Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract
Abstract
1. Introduction
2. Results
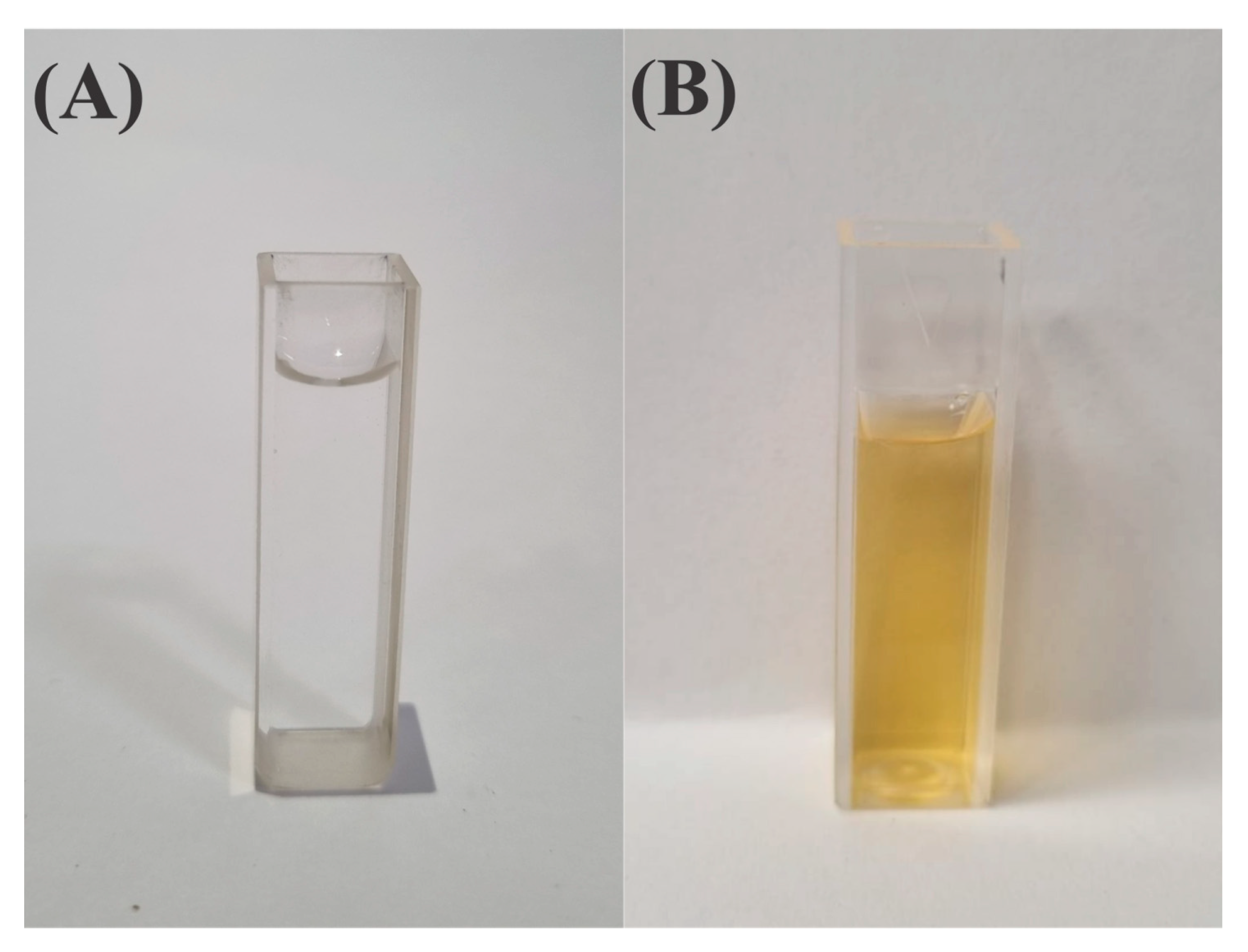
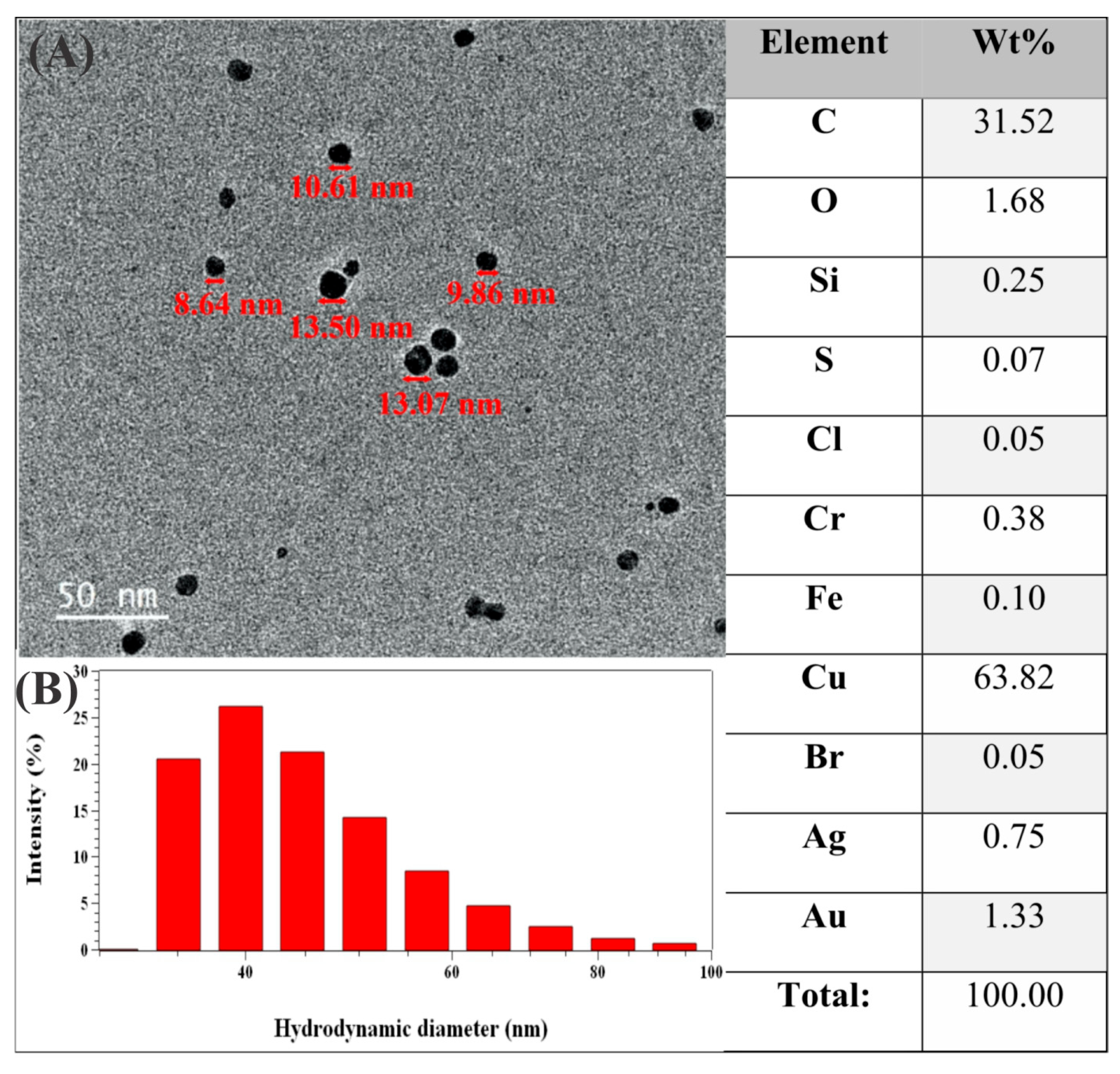
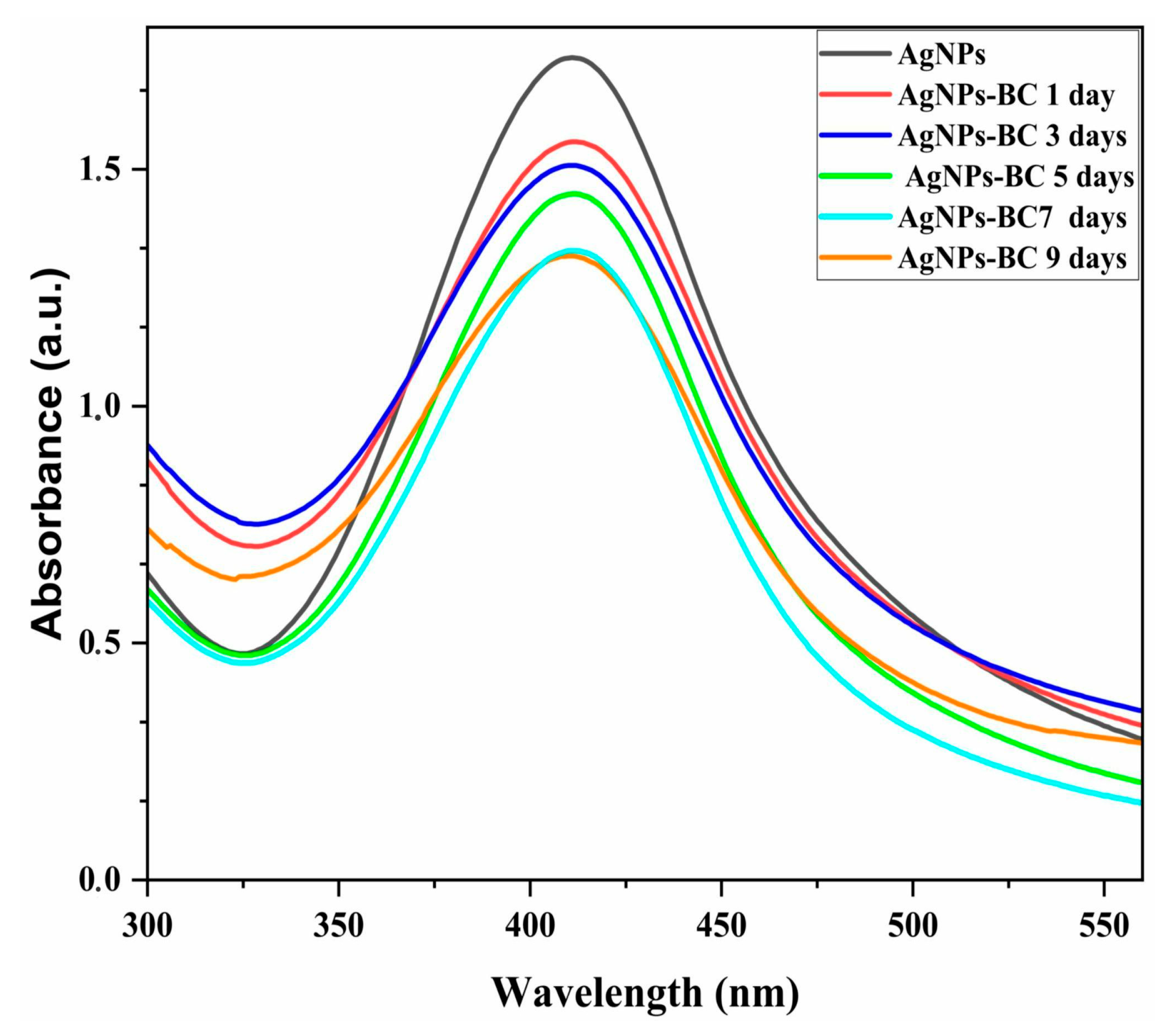
2.1. Production and Characterization of AgNPs
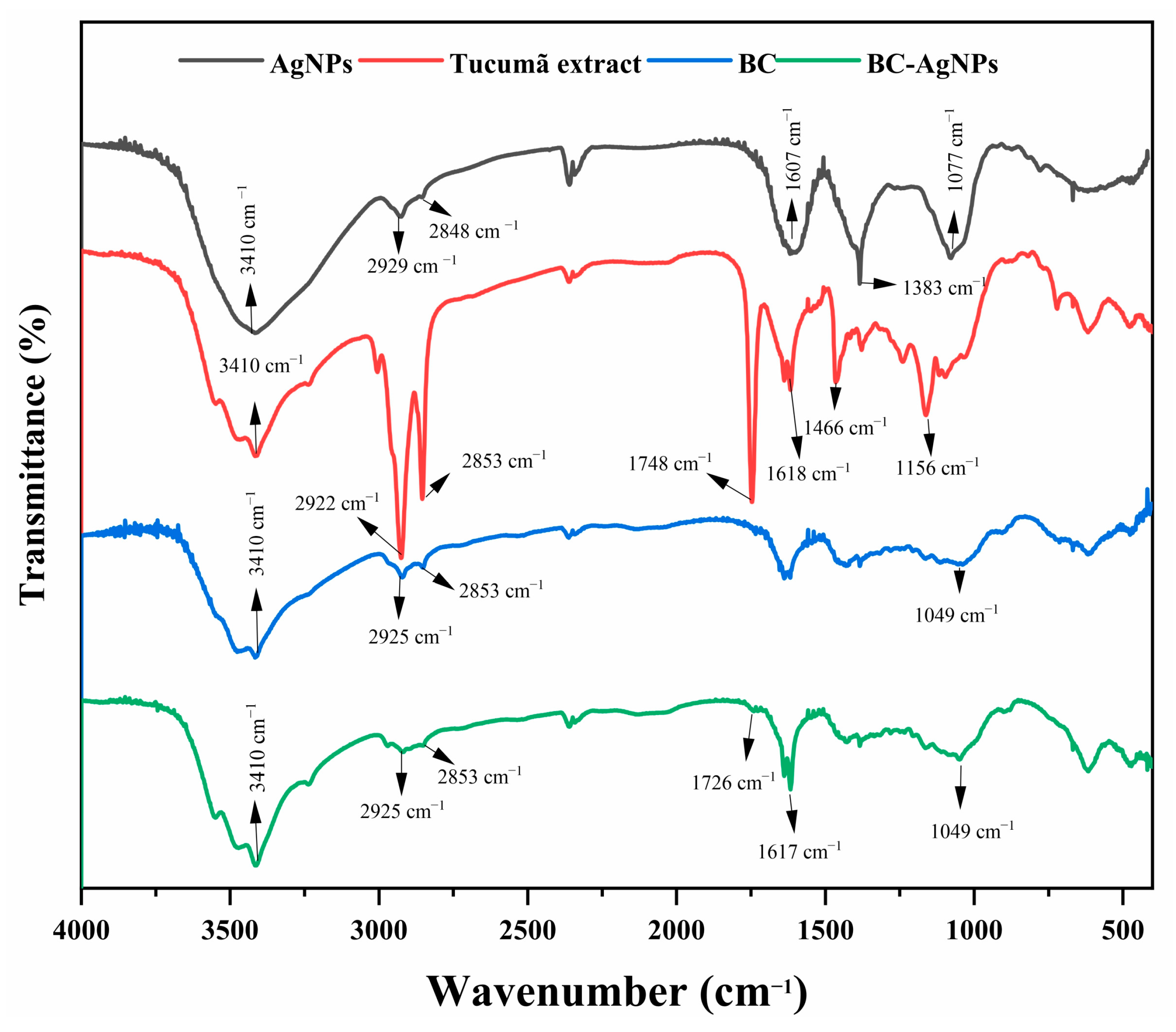
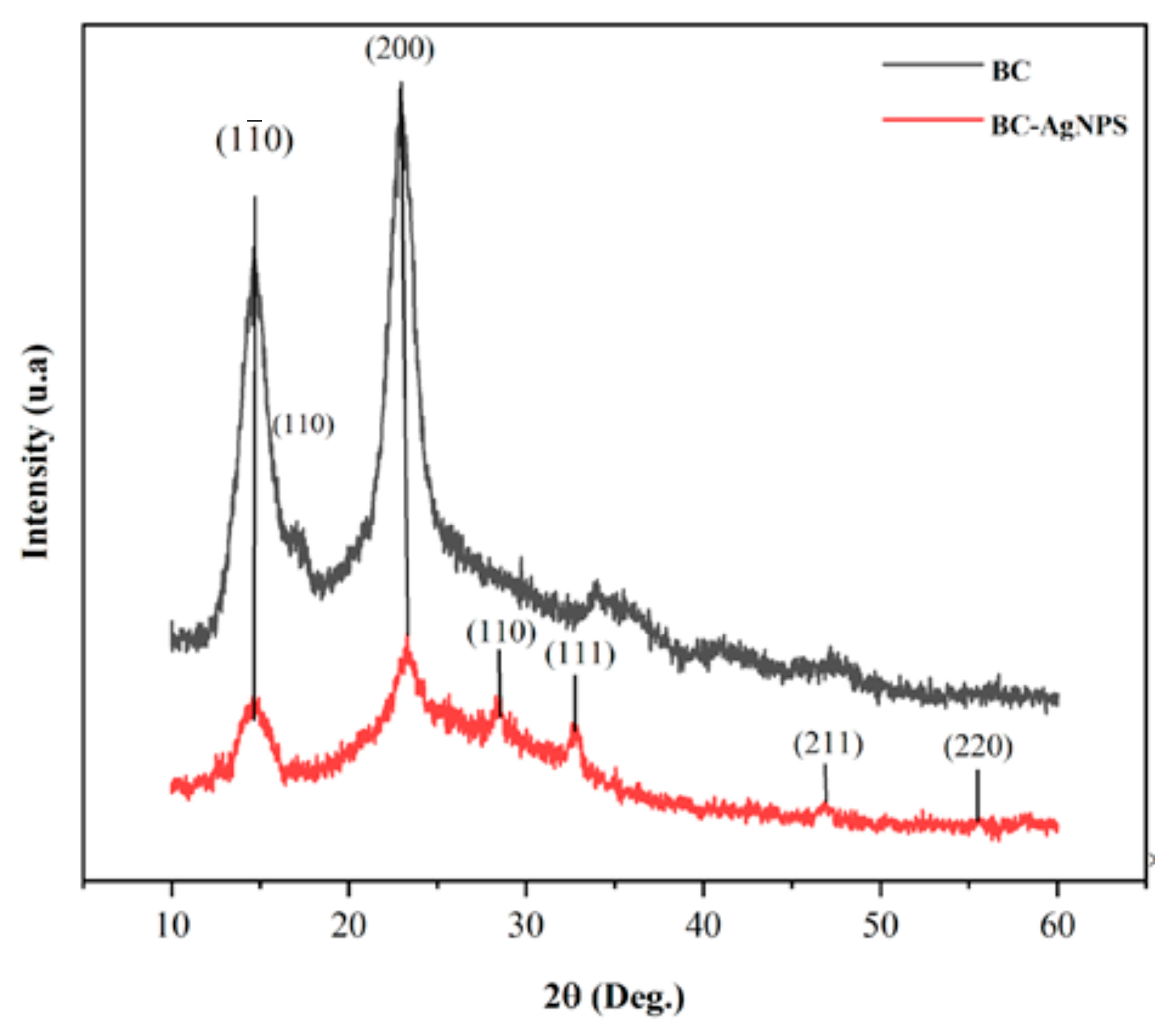
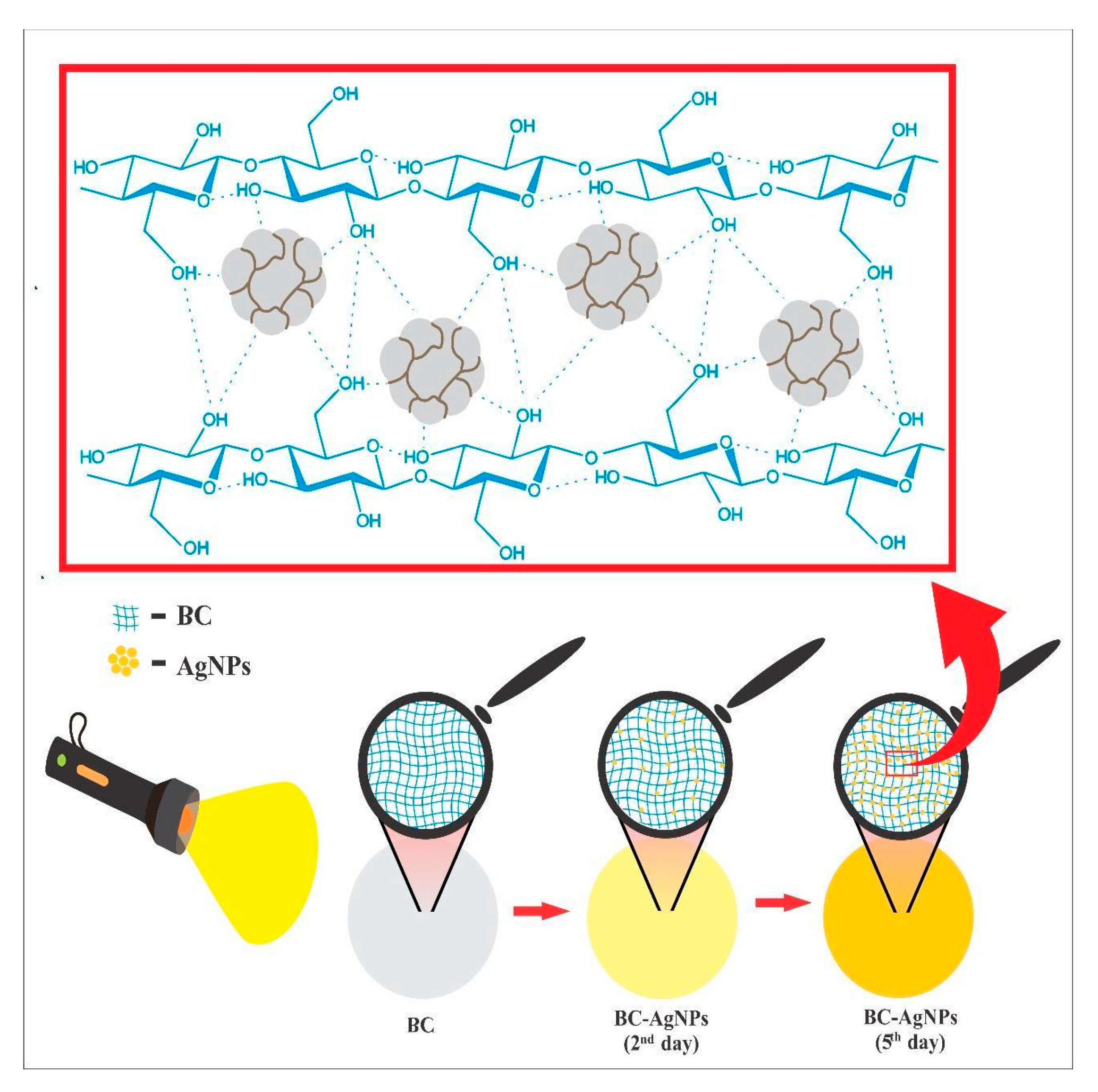
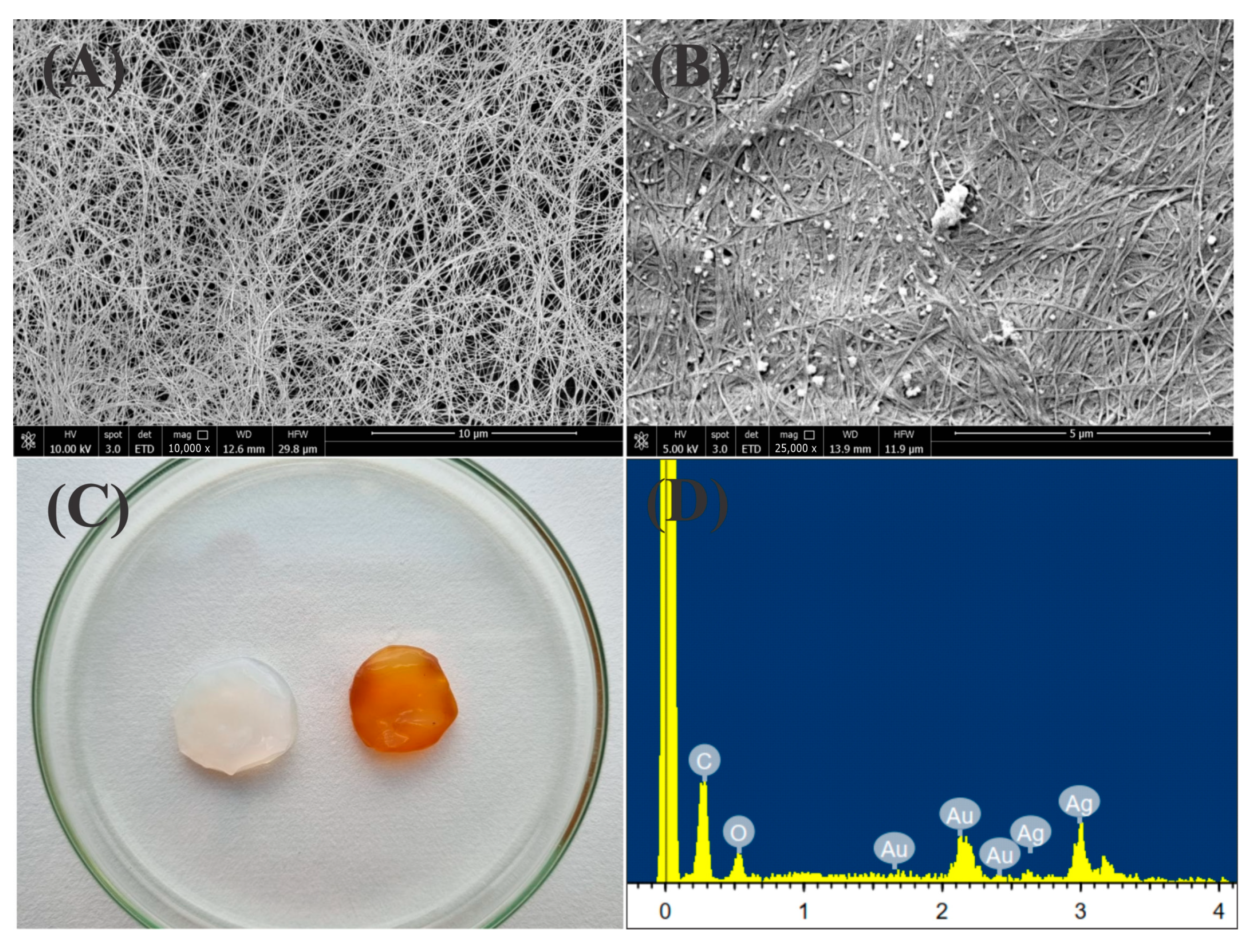
2.2. Chemical and Morphological Characterization of the BC-AgNP Nanocomposite
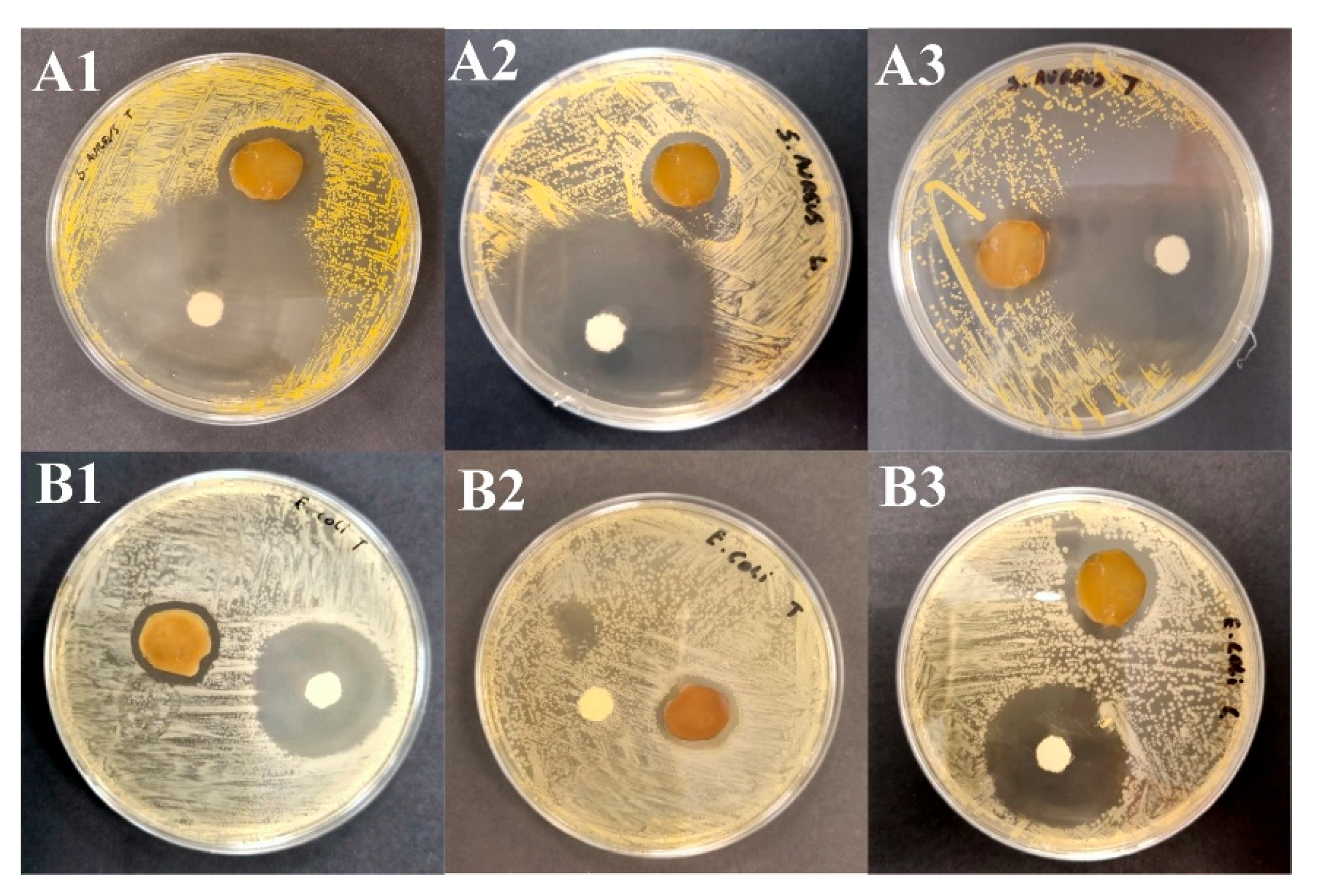
2.3. Assay of Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Preparation of Bacterial Cellulose (BC)
4.3. Production of Colloidal Dispersion of Silver Nanoparticles
4.4. Characterization of AgNPs
4.4.1. UV-Vis Spectroscopy
4.4.2. Dynamic Light Scattering
4.4.3. Transmission Electron Microscopy (TEM)
4.5. Incorporation of AgNPs into BC
4.6. Characterization of the BC-AgNP Nanocomposite
4.6.1. Morphological Study by Scanning Electron Microscopy (SEM)
4.6.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.6.3. X-Ray Diffraction Analysis (XRD)
4.6.4. Degree of Swelling
4.7. Inductively Coupled Plasma Analysis
4.8. Antimicrobial Activity
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNO3 | Silver nitrate |
| AgNPs | Silver nanoparticles |
| BC | Bacterial cellulose |
| BC-AgNP | Nanocomposite of silver nanoparticles and bacterial cellulose |
| DLS | Dynamic light scattering |
| EDS | Energy dispersive spectrometer |
| FTIR | Fourier transform infrared spectroscopy |
| ICP | Inductively coupled plasma analysis |
| NaOH | Sodium hypochlorite |
| NPs | Nanoparticles |
| OH− | Hydroxyl ion |
| PDI | Polydispersity index |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| UV-Vis | Ultraviolet–visible microscopy |
| XRD | X-ray diffraction analysis |
References
- Rozman, U.; Pušnik, M.; Kmetec, S.; Duh, D.; Šostar Turk, S. Reduced Susceptibility and Increased Resistance of Bacteria Against Disinfectants: A Systematic Review. Microorganisms 2021, 9, 2550. [Google Scholar] [CrossRef]
