Cardiac Electrophysiological Effects of the Sodium Channel-Blocking Antiepileptic Drugs Lamotrigine and Lacosamide
Abstract
1. Introduction
2. Results
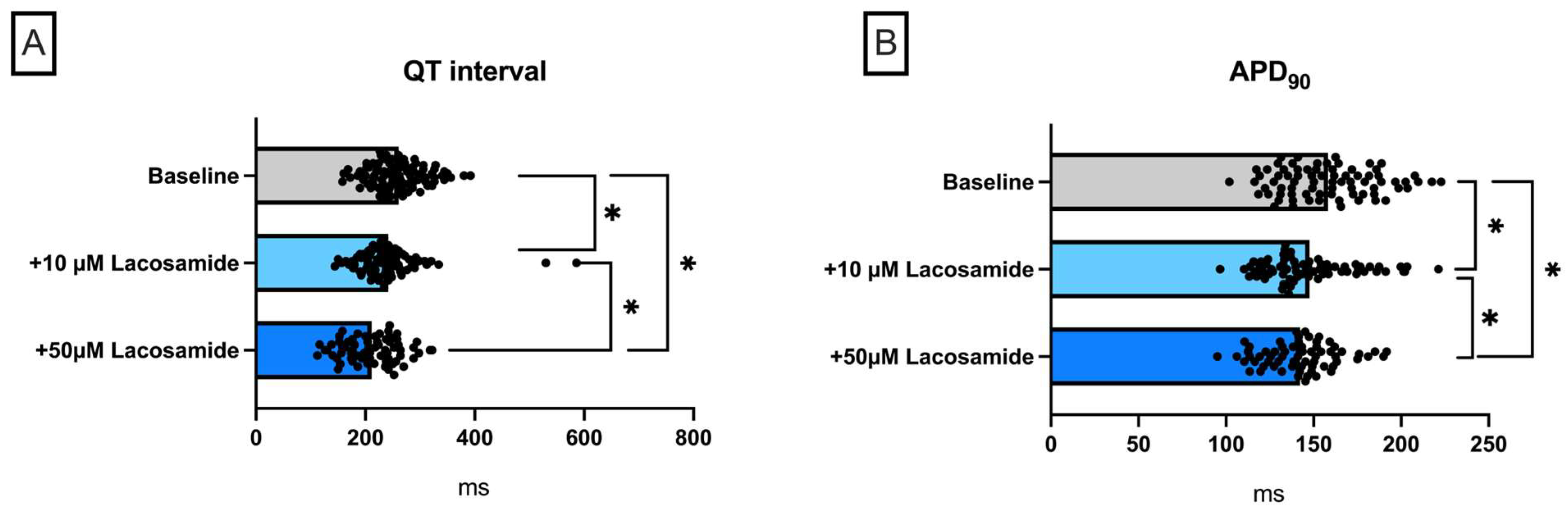
2.1. Lacosamide
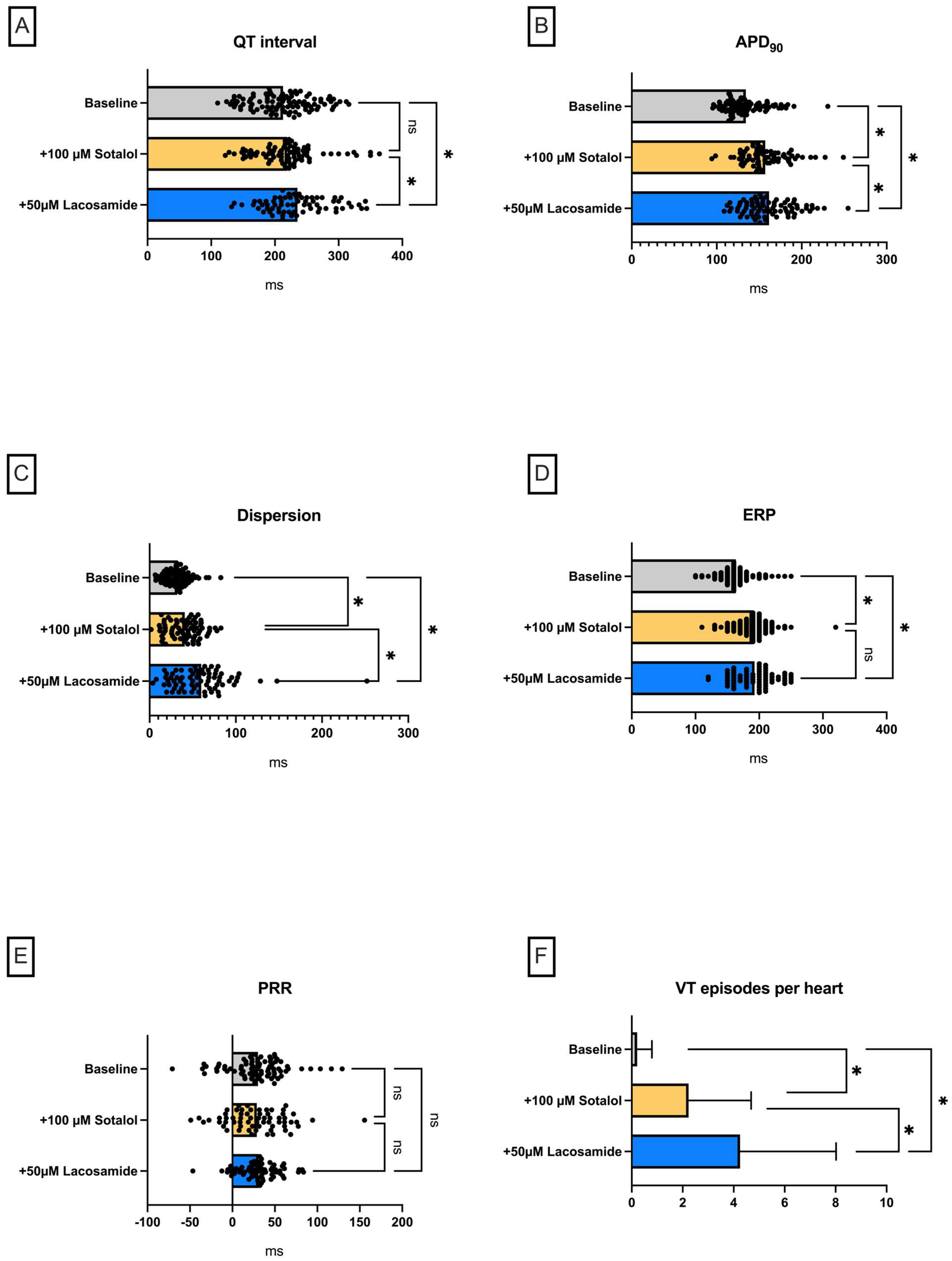
