Does Green Brazilian Propolis Extract Improve Functional Capacity in Symptomatic Chronic Coronary Disease?—A Pilot Randomized Trial
Abstract
1. Introduction
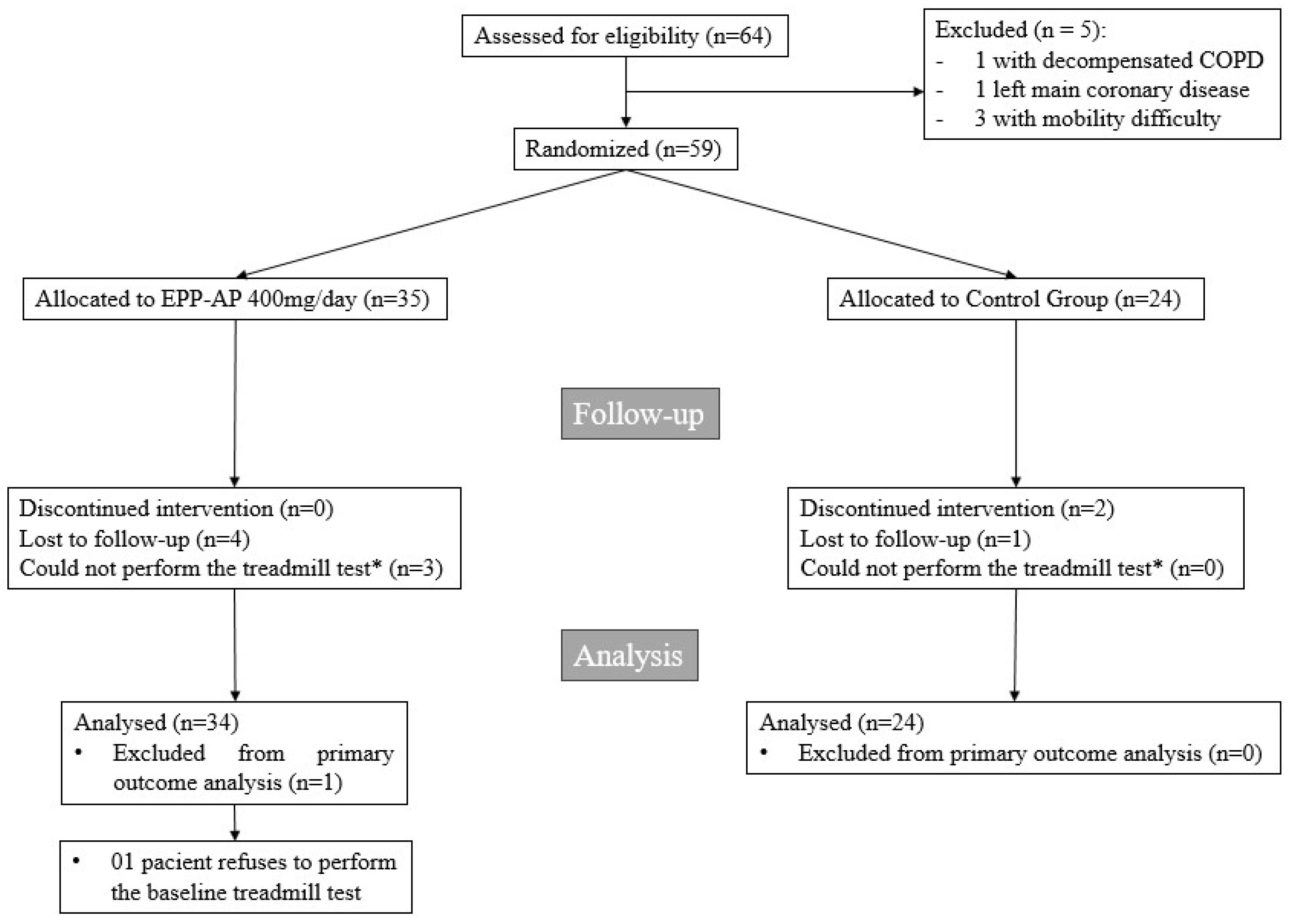
2. Results
Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Designed and Patients
4.2. Randomization and Follow-Up
4.3. End Points
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, E9–E119. [Google Scholar] [CrossRef] [PubMed]
- Malta, D.C.; Pinheiro, P.C.; de Vasconcelos, N.M.; Stopa, S.R.; Vieira, M.L.F.P.; Lotufo, P.A. Prevalence of Angina Pectoris and Associated Factors in the Adult Population of Brazil: National Survey of Health, 2019. Rev. Bras. Epidemiol. 2021, 24, e210012. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.S.; Roseira, E.S.; Viana, T.T.; Silveira, M.A.D.; de Melo, R.M.V.; Fernandez, M.G.; Lemos, L.M.G.; Passos, L.C.S. Inflammation in Coronary Atherosclerosis: Insights into Pathogenesis and Therapeutic Potential of Anti-Inflammatory Drugs. Pharmaceuticals 2023, 16, 1242. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Andreis, A.; Imazio, M.; Piroli, F.; Avondo, S.; Casula, M.; Paneva, E.; De Ferrari, G.M. Efficacy and safety of colchicine for the prevention of major cardiovascular and cerebrovascular events in patients with coronary artery disease: A systematic review and meta-analysis on 12 869 patients. Eur. J. Prev. Cardiol. 2021, 28, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Fuster, V.; Ridker, P.M. Low-Dose Colchicine for Secondary Prevention of Coronary Artery Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 82, 648–660. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vuppu, S.; Balar, P.C.; Mishra, T.; Bezbaruah, R.; Teli, D.; Sharma, N.; Alom, S. Propolis in the management of cardiovascular disease. Int. J. Biol. Macromol. 2024, 266, 131219. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The cardiovascular therapeutic potential of propolis—A comprehensive review. Biology 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Niida, T.; Kinoshita, D.; Suzuki, K.; Yuki, H.; Fujimoto, D.; Dey, D.; Lee, H.; McNulty, I.; Ferencik, M.; Yonetsu, T.; et al. Layered plaque is associated with high levels of vascular inflammation and vulnerability in patients with stable angina pectoris. J. Thromb. Thrombolysis. 2024, 57, 880–887. [Google Scholar] [CrossRef]
- Zouridakis, E.; Avanzas, P.; Arroyo-Espliguero, R.; Fredericks, S.; Kaski, J.C. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation 2004, 110, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Xiang, L. The correlation between stable angina and inflammatory factors and blood lipids: A case-control study. Front. Cardiovasc. Med. 2024, 11, 1443450. [Google Scholar] [CrossRef]
- Machado, J.L.; Assunção, A.K.M.; da Silva, M.C.P.; Reis, A.S.D.; Costa, G.C.; de Sousa Arruda, D.; Rocha, B.A.; de Oliveira Lima Leite Vaz, M.M.; de Andrade Paes, A.M.; Guerra, R.N.M.; et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid.-Based Complement. Altern. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef]
- Jalali, M.; Ranjbar, T.; Mosallanezhad, Z.; Mahmoodi, M.; Moosavian, S.P.; Ferns, G.A.; Jalali, R.; Sohrabi, Z. Effect of Propolis Intake on Serum C-Reactive Protein (CRP) and Tumor Necrosis Factor-alpha (TNF-α) Levels in Adults: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Med. 2020, 50, 102380. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Malta-Santos, H.; Rebouças-Silva, J.; Teles, F.; Galvão, E.B.d.S.; de Souza, S.P.; Dutra, F.R.D.; Gomes, M.M.D.; Teixeira, M.B.; da Conceição, L.F.M.R.; et al. Effects of Standardized Brazilian Green Propolis Extract (EPP-AF®) on Inflammation in Haemodialysis Patients: A Clinical Trial. Int. J. Nephrol. 2022, 2022, 1035475. [Google Scholar]