- Kumar, Y. Antimicrobial Resistance: A Global Threat; BoD–Books on Demand: Norderstedt, Germany, 2019; ISBN 178985783X. [Google Scholar]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial Cellulose in Food Industry: Current Research and Future Prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef]
- Sabio, L.; Sosa, A.; Delgado-López, J.M.; Dominguez-Vera, J.M. Two-Sided Antibacterial Cellulose Combining Probiotics and Silver Nanoparticles. Molecules 2021, 26, 2848. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Carnero Canales, C.S.; Primo, L.M.D.G.; Colturato, V.M.M.; Polinário, G.; Di Filippo, L.D.; Duarte, J.L.; Chorilli, M.; da Silva Barud, H.; Pavan, F.R. Cellulose from Bacteria as a Delivery System for Improved Treatment of Infectious Diseases: A Review of Updates and Prospects. Int. J. Biol. Macromol. 2024, 277, 133831. [Google Scholar] [CrossRef]
- Wahid, F.; Huang, L.H.; Zhao, X.Q.; Li, W.C.; Wang, Y.Y.; Jia, S.R.; Zhong, C. Bacterial Cellulose and Its Potential for Biomedical Applications. Biotechnol. Adv. 2021, 53, 7856. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Treviño-Garza, M.; Guerrero-Medina, A.; González-Sánchez, R.; García-Gómez, C.; Guzmán-Velasco, A.; Báez-González, J.; Márquez-Reyes, J. Production of Microbial Cellulose Films from Green Tea (Camellia Sinensis) Kombucha with Various Carbon Sources. Coatings 2020, 10, 1132. [Google Scholar] [CrossRef]
- Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa Carandas l. And Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules 2021, 11, 299. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. In Situ Development of Nanosilver-Impregnated Bacterial Cellulose for Sustainable Released Antimicrobial Wound Dressing. J. Appl. Biomater. Funct. Mater. 2016, 14, 53–58. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Carson, L.; Bandara, S.; Joseph, M.; Green, T.; Grady, T.; Osuji, G.; Weerasooriya, A.; Ampim, P.; Woldesenbet, S. Green Synthesis of Silver Nanoparticles with Antimicrobial Properties Using Phyla Dulcis Plant Extract. Foodborne Pathog. Dis. 2020, 17, 504–511. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Oves, M.; Ahmar Rauf, M.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Sarwar Zaman, G.; Saeed, M. Green Synthesis of Silver Nanoparticles by Conocarpus Lancifolius Plant Extract and Their Antimicrobial and Anticancer Activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef]