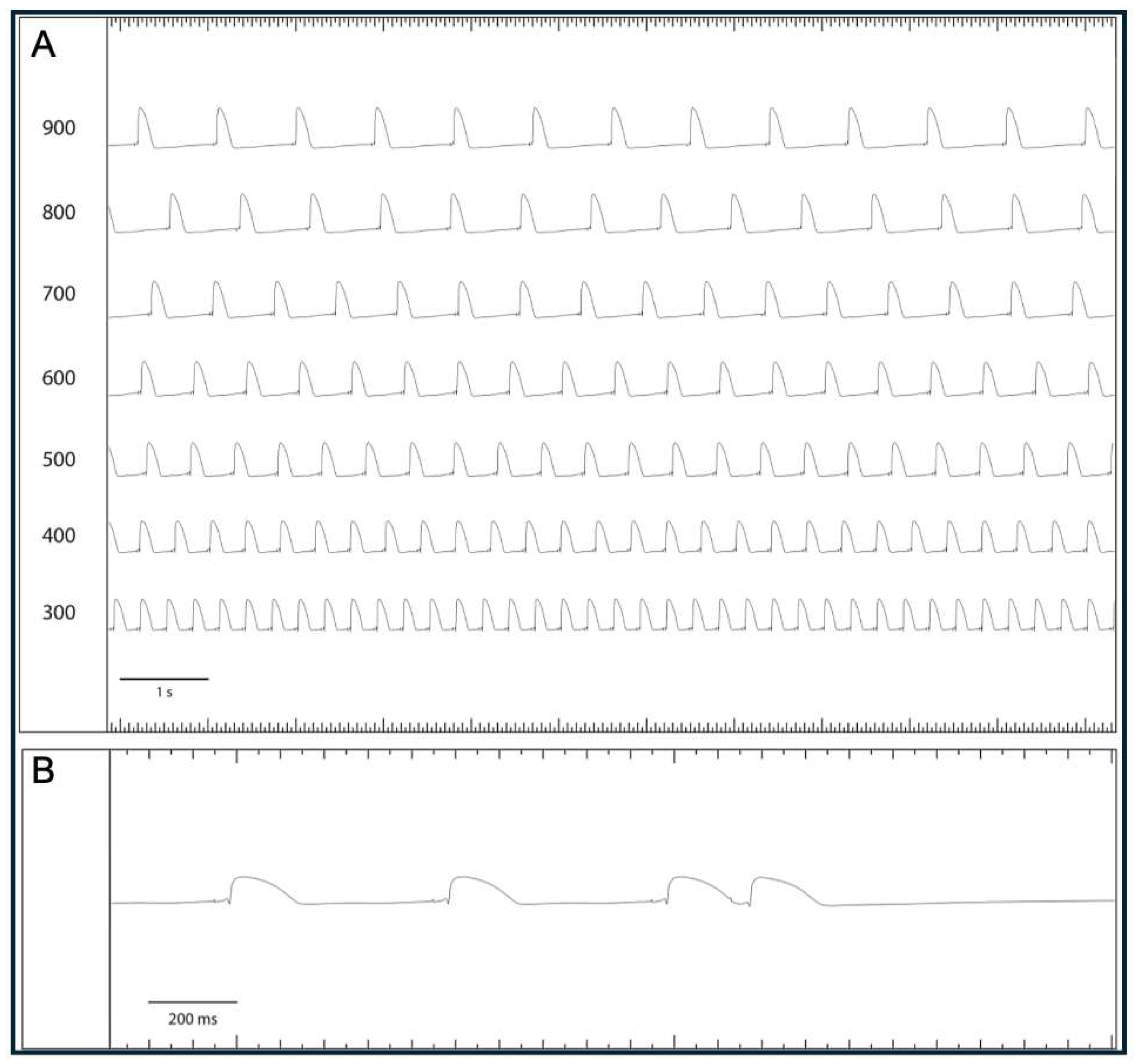
Lacosamide in a Model of QT Prolongation
2.2. Lamotrigine
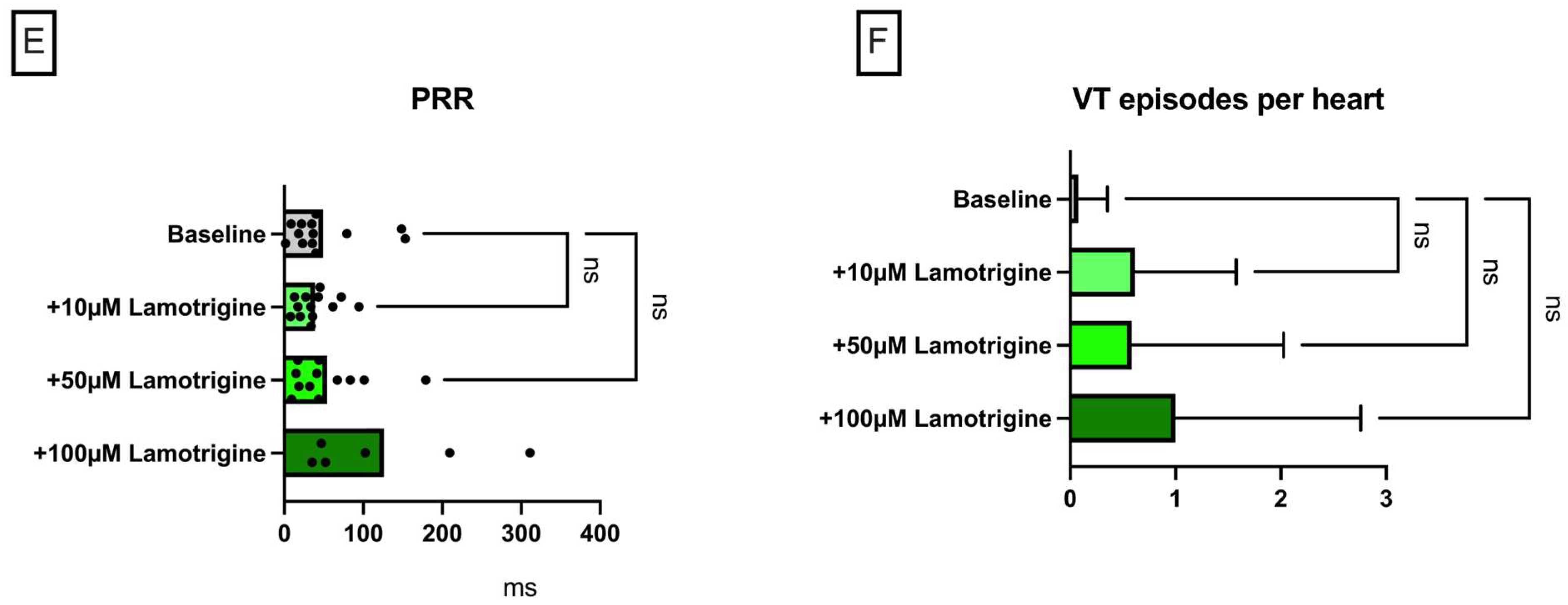
