fadda, L.M.; Alqahtani, Q.H.; Sarawi, W.S.; Hasan, I.H. The beneficial efficacy of liposomal resveratrol against doxorubicin-induced hepatotoxicity in rats: Role of TGF-β1 and SIRT1. J. King Saud. Univ. Sci. 2021, 33, 101640. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef]
- Renatino, C.R.; de Souza, M.C.P.; Moreno, C.N.; Luiz, A.L.; Ronchi, C.J.; Tonhasca, L.Y.; Sousa, M.S.; Reddy, S.P.; Regina, B.S.; de Mattos, Z.A.C.; et al. Glutathione levels are associated with methotrexate resistance in acute lymphoblastic leukemia cell lines. Front. Oncol. 2022, 12, 1032336. [Google Scholar] [CrossRef]
- Schmidt, S.; Messner, C.J.; Gaiser, C.; Hämmerli, C.; Suter-Dick, L. Methotrexate-Induced Liver Injury Is Associated with Oxidative Stress, Impaired Mitochondrial Respiration, and Endoplasmic Reticulum Stress In Vitro. Int. J. Mol. Sci. 2022, 23, 15116. [Google Scholar] [CrossRef]
- Hawwa, A.F.; AlBawab, A.Q.; Rooney, M.; Wedderburn, L.R.; Beresford, M.W.; McElnay, J.C. Methotrexate polyglutamates as a potential marker of adherence to long-term therapy in children with juvenile idiopathic arthritis and juvenile dermatomyositis: An observational, cross-sectional study. Arthritis Res. Ther. 2015, 17, 295. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, W.; Liang, Y.; Sun, L.; Yin, Y.; Zhang, W. Anti-inflammatory and anti-oxidative activity of indole-3-acetic acid involves induction of HO-1 and neutralization of free radicals in RAW264.7 cells. Int. J. Mol. Sci. 2020, 21, 1579. [Google Scholar] [CrossRef]
- Perez-Gonzalez, A.; Muñoz-Rugeles, L.; Alvarez-Idaboy, J.R. Tryptophan: Antioxidant or target of oxidative stress? A quantum chemistry elucidation. RSC Adv. 2014, 4, 56128–56131. [Google Scholar] [CrossRef]
- Pérez-González, A.; Alvarez-Idaboy, J.R.; Galano, A. Free-radical scavenging by tryptophan and its metabolites through electron transfer based processes. J. Mol. Model. 2015, 21, 213. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Aljarboa, A.S.; Alhusaini, A.M.; Sarawi, W.S.; Mohammed, R.; Ali, R.A.; Hasan, I.H. The implication of LPS/TLR4 and FXR receptors in hepatoprotective efficacy of indole-3-acetic acid and chenodeoxycholic acid. Life Sci. 2023, 334, 122182. [Google Scholar] [CrossRef]
- Chu, Y.; Jing, Y.; Zhao, X.; Wang, M.; Zhang, M.; Ma, R.; Ma, W.; Lv, Y.; Zhu, L. Modulation of the HMGB1/TLR4/NF-κB signaling pathway in the CNS by matrine in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2021, 352, 577480. [Google Scholar] [CrossRef]
- Arab, H.H.; Abd El-Aal, S.A.; Eid, A.H.; Arafa, E.S.A.; Mahmoud, A.M.; Ashour, A.M. Targeting inflammation, autophagy, and apoptosis by troxerutin attenuates methotrexate-induced renal injury in rats. Int. Immunopharmacol. 2022, 103, 108284. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.K.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of Thymoquinone Hepatorenal Protection in Methotrexate-Induced Toxicity in Rats. Mediators Inflamm. 2015, 2015, 859383. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadvand, H.; Sotoodehnejadnematalahi, F.; Yaghmaei, P. The Protective Effects of Indole-Acetic Acid on the Renal Ischemia-Reperfusion Injury via Antioxidant and Anti-apoptotic Properties in A Rat Model. Iran. J. Kidney Dis. 2022, 16, 125–134. [Google Scholar]
- Taskin, B.; Erdoǧan, M.A.; Yiǧittürk, G.; Alper, S.; Erbaş, O. Benefits of Digoxin on Hepatotoxicity Induced by Methotrexate Treatment. Gastroenterol. Res. Pract. 2021, 2021, 6619844. [Google Scholar] [CrossRef] [PubMed]
- El Bana, E.; Kamal, K.M. Protective and therapeutic effects of quercetin on methotrexate induced hepatotoxicity in adult albino rats: Biochemical, histological and immunohistochemical study. Egypt. J. Histol. 2020, 43, 220–229. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alturaif, S.A.; Alhusaini, A.; Sarawi, W.; Hasan, I.; Alsaab, J.; Ali, R.; Mohammed, R.; Alotaibi, S.S.; Almutairi, F.; Alsaif, S.; et al. Indole-3-Acetic Acid: Promising Protective Agent Against Methotrexate-Induced Liver Injury via Modulation of TLR4/NF-κB/Caspase-3 Pathway. Pharmaceuticals 2025, 18, 828. https://doi.org/10.3390/ph18060828
Alturaif SA, Alhusaini A, Sarawi W, Hasan I, Alsaab J, Ali R, Mohammed R, Alotaibi SS, Almutairi F, Alsaif S, et al. Indole-3-Acetic Acid: Promising Protective Agent Against Methotrexate-Induced Liver Injury via Modulation of TLR4/NF-κB/Caspase-3 Pathway. Pharmaceuticals. 2025; 18(6):828. https://doi.org/10.3390/ph18060828
Chicago/Turabian StyleAlturaif, Sumayya A., Ahlam Alhusaini, Wedad Sarawi, Iman Hasan, Juman Alsaab, Rehab Ali, Raeesa Mohammed, Sahar S. Alotaibi, Faris Almutairi, Shaikha Alsaif, and et al. 2025. "Indole-3-Acetic Acid: Promising Protective Agent Against Methotrexate-Induced Liver Injury via Modulation of TLR4/NF-κB/Caspase-3 Pathway" Pharmaceuticals 18, no. 6: 828. https://doi.org/10.3390/ph18060828
APA StyleAlturaif, S. A., Alhusaini, A., Sarawi, W., Hasan, I., Alsaab, J., Ali, R., Mohammed, R., Alotaibi, S. S., Almutairi, F., Alsaif, S., Alsultan, E., Aljasas, E., & Alsanea, S. (2025). Indole-3-Acetic Acid: Promising Protective Agent Against Methotrexate-Induced Liver Injury via Modulation of TLR4/NF-κB/Caspase-3 Pathway. Pharmaceuticals, 18(6), 828. https://doi.org/10.3390/ph18060828