- Cárdenas Sierra, D.M.; Gómez Rave, L.J.; Soto, J.A. Biological Activity of Sacha Inchi (Plukenetia Volubilis Linneo) and Potential Uses in Human Health: A Review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef]
- Lopez-Vega, H.; Lakemond, N. Tapping into Emerging Markets: EMNEs Strategies for Innovation Capability Building. Glob. Strategy J. 2022, 12, 394–417. [Google Scholar] [CrossRef]
- Fine, P.V.A.; Salazar, D.; Martin, R.E.; Metz, M.R.; Misiewicz, T.M.; Asner, G.P. Exploring the Links between Secondary Metabolites and Leaf Spectral Reflectance in a Diverse Genus of Amazonian Trees. Ecosphere 2021, 12, 33–62. [Google Scholar] [CrossRef]
- Garrett, R.D.; Cammelli, F.; Ferreira, J.; Levy, S.A.; Valentim, J.; Vieira, I. Annual Review of Environment and Resources Forests and Sustainable Development in the Brazilian Amazon: History, Trends, and Future Prospects. Annu. Rev. Environ. 2021, 46, 525–552. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M.; Iqbal, T. Green Synthesis of Silver Nanoparticles by Using Various Extracts: A Review. Inorg. Nano-Metal Chem. 2020, 51, 744–755. [Google Scholar] [CrossRef]
- dos Santos, S.S.; de Couto, R.A.A.; da Silva, I.R.; Aouada, M.R.M.; Costantino, V.R.L.; da Costa, L.P.; Perotti, G.F. Production of Silver Nanoparticles Mediated by Aqueous Extracts of Tucumã (Astrocaryum aculeatum) Pulp. J. Braz. Chem. Soc. 2023, 34, 705–712. [Google Scholar] [CrossRef]
- Machado, A.P.d.F.; Nascimento, R.d.P.d.; Alves, M.d.R.; Reguengo, L.M.; Marostica Junior, M.R. Brazilian Tucumã-Do-Amazonas (Astrocaryum aculeatum) and Tucumã-Do-Pará (Astrocaryum Vulgare) Fruits: Bioactive Composition, Health Benefits, and Technological Potential. Food Res. Int. 2022, 151, 110902. [Google Scholar] [CrossRef]
