Near-Infrared Photoimmunotherapy in Brain Tumors—An Unexplored Frontier
Abstract
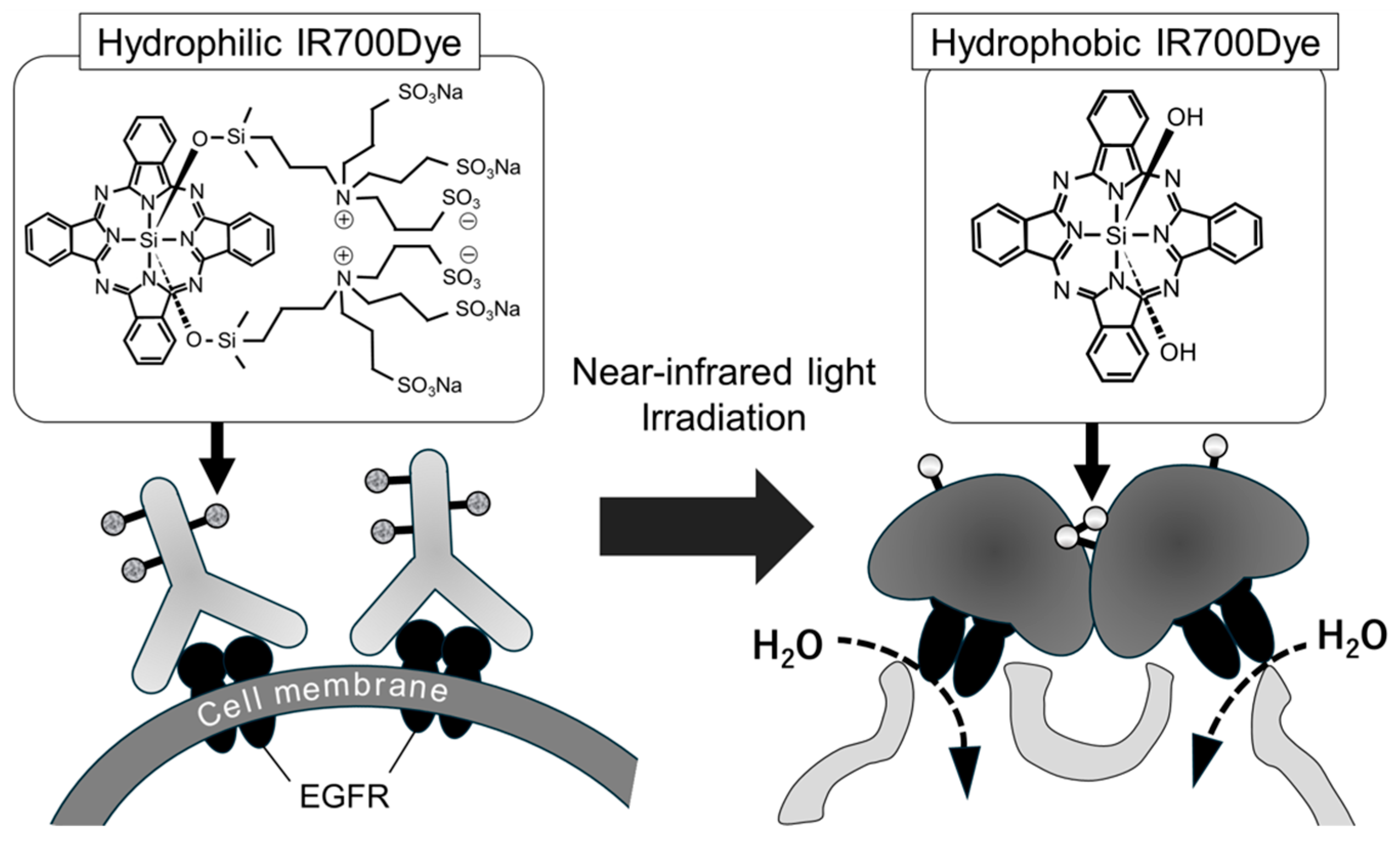
1. Introduction
2. PDT vs. NIR-PIT
3. Therapeutic Potential of NIR-PIT: Consideration of Targetable Surface Antigens Based on Brain Tumor Type
3.1. Glioblastoma
3.2. Glioma Stem Cells
3.3. Brain Metastasis
3.4. Malignant Meningioma
3.5. Functional Pituitary Neuroendocrine Tumor (PitNET)
4. Designing the Optimal Partner for IR700 Conjugate
4.1. Challenges in Developing Ideal Conjugates for NIR-PIT in Brain Tumor
4.2. The Epitope of Antibody for NIR-PIT Is in the Extracellular Domain
4.3. New Strategies to Deliver IR700 Conjugate to the Brain
4.4. Preliminary Data Showing Rapid Uptake of Affibody Conjugate in an Intracranial Xenograft Model
5. Immune Response After NIR-PIT
5.1. Mechanisms of NIR-PIT-Induced Immunogenic Cell Death in Brain Tumors
5.2. Unique Challenges and Potential of NIR-PIT for Brain Tumors
6. Treating Adverse Events in NIR-PIT for Brain Tumors
7. NIR-PIT for Brain Tumors
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH | Aldehyde dehydrogenases |
| BBB | Blood–brain barrier |
| BBTB | Blood–brain–tumor barrier |
| CAR-T | Chimeric antigen receptor-T |
| CLEC-2 | C-type lectin-like receptor-2 |
| DAMPs | Damage-associated molecular patterns |
| DB | Diabody |
| EGFRvIII | Epidermal growth factor variant III |
| H&E | Hematoxylin and eosin |
| HNSCC | Head and neck squamous cell carcinoma |
| ICD | Immunogenic cell death |
| LDL | Low-density lipoproteins |
| MB | Minibody |
| NEC | Neuroendocrine carcinoma |
| NEN | Neuroendocrine neoplasm |
| NET | Neuroendocrine tumor |
| NIR-PIT | Near-infrared photoimmunotherapy |
| NSCLC | Non-small cell lung cancer |
| PDT | Photodynamic therapy |
| PET | Positron emission tomography |
| Pit-NEC | Pituitary neuroendocrine carcinoma |
| Pit-NET | Pituitary neuroendocrine tumor |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PRRT | Peptide receptor radionuclide therapy |
| PSMA | Prostate-specific membrane antigen |
| ROS | Reactive oxygen species |
| SSA | Somatostatin analogue |
| SSTR-2 | Somatostatin receptor type 2 |
| SSTR-5 | Somatostatin receptor type 5 |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Fukuya, Y.; Ikuta, S.; Maruyama, T.; Nitta, M.; Saito, T.; Tsuzuki, S.; Chernov, M.; Kawamata, T.; Muragaki, Y. Tumor recurrence patterns after surgical resection of intracranial low-grade gliomas. J. Neuro-Oncol. 2019, 144, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors-A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef]
- Dougherty, T.J. Photodynamic therapy. Photochem. Photobiol. 1993, 58, 895–900. [Google Scholar] [CrossRef]
- Nwogu, C.; Kloc, A.; Attwood, K.; Bshara, W.; Durrani, F.; Pandey, R. Porfimer Sodium Versus PS785 for Photodynamic Therapy (PDT) of Lung Cancer Xenografts in Mice. J. Surg. Res. 2021, 263, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Tokashiki, R.; Ito, H.; Shimizu, A.; Nakamura, K.; Hiramatsu, H.; Tsukahara, K.; Shimizu, S.; Takata, D.; Okamoto, I.; et al. Therapeutic effects of a new photosensitizer for photodynamic therapy of early head and neck cancer in relation to tissue concentration. Auris Nasus Larynx 2008, 35, 545–551. [Google Scholar] [CrossRef]
- Matsumura, H.; Akimoto, J.; Haraoka, J.; Aizawa, K. Uptake and retention of the photosensitizer mono-L-asparthyl chlorine e6 in experimental malignant glioma. Lasers Med. Sci. 2008, 23, 237–245. [Google Scholar] [CrossRef]
- Miki, Y.; Akimoto, J.; Yokoyama, S.; Homma, T.; Tsutsumi, M.; Haraoka, J.; Hirano, K.; Beppu, M. Photodynamic therapy in combination with talaporfin sodium induces mitochondrial apoptotic cell death accompanied with necrosis in glioma cells. Biol. Pharm. Bull. 2013, 36, 215–221. [Google Scholar] [CrossRef]
- Namatame, H.; Akimoto, J.; Matsumura, H.; Haraoka, J.; Aizawa, K. Photodynamic therapy of C6-implanted glioma cells in the rat brain employing second-generation photosensitizer talaporfin sodium. Photodiagn. Photodyn. Ther. 2008, 5, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Maebayashi, K.; Saito, T.; Okada, Y.; Kaneko, S.; et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors: Clinical article. J. Neurosurg. JNS 2013, 119, 845–852. [Google Scholar] [CrossRef]
- Nitta, M.; Muragaki, Y.; Maruyama, T.; Iseki, H.; Komori, T.; Ikuta, S.; Saito, T.; Yasuda, T.; Hosono, J.; Okamoto, S.; et al. Role of photodynamic therapy using talaporfin sodium and a semiconductor laser in patients with newly diagnosed glioblastoma. J. Neurosurg. 2019, 131, 1361–1368. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nitta, M.; Shimizu, K.; Saito, T.; Tsuzuki, S.; Fukui, A.; Koriyama, S.; Kuwano, A.; Komori, T.; Masui, K.; et al. Therapeutic Options for Recurrent Glioblastoma—Efficacy of Talaporfin Sodium Mediated Photodynamic Therapy. Pharmaceutics 2022, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Nitta, M.; Komori, T.; Maruyama, T.; Yasuda, T.; Fujii, Y.; Masamune, K.; Kawamata, T.; Maehara, T.; Muragaki, Y. Intraoperative Photodynamic Diagnosis Using Talaporfin Sodium Simultaneously Applied for Photodynamic Therapy against Malignant Glioma: A Prospective Clinical Study. Front. Neurol. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.M.; Mo, H.; McConathy, W.J.; Sabnis, N.; Lacko, A.G. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 2013, 4, 119. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Vilchez, M.L.; Caverzán, M.D.; Milla Sanabria, L.N. Understanding the glioblastoma tumor biology to optimize photodynamic therapy: From molecular to cellular events. J. Neurosci. Res. 2021, 99, 1024–1047. [Google Scholar] [CrossRef]