- Kerpel-Fronius, S.; Teschke, R.; Hanau, H.; Ming Yang, G.; Miao, M.; Huang, J. Characteristic analysis of clinical trials for new traditional Chinese medicines in mainland China from 2013 to 2021. Front. Med. 2022, 9, 1008683. [Google Scholar] [CrossRef]
- Kapoor, D.; Vijayvergiya, R.; Dhawan, V. Terminalia arjuna in coronary artery disease: Ethnopharmacology, pre-clinical, clinical & safety evaluation. J. Ethnopharmacol. 2014, 155, 1029–1045. [Google Scholar] [PubMed]
- Jolly, S.S.; D’entremont, M.-A.; Lee, S.F.; Mian, R.; Tyrwhitt, J.; Kedev, S.; Montalescot, G.; Cornel, J.H.; Stanković, G.; Moreno, R.; et al. Colchicine in Acute Myocardial Infarction. N. Engl. J. Med. 2025, 392, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Camici, P.G.; Crea, F.; Danchin, N.; Fox, K.; Manolis, A.J.; Marzilli, M.; Rosano, G.M.; Lopez-Sendon, J.L.; Pinto, F.; et al. Anti-anginal drugs: Systematic review and clinical implications. Int. J. Cardiol. 2019, 283, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.C.; Krishnaswami, S. Combination treatment with trimetazidine and diltiazem in stable angina pectoris. Heart 1997, 78, 353–357. [Google Scholar] [CrossRef]
- Chan, P.S.; Jones, P.G.; Arnold, S.A.; Spertus, J.A. Development and Validation of a Short Version of the Seattle Angina Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 640–647. [Google Scholar] [CrossRef]
| Propolis (N = 35) | Placebo (N = 24) | |
|---|---|---|
| Age (IQR) | 59 (53–65) | 64 (59–71) |
| Male (%) | 68.6% | 54.2% |
| Body mass index (IQR) | 27.3 (25.3–28.3) | 26.7 (26–28.6) |
| SBP (IQR) | 130 (112–154) | 131 (120–150) |
| Heart rate (IQR) | 71 (64–77) | 65 (62–69) |
| Total cholesterol (IQR) | 140 (93–171) | 132 (114–181) |
| LDLc (IQR) | 73 (45–104) | 76 (58–90) |
| Creatinine clearance (IQR) | 87 (75–101) | 86 (60–98) |
| Hypertension (n) | 31 (88.6%) | 22 (91.7%) |
| Diabetes (n) | 17 (48.6%) | 13 (54.2%) |
| LVEF (IQR) | 63 (53–67) | 66 (55–74) |
| LVEDd (IQR) | 50 (46–53) | 47 (44–51) |
| LVESd (IQR) | 31 (31–37) | 31 (26–33) |
| STEMI (n) | 15 (42.9%) | 11 (45.8%) |
