Phytochemical Analysis and Neuroprotective Effect of Salvia castanea Diels f. Tomentosa Stib Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Process Optimization of Total Tanshinones and Total Phenolic Acids
2.2. The Chemical Compositions of Total Tanshinones and Total Phenolic Acids Extracts
2.3. The Neuroprotective Effect of Total Tanshinone Combined with Total Phenolic Acid Extracts of SCT on IS
2.3.1. SCT Reduces CIRI and Alleviates Neurological Deficits
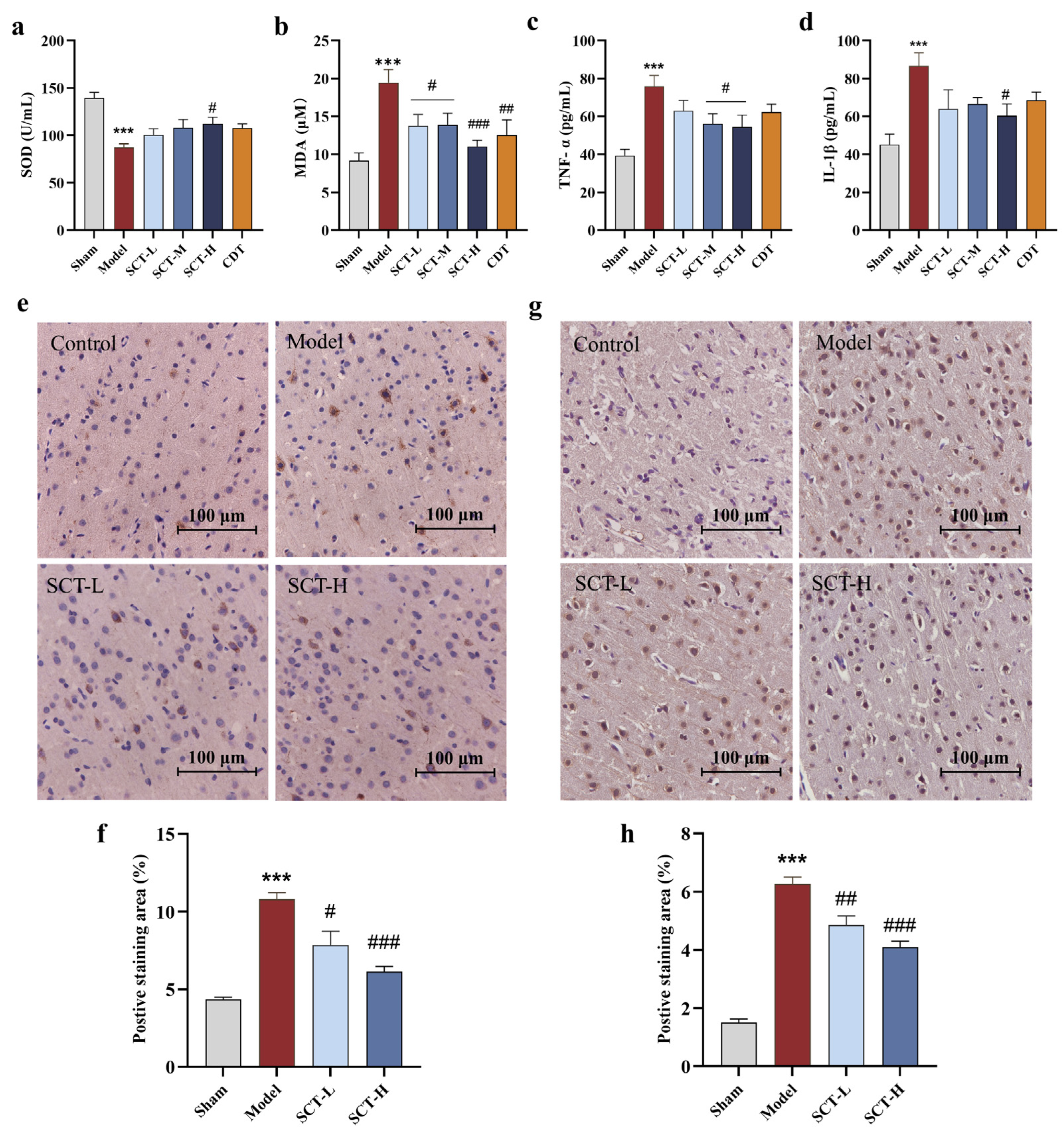
2.3.2. SCT Exerts Neuroprotective Effect via Attenuating Oxidative Stress and Neuroinflammation
2.4. RNA-Seq and Network Pharmacology Combined Analysis Reveal the Potential Therapeutic Mechanism
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Extraction Optimization of Total Tanshinones and Total Phenolic Acids from SCT
3.3. Qualitative and Quantitative Analysis of Total Tanshinone and Total Phenolic Acid Extracts
3.4. The Neuroprotective Effect of Total Tanshinones Combined with Total Phenolic Acid Extracts of SCT Against IS
3.4.1. Animal and Administration
3.4.2. Establishment of the MCAO Model
3.4.3. Evaluation of Neurological Behaviors and Function Deficits
3.4.4. Measurement of Inflammatory Cytokines and Oxidative Stress
3.4.5. Determination of Brain Infarct Volume
3.4.6. Assessment of Cerebral Histopathology
3.4.7. Immunohistochemistry Staining
3.5. The Potential Therapeutic Mechanism of Total Tanshinones Combined with Total Phenolic Acid Extracts of SCT Against IS
3.5.1. DEG Screening and Enrichment
3.5.2. Potential Treatment Targets and Interaction Network Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T-IIA | Tanshinone IIA |
| RA | Rosmarinic acid. |
| SCT | Salvia castanea Diels f. Tomentosa Stib. |
| IS | Ischemic stroke. |
