Protective Effects of Xanthorrhizol-Rich Extracts Against PM-Induced Skin Damage in Human Keratinocytes and 3D-Reconstructed Skin Models
Abstract
1. Introduction
2. Results
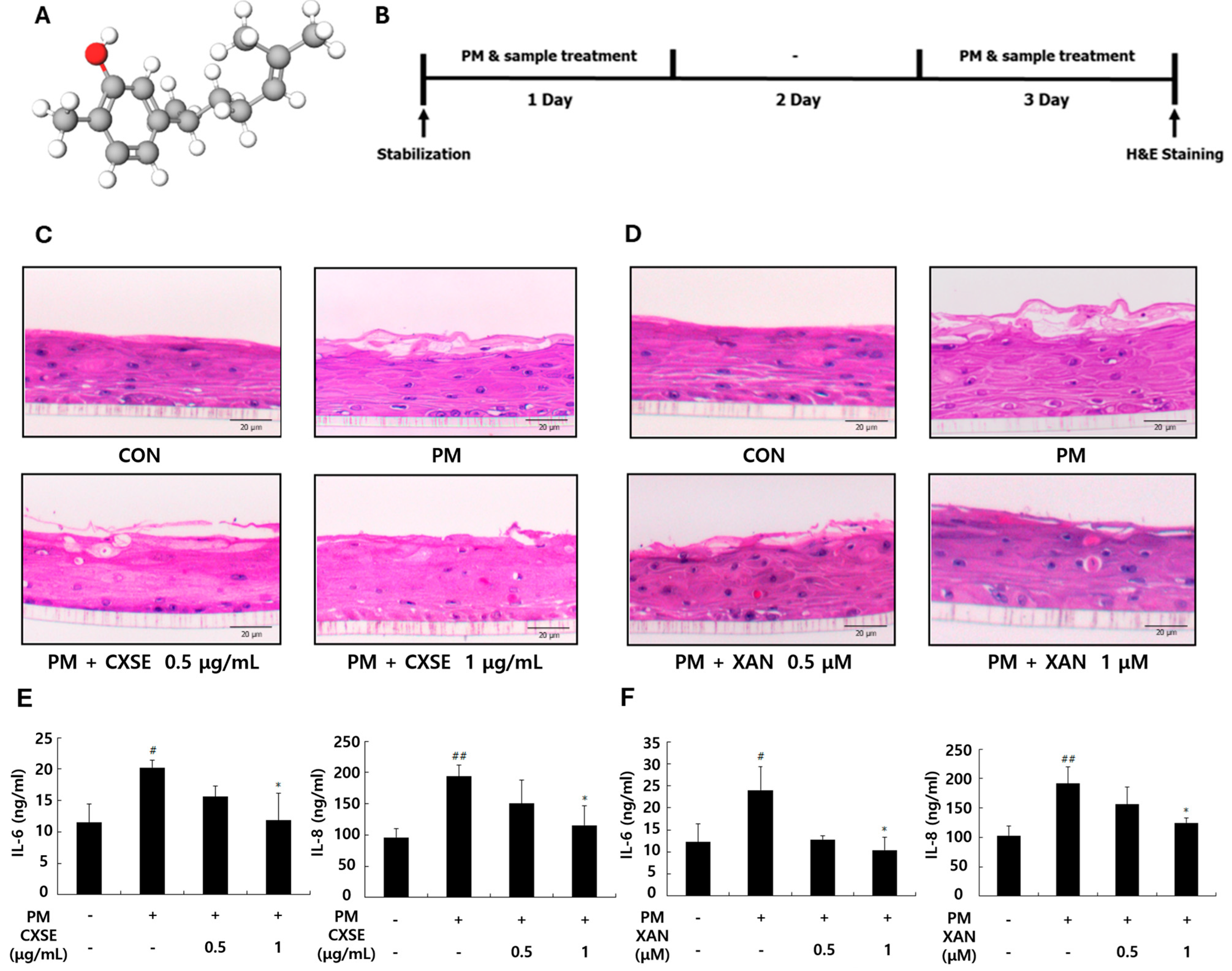
2.1. CXSE and XAN Mitigate PM-Induced Histological Changes and Suppress Inflammatory Cytokines in 3D-Reconstructed Skin
2.2. CXSE and XAN Modulate the Expression of AhR and Attenuate Oxidative Responses After PM Exposure
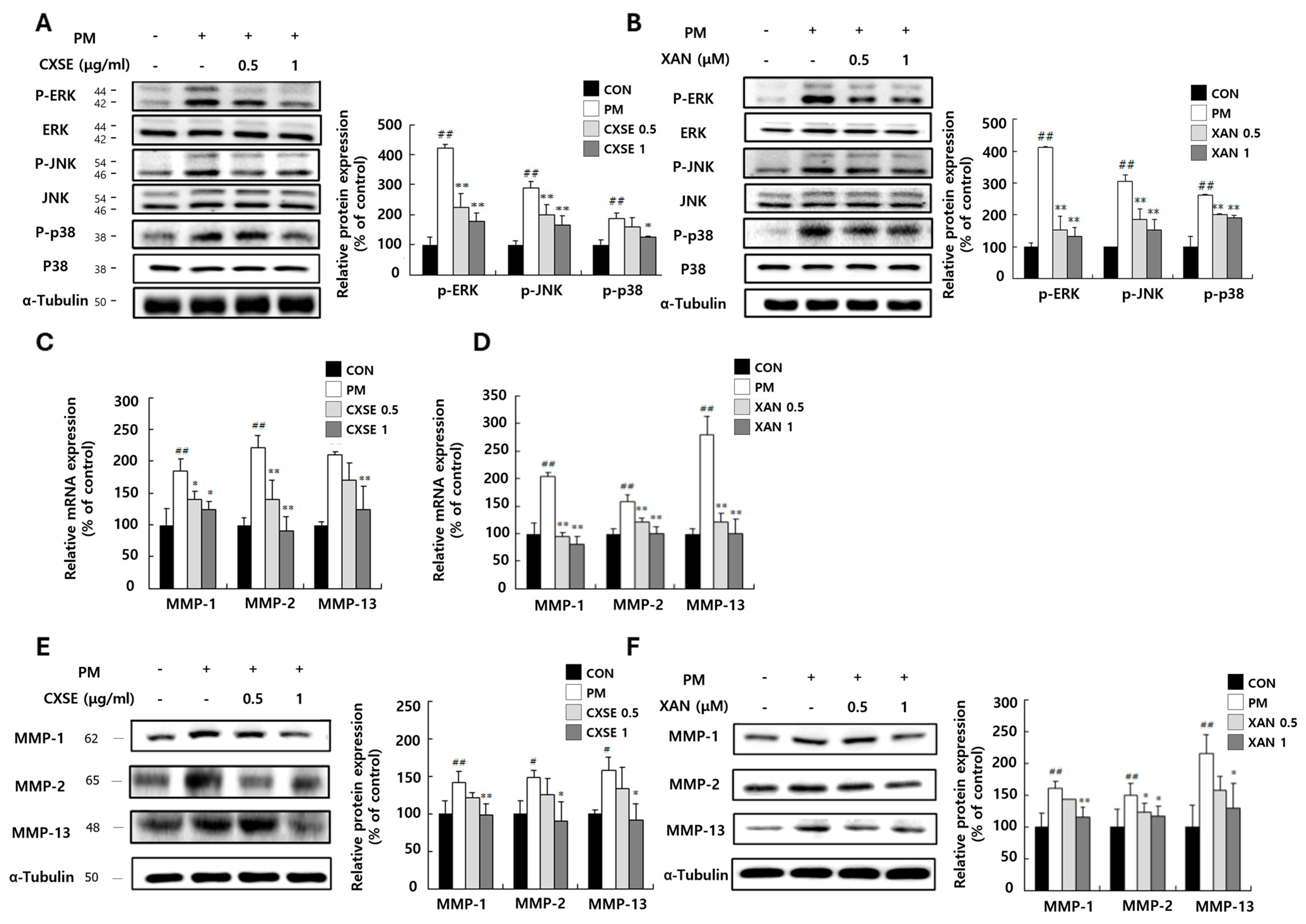
2.3. CXSE and XAN Downregulate MAPK Pathway and Suppress MMP mRNA Expression in PM-Treated HaCaT Cells
2.4. CXSE and XAN Reduce Inflammatory Responses in PM-Treated Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Preparation of CXSE and XAN
4.2. Preparation of Fine Particulate Matter Samples
4.3. Cell Culture
4.4. AhR Reporter Gene Luciferase Assay
4.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.6. Western Blot Analysis
4.7. Measurement of ROS
4.8. 3D-Reconstructed Human Skin Model Culture
4.9. Histological Analysis
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CXSE | Curcuma xanthorrhiza supercritical extract |
| XAN | Xanthorrhizol |
| PM | Particulate matter |
| AhR | Aryl hydrocarbon receptor |
| ECM | Extracellular matrix |
| Res | Resveratrol |
| BaP | Benzo[a]pyrene |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| MMPs | Matrix metalloproteinases |
| ROS | Reactive oxygen species |
| PAHsMAPK | Polycyclic aromatic hydrocarbonsMitogen-activated protein kinases |
References
- Salleh, N.A.; Ismail, S.; Ab Halim, M.R. Effects of Curcuma xanthorrhiza Extracts and Their Constituents on Phase II Drug-metabolizing Enzymes Activity. Pharmacogn. Res. 2016, 8, 309–315. [Google Scholar] [CrossRef]
- Oon, S.F.; Nallappan, M.; Tee, T.T.; Shohaimi, S.; Kassim, N.K.; Sa’ariwijaya, M.S.; Cheah, Y.H. Xanthorrhizol: A review of its pharmacological activities and anticancer properties. Cancer Cell Int. 2015, 15, 100. [Google Scholar] [CrossRef]
- Rahmat, E.; Lee, J.; Kang, Y. Javanese Turmeric (Curcuma xanthorrhiza Roxb.): Ethnobotany, Phytochemistry, Biotechnology, and Pharmacological Activities. Evid. Based Complement. Alternat. Med. 2021, 2021, 9960813. [Google Scholar] [CrossRef]
- Lim, C.S.; Jin, D.Q.; Mok, H.; Oh, S.J.; Lee, J.U.; Hwang, J.K.; Ha, I.; Han, J.S. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J. Neurosci. Res. 2005, 82, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.A.; Kim, S.H.; Chung, W.Y.; Hwang, J.K.; Park, K.K. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem. Biophys. Res. Commun. 2005, 326, 210–217. [Google Scholar] [CrossRef]