- Guex, C.G.; Cassanego, G.B.; Dornelles, R.C.; Casoti, R.; Engelmann, A.M.; Somacal, S.; Maciel, R.M.; Duarte, T.; Borges, W.d.S.; Andrade, C.M.d.; et al. Tucumã (Astrocaryum aculeatum) Extract: Phytochemical Characterization, Acute and Subacute Oral Toxicity Studies in Wistar Rats. Drug Chem. Toxicol. 2022, 45, 810–821. [Google Scholar] [CrossRef]
- Wallner, C.; Moormann, E.; Lulof, P.; Drysch, M.; Lehnhardt, M.; Behr, B. Burn Care in the Greek and Roman Antiquity. Medicina 2020, 56, 657. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite Hydrogels with Embedded Silver Nanoparticles and Ibuprofen as Wound Dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Chen, S.; Feng, C.; Chen, S.; Yin, N.; Yang, J.; Wang, H.; Xu, Y. Facilely Green Synthesis of Silver Nanoparticles into Bacterial Cellulose. Cellulose 2015, 22, 373–383. [Google Scholar] [CrossRef]
- Berndt, S.; Wesarg, F.; Wiegand, C.; Kralisch, D.; Müller, F.A. Antimicrobial Porous Hybrids Consisting of Bacterial Nanocellulose and Silver Nanoparticles. Cellulose 2013, 20, 771–783. [Google Scholar] [CrossRef]
- Zhou, S.; Peng, H.; Zhao, A.; Zhang, R.; Li, T.; Yang, X.; Lin, D. Synthesis of Bacterial Cellulose Nanofibers/Ag Nanoparticles: Structure, Characterization and Antibacterial Activity. Int. J. Biol. Macromol. 2024, 259, 129392. [Google Scholar] [CrossRef]
- Anastas, P.T.; Williamson, T.C. Green Chemistry: An Overview; ACS Publications: Washington, DC, USA, 1996. [Google Scholar]
- Ruby; Aryan; Mehata, M.S. Surface Plasmon Resonance Allied Applications of Silver Nanoflowers Synthesized from Breynia Vitis-Idaea Leaf Extract. Dalton Trans. 2022, 51, 2726–2736. [Google Scholar] [CrossRef]
- Fadakar Sarkandi, A.; Montazer, M.; Harifi, T.; Mahmoudi Rad, M. Innovative Preparation of Bacterial Cellulose/Silver Nanocomposite Hydrogels: In Situ Green Synthesis, Characterization, and Antibacterial Properties. J. Appl. Polym. Sci. 2021, 138, 49824. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Pu, S.; Gong, C.; Robertson, A.W. Liquid Cell Transmission Electron Microscopy and Its Applications. R. Soc. Open Sci. 2020, 7, 191204. [Google Scholar] [CrossRef]