| OED | Orthogonal experimental design. |
| MCAO | Middle cerebral artery occlusion. |
| SMB | Salvia miltiorrhiza Bunge. |
| HPSFE | High-pressure supercritical fluid extraction. |
| HPDE | High-pressure disruption extraction. |
| VEGF | Vascular Endothelial Growth Factor. |
| p-Akt | Phosphorylation of Akt. |
| DSS | Danshensu sodium. |
| CA | Caffeic acid. |
| FA | Ferulic acid. |
| SAB | Salvianolic acid B. |
| DT-I | Dihydrotanshinone I. |
| CT | Cryptotanshinone. |
| T-I | Tanshinone I. |
| mNSS | Modified neurological severity score. |
| TTC | 2,3,5-triphenyltetrazolium chloride. |
| HE | Hematoxylin and eosin. |
| CDTs | Compound Danshen Tablets. |
| DEG | Differential expression gene. |
| GO | Gene Ontology. |
| KEGG | Kyoto Encyclopedia of Genes and Genomes. |
References
- Manolescu, B.N.; Oprea, E.; Mititelu, M.; Ruta, L.L.; Farcasanu, H.C. Dietary anthocyanins and stroke: A review of pharmacokinetic and pharmacodynamic studies. Nutrients 2019, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global burden of stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- De Lima, L.G.; Soares, B.G.O.; Saconato, H.; Atallah, Á.N.; da Silva, E.M.K. Beta-blockers for preventing stroke recurrence. Cochrane Db. Syst. Rev. 2013, 5, CD007890. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, J. Research progress in the treatment of acute ischemic stroke. Chin. J. Pract. Nerv. Dis. 2022, 25, 1146–1153. Available online: https://kns.cnki.net/kcms2/article/abstract?v=mtmIrHeyR2u84G5bq8-7weZcI5LfcVyh3bW3N5M22kcb4HmuYcFQ3XAAQJPeTP78PPiiFNmlv9yMQG855-OD2oKTHVM-8CycOiV1m9xoITtBmZzxlsB4kdk3r2MS1UCEw--4MTyffcuGLZfwSgOi25w4nDnTxkZUuMRNjJKdLmKeY7eFFCEtAAR0azDh-C5cuYH5nrf8xJA=&uniplatform=NZKPT&language=CHS (accessed on 21 December 2023).