- Liao, W.P.; Khoo, Y.W.; Wen, K.G.J.; Wai-Shiu, F.W. Anti-oxidative and anti-inflammatory effects of Xanthorrhizol on aeroallergens-induced biological responses in vitro and ex vivo. Eur. Respir. J. 2019, 54, PA4206. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, M.S.; Hunter, R.P. Health Outcomes of Exposure to Biological and Chemical Components of Inhalable and Respirable Particulate Matter. Int. J. Environ. Res. Public Health 2016, 13, 593. [Google Scholar] [CrossRef]
- Schafer, T.; Ring, J. Epidemiology of allergic diseases. Allergy 1997, 52, 14–22; discussion 35–36. [Google Scholar] [CrossRef]
- Surawski, N.C.; Miljevic, B.; Ayoko, G.A.; Elbagir, S.; Stevanovic, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Ristovski, Z.D. Physicochemical characterization of particulate emissions from a compression ignition engine: The influence of biodiesel feedstock. Environ. Sci. Technol. 2011, 45, 10337–10343. [Google Scholar] [CrossRef]
- McCormack, M.C.; Breysse, P.N.; Hansel, N.N.; Matsui, E.C.; Tonorezos, E.S.; Curtin-Brosnan, J.; Williams, D.L.; Buckley, T.J.; Eggleston, P.A.; Diette, G.B. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ. Res. 2008, 106, 148–155. [Google Scholar] [CrossRef]
- Leikauf, G.D.; Kim, S.H.; Jang, A.S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Orellano, P.; Kasdagli, M.I.; Perez Velasco, R.; Samoli, E. Long-Term Exposure to Particulate Matter and Mortality: An Update of the WHO Global Air Quality Guidelines Systematic Review and Meta-Analysis. Int. J. Public Health 2024, 69, 1607683. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Thurston, G.D.; Virani, S.; Lavie, C.J. PM2.5 and cardiovascular diseases: State-of-the-Art review. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200217. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Pervaiz, S. Mitochondria: Redox metabolism and dysfunction. Biochem. Res. Int. 2012, 2012, 896751. [Google Scholar] [CrossRef]
- Ghio, A.J.; Devlin, R.B. Inflammatory lung injury after bronchial instillation of air pollution particles. Am. J. Respir. Crit. Care Med. 2001, 164, 704–708. [Google Scholar] [CrossRef]