- Wang, P.; Xu, F.; Gao, P.; Cai, S.; Bai, X. In-Situ Optical TEM. In In-Situ Transmission Electron Microscopy; Springer Nature: Singapore, 2023; pp. 151–186. [Google Scholar] [CrossRef]
- Patil, R.M.; Deshpande, P.P.; Aalhate, M.; Gananadhamu, S.; Singh, P.K. An Update on Sophisticated and Advanced Analytical Tools for Surface Characterization of Nanoparticles. Surf. Interfaces 2022, 33, 102165. [Google Scholar] [CrossRef]
- Shumail, H.; Khalid, S.; Ahmad, I.; Khan, H.; Amin, S.; Ullah, B. Review on Green Synthesis of Silver Nanoparticles through Plants. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 994–1007. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Wamunyokoli, F.; Magoma, G. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona Muricata. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1231–1242. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic Light Scattering and Transmission Electron Microscopy in Drug Delivery: A Roadmap for Correct Characterization of Nanoparticles and Interpretation of Results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Maheshwari, D.; Chawathe, A.; Sharma, N. Current Analytical Approaches for Characterizing Nanoparticle Sizes in Pharmaceutical Research. J. Nanoparticle Res. 2024, 26, 19. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver Nanoparticles: Toxicity in Model Organisms as an Overview of Its Hazard for Human Health and the Environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Hoang, S.A.; Bolan, N.S.; Kirkham, M.B.; Liu, J.; Xia, X.; Li, Y. Silver Nanoparticles in Aquatic Sediments: Occurrence, Chemical Transformations, Toxicity, and Analytical Methods. J. Hazard. Mater. 2021, 418, 126368. [Google Scholar] [CrossRef] [PubMed]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of Plant Extracts on the Characteristics of Silver Nanoparticles for Topical Application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.K.O.; Vasconcelos, A.A.; Kobayashi, R.K.T.; Nakazato, G.; Braga, H.d.C.; Taube, P.S. Green Synthesis: Characterization and Biological Activity of Silver Nanoparticles Using Aqueous Extracts of Plants from the Arecaceae Family. Acta Sci. Technol. 2021, 43, 52011. [Google Scholar] [CrossRef]