- Shen, Q.; Zhang, S.; Tu, Y.; Wang, Z.; Liu, Y. Research progress in clinical treatment of acute ischemic stroke. Chin. Med. 2020, 15, 633–636. [Google Scholar]
- Kakarla, R.; Vinjavarapu, L.A.; Krishnamurthy, S. Diet and Nutraceuticals for treatment and prevention of primary and secondary stroke: Emphasis on nutritional antiplatelet and antithrombotic agents. Neurochem. Int. 2024, 179, 105823. [Google Scholar] [CrossRef]
- Liao, Y.; Qi, W.; Li, S.; Shi, X.; Wu, X.; Chi, F.; Xia, R.; Qin, L.; Cao, L.; Ren, L. Analysis of onset-to-door time and its influencing factors in Chinese patients with acute ischemic stroke during the 2020 COVID-19 epidemic: A preliminary, prospective, multicenter study. BMC Health Serv. Res. 2024, 24, 615. [Google Scholar] [CrossRef]
- Liu, H.; Tang, Y.; Zhou, Q.; Zhang, J.; Li, X.; Gu, H.; Hu, B.; Li, Y. The Interrelation of Blood Urea Nitrogen-to-Albumin Ratio with Three-Month Clinical Outcomes in Acute Ischemic Stroke Cases: A Secondary Analytical Exploration Derived from a Prospective Cohort Study. Int. J. Gen. Med. 2024, 17, 5333–5347. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Zhu, B.; Yang, Y.; Cai, C.; Wang, X.; Deng, L.; He, B.; Cui, Y.; Zhou, W. A comparative study of the neuroprotective effects of dl-3-n-butylphthalide and edaravone dexborneol on cerebral ischemic stroke rats. Eur. J. Pharmacol. 2023, 951, 175801. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tan, B.K.H.; Zhu, Y. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol. Sin. 2000, 21, 1089–1094. Available online: https://webofscience.clarivate.cn/wos/woscc/full-record/WOS:000165828500005 (accessed on 21 December 2023). [PubMed]
- Wang, Z.; Sun, Y.; Deng, X.; Wang, C.; Tian, J.; Lu, T. Research progress on liposoluble constituents of tanshinone and their intervention mechanisms in ischemic stroke. Chin. Tradit. Herb. Drugs 2022, 53, 1181–1190. [Google Scholar]
- Liu, C.; Du, L.; Zhang, S.; Wang, H.; Kong, L.; Du, G. Network pharmacology and experimental study of phenolic acids in salvia miltiorrhiza bung in preventing ischemic stroke. Front. Pharmacol. 2023, 14, 1108518. [Google Scholar] [CrossRef]
- Huang, Y.; Long, X.; Tang, J.; Li, X.; Zhang, X.; Luo, C.; Zhou, Y.; Zhang, P. The Attenuation of Traumatic Brain Injury via Inhibition of Oxidative Stress and Apoptosis by Tanshinone IIA. Oxid. Med. Cell. Longev. 2020, 2020, 4170156. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Guo, H.; Li, Y.; Jiang, Z.; Gu, T.; Su, B.; Hou, W.; Zhong, H.; Cheng, D.; et al. Rosmarinic acid protects rats against post-stroke depression after transient focal cerebral ischemic injury through enhancing antioxidant response. Brain Res. 2021, 1757, 147336. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P. Tanshinone IIA: A phytochemical as a promising drug candidate for neurodegenerative diseases. Pharmacol. Res. 2021, 169, 105661. [Google Scholar] [CrossRef]
- Hua, H.; Zhu, H.; Guo, Y.; Cheng, Y.; Qiao, H. The neuroprotective functions of petroleum ether extract from Tibetan turnip (Brassica rapa L.) against cerebral ischemic stroke. Pharmacol. Res.-Mod. Chin. Med. 2023, 7, 100248. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag+, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef]
- Yang, D.; Ma, P.; Liang, X.; Liang, Z.; Zhang, M.; Shen, S.; Liu, H.; Liu, Y. Metabolic Profiles and cDNA-AFLP Analysis of Salvia miltiorrhiza and Salvia castanea Diel f. tomentosa Stib. PLoS ONE 2012, 7, e29678. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Su, F.; Chen, J.; Zhang, X.; Xu, L.; Yang, D.; Liang, Z. Application of 1H-NMR combined with qRT-PCR technology in the exploration of rosmarinic acid biosynthesis in hair roots of Salvia miltiorrhiza Bunge and Salvia castanea f. tomentosa Stib. Planta 2021, 253, 2. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, R.; Tang, L.; Long, G.; Yan, H.; Yang, Y.; Guo, Z.; Zheng, Y.; Wang, Y.; Jia, J.; et al. (±)-Salvicatone A: A Pair of C27-Meroterpenoid Enantiomers with Skeletons from the Roots and Rhizomes of Salvia castanea Diels f. tomentosa Stib. J. Org. Chem. 2023, 89, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, Y.; Su, F.; Wang, Y.; Zhang, X.; Xu, L.; Yang, D.; Liang, Z. The exploration of methyl jasmonate on the tanshinones biosynthesis in hair roots of Salvia miltiorrhiza Bunge and Salvia castanea f. tomentosa Stib. Ind. Crops Prod. 2021, 167, 113563. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Guo, J.; Yang, J.; Zhang, X.; Wang, Z.; Cheng, Y.; Du, Z.; Qi, Z.; Huang, Y.; et al. Integrated Transcriptomics and Proteomics to Reveal Regulation Mechanism and Evolution of SmWRKY61 on Tanshinone Biosynthesis in Salvia miltiorrhiza and Salvia castanea. Front. Plant. Sci. 2022, 12, 820582. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, N.M.; Radwan, R.A.; Bakry, S.M.; Saad, H.H. Molecular networking aided metabolomic profiling of beet leaves using three extraction solvents and in relation to its anti-obesity effects. J. Adv. Res. 2020, 24, 545–555. [Google Scholar] [CrossRef]
- Patil, A.A.; Sachin, B.S.; Wakte, P.S.; Shinde, D.B. Optimization of supercritical fluid extraction and HPLC identification of wedelolactone from Wedelia calendulacea by orthogonal array design. J. Adv. Res. 2014, 5, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Fang, T.; Ma, J.; Shi, Q.; Peng, Y.; Ge, F.; Wang, X. Homogenate-assisted high-pressure disruption extraction for determination of phenolic acids in Lonicerae Japonicae Flos. J. Chrom. B 2018, 1097–1098, 119–127. [Google Scholar] [CrossRef]
- Zheng, X.; Xiang, A.; Lian, Z.; Zhou, X.; Zhou, D.; Jia, J.; Ge, F. Efficient isolation of polyunsaturated amides from Zanthoxylum bungeanum and assessment of their anti-inflammatory potential. Sep. Purif. Technol. 2024, 330, 125298. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, X.; Shi, Q.; Pan, J.; Zhan, H.; Ge, F. High-pressure supercritical carbon dioxide extraction of Idesia polycarpa oil: Evaluation the influence of process parameters on the extraction yield and oil quality. Ind. Crops Prod. 2022, 188, 115586. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Wang, X.; Deng, L.; He, B.; Yi, X.; Li, J. Mangiferin alleviated poststroke cognitive impairment by modulating lipid metabolism in cerebral ischemia/reperfusion rats. Eur. J. Pharmacol. 2024, 977, 176724. [Google Scholar] [CrossRef]
- James, J.M.; Gewolb, C.; Bautch, V.L. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 2009, 136, 833–841. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.; Li, X.; Chen, Y.; Mu, F.; Wen, A.; Liu, M.; Ding, Y. Advances of phytotherapy in ischemic stroke targeting PI3K/Akt signaling. Phytother. Res. 2023, 37, 5509–5528. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Zhou, X.; Chen, W.; Wen, A.; Liu, M.; Ding, Y. PI3K/AKT signaling and neuroprotection in ischemic stroke: Molecular mechanisms and therapeutic perspectives. Neural. Regen. Res. 2025, 20, 2758–2775. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Li, J.; Shi, Q.; Ge, F. Study on the Extraction of Liposoluble Active Components from Salvia castanea Diels f. Tomentosa Stib by Supercritical CO2. Chin. Med. Mater. 2004, 10, 735–736. [Google Scholar] [CrossRef]
- He, R.; Lian, Z.; Cheng, Z.; Liu, Y.; Peng, X.; Wang, Y.; Ma, H.; Zhou, X.; Ge, F. The Phytochemical Characterization of a Cili (Rosa roxburghii) Fruit Low-Temperature Extract with Hepatoprotective Effects. Foods 2025, 14, 1301. [Google Scholar] [CrossRef]
- Cao, J.; Hu, J.; Wei, J.; Li, B.; Zhang, M.; Xiang, C.; Li, P. Optimization of Micellar Electrokinetic Chromatography Method for the Simultaneous Determination of Seven Hydrophilic and Four Lipophilic Bioactive Components in Three Salvia Species. Molecules 2015, 20, 15304–15318. [Google Scholar] [CrossRef]
- Qiu, G.; Rui, Y. Determination of cryptotanshinone content in Salvia Castanea Diels f. Tomentosa Stib and optimization of ultrasonic extraction process by HPLC. Jiangsu Agric. Sci. 2012, 41, 280–282. [Google Scholar] [CrossRef]
- Rui, Y.; Qiu, G. Determination of tanshinone IIA content in Salvia Castanea Diels f. Tomentosa Stib and optimization of ultrasonic extraction process by HPLC. Jiangsu Agric. Sci. 2013, 41, 252–254. [Google Scholar] [CrossRef]
- Qu, G.; Yue, X.; An, F.; Dai, S.; Li, G.; Li, B. Chemical constituents contained in Salvia castanea. China J. Chin. Mater. Medica 2012, 37, 1985–1989. Available online: https://kns.cnki.net/kcms2/article/abstract?v=amOBmv6QLtosExUWRYkpezN3yRm_tRI_0M76hBB0YVLxElY4FZw2BcGLe0HdwjqkIY4JBiFdH7e38-IXjoIB0g-BUQKKRQBKOQDd3b_b9uWdgSKHaFg8uEgKWzbCPftTQJFTAP7dKbAxh1mFU0OGTMp5i87rInvDbx1ZsqEy_WPfZoHhu0dLf5WMo4MjG_vy&uniplatform=NZKPT&language=CHS (accessed on 15 December 2021).