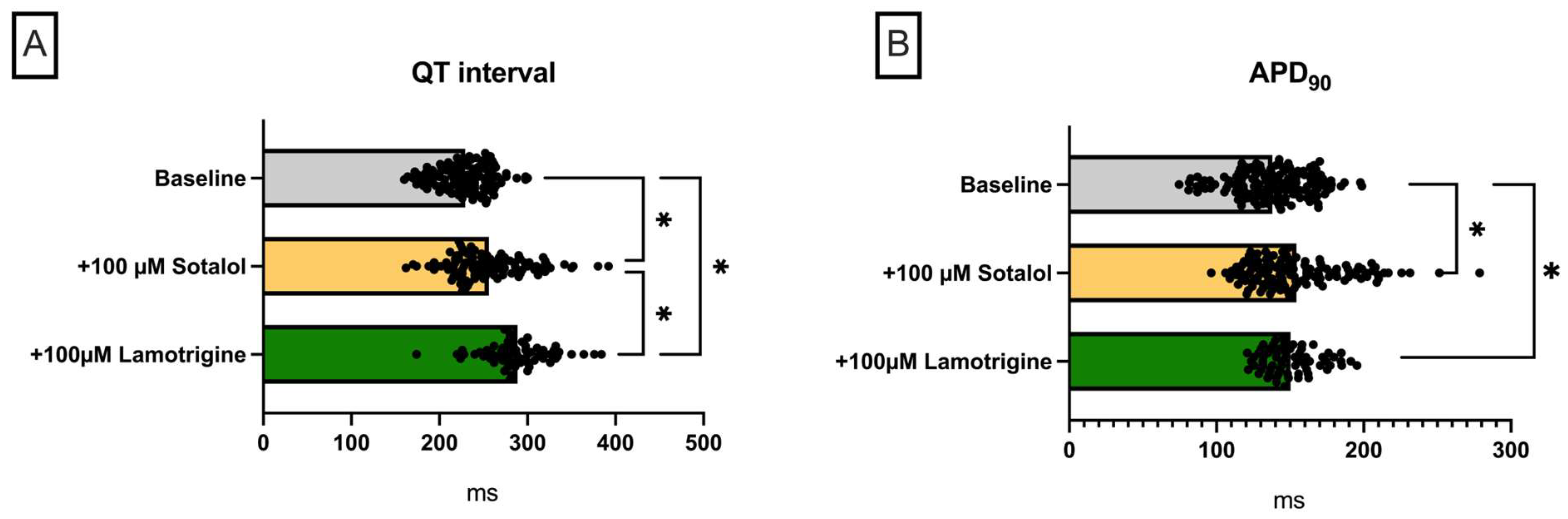
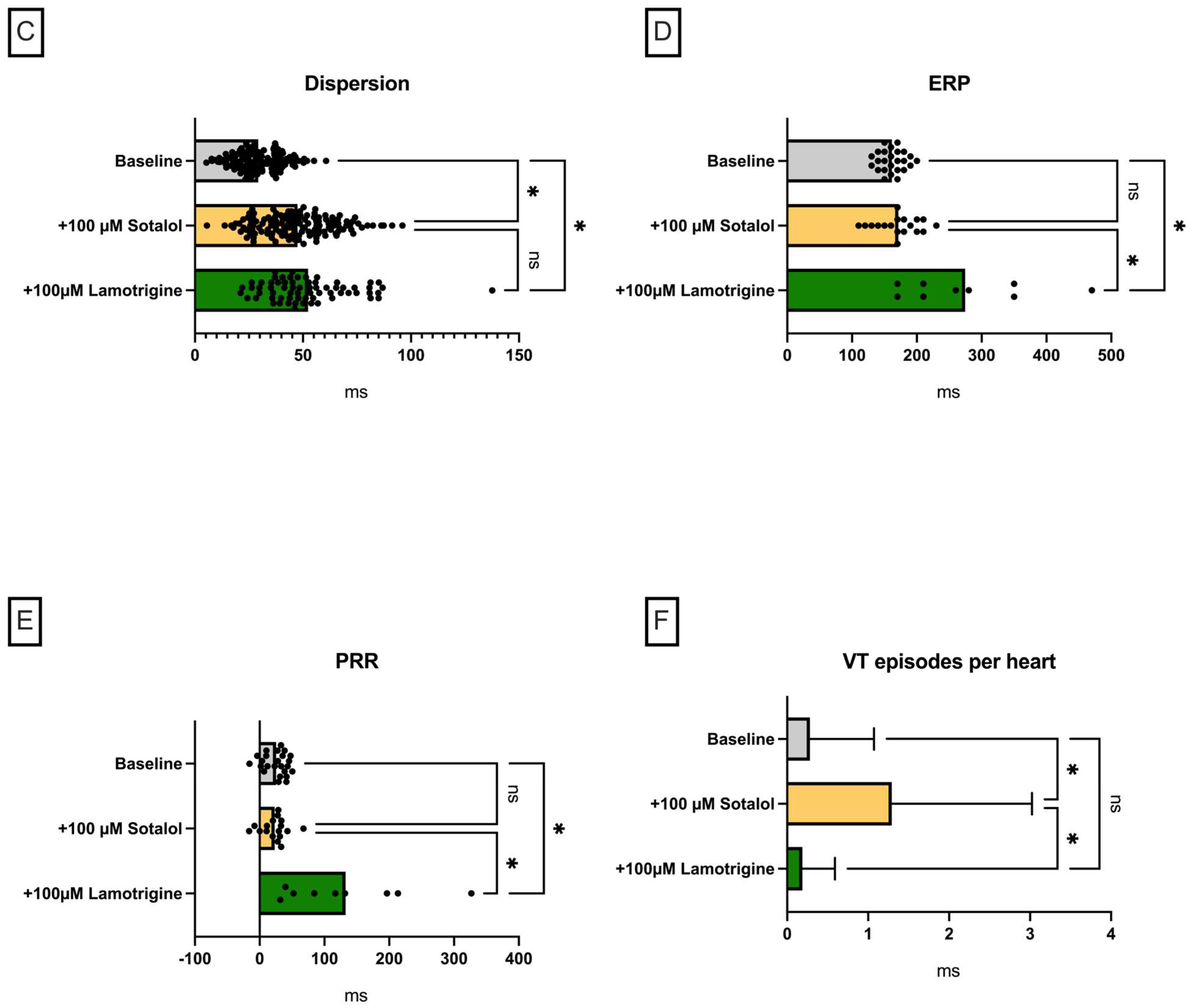
Lamotrigine in a Model of QT Prolongation
3. Discussion
- (I) Both substances led to a trend towards a decrease in action potential duration and/or QT interval under baseline conditions.