- Nowak-Stepniowska, A.; Pergoł, P.; Padzik-Graczyk, A. Photodynamic method of cancer diagnosis and therapy--mechanisms and applications. Postepy Biochem. 2013, 59, 53–63. [Google Scholar]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Harada, M.; Takakura, H.; Ando, K.; Goto, Y.; Tsuneda, T.; Ogawa, M.; Taketsugu, T. Theoretical and Experimental Studies on the Near-Infrared Photoreaction Mechanism of a Silicon Phthalocyanine Photoimmunotherapy Dye: Photoinduced Hydrolysis by Radical Anion Generation. Chempluschem 2020, 85, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Griffiths, G.L.; Choyke, P.L. Near-Infrared Photoimmunotherapy: Photoactivatable Antibody-Drug Conjugates (ADCs). Bioconjug. Chem. 2020, 31, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Wakiyama, H.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy for cancers: A translational perspective. EBioMedicine 2021, 70, 103501. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, N.L.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Review of RM-1929 Near-Infrared Photoimmunotherapy Clinical Efficacy for Unresectable and/or Recurrent Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 5117. [Google Scholar] [CrossRef]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. J. Photochem. Photobiol. B 2023, 248, 112796. [Google Scholar] [CrossRef]
- Inagaki, F.F.; Fujimura, D.; Furusawa, A.; Okada, R.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Fluorescence Imaging of Tumor-Accumulating Antibody-IR700 Conjugates Prior to Near-Infrared Photoimmunotherapy (NIR-PIT) Using a Commercially Available Camera Designed for Indocyanine Green. Mol. Pharm. 2021, 18, 1238–1246. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Li, B.; Lin, L. Internal light source for deep photodynamic therapy. Light. Sci. Appl. 2022, 11, 85. [Google Scholar] [CrossRef]
- Kiesslich, T.; Neureiter, D.; Alinger, B.; Jansky, G.L.; Berlanda, J.; Mkrtchyan, V.; Ocker, M.; Plaetzer, K.; Berr, F. Uptake and phototoxicity of meso-tetrahydroxyphenyl chlorine are highly variable in human biliary tract cancer cell lines and correlate with markers of differentiation and proliferation. Photochem. Photobiol. Sci. 2010, 9, 734–743. [Google Scholar] [CrossRef]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Fabbro-Peray, P.; Zouaoui, S.; Darlix, A.; Fabbro, M.; Pallud, J.; Rigau, V.; Mathieu-Daude, H.; Bessaoud, F.; Bauchet, F.; Riondel, A.; et al. Association of patterns of care, prognostic factors, and use of radiotherapy-temozolomide therapy with survival in patients with newly diagnosed glioblastoma: A French national population-based study. J. Neuro-Oncol. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Chen, T.; Chen, J.; Zhu, Y.; Li, Y.; Wang, Y.; Chen, H.; Wang, J.; Li, X.; Liu, Y.; Li, B.; et al. CD163, a novel therapeutic target, regulates the proliferation and stemness of glioma cells via casein kinase 2. Oncogene 2019, 38, 1183–1199. [Google Scholar] [CrossRef]
- Dutoit, V.; Herold-Mende, C.; Hilf, N.; Schoor, O.; Beckhove, P.; Bucher, J.; Dorsch, K.; Flohr, S.; Fritsche, J.; Lewandrowski, P.; et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain 2012, 135, 1042–1054. [Google Scholar] [CrossRef]
- Breiteneder-Geleff, S.; Soleiman, A.; Kowalski, H.; Horvat, R.; Amann, G.; Kriehuber, E.; Diem, K.; Weninger, W.; Tschachler, E.; Alitalo, K.; et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999, 154, 385–394. [Google Scholar] [CrossRef]
- Wicki, A.; Christofori, G. The potential role of podoplanin in tumour invasion. Br. J. Cancer 2007, 96, 1–5. [Google Scholar] [CrossRef]
- Riedl, J.; Preusser, M.; Nazari, P.M.; Posch, F.; Panzer, S.; Marosi, C.; Birner, P.; Thaler, J.; Brostjan, C.; Lötsch, D.; et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017, 129, 1831–1839. [Google Scholar] [CrossRef]
- Watanabe, J.; Natsumeda, M.; Okada, M.; Kanemaru, Y.; Tsukamoto, Y.; Oishi, M.; Kakita, A.; Fujii, Y. Podoplanin Expression and IDH-Wildtype Status Predict Venous Thromboembolism in Patients with High-Grade Gliomas in the Early Postoperative Period. World Neurosurg. 2019, 128, e982–e988. [Google Scholar] [CrossRef] [PubMed]
- Mir Seyed Nazari, P.; Riedl, J.; Preusser, M.; Posch, F.; Thaler, J.; Marosi, C.; Birner, P.; Ricken, G.; Hainfellner, J.A.; Pabinger, I.; et al. Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism. J. Thromb. Haemost. 2018, 16, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Natsumeda, M.; Kawamura, M.; Shirakawa, K.; Okada, M.; Tsukamoto, Y.; Eda, T.; Watanabe, J.; Saito, S.; Takahashi, H.; et al. Elevated ratio of C-type lectin-like receptor 2 level and platelet count (C2PAC) aids in the diagnosis of post-operative venous thromboembolism in IDH-wildtype gliomas. Thromb. Res. 2023, 223, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xiao, L.; Zhao, Y.; Shi, J.; Yuan, Y.; Gu, Y.; Zhang, F.; Gao, X.; Yang, Y.; Yang, R.; et al. Wild-Type IDH1 and Mutant IDH1 Opposingly Regulate Podoplanin Expression in Glioma. Transl. Oncol. 2020, 13, 100758. [Google Scholar] [CrossRef]