- Hu, E.; Li, T.; Li, Z.; Su, H.; Yan, Q.; Wang, L.; Li, H.; Zhang, W.; Tang, T.; Wang, Y. Metabolomics reveals the effects of hydroxysafflor yellow A on neurogenesis and axon regeneration after experimental traumatic brain injury. Pharm. Biol. 2023, 61, 1054–1064. [Google Scholar] [CrossRef]
- Feng, D.; Li, P.; Xiao, W.; Pei, Z.; Chen, P.; Hu, M.; Yang, Z.; Li, T.; Xia, Z.; Cui, H.; et al. N6-methyladenosine profiling reveals that Xuefu Zhuyu decoction upregulates METTL14 and BDNF in a rat model of traumatic brain injury. J. Ethnopharmacol. 2023, 317, 116823. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Z.; Hou, J.; Guo, D. Comparison of different purification processes for total tanshinones. Chin. Tradit. Herb. Drugs 2008, 39, 1815–1818. Available online: https://kns.cnki.net/kcms2/article/abstract?v=amOBmv6QLtp6H9R7Uu3D23Mn9UomKZXR825Dbw2plocupNcTfYVQlDq7zwLVk5zGxf1LOXQjIfQCU1hoYLNmKex5IKJZHppcN-xFuqnKlyElNsOs7i4Xhsp2WboZjr_FZuWlyegvEloPGX5SSwKlDT1wYyFPUNXS_qMfK0uHQMpqL_zUhjAR6BPoUc3hoBSF&uniplatform=NZKPT&language=CHS (accessed on 28 April 2022).
- Wu, W.; Guo, D. Separation, purification and enrichment of total phenolic acids from Salvia miltiorrhiza Bunge with macroporous adsorbent resin. Chin. Tradit. Herb. Drugs 2008, 6, 862–864. Available online: https://kns.cnki.net/kcms2/article/abstract?v=amOBmv6QLtpqOcfhd7s25br0fQCmchp-ThQCe56cKm5n_6CTsNiYLPaf3NNByFryivOXzsW3D_6Rpd1edkwHoCO_2dvXNSdo-LRoahBcIPFjT7IaUp08FkCHFNjitnpfvpvDd2vba1I6fkB76v4MEXUiRHstGvEJFQ4HLtvkjx00_k9QzSIrGo9hQUNUPRvu&uniplatform=NZKPT&language=CHS (accessed on 28 April 2022).