- Arias-Perez, R.D.; Taborda, N.A.; Gomez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Fujii, T.; Hayashi, S.; Hogg, J.C.; Vincent, R.; Van Eeden, S.F. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 25, 265–271. [Google Scholar] [CrossRef]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-dependent transcriptional regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.H.; Nguyen, U.T.; Kim, Y.H.; Shin, H.M.; Yang, I.J. Astragali Radix and its compound formononetin ameliorate diesel particulate matter-induced skin barrier disruption by regulation of keratinocyte proliferation and apoptosis. J. Ethnopharmacol. 2019, 228, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, S.H.; Yoo, J.A.; Kwon, K.; Kim, J.W.; Oh, S.W.; Park, S.J.; Kim, J.; Yu, E.; Han, B.S.; et al. Antagonizing Effects of Clematis apiifolia DC. Extract against Benzo[a]pyrene-Induced Damage to Human Keratinocytes. Oxid. Med. Cell. Longev. 2019, 2019, 2386163. [Google Scholar] [CrossRef]
- Moon, J.Y.; Ngoc, L.T.N.; Chae, M.; Tran, V.V.; Lee, Y.C. Effects of Microwave-Assisted Opuntia humifusa Extract in Inhibiting the Impacts of Particulate Matter on Human Keratinocyte Skin Cell. Antioxidants 2020, 9, 271. [Google Scholar] [CrossRef]
- Paik, K.; Na, J.I.; Huh, C.H.; Shin, J.W. Particulate Matter and Its Molecular Effects on Skin: Implications for Various Skin Diseases. Int. J. Mol. Sci. 2024, 25, 9888. [Google Scholar] [CrossRef]
- Park, T.H.; Park, S.; Cho, M.K.; Kim, S. Associations of particulate matter with atopic dermatitis and chronic inflammatory skin diseases in South Korea. Clin. Exp. Dermatol. 2022, 47, 325–334. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Yang, R.; Yu, H.; Shang, S.; Hu, Y. Short-term exposure to ambient fine particulate matter and psoriasis: A time-series analysis in Beijing, China. Front. Public Health 2022, 10, 1015197. [Google Scholar] [CrossRef]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef] [PubMed]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Potprommanee, S.; Kiattisin, K. Comparison of Biological Activities and Protective Effects on PAH-Induced Oxidative Damage of Different Coffee Cherry Pulp Extracts. Foods 2023, 12, 4292. [Google Scholar] [CrossRef]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Duangnin, N.; Kiattisin, K. Potential of Coffee Cherry Pulp Extract against Polycyclic Aromatic Hydrocarbons in Air Pollution Induced Inflammation and Oxidative Stress for Topical Applications. Int. J. Mol. Sci. 2024, 25, 9416. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hsu, L.F.; Fang, J.Y.; Chiang, Y.C.; Tsai, M.H.; Lee, M.H.; Li, S.Y.; Hu, S.C.