- Abe, S.; Kaneko, M.K.; Tsuchihashi, Y.; Izumi, T.; Ogasawara, S.; Okada, N.; Sato, C.; Tobiume, M.; Otsuka, K.; Miyamoto, L.; et al. Antitumor effect of novel anti-podoplanin antibody NZ-12 against malignant pleural mesothelioma in an orthotopic xenograft model. Cancer Sci. 2016, 107, 1198–1205. [Google Scholar] [CrossRef]
- Ji, R.C.; Eshita, Y.; Xing, L.; Miura, M. Multiple expressions of lymphatic markers and morphological evolution of newly formed lymphatics in lymphangioma and lymph node lymphangiogenesis. Microvasc. Res. 2010, 80, 195–201. [Google Scholar] [CrossRef]
- Shibahara, J.; Kashima, T.; Kikuchi, Y.; Kunita, A.; Fukayama, M. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006, 448, 493–499. [Google Scholar] [CrossRef]
- Inoue, H.; Tsuchiya, H.; Miyazaki, Y.; Kikuchi, K.; Ide, F.; Sakashita, H.; Kusama, K. Podoplanin expressing cancer-associated fibroblasts in oral cancer. Tumour Biol. 2014, 35, 11345–11352. [Google Scholar] [CrossRef]
- Yurugi, Y.; Wakahara, M.; Matsuoka, Y.; Sakabe, T.; Kubouchi, Y.; Haruki, T.; Nosaka, K.; Miwa, K.; Araki, K.; Taniguchi, Y.; et al. Podoplanin Expression in Cancer-associated Fibroblasts Predicts Poor Prognosis in Patients with Squamous Cell Carcinoma of the Lung. Anticancer. Res. 2017, 37, 207–213. [Google Scholar] [CrossRef]
- Ramos-Vega, V.; Venegas Rojas, B.; Donoso Torres, W. Immunohistochemical analysis of cancer-associated fibroblasts and podoplanin in head and neck cancer. Med. Oral. Patol. Oral. Cir. Bucal 2020, 25, e268–e276. [Google Scholar] [CrossRef]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Furusawa, A.; Okada, R.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Fukushima, H.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Podoplanin-Expressing Cancer Cells and Cancer-Associated Fibroblasts. Mol. Cancer Ther. 2023, 22, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kaneko, M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci. Rep. 2014, 4, 5924. [Google Scholar] [CrossRef]
- Tanaka, T.; Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Anti-Podoplanin Monoclonal Antibody, PMab-117-mG2a Exerts Antitumor Activities in Human Tumor Xenograft Models. Cells 2024, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ogasawara, S.; Kaneko, M.K.; Kato, Y. LpMab-23: A Cancer-Specific Monoclonal Antibody Against Human Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 72–76. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. A Humanized and Defucosylated Antibody against Podoplanin (humLpMab-23-f) Exerts Antitumor Activities in Human Lung Cancer and Glioblastoma Xenograft Models. Cancers 2023, 15, 5080. [Google Scholar] [CrossRef]
- Ishikawa, A.; Waseda, M.; Ishii, T.; Kaneko, M.K.; Kato, Y.; Kaneko, S. Improved anti-solid tumor response by humanized anti-podoplanin chimeric antigen receptor transduced human cytotoxic T cells in an animal model. Genes Cells 2022, 27, 549–558. [Google Scholar] [CrossRef]
- Wikstrand, C.J.; Hale, L.P.; Batra, S.K.; Hill, M.L.; Humphrey, P.A.; Kurpad, S.N.; McLendon, R.E.; Moscatello, D.; Pegram, C.N.; Reist, C.J.; et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995, 55, 3140–3148. [Google Scholar]
- Nishikawa, R.; Ji, X.D.; Harmon, R.C.; Lazar, C.S.; Gill, G.N.; Cavenee, W.K.; Huang, H.J. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl. Acad. Sci. USA 1994, 91, 7727–7731. [Google Scholar] [CrossRef]
- Gupta, P.; Han, S.Y.; Holgado-Madruga, M.; Mitra, S.S.; Li, G.; Nitta, R.T.; Wong, A.J. Development of an EGFRvIII specific recombinant antibody. BMC Biotechnol. 2010, 10, 72. [Google Scholar] [CrossRef]
- Fan, Q.W.; Cheng, C.K.; Gustafson, W.C.; Charron, E.; Zipper, P.; Wong, R.A.; Chen, J.; Lau, J.; Knobbe-Thomsen, C.; Weller, M.; et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 2013, 24, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Gong, K.; Wohlfeld, B.; Hatanpaa, K.J.; Zhao, D.; Habib, A.A. Ligand-Independent EGFR Signaling. Cancer Res. 2015, 75, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Katanasaka, Y.; Kodera, Y.; Kitamura, Y.; Morimoto, T.; Tamura, T.; Koizumi, F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol. Cancer 2013, 12, 31. [Google Scholar] [CrossRef]
- Inda, M.M.; Bonavia, R.; Mukasa, A.; Narita, Y.; Sah, D.W.; Vandenberg, S.; Brennan, C.; Johns, T.G.; Bachoo, R.; Hadwiger, P.; et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010, 24, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Zanca, C.; Villa, G.R.; Benitez, J.A.; Thorne, A.H.; Koga, T.; D’Antonio, M.; Ikegami, S.; Ma, J.; Boyer, A.D.; Banisadr, A.; et al. Glioblastoma cellular cross-talk converges on NF-κB to attenuate EGFR inhibitor sensitivity. Genes Dev. 2017, 31, 1212–1227. [Google Scholar] [CrossRef]