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible Middle Cerebral Artery Occlusion Without Craniectomy in Rats. Stroke 1989, 1, 84–91. [Google Scholar] [CrossRef]
- You, J.; Liu, X.; Bao, Y.; Shen, Z.; Wang, Q.; He, G.; Lu, J.; Zhang, J.; Chen, J.; Liu, P. A novel phosphodiesterase 9A inhibitor LW33 protects against ischemic stroke through the cGMP/PKG/CREB pathway. Eur. J. Pharmacol. 2022, 925, 174987. [Google Scholar] [CrossRef]




| Run No. | A (MPa) | B (°C) | C (mL/g) | D (MPa) | Combined Score (Extraction Yield and Yield of Four Tanshinones Were Combined at 1:1) |
|---|---|---|---|---|---|
| 1 | 60 (1) | 60 (1) | 0.5 (1) | 6 (1) | 0.437 |
| 2 | 60 (1) | 55 (2) | 1.5 (2) | 9 (2) | 0.695 |
| 3 | 60 (1) | 50 (3) | 2.5 (3) | 12 (3) | 0.695 |
| 4 | 50 (2) | 60 (1) | 1.5 (2) | 12 (3) | 0.463 |
| 5 | 50 (2) | 55 (2) | 2.5 (3) | 6 (1) | 1.000 |
| 6 | 50 (2) | 50 (3) | 0.5 (1) | 9 (2) | 0.184 |
| 7 | 40 (3) | 60 (1) | 2.5 (3) | 9 (2) | 0.535 |
| 8 | 40 (3) | 55 (2) | 0.5 (1) | 12 (3) | 0.480 |
| 9 | 40 (3) | 50 (3) | 1.5 (2) | 6 (1) | 0.576 |
| a k1 | 0.609 | 0.478 | 0.367 | 0.671 | |
| a k2 | 0.549 | 0.725 | 0.578 | 0.471 | |
| a k3 | 0.530 | 0.485 | 0.743 | 0.546 | |
| b R | 0.079 | 0.247 | 0.376 | 0.200 | |
| Optimal level | A1 | B2 | C3 | D1 |
| Run No. | A (g/mL) | B (min) | C (°C) | Combined Score (Extraction Yield and Yield of Five Phenolic Acids Were Combined at 1:1) |
|---|---|---|---|---|
| 1 | 1:20 (1) | 5 (1) | 4 (1) | 0.712 |
| 2 | 1:20 (1) | 15 (2) | 14 (2) | 0.589 |
| 3 | 1:20 (1) | 25 (3) | 24 (3) | 0.662 |
| 4 | 1:30 (2) | 5 (1) | 24 (3) | 0.827 |
| 5 | 1:30 (2) | 15 (2) | 4 (1) | 0.664 |
| 6 | 1:30 (2) | 25 (3) | 14 (2) | 0.771 |
| 7 | 1:40 (3) | 5 (1) | 14 (2) | 0.632 |
| 8 | 1:40 (3) | 15 (2) | 24 (3) | 0.754 |
| 9 | 1:40 (3) | 25 (3) | 4 (1) | 0.911 |
| a k1 | 0.654 | 0.724 | 0.762 | |
| a k2 | 0.754 | 0.669 | 0.664 | |
| a k3 | 0.766 | 0.781 | 0.748 | |
| b R | 0.112 | 0.112 | 0.098 | |
| Optimal level | A3 | B3 | C1 |
| Factor | Sum of Squares | Degrees of Freedom | F-Ratio | F Critical Value | Significance |
|---|---|---|---|---|---|
| A | 0.010 | 2 | 0.100 | 4.460 | - |
| B | 0.118 | 2 | 1.174 | 4.460 | - |
| C | 0.213 | 2 | 2.119 | 4.460 | - |
| D | 0.061 | 2 | 0.607 | 4.460 | - |
| Pure error | 0.400 | 8 | - | - |
| Factor | Sum of Squares | Degrees of Freedom | F-Ratio | F Critical Value | Significance |
|---|---|---|---|---|---|
| A | 0.022 | 2 | 1.138 | 5.140 | - |
| B | 0.019 | 2 | 0.983 | 5.140 | - |
| C | 0.017 | 2 | 0.879 | 5.140 | - |
| Pure error | 0.060 | 6 | - | - |
| Sample | Extraction Yield (%) | Content of Total Phenolic Acid (mg/g) | Content of Five Phenolic Acid Components (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| DSS | CA | FA | RA | SAB | |||
| total phenolic acid extract | 26.13 ± 1.09 | 189.10 ± 1.47 | 0.74 ± 0.08 | 1.93 ± 0.22 | 0.54 ± 0.06 | 21.26 ± 0.29 | 4.54 ± 0.18 |
| Sample | Extraction Yield (%) | Content of Total Tanshinone (mg/g) | Content of Four Tanshinone Components (mg/g) | |||
|---|---|---|---|---|---|---|
| DT−I | CT | T−I | T−IIA | |||