- Csapó, E.; Patakfalvi, R.; Hornok, V.; Tóth, L.T.; Sipos, Á.; Szalai, A.; Csete, M.; Dékány, I. Effect of PH on Stability and Plasmonic Properties of Cysteine-Functionalized Silver Nanoparticle Dispersion. Colloids Surf. B Biointerfaces 2012, 98, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental Methods in Chemical Engineering: Zeta Potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Strong Antimicrobial Activity of Silver Nanoparticles Obtained by the Green Synthesis in Viridibacillus Sp. Extracts. Front. Microbiol. 2022, 13, 48. [Google Scholar] [CrossRef]
- Barzan, G.; Rocchetti, L.; Portesi, C.; Pellegrino, F.; Taglietti, A.; Rossi, A.M.; Giovannozzi, A.M. Surface Minimal Bactericidal Concentration: A Comparative Study of Active Glasses Functionalized with Different-Sized Silver Nanoparticles. Colloids Surf. B Biointerfaces 2021, 204, 111800. [Google Scholar] [CrossRef]
- Meroni, G.; Filipe, J.F.S.; Martino, P.A. In Vitro Antibacterial Activity of Biological-Derived Silver Nanoparticles: Preliminary Data. Vet. Sci. 2020, 7, 12. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, L.; Fang, H.; Yang, P.; Wang, B.; Li, L.; Wang, M.; Feng, W. Single Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry—A Powerful Tool for the Analysis of Nanoparticles in the Environment. Processes 2023, 11, 1237. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Zayed, M.; Salama, H.S. Antibacterial Potential of Bacterial Cellulose Impregnated with Green Synthesized Silver Nanoparticle Against S. aureus and P. aeruginosa. Curr. Microbiol. 2023, 80, 3182. [Google Scholar] [CrossRef] [PubMed]
- Mutiara, T.; Fahrurrozi, M.; Sulistyo, H.; Hidayat, M. Green Synthesis Methods and Characterization of Bacterial Cellulose/Silver Nanoparticle Composites. Green. Process. Synth. 2023, 12, 67. [Google Scholar] [CrossRef]