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Feng, B.; Liang, H.; Zhao, X.; Song, J. Particulate matter and inflammatory skin diseases: From epidemiological and mechanistic studies. Sci. Total Environ. 2023, 905, 167111. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; Lee, C.W.; Tsai, M.H.; Ko, H.H.; Fang, J.Y.; Chiang, Y.C.; Liang, C.J.; Hsu, L.F.; Hu, S.C.; Yen, F.L. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int. J. Nanomed. 2016, 11, 3907–3926. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Madushan Fernando, P.D.S.; Kang, H.K.; Ahn, Y.S.; Hyun, J.W. Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Mar. Drugs 2019, 17, 61. [Google Scholar] [CrossRef]
- Fribourg, S. Amenorrhea and endometrial atrophy. Obstet. Gynecol. 1985, 66, 836–837. [Google Scholar]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Kim, S.; Kook, K.E.; Kim, C.; Hwang, J.K. Inhibitory Effects of Curcuma xanthorrhiza Supercritical Extract and Xanthorrhizol on LPS-Induced Inflammation in HGF-1 Cells and RANKL-Induced Osteoclastogenesis in RAW264.7 Cells. J. Microbiol. Biotechnol. 2018, 28, 1270–1281. [Google Scholar] [CrossRef]
- Kook, K.E.; Kim, C.; Kang, W.; Hwang, J.K. Inhibitory Effect of Standardized Curcuma xanthorrhiza Supercritical Extract on LPS-Induced Periodontitis in Rats. J. Microbiol. Biotechnol. 2018, 28, 1614–1625. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.; Oh, B.; Lee, D.W.; Hwang, J.K. Standardized Curcuma xanthorrhiza Extract and Its Major Compound, Xanthorrhizol, Mitigate Cancer-Associated Muscle Atrophy in CT26-Bearing Mice by Inhibiting Catabolic Signaling Pathways. J. Med. Food 2025, 28, 377–384. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.W.; Hwang, J.K. Curcuma xanthorrhiza extract and xanthorrhizol ameliorate cancer-induced adipose wasting in CT26-bearing mice by regulating lipid metabolism and adipose tissue browning. Integr. Med. Res. 2024, 13, 101020. [Google Scholar] [CrossRef]
- Kim, M.B.; Kim, C.; Song, Y.; Hwang, J.K. Antihyperglycemic and Anti-Inflammatory Effects of Standardized Curcuma xanthorrhiza Roxb. Extract and Its Active Compound Xanthorrhizol in High-Fat Diet-Induced Obese Mice. Evid. Based Complement. Alternat. Med. 2014, 2014, 205915. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Hung, K.F.; Yen, C.C.; Laio, C.H.; Wang, J.L.; Lan, Y.W.; Chong, K.Y.; Fan, H.C.; Chen, C.M. Kefir peptides alleviate particulate matter <4 μm (PM4.0)-induced pulmonary inflammation by inhibiting the NF-KB pathway using luciferase transgenic mice. Sci. Rep. 2019, 9, 11529. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix Metalloproteinases on Skin Photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Tsai, J.H.; Chen, S.J.; Huang, K.L.; Lin, Y.C.; Lee, W.J.; Lin, C.C.; Lin, W.Y. PM, carbon, and PAH emissions from a diesel generator fuelled with soy-biodiesel blends. J. Hazard. Mater. 2010, 179, 237–243. [Google Scholar] [CrossRef]
- Huang, P.; Han, J.; Hui, L. MAPK signaling in inflammation-associated cancer development. Protein Cell 2010, 1, 218–226. [Google Scholar] [CrossRef]


| Gene | Direction | Sequence (5′-3′) |
|---|---|---|
| CYP1A1 | Forward | CTACCCAACCCTTCCCTGAAT |
| Reverse | CGCCCCTTGGGGATGTAAAA | |
| CYP1B1 | Forward | CTGCGACTCCAGTTGTGAGA |
| Reverse | AAGGAACTGGGACCTTTGCC | |
| MMP-1 | Forward | AAGTCAAGTTTGTGGCTTAT |
| Reverse | GACTCATGTCTCCTGTCTCT | |
| Catalase | Forward | GCCACAGGAAAGTACCCCTC |
| Reverse | CGGTGAGTGTCAGGATAGGC | |
| IL-6 | Forward | ATGAGGAGACTTGCCTGGTG |
| Reverse | ACAACAATCTGAGGTGCCCA | |
| IL-8 | Forward | CCAGGAAGAAACCACCGGAA |
| Reverse | CCTCTGCACCCAGTTTTCCT | |
| GAPDH | Forward | CTCCTGTTCGACAGTCAGCC |
| Reverse | TCGCCCCACTTGATTTTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Ko, E.-J.; Lee, D.; Kang, J.; Hwang, J.-K.; Kim, E. Protective Effects of Xanthorrhizol-Rich Extracts Against PM-Induced Skin Damage in Human Keratinocytes and 3D-Reconstructed Skin Models. Pharmaceuticals 2025, 18, 808. https://doi.org/10.3390/ph18060808
Kang H, Ko E-J, Lee D, Kang J, Hwang J-K, Kim E. Protective Effects of Xanthorrhizol-Rich Extracts Against PM-Induced Skin Damage in Human Keratinocytes and 3D-Reconstructed Skin Models. Pharmaceuticals. 2025; 18(6):808. https://doi.org/10.3390/ph18060808
Chicago/Turabian StyleKang, Haneul, Eun-Ji Ko, Dahye Lee, Junhui Kang, Jae-Kwan Hwang, and Eunsoo Kim. 2025. "Protective Effects of Xanthorrhizol-Rich Extracts Against PM-Induced Skin Damage in Human Keratinocytes and 3D-Reconstructed Skin Models" Pharmaceuticals 18, no. 6: 808. https://doi.org/10.3390/ph18060808
APA StyleKang, H., Ko, E.-J., Lee, D., Kang, J., Hwang, J.-K., & Kim, E. (2025). Protective Effects of Xanthorrhizol-Rich Extracts Against PM-Induced Skin Damage in Human Keratinocytes and 3D-Reconstructed Skin Models. Pharmaceuticals, 18(6), 808. https://doi.org/10.3390/ph18060808