- (II) Perfusion with both sodium channel blockers did not lead to a significant increase in arrhythmia incidence under baseline conditions.
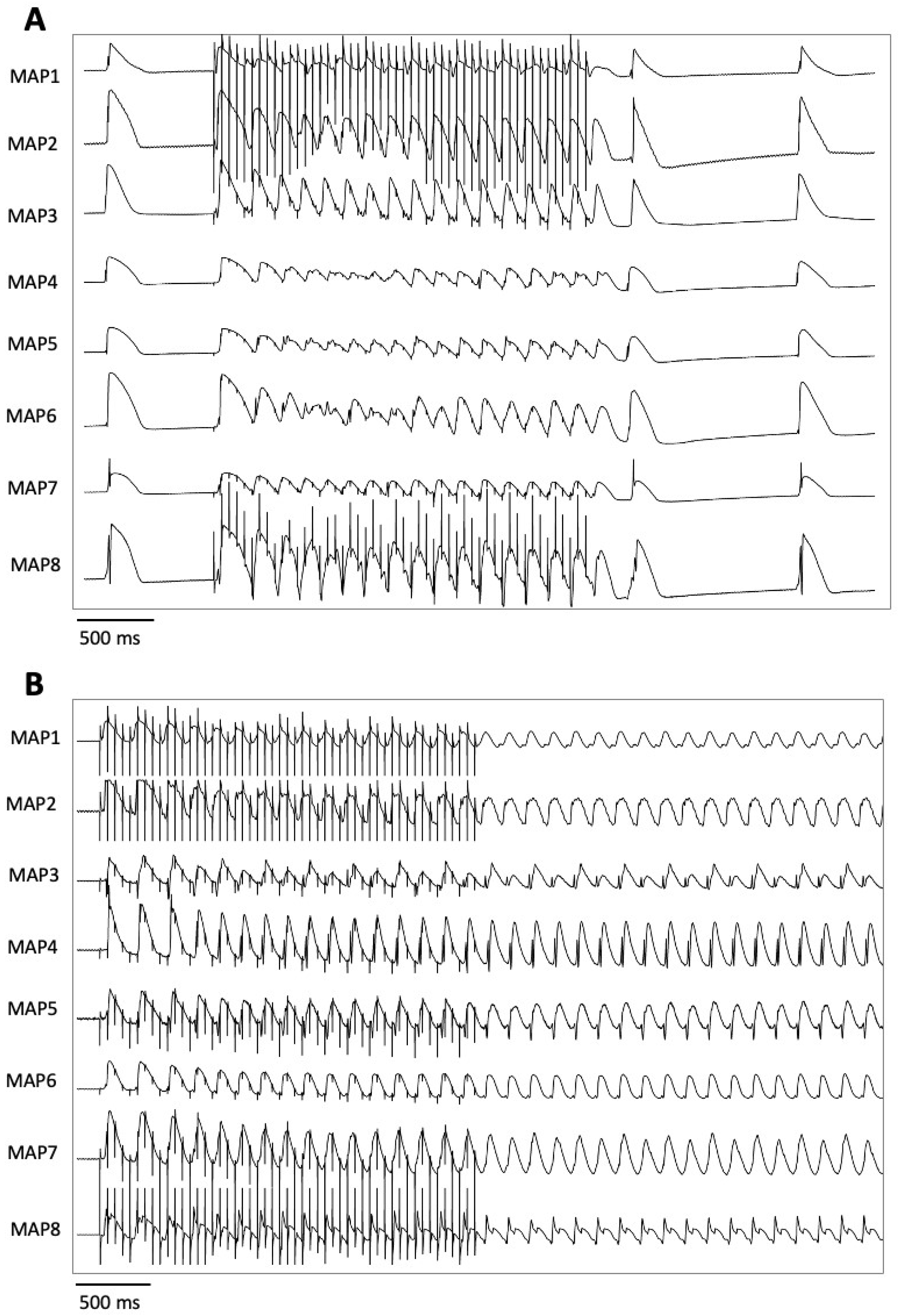
- (III) In the course of a drug-induced LQT syndrome, perfusion with lacosamide led to a significant increase in QT interval and arrhythmia incidence.