- Rengarajan, S.; Thangavel, N.; Sivalingam, A.M.; Lakshmanan, G.; Selvakumari, J.; Pandian, A. Green Synthesis and Characterization of Silver Nanoparticles with Different Solvent Extracts of Sesbania grandiflora (L.) Poiret and Assessment of Their Antibacterial and Antioxidant Potentials. Biomass Convers. Biorefin 2023, 23, 1–17. [Google Scholar] [CrossRef]
- Rizwan, M.; Amin, S.; Kudaibergenova, B.M.; Rauf, A.; Siddique, M.; Ullah, K.; Bawazeer, S.; Farooq, U.; Mabkhot, Y.N.; Ramadan, M.F. Green Synthesis and Antimicrobial Potential of Silver Nanoparticles with Boerhavia Procumbens Extract. J. Pure Appl. Microbiol. 2020, 14, 1437–1451. [Google Scholar] [CrossRef]
- Jenkhongkarn, R.; Phisalaphong, M. Effect of Reduction Methods on the Properties of Composite Films of Bacterial Cellulose-Silver Nanoparticles. Polymers 2023, 15, 142996. [Google Scholar] [CrossRef]
- Mohammad, N.H.; Hammad, A.A.; Askar, A.A.; Abou, S.A.; Nour, E. Gamma Ray-Induced Synthesis of Silver Nanoparticles Using Bacterial Cellulose as a Multi-Functional Agent and Its Application. Res. Sq. 2021, 1, 522859. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Silver Decorated Bacterial Cellulose Nanocomposites as Antimicrobial Food Packaging Materials. ES Food Agrofor. 2021, 6, 12–26. [Google Scholar] [CrossRef]
- Khina, A.G.; Krutyakov, Y.A. Similarities and Differences in the Mechanism of Antibacterial Action of Silver Ions and Nanoparticles. Appl. Biochem. Microbiol. 2021, 57, 683–693. [Google Scholar] [CrossRef]
- Jiang, X.; Foldbjerg, R.; Miclaus, T.; Wang, L.; Singh, R.; Hayashi, Y.; Sutherland, D.; Chen, C.; Autrup, H.; Beer, C. Multi-Platform Genotoxicity Analysis of Silver Nanoparticles in the Model Cell Line CHO-K1. Toxicol. Lett. 2013, 222, 55–63. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Żyro, D.; Sikora, J.; Szynkowska-Jóźwik, M.I.; Ochocki, J. Silver, Its Salts and Application in Medicine and Pharmacy. Int. J. Mol. Sci. 2023, 24, 15723. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Narayanan, M.; Basha, S.; Elfasakhany, A.; Brindhadevi, K.; Xia, C.; Pugazhendhi, A. In Vitro and in Vivo Efficacy of Green Synthesized AgNPs against Gram Negative and Gram Positive Bacterial Pathogens. Process Biochem. 2022, 112, 241–247. [Google Scholar] [CrossRef]