| PCI (n) | 14 (40%) | 8 (33.3%) |
| CABG surgery (n) | 1 (2.9%) | 0 |
| ADA disease (n) | 30 (85.7%) | 20 (83.3%) |
| CAD multiarterial (n) | 20 (57.1%) | 14 (58.3%) |
Angina symptoms (n)
| 13 (37.1%) 11 (31.4%) 7 (20%) 4 (4.2%) | 8 (33.3%) 8 (33.3%) 7 (29.2%) 1 (11.4%) |
| Aspirin (n) | 34 (97.1%) | 22 (91.7%) |
| Clopidogrel (n) | 10 (28.6%) | 8 (33.3%) |
| Statins (n) | 34 (97.1%) | 24 (100%) |
| Ezetimibe (n) | 12 (34.3%) | 7 (29.2%) |
| IECA/ARB (n) | 33 (94.3%) | 19 (79.2%) |
Anti-anginal medications
| 13 (37.1%) 34 (97.1%) 25 (71.4%) 14 (40%) | 14 (58.3%) 23 (95.8%) 17 (70.8%) 11 (45.8%) |
| hs-CRP (IQR) | 1.43 (0.38–2.90) | 1.22 (0.49–3.62) |
| hs-CRP ≥ 2mg/L | 9 (25%) | 10 (42%) |
| Propolis | Placebo | |||||
| Before Treatment (n = 34) | After Treatment (n = 31) | p | Before Treatment (n = 24) | After Treatment (n = 23) | p | |
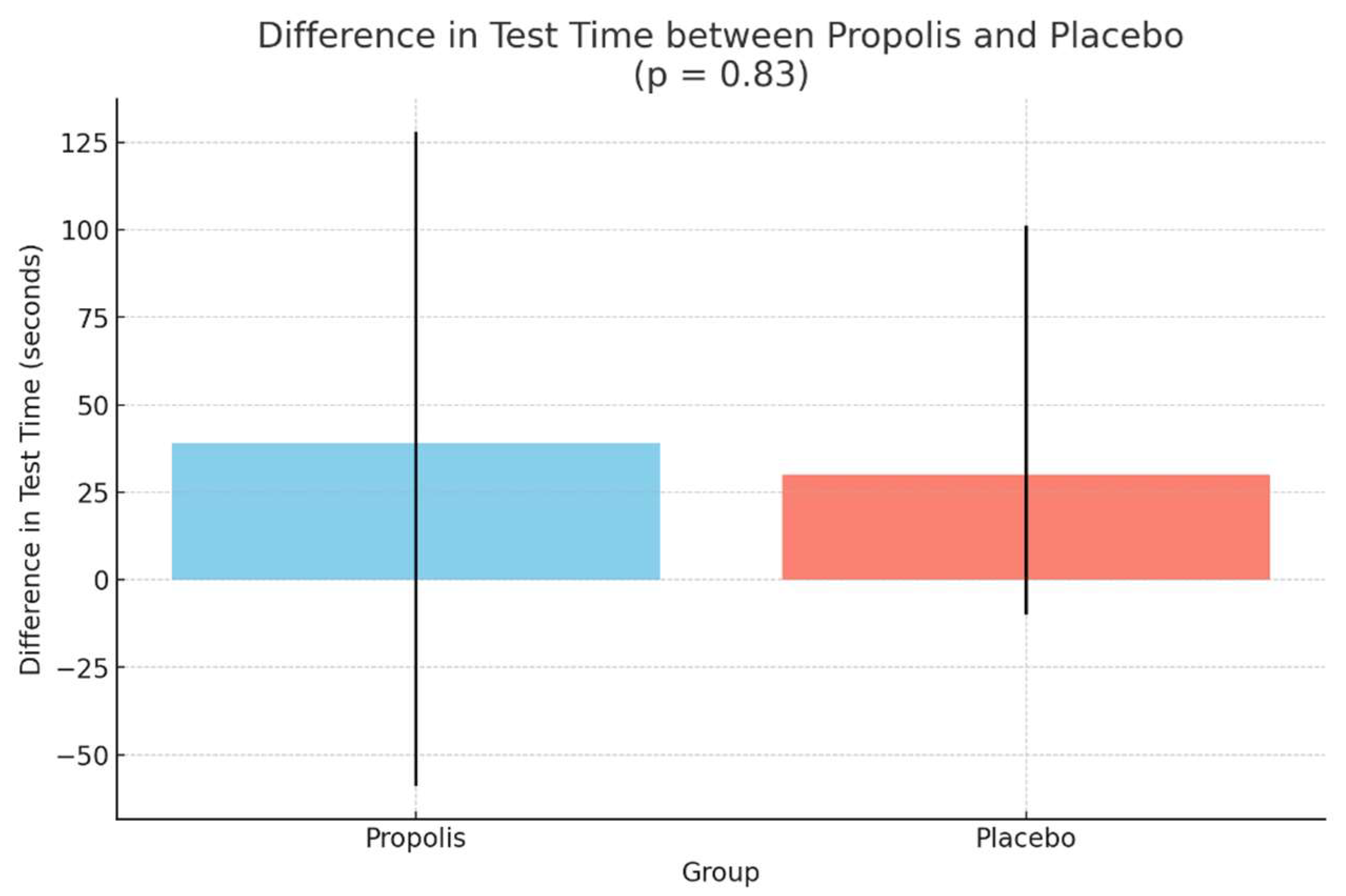
| Duration on the treadmill test (seconds) (IQR) | 367 (273–555) | 378 (232–558) | 0.24 | 341 (217–380) | 355 (232–443) | 0.03 |
| Functional capacity in METs (IQR) | 6.70 (4.93–9.80) | 7.16 (4.98–9.98) | 0.36 | 5.98 (3.94–7.16) | 6.36 (4.31–7.75) | 0.06 |
| Propolis | Placebo | |||||
|
Before Treatment (n = 35) |
After Treatment (n = 31) | p |
Before Treatment (n = 24) |
After Treatment (n = 23) | p | |
| hsCRP (IQR) | 0.92 (0.44–2.45) | 1.14 (0.34–3.99) | 0.65 | 1.53 (0.735–4.40) | 1.24 (0.68–5.59) | 0.42 |
| Propolis | Placebo | |||||
|---|---|---|---|---|---|---|
| Before Treatment (n = 35) | After Treatment (n = 31) | p | Before Treatment (n = 24) | After Treatment (n = 23) | p | |
| SAQ-7 Angina Frequency Score, Median (IQR) | 80 (70–85) | 90 (80–100) | 0.43 | 80 (60–80) | 90 (80–100) | 0.002 |
| SAQ-7 Physical Limitation Score, Median (IQR) | 55.5 (41.6–88.8) | 62.5 (40.3–87.5) | 0.84 | 66.6 (41.6–85.1) | 69.4 (55.5–91.6) | 0.36 |
| SAQ-7 Quality-of-Life Score, Median (IQR) | 58.3 (41.7–66.7) | 50 (33.3–66.7) | 0.52 | 50 (41.6–70.8) | 66.6 (50–75) | 0.055 |