- Nozawa, T.; Okada, M.; Natsumeda, M.; Eda, T.; Abe, H.; Tsukamoto, Y.; Okamoto, K.; Oishi, M.; Takahashi, H.; Fujii, Y.; et al. EGFRvIII Is Expressed in Cellular Areas of Tumor in a Subset of Glioblastoma. Neurol. Med. Chir. 2019, 59, 89–97. [Google Scholar] [CrossRef]
- Maire, C.L.; Ligon, K.L. Molecular pathologic diagnosis of epidermal growth factor receptor. Neuro Oncol. 2014, 16 (Suppl. S8), viii1–viii6. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin. Cancer Res. 2020, 26, 1586–1594. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Burley, T.A.; Mączyńska, J.; Shah, A.; Szopa, W.; Harrington, K.J.; Boult, J.K.R.; Mrozek-Wilczkiewicz, A.; Vinci, M.; Bamber, J.C.; Kaspera, W.; et al. Near-infrared photoimmunotherapy targeting EGFR-Shedding new light on glioblastoma treatment. Int. J. Cancer 2018, 142, 2363–2374. [Google Scholar] [CrossRef]
- Mączyńska, J.; Raes, F.; Da Pieve, C.; Turnock, S.; Boult, J.K.R.; Hoebart, J.; Niedbala, M.; Robinson, S.P.; Harrington, K.J.; Kaspera, W.; et al. Triggering anti-GBM immune response with EGFR-mediated photoimmunotherapy. BMC Med. 2022, 20, 16. [Google Scholar] [CrossRef]
- Anderson, H.J.; Galileo, D.S. Small-molecule inhibitors of FGFR, integrins and FAK selectively decrease L1CAM-stimulated glioblastoma cell motility and proliferation. Cell. Oncol. 2016, 39, 229–242. [Google Scholar] [CrossRef]
- Zeng, J.; Xi, S.-Y.; Wang, F.; Liao, H.-D.; Yang, Y.-Z.; Hu, W.-M. L1CAM High Expression Associates with Poor Prognosis in Glioma but Does Not Correlate with C11orf95-RELA Fusion. BioMed Res. Int. 2020, 2020, 1353284. [Google Scholar] [CrossRef] [PubMed]
- Malgulwar, P.B.; Nambirajan, A.; Pathak, P.; Faruq, M.; Rajeshwari, M.; Singh, M.; Suri, V.; Sarkar, C.; Sharma, M.C. C11orf95-RELA fusions and upregulated NF-KB signalling characterise a subset of aggressive supratentorial ependymomas that express L1CAM and nestin. J. Neuro-Oncol. 2018, 138, 29–39. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mahdi, J.; Ramakrishna, S.; Patel, S.; Chinnasamy, H.; Yeom, K.; Schultz, L.; Barsan, V.; Richards, R.; Campen, C.; et al. Major tumor regression in H3K27M-mutated diffuse midline glioma (DMG) following sequential intravenous (IV) and intracerbroventricular (ICV) delivery of GD2-CAR T cells. Cancer Res. 2022, 24 (Suppl. S1), i20–i21. [Google Scholar]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Hatano, M.; Eguchi, J.; Tatsumi, T.; Kuwashima, N.; Dusak, J.E.; Kinch, M.S.; Pollack, I.F.; Hamilton, R.L.; Storkus, W.J.; Okada, H. EphA2 as a glioma-associated antigen: A novel target for glioma vaccines. Neoplasia 2005, 7, 717–722. [Google Scholar] [CrossRef]
- Hou, J.; Lv, A.; Deng, Q.; Zhang, G.; Hu, X.; Cui, H. TROP2 promotes the proliferation and metastasis of glioblastoma cells by activating the JAK2/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 753–764. [Google Scholar] [CrossRef]
- Park, D.H.; Bhojnagarwala, P.S.; Liaw, K.; Bordoloi, D.; Tursi, N.J.; Zhao, S.; Binder, Z.A.; O’Rourke, D.; Weiner, D.B. Novel tri-specific T-cell engager targeting IL-13Rα2 and EGFRvIII provides long-term survival in heterogeneous GBM challenge and promotes antitumor cytotoxicity with patient immune cells. J. Immunother. Cancer 2024, 12, e009604. [Google Scholar] [CrossRef]
- Newman, J.P.; Wang, G.Y.; Arima, K.; Guan, S.P.; Waters, M.R.; Cavenee, W.K.; Pan, E.; Aliwarga, E.; Chong, S.T.; Kok, C.Y.L.; et al. Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat. Commun. 2017, 8, 1913. [Google Scholar] [CrossRef]
- Tachi, T.; Kijima, N.; Kuroda, H.; Ikeda, S.; Murakami, K.; Nakagawa, T.; Yaga, M.; Nakagawa, K.; Utsugi, R.; Hirayama, R.; et al. Antitumor effects of intracranial injection of B7-H3-targeted Car-T and Car-Nk cells in a patient-derived glioblastoma xenograft model. Cancer Immunol. Immunother. 2024, 73, 256. [Google Scholar] [CrossRef]
- Inthanachai, T.; Boonkrai, C.; Phakham, T.; Pisitkun, T.; Thaiwong, R.; Chuthaphakdikun, V.; Sakunrangsit, N.; Limprasutr, V.; Chinsuwan, T.; Hirankarn, N.; et al. Novel B7-H3 CAR T cells show potent antitumor effects in glioblastoma: A preclinical study. J. Immunother. Cancer 2025, 13, e010083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Li, F.; Shen, Z.; Qiao, Y.; Li, L.; Liu, S.; Song, M.; Zhao, X.; Ren, F.; et al. Large-scale analysis reveals the specific clinical and immune features of B7-H3 in glioma. Oncoimmunology 2018, 7, e1461304. [Google Scholar] [CrossRef] [PubMed]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer stem cells: An old idea--a paradigm shift. Cancer Res. 2006, 66, 1883–1890, discussion 1895–1896. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Kahlert, U.D.; Suwala, A.K.; Koch, K.; Natsumeda, M.; Orr, B.A.; Hayashi, M.; Maciaczyk, J.; Eberhart, C.G. Pharmacologic Wnt Inhibition Reduces Proliferation, Survival, and Clonogenicity of Glioblastoma Cells. J. Neuropathol. Exp. Neurol. 2015, 74, 889–900. [Google Scholar] [CrossRef]
- Joyce, T.; Jagasia, S.; Tasci, E.; Camphausen, K.; Krauze, A.V. An Overview of CD133 as a Functional Unit of Prognosis and Treatment Resistance in Glioblastoma. Curr. Oncol. 2023, 30, 8278–8293. [Google Scholar] [CrossRef]