| total tanshinone extract | 3.13 ± 0.42 | 369.43 ± 0.11 | 5.97 ± 0.34 | 39.13 ± 0.97 | 20.73 ± 0.27 | 151.27 ± 0.56 |
| No. | Ion Model | RT. (Min) | [M + H]+/[M − H]− (m/z) | Fragment Ions (m/z) | Formula | PubChem CID | Compound Analyzed | |
|---|---|---|---|---|---|---|---|---|
| Calculated | Calculated | |||||||
| * 1 | − | 8.743 | 395.0984 | 395.0987 | 197.054 | C9H10O5 | 23693207 | Danshensu |
| 2 | + | 10.450 | 463.1235 | 463.1184 | 301.0506, 286.1035 | C22H22O11 | 11016019 | Diosmetin-7-o-β-D-glucopyranoside |
| 3 | + | 18.790 | 275.1599 | 475.1598 | 313.1112, 295.0471 | C24H26O10 | - | 2-[2-(4-Hydroxybenyl)-5-oxo-2,5-dihydro-3-furanyl]-5-meth-oxyphenyl beta-D-glucopyranoside |
| 4 | + | 20.400 | 497.0867 | 497.0889 | 139.5718 | C21H20O14 | 460896 | 3,5-Di-O-galloyquinic acid |
| 5 | + | 21.028 | 517.1341 | 517.1411 | 325.2438 | C25H24O12 | 5281780 | Isochlorogenic acid B |
| * 6 | + | 22.457 | 181.0495 | 181.0521 | 181.0521, 163.0297 | C9H8O4 | 689043 | Caffeic acid |
| 7 | + | 22.502 | 479.1548 | 479.1733 | 325.0827, 163.0440 | C23H26H11 | 5273567 | Calceolarioside B |
| 8 | + | 24.670 | 447.1286 | 447.1388 | 285.1189 | C22H22O10 | 5318267 | Calycosin 7-o-β-D-glucoside |
| * 9 | − | 26.790 | 359.0772 | 359.0780 | 235.5024, 223.0207, 179.0340 | C18H16O8 | 5281792 | Rosmarinic acid |
| 10 | + | 26.910 | 479.1548 | 479.1453 | 163.0443 | C23H26H11 | 5273566 | Calceolarioside A |
| 11 | + | 27.838 | 315.0863 | 315.1038 | 300.0916, 243.0992, 184.0822 | C17H14O6 | 13965473 | 3′,7-Dihydroxy-4′,6-dimethoxyisoflavone |
| 12 | + | 28.293 | 313.1071 | 313.1176 | 253.6042 | C18H16O5 | 5321620 | Tanshiniol B |
| 13 | + | 28.772 | 539.1184 | 539.1186 | 295.0967, 251.0219 | C27H22O12 | 6441498 | Lithospermic acid |
| * 14 | + | 28.802 | 741.1426 ([M + Na]+) | 741.1454 ([M + Na]+) | 727.1454, 519.0850 | C36H30O16 | 6451084 | Salvianolic acid B |
| 15 | − | 28.973 | 717.1461 | 717.1425 | 519.795 | C36H30O16 | 86278266/ 11765414 | Salvianolic acid E/L |
| 16 | − | 30.427 | 357.0616 | 357.0642 | 339.1991 | C18H14O8 | 10052949/10459878 | Salvianolic acid H/I |
| 17 | + | 30.442 | 285.0758 | 285.0713 | 225.1599, 213.1902 | C16H12O5 | 5280448 | Calycosin |
| 18 | + | 30.885 | 457.3829 | 457.3857 | 411.3058, 352.1478 | C30H48O3 | 64945 | Ursolic acid |
| 19 | + | 34.310 | 269.0808 | 269.0777 | 226.2148 | C16H12O4 | 5280378 | Formononetin |
| No. | Ion Model | RT. (Min) | [M + H]+/[M − H]− (m/z) | Fragment Ions (m/z) | Formula | PubChem CID | Compound Analyzed | |
|---|---|---|---|---|---|---|---|---|
| Calculated | Calculated | |||||||
| 20 | + | 37.148 | 311.1278 | 311.1272 | 293.1193, 278.0720, 275.0944, 267.1271, 252.1204, 247.1112 | C19H18O4 | 318797 | Tanshinone IIB |
| 21 | − | 38.787 | 295.0976 | 295.0944 | 265.8566 | C18H16O4 | 149138 | Danshenxinkun A |
| 22 | − | 38.897 | 295.0976 | 295.1044 | 280.9173 | C18H16O4 | 126071 | Tanshinol B |
| 23 | − | 39.620 | 313.1445 | 313.1484 | 269.2219 | C19H22O4 | 389888 | Neocryptotanshinone |
| 24 | + | 39.635 | 297.1485 | 297.1493 | 269.1546, 254.1453, 253.1596, 237.0480, 223.1292 | C19H20O3 | 626608 | Isocryptotanshinone |