| SAQ-7 Angina Stability Score, Median (IQR) | 75 (50–87.5) | 75 (50–100) | 0.77 | 50 (50–100) | 100 (75–100) | 0.015 |
| SAQ-7 Treatment Satisfaction Score, Median (IQR) | 93.7 (78.1–100) | 93.7 (78.1–100) | 0.73 | 87.5 (75–100) | 100 (87.5–100) | 0.162 |
| SAQ-7 Summary Score, Median (IQR) | 61.3 (47.4–75.3) | 62.7 (50.2–75.2) | 0.38 | 63.7 (50.2–70.5) | 76.3 (59.5–84.2) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, C.S.; Passos, L.C.S.; Cafezeiro, C.R.F.; de Melo, R.M.V.; Viana, T.T.; de Oliveira, E.J.G.; Berretta, A.A.; Silveira, M.A.D. Does Green Brazilian Propolis Extract Improve Functional Capacity in Symptomatic Chronic Coronary Disease?—A Pilot Randomized Trial. Pharmaceuticals 2025, 18, 827. https://doi.org/10.3390/ph18060827
Figueiredo CS, Passos LCS, Cafezeiro CRF, de Melo RMV, Viana TT, de Oliveira EJG, Berretta AA, Silveira MAD. Does Green Brazilian Propolis Extract Improve Functional Capacity in Symptomatic Chronic Coronary Disease?—A Pilot Randomized Trial. Pharmaceuticals. 2025; 18(6):827. https://doi.org/10.3390/ph18060827
Chicago/Turabian StyleFigueiredo, Clara Salles, Luiz Carlos Santana Passos, Caio Rebouças Fonseca Cafezeiro, Rodrigo Morel Vieira de Melo, Tainá Teixeira Viana, Eduardo Jorge Gomes de Oliveira, Andresa Aparecida Berretta, and Marcelo Augusto Duarte Silveira. 2025. "Does Green Brazilian Propolis Extract Improve Functional Capacity in Symptomatic Chronic Coronary Disease?—A Pilot Randomized Trial" Pharmaceuticals 18, no. 6: 827. https://doi.org/10.3390/ph18060827
APA StyleFigueiredo, C. S., Passos, L. C. S., Cafezeiro, C. R. F., de Melo, R. M. V., Viana, T. T., de Oliveira, E. J. G., Berretta, A. A., & Silveira, M. A. D. (2025). Does Green Brazilian Propolis Extract Improve Functional Capacity in Symptomatic Chronic Coronary Disease?—A Pilot Randomized Trial. Pharmaceuticals, 18(6), 827. https://doi.org/10.3390/ph18060827