- Behnan, J.; Isakson, P.; Joel, M.; Cilio, C.; Langmoen, I.A.; Vik-Mo, E.O.; Badn, W. Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells 2014, 32, 1110–1123. [Google Scholar] [CrossRef]
- Mao, P.; Joshi, K.; Li, J.; Kim, S.H.; Li, P.; Santana-Santos, L.; Luthra, S.; Chandran, U.R.; Benos, P.V.; Smith, L.; et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc. Natl. Acad. Sci. USA 2013, 110, 8644–8649. [Google Scholar] [CrossRef]
- Jing, H.; Weidensteiner, C.; Reichardt, W.; Gaedicke, S.; Zhu, X.; Grosu, A.L.; Kobayashi, H.; Niedermann, G. Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies. Theranostics 2016, 6, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, M.; Kita, S.; Higuchi, H. Quantitative evaluation of malignant gliomas damage induced by photoactivation of IR700 dye. Sci. Technol. Adv. Mater. 2016, 17, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; Schultze-Seemann, S.; Gratzke, C.; Wolf, P. Targeting CD44 and EpCAM with Antibody Dye Conjugates for the Photoimmunotherapy of Prostate Cancer. Antibodies 2025, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Yaku, H.; Takahashi, K.; Okada, H.; Kobiyama, K.; Shiokawa, M.; Uza, N.; Kodama, Y.; Ishii, K.J.; Seno, H. Near-infrared photoimmunotherapy as a complementary modality to in situ vaccine in a preclinical pancreatic cancer model. Biochem. Biophys. Res. Commun. 2024, 737, 150534. [Google Scholar] [CrossRef]
- Mungra, N.; Nsole Biteghe, F.A.; Huysamen, A.M.; Hardcastle, N.S.; Bunjun, R.; Naran, K.; Lang, D.; Richter, W.; Hunter, R.; Barth, S. An Investigation into the In Vitro Targeted Killing of CD44-Expressing Triple-Negative Breast Cancer Cells Using Recombinant Photoimmunotherapeutics Compared to Auristatin-F-Based Antibody-Drug Conjugates. Mol. Pharm. 2024, 21, 4098–4115. [Google Scholar] [CrossRef]
- Jin, J.; Barnett, J.D.; Mironchik, Y.; Gross, J.; Kobayashi, H.; Levin, A.; Bhujwalla, Z.M. Photoimmunotheranostics of epithelioid sarcoma by targeting CD44 or EGFR. Transl. Oncol. 2024, 45, 101966. [Google Scholar] [CrossRef]
- Liao, F.; Zhang, J.; Hu, Y.; Najafabadi, A.H.; Moon, J.J.; Wicha, M.S.; Kaspo, B.; Whitfield, J.; Chang, A.E.; Li, Q. Efficacy of an ALDH peptide-based dendritic cell vaccine targeting cancer stem cells. Cancer Immunol. Immunother. 2022, 71, 1959–1973. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I.A. Role of the blood-brain barrier in the formation of brain metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.D.; Zheng, S.; Xiu, J.; Zhou, S.; Khasraw, M.; Brastianos, P.K.; Kesari, S.; Hu, J.; Rudnick, J.; Salacz, M.E.; et al. Profiles of brain metastases: Prioritization of therapeutic targets. Int. J. Cancer 2018, 143, 3019–3026. [Google Scholar] [CrossRef]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Clark, J.W.; Shih, H.A.; Oh, K.S.; Brastianos, P.K.; Wo, J.Y.; Strickland, M.R.; Curry, W.T.; Parikh, A.R.; Corcoran, R.B.; et al. Enrichment of HER2 Amplification in Brain Metastases from Primary Gastrointestinal Malignancies. Oncologist 2019, 24, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 1999, 36, 179–194. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Hodge, J.W.; Schlom, J.; Kobayashi, H. Near infrared photoimmunotherapy with avelumab, an anti-programmed death-ligand 1 (PD-L1) antibody. Oncotarget 2017, 8, 8807–8817. [Google Scholar] [CrossRef]
- Mao, C.; Qu, P.; Miley, M.J.; Zhao, Y.; Li, Z.; Ming, X. P-glycoprotein targeted photodynamic therapy of chemoresistant tumors using recombinant Fab fragment conjugates. Biomater. Sci. 2018, 6, 3063–3074. [Google Scholar] [CrossRef]
- Yamashita, S.; Kojima, M.; Onda, N.; Yoshida, T.; Shibutani, M. Trastuzumab-based near-infrared photoimmunotherapy in xenograft mouse of breast cancer. Cancer Med. 2023, 12, 4579–4589. [Google Scholar] [CrossRef]
- Kurihara, H.; Hamada, A.; Yoshida, M.; Shimma, S.; Hashimoto, J.; Yonemori, K.; Tani, H.; Miyakita, Y.; Kanayama, Y.; Wada, Y.; et al. (64)Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015, 5, 8. [Google Scholar] [CrossRef]
- Nienhuis, P.H.; Antunes, I.F.; Glaudemans, A.; Jalving, M.; Leung, D.; Noordzij, W.; Slart, R.; de Vries, E.F.J.; Hospers, G.A.P. (18)F-BMS986192 PET Imaging of PD-L1 in Metastatic Melanoma Patients with Brain Metastases Treated with Immune Checkpoint Inhibitors: A Pilot Study. J. Nucl. Med. 2022, 63, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Mitsogianni, M.; Trontzas, I.P.; Gomatou, G.; Ioannou, S.; Syrigos, N.K.; Kotteas, E.A. The changing treatment of metastatic her2-positive breast cancer. Oncol. Lett. 2021, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 12 (Suppl. S2), iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro Oncol. 2021, 24, 796–808. [Google Scholar] [CrossRef]
- Louis, D.N. World Health Organization Classification of Tumours of the Central Nervous System, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Sahm, F.; Aldape, K.D.; Brastianos, P.K.; Brat, D.J.; Dahiya, S.; von Deimling, A.; Giannini, C.; Gilbert, M.R.; Louis, D.N.; Raleigh, D.R.; et al. cIMPACT-NOW update 8: Clarifications on molecular risk parameters and recommendations for WHO grading of meningiomas. Neuro Oncol. 2024, 27, 319–330. [Google Scholar] [CrossRef]