- Ciftci, F. Bioadhesion, Antimicrobial Activity, and Biocompatibility Evaluation Bacterial Cellulose Based Silver Nanoparticle Bioactive Composite Films. Process Biochem. 2023, 12, 21. [Google Scholar] [CrossRef]
- Elayaraja, S.; Liu, G.; Zagorsek, K.; Mabrok, M.; Ji, M.; Ye, Z.; Zhu, S.; Rodkhum, C. TEMPO-Oxidized Biodegradable Bacterial Cellulose (BBC) Membrane Coated with Biologically-Synthesized Silver Nanoparticles (AgNPs) as a Potential Antimicrobial Agent in Aquaculture (In Vitro). Aquaculture 2021, 530, 735–746. [Google Scholar] [CrossRef]
| Samples | Dry Weight (g) | Wet Weight (g) | Average Water Absorption (%) |
|---|---|---|---|
| BC | 0.012 ± 0.0008 | 0.105 ± 0.006 | 777 |
| BC–AgNP | 0.018 ± 0.0007 | 0.118 ± 0.0015 | 556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, S.S.; Cerqueira, M.Â.; Azevedo, A.G.; Pastrana, L.M.; Aouada, F.A.; Tanaka, F.C.; Perotti, G.F.; de Moura, M.R. Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract. Pharmaceuticals 2025, 18, 799. https://doi.org/10.3390/ph18060799
dos Santos SS, Cerqueira MÂ, Azevedo AG, Pastrana LM, Aouada FA, Tanaka FC, Perotti GF, de Moura MR. Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract. Pharmaceuticals. 2025; 18(6):799. https://doi.org/10.3390/ph18060799
Chicago/Turabian Styledos Santos, Sidney S., Miguel Ângelo Cerqueira, Ana Gabriela Azevedo, Lorenzo M. Pastrana, Fauze Ahmad Aouada, Fabrício C. Tanaka, Gustavo Frigi Perotti, and Marcia Regina de Moura. 2025. "Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract" Pharmaceuticals 18, no. 6: 799. https://doi.org/10.3390/ph18060799
APA Styledos Santos, S. S., Cerqueira, M. Â., Azevedo, A. G., Pastrana, L. M., Aouada, F. A., Tanaka, F. C., Perotti, G. F., & de Moura, M. R. (2025). Biotechnological Utilization of Amazonian Fruit: Development of Active Nanocomposites from Bacterial Cellulose and Silver Nanoparticles Based on Astrocaryum aculeatum (Tucumã) Extract. Pharmaceuticals, 18(6), 799. https://doi.org/10.3390/ph18060799