| 25 | + | 39.683 | 337.1434 | 337.1404 | 309.1508, 263.0817 | C21H20O4 | 127172 | Danshenxinkun D |
| 26 | + | 41.547 | 311.1278 | 311.1273 | 296.1552, 293.1090, 278.1037 | C19H18O4 | 5318349 | Hydroxytanshinone IIA |
| 27 | + | 42.202 | 309.1121 | 309.1124 | 310.0717, 282.1034, 252.0975 | C19H16O4 | 124268 | Tanshinaldehyde |
| * 28 | + | 42.705 | 279.1016 | 279.1016 | 261.0982, 233.1394, 218.0920, 205.1570, 189.0836 | C18H14O3 | 11425923 | Dihydrotanshinone I |
| 29 | + | 44.075 | 339.1227 | 339.1249 | 281.1223, 235.1182 | C20H18O5 | 14610613 | Methyl tanshinonate |
| 30 | + | 44.325 | 281.1172 | 281.1206 | 253.1448, 235.1072 | C18H16O3 | 5320113 | Danshenxinkun B |
| 31 | − | 44.438 | 309.1132 | 309.1227 | 290.3985 | C19H18O4 | 619402 | Przewaquinone A |
| 32 | + | 44.972 | 295.1329 | 295.1352 | 295.1543, 277.1002, 249.1128, 225.0589 | C19H18O3 | 626354 | Isotanshinone IIA |
| 33 | − | 46.080 | 279.0569 | 279.0592 | 251.0661 | C17H12O4 | 10062187 | Nortanshinone |
| * 34 | + | 48.518 | 297.1485 | 297.1476 | 297.1476, 282.1300, 279.1407, 254.0798, 251.1457 | C19H20O3 | 160254 | Cryptotanshinone |
| 35 | − | 48.635 | 299.2017 | 299.1943 | 281.1008 | C20H28O2 | 94162 | Sugiol |
| 36 | − | 48.718 | 299.1653 | 299.1891 | 284.0926, 281.0704 | C19H24O3 | 10789 | Tropolone |
| * 37 | + | 51.052 | 277.0880 | 277.0880 | 277.0880, 249.0949, 202.1097, 178.1275 | C18H12O3 | 114917 | Tanshinone I |
| 38 | + | 55.345 | 279.1016 | 279.1016 | 261.0932, 233.1030, 205.1061, 205.1061, 190.0726, 189.0778 | C18H14O3 | 44425164 | Dihydroisotanshinone II |
| 39 | + | 56.073 | 299.1289 | 301.1535, 283.2216 | 269.0800 | C18H20O4 | 3083514 | Danshenol A |
| * 40 | + | 60.282 | 295.1329 | 295.1365 | 295.1312, 277.1254, 249.1288, 206.1100 | C19H18O3 | 164676 | Tanshinone IIA |
| 41 | + | 61.322 | 281.1536 | 281.1532 | 266.0938, 253.0989 | C19H20O2 | 3082765 | Dehydromiltirone |
| 42 | + | 62.540 | 283.1693 | 283.1737 | 265.1229, 241.1234, 195.1415 | C19H22O2 | 160142 | Miltirone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Dai, Y.; Chen, J.; Lu, J.; Zhou, D.; Ge, F.; Liu, P.; Zhou, X. Phytochemical Analysis and Neuroprotective Effect of Salvia castanea Diels f. Tomentosa Stib Extracts. Pharmaceuticals 2025, 18, 728. https://doi.org/10.3390/ph18050728
Peng X, Dai Y, Chen J, Lu J, Zhou D, Ge F, Liu P, Zhou X. Phytochemical Analysis and Neuroprotective Effect of Salvia castanea Diels f. Tomentosa Stib Extracts. Pharmaceuticals. 2025; 18(5):728. https://doi.org/10.3390/ph18050728
Chicago/Turabian StylePeng, Xiaoyan, Yuxing Dai, Jianwen Chen, Jing Lu, Dan Zhou, Fahuan Ge, Peiqing Liu, and Xue Zhou. 2025. "Phytochemical Analysis and Neuroprotective Effect of Salvia castanea Diels f. Tomentosa Stib Extracts" Pharmaceuticals 18, no. 5: 728. https://doi.org/10.3390/ph18050728
APA StylePeng, X., Dai, Y., Chen, J., Lu, J., Zhou, D., Ge, F., Liu, P., & Zhou, X. (2025). Phytochemical Analysis and Neuroprotective Effect of Salvia castanea Diels f. Tomentosa Stib Extracts. Pharmaceuticals, 18(5), 728. https://doi.org/10.3390/ph18050728