- Walsh, K.A.-O.; Price, M.; Neff, C.; Komisarow, J.M.; Wimberly, C.E.; Kruchko, C.A.-O.X.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. The joint impacts of sex and race/ethnicity on incidence of grade 1 versus grades 2–3 meningioma across the lifespan. Neuro-Oncol. Adv. 2023, 5, i5–i12. [Google Scholar] [CrossRef]
- Maier, A.D.; Meddis, A.; Mirian, C.; Haslund-Vinding, J.; Bartek, J.; Krog, S.M.; Nguyen, T.U.P.; Areškevičiūtė, A.; Melchior, L.C.; Heegaard, S.; et al. Gene expression analysis during progression of malignant meningioma compared to benign meningioma. J. Neurosurg. 2022, 138, 1302–1312. [Google Scholar] [CrossRef]
- Tosefsky, K.; Rebchuk, A.D.; Wang, J.Z.; Ellenbogen, Y.; Drexler, R.; Ricklefs, F.L.; Sauvigny, T.; Schüller, U.; Cutler, C.B.; Lucke-Wold, B.; et al. Grade 3 meningioma survival and recurrence outcomes in an international multicenter cohort. J. Neurosurg. 2024, 140, 393–403. [Google Scholar] [CrossRef]
- Klungtvedt, V.; Aunan-Diop, J.S.; Poulsen, F.R.; Pedersen, C.B.; Wismann, J.; Wang, E.W.; Dahlrot, R.H.; Halle, B. Radiotherapy for World Health Organization Grade 1 and 2 Intracranial Meningiomas: A Retrospective Analysis of Efficacy. World Neurosurg. 2025, 197, 123858. [Google Scholar] [CrossRef]
- Silva, C.B.; Ongaratti, B.R.; Trott, G.; Haag, T.; Ferreira, N.P.; Leães, C.G.; Pereira-Lima, J.F.; Oliveira Mda, C. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int. J. Clin. Exp. Pathol. 2015, 8, 13185–13192. [Google Scholar] [PubMed]
- Dutour, A.; Kumar, U.; Panetta, R.; Ouafik, L.; Fina, F.; Sasi, R.; Patel, Y.C. Expression of somatostatin receptor subtypes in human brain tumors. Int. J. Cancer 1998, 76, 620–627. [Google Scholar] [CrossRef]
- Arena, S.; Barbieri, F.; Thellung, S.; Pirani, P.; Corsaro, A.; Villa, V.; Dadati, P.; Dorcaratto, A.; Lapertosa, G.; Ravetti, J.L.; et al. Expression of somatostatin receptor mRNA in human meningiomas and their implication in in vitro antiproliferative activity. J. Neuro-Oncol. 2004, 66, 155–166. [Google Scholar] [CrossRef]
- Schulz, S.; Pauli, S.U.; Schulz, S.; Händel, M.; Dietzmann, K.; Firsching, R.; Höllt, V. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin. Cancer Res. 2000, 6, 1865–1874. [Google Scholar] [PubMed]
- Durand, A.; Champier, J.; Jouvet, A.; Labrousse, F.; Honnorat, J.; Guyotat, J.; Fèvre-Montange, M. Expression of c-Myc, neurofibromatosis Type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: Relation to grades or histotypes. Clin. Neuropathol. 2008, 27, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Alafaci, C.; Salpietro, F.; Tuccari, G. Sstr2A immunohistochemical expression in human meningiomas: Is there a correlation with the histological grade, proliferation or microvessel density? Oncol. Rep. 2008, 20, 485–492. [Google Scholar] [CrossRef]
- Agaimy, A.; Buslei, R.; Coras, R.; Rubin, B.P.; Mentzel, T. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology 2014, 65, 60–70. [Google Scholar] [CrossRef]
- Dijkstra, B.M.; Motekallemi, A.; den Dunnen, W.F.A.; Jeltema, J.R.; van Dam, G.M.; Kruyt, F.A.E.; Groen, R.J.M. SSTR-2 as a potential tumour-specific marker for fluorescence-guided meningioma surgery. Acta Neurochir. 2018, 160, 1539–1546. [Google Scholar] [CrossRef]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L.; Bartenstein, P.; et al. Improved Detection of Transosseous Meningiomas Using (68)Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Giesel, F.L.; Linhart, H.G.; Haberkorn, U.; Haufe, S.; Combs, S.E.; Podlesek, D.; Eisenhut, M.; Kratochwil, C. Detection of cranial meningiomas: Comparison of ⁶⁸Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1409–1415. [Google Scholar] [CrossRef]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Romano, D.; Defilles, C.; Saveanu, A.; Mohamed, A.; Figarella-Branger, D.; Roche, P.H.; Fuentes, S.; Chinot, O.; Dufour, H.; et al. Octreotide therapy in meningiomas: In vitro study, clinical correlation, and literature review. J. Neurosurg. 2017, 127, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Marincek, N.; Radojewski, P.; Dumont, R.A.; Brunner, P.; Müller-Brand, J.; Maecke, H.R.; Briel, M.; Walter, M.A. Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: Long-term results of a phase II clinical trial. J. Nucl. Med. 2015, 56, 171–176. [Google Scholar] [CrossRef]
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Miki, Y. Imaging of pituitary tumors: An update with the 5th WHO Classifications-part 1. Pituitary neuroendocrine tumor (PitNET)/pituitary adenoma. Jpn. J. Radiol. 2023, 41, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, S.; Costa, D.; Lauretta, R.; Mercuri, V.; Sbardella, E.; Samperi, I.; Appetecchia, M.; Bianchi, A.; Giampietro, A.; Gargiulo, P.; et al. Partial response to first generation SSA guides the choice and predict the outcome of second line therapy in acromegaly. Endocrine 2022, 78, 343–353. [Google Scholar] [CrossRef]
- Puig Domingo, M. Treatment of acromegaly in the era of personalized and predictive medicine. Clin. Endocrinol. 2015, 83, 3–14. [Google Scholar] [CrossRef]
- Marques, P. The Effects of Peptide Receptor Radionuclide Therapy on the Neoplastic and Normal Pituitary. Cancers 2023, 15, 2710. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Brizzi, M.P.; Faggiano, A.; La Rosa, S.; Rapa, I.; Ferrero, A.; Mansueto, G.; Righi, L.; Garancini, S.; Capella, C.; et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: A proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod. Pathol. 2007, 20, 1172–1182. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Khawli, L.A.; Prabhu, S. Drug delivery across the blood-brain barrier. Mol. Pharm. 2013, 10, 1471–1472. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood-Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Nam, L.; Coll, C.; Erthal, L.C.S.; de la Torre, C.; Serrano, D.; Martínez-Máñez, R.; Santos-Martínez, M.J.; Ruiz-Hernández, E. Drug Delivery Nanosystems for the Localized Treatment of Glioblastoma Multiforme. Materials 2018, 11, 779. [Google Scholar] [CrossRef]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; McCarty, J.H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267. [Google Scholar] [CrossRef] [PubMed]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates. 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Sasaki, K.; Sugii, N.; Yamaguchi, S.; Inoue, H.; Oshima, A.; Tanaka, K.; Otani, Y.; Shirahata, M.; Shibahara, I.; et al. Cost of medical care for malignant brain tumors at hospitals in the Japan Clinical Oncology Group brain-tumor study group. Jpn. J. Clin. Oncol. 2024, 54, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of B-cell lymphoma. Mol. Oncol. 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Gao, C.; Bian, H.; Ma, Y.; Jing, F.; Zhao, X. Role of Rituximab in Treatment of Patients with Primary Central Nervous System Lymphoma (PCNSL): A Systematic Review and Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 23, 733–741. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Perez, E.; Haddad, A.F.; Young, J.S.; Savastano, L.; Villanueva-Meyer, J.E.; Winkler, E.; de Groot, J. Strategies to Improve Drug Delivery Across the Blood-Brain Barrier for Glioblastoma. Curr. Neurol. Neurosci. Rep. 2024, 24, 123–139. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Preda, M.D.; Niculescu, A.G.; Vladâcenco, O.; Radu, C.I.; Grumezescu, A.M.; Teleanu, D.M. Current Strategies to Enhance Delivery of Drugs across the Blood-Brain Barrier. Pharmaceutics 2022, 14, 987. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Sethi, B.; Kumar, V.; Mahato, K.; Coulter, D.W.; Mahato, R.I. Recent advances in drug delivery and targeting to the brain. J. Control. Release 2022, 350, 668–687. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Sun, Y.; Tang, X.; Duan, X.; Zhao, Y.; Xia, H.; Xu, L.; Zhang, P.; Sun, K.; Yang, G.; et al. The Tumor-Derived Exosomes Enhanced Bevacizumab across the Blood-Brain Barrier for Antiangiogenesis Therapy against Glioblastoma. Mol. Pharm. 2025, 22, 972–983. [Google Scholar] [CrossRef]
- Wen, J.; Wu, D.; Qin, M.; Liu, C.; Wang, L.; Xu, D.; Vinters, H.V.; Liu, Y.; Kranz, E.; Guan, X.; et al. Sustained delivery and molecular targeting of a therapeutic monoclonal antibody to metastases in the central nervous system of mice. Nat. Biomed. Eng. 2019, 3, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Tao, Z.; Shi, Q.; Wang, L.; Liu, L.; She, T.; Yi, Q.; Wen, X.; Liu, L.; Li, S.; et al. Selective Photokilling of Colorectal Tumors by Near-Infrared Photoimmunotherapy with a GPA33-Targeted Single-Chain Antibody Variable Fragment Conjugate. Mol. Pharm. 2020, 17, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, F.; Mao, C.; Ming, X. Multiarm Nanoconjugates for Cancer Cell-Targeted Delivery of Photosensitizers. Mol. Pharm. 2018, 15, 2559–2569. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Suzuki, T.; Okada, Y.; Ono, J.; Sano, H.; Banba, A.; Sakata, H.; Ishikawa, A.; Morita, T. Near-Infrared Photoimmunotherapy Using a Protein Mimetic for EGFR-Positive Salivary Gland Cancer. Int. J. Mol. Sci. 2024, 25, 3233. [Google Scholar] [CrossRef]
- Yamaguchi, H.; On, J.; Morita, T.; Suzuki, T.; Okada, Y.; Ono, J.; Evdokiou, A. Combination of Near-Infrared Photoimmunotherapy Using Trastuzumab and Small Protein Mimetic for HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 12213. [Google Scholar] [CrossRef]
- Watanabe, R.; Hanaoka, H.; Sato, K.; Nagaya, T.; Harada, T.; Mitsunaga, M.; Kim, I.; Paik, C.H.; Wu, A.M.; Choyke, P.L.; et al. Photoimmunotherapy targeting prostate-specific membrane antigen: Are antibody fragments as effective as antibodies? J. Nucl. Med. 2015, 56, 140–144. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef]
- Mączyńska, J.; Da Pieve, C.; Burley, T.A.; Raes, F.; Shah, A.; Saczko, J.; Harrington, K.J.; Kramer-Marek, G. Immunomodulatory activity of IR700-labelled affibody targeting HER2. Cell Death Dis. 2020, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Ogawa, M. Phototoxicity in near-infrared photoimmunotherapy is influenced by the subcellular localization of antibody-IR700. Photodiagnosis Photodyn. Ther. 2020, 31, 101926. [Google Scholar] [CrossRef]
- Yang, J.K.; Kwon, H.; Kim, S. Recent advances in light-triggered cancer immunotherapy. J. Mater. Chem. B 2024, 12, 2650–2669. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Takezawa, H.; Suzuki, T.; Banba, A.; Shibata, S.; Sakata, H.; Ishikawa, A.; Morita, T. Near-infrared photoimmunotherapy using a small protein mimetic for brain metastasis of HER2-positive breast cancer. J. Immunother. Cancer 2024, 12, A15–A16. [Google Scholar] [CrossRef]
- Kyrysyuk, O.; Wucherpfennig, K.W. Designing Cancer Immunotherapies That Engage T Cells and NK Cells. Annu. Rev. Immunol. 2023, 41, 17–38. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, J.; Zhang, B.; Li, X.; Chen, X.; Cui, B.; Li, S.; Guo, G. Efficacy and Safety of Anti-PD1/PDL1 in Advanced Biliary Tract Cancer: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 801909. [Google Scholar] [CrossRef]
- Miliotou, A.N.; Papadopoulou, L.C. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr. Pharm. Biotechnol. 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Caspi, R.R. Immunotherapy of autoimmunity and cancer: The penalty for success. Nat. Rev. Immunol. 2008, 8, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Maruoka, Y.; Furusawa, A.; Inagaki, F.; Nagaya, T.; Fujimura, D.; Choyke, P.L.; Kobayashi, H. The Effect of Antibody Fragments on CD25 Targeted Regulatory T Cell Near-Infrared Photoimmunotherapy. Bioconjugate Chem. 2019, 30, 2624–2633. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 2021, 373, eabf7844. [Google Scholar] [CrossRef]
- Nakajima, K.; Sugikawa, A.; Yasui, H.; Higashikawa, K.; Suzuki, C.; Natsume, T.; Suzuki, M.; Takakura, H.; Tomita, M.; Takahashi, S.; et al. In vivo imaging of acute physiological responses after treatment of cancer with near-infrared photoimmunotherapy. Mol. Imaging Biol. 2023, 25, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Gordon, M.; Lapetoda, J.; Lozano, A.; Amantea, C.M.; Osada, T.; Thorne, A.H. Reduction in photoimmunotherapy-induced edema with COX-2 inhibition: Combatting clinically relevant adverse events without compromising efficacy. In Proceedings of the American Association for Cancer Research, San Diego, CA, USA, 5–10 April 2024. [Google Scholar]
- Layard Horsfall, H.; Mohan, M.; Devi, B.I.; Adeleye, A.O.; Shukla, D.P.; Bhat, D.; Khan, M.; Clark, D.J.; Chari, A.; Servadei, F.; et al. Hinge/floating craniotomy as an alternative technique for cerebral decompression: A scoping review. Neurosurg. Rev. 2020, 43, 1493–1507. [Google Scholar] [CrossRef]
- Kumar Sengupta, S. Expansive Cranioplasty a Single Stage Surgery Avoiding Cranioplasty at a Later Date Following Decompressive Hemicraniectomy. World Neurosurg. 2019, 128, 631. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Zhou, L.-J.; Kan, L.-D.; Fan, H.; Fang, H.-M. Glycerol Infusion Versus Mannitol for Cerebral Edema: A Systematic Review and Meta-analysis. Clin. Ther. 2021, 43, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Cloughesy, T.; Samant, M.; Prados, M.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist 2010, 15, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Okada, T.; Tokashiki, K.; Tsukahara, K. Photoimmunotherapy for Managing Recurrent Laryngeal Cancer Cervical Lesions: A Case Report. Case Rep. Oncol. 2022, 15, 34–39. [Google Scholar] [CrossRef]
- Omura, G.; Honma, Y.; Matsumoto, Y.; Shinozaki, T.; Itoyama, M.; Eguchi, K.; Sakai, T.; Yokoyama, K.; Watanabe, T.; Ohara, A.; et al. Transnasal photoimmunotherapy with cetuximab sarotalocan sodium: Outcomes on the local recurrence of nasopharyngeal squamous cell carcinoma. Auris Nasus Larynx 2023, 50, 641–645. [Google Scholar] [CrossRef]

| Characteristics | NIR-PIT | PDT |
|---|---|---|
| Photosensitizer | IR700 conjugated to antibodies | Talaporfin sodium |
| Excitation wavelength | 690 nm | 664 nm |
| Penetration depth | Approximately 2 cm [24] | <10 mm [29,30] |
| Cell death mechanism | Disruption of cell membranes | Apoptosis via ROS generation |
| Specificity | High (targets only cells expressing specific antigens) | Moderate (preferential accumulation in tumors) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, H.; Okada, M.; Otani, T.; On, J.; Shibuma, S.; Takino, T.; Watanabe, J.; Tsukamoto, Y.; Ogura, R.; Oishi, M.; et al. Near-Infrared Photoimmunotherapy in Brain Tumors—An Unexplored Frontier. Pharmaceuticals 2025, 18, 751. https://doi.org/10.3390/ph18050751
Yamaguchi H, Okada M, Otani T, On J, Shibuma S, Takino T, Watanabe J, Tsukamoto Y, Ogura R, Oishi M, et al. Near-Infrared Photoimmunotherapy in Brain Tumors—An Unexplored Frontier. Pharmaceuticals. 2025; 18(5):751. https://doi.org/10.3390/ph18050751
Chicago/Turabian StyleYamaguchi, Haruka, Masayasu Okada, Takuya Otani, Jotaro On, Satoshi Shibuma, Toru Takino, Jun Watanabe, Yoshihiro Tsukamoto, Ryosuke Ogura, Makoto Oishi, and et al. 2025. "Near-Infrared Photoimmunotherapy in Brain Tumors—An Unexplored Frontier" Pharmaceuticals 18, no. 5: 751. https://doi.org/10.3390/ph18050751
APA StyleYamaguchi, H., Okada, M., Otani, T., On, J., Shibuma, S., Takino, T., Watanabe, J., Tsukamoto, Y., Ogura, R., Oishi, M., Suzuki, T., Ishikawa, A., Sakata, H., & Natsumeda, M. (2025). Near-Infrared Photoimmunotherapy in Brain Tumors—An Unexplored Frontier. Pharmaceuticals, 18(5), 751. https://doi.org/10.3390/ph18050751