- (IV) In the course of a drug-induced LQT syndrome, perfusion with lamotrigine did not lead to a significant increase in QT interval. However, the incidence of arrhythmia was significantly reduced. This observation was accompanied by an increase in PRR.
3.1. Sodium Channel Inactivation
3.2. Simulation of a Model of Reduced Repolarization Reserve
3.3. Electrophysiological Effects of Lacosamide
3.4. Electrophysiological Effects of Lamotrigine
3.5. Limitations
3.6. Pharmacokinetic Suitability
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APD90 | Action potential duration at 90% repolarization |
| ARRIVE | Animals in Research: Reporting In Vivo Experiments |
| ECG | Electrocardiogram |
| ERP | Effective refractory period |
| FDA | Food and Drug Administration |
| ICH | International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use |
| IFM | Ile-Phe-Met |
| IKr | Rapid component of the delayed rectifier potassium current |
| INa,L | Late sodium current |
| KHB | Krebs–Henseleit buffer |
| LQTS | Long-QT syndrome |
| MAP | Monophasic action potential |
| NaV | Voltage-gated sodium channel |
| NCX | Na+/Ca2+ exchanger |
| NIH | National Institutes of Health |
| PRR | Post-repolarization refractoriness |
| SUDEP | Sudden unexpected death in epilepsy |
| VF | Ventricular fibrillation |
| VT | Ventricular tachycardia |
References
- Nashef, L.; So, E.L.; Ryvlin, P.; Tomson, T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012, 53, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P.; Nashef, L.; Lhatoo, S.D.; Bateman, L.M.; Bird, J.; Bleasel, A.; Boon, P.; Crespel, A.; Dworetzky, B.A.; Hogenhaven, H.; et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): A retrospective study. Lancet Neurol. 2013, 12, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, R.D.; Crompton, D.E.; Semsarian, C. Genetic Basis of Sudden Unexpected Death in Epilepsy. Front. Neurol. 2017, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, R.D.; Crompton, D.E.; Petrovski, S.; Lam, L.; Cutmore, C.; Garry, S.I.; Sadleir, L.G.; Dibbens, L.M.; Cairns, A.; Kivity, S.; et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann. Neurol. 2016, 79, 522–534. [Google Scholar] [CrossRef]
- Lu, Y.T.; Lin, C.H.; Ho, C.J.; Hsu, C.W.; Tsai, M.H. Evaluation of Cardiovascular Concerns of Intravenous Lacosamide Therapy in Epilepsy Patients. Front. Neurol. 2022, 13, 891368. [Google Scholar] [CrossRef]
- Li, Y.; Su, S.; Zhang, M.; Yu, L.; Miao, X.; Li, H.; Sun, Y. Risk assessment of arrhythmias related to three antiseizure medications: A systematic review and single-arm meta-analysis. Front. Neurol. 2024, 15, 1295368. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, H.; Bae, E.K.; Kim, D.W. Cardiac effects of rapid intravenous loading of lacosamide in patients with epilepsy. Epilepsy Res. 2021, 176, 106710. [Google Scholar] [CrossRef]
- Aboukaoud, M.; Wilf-Yarkoni, A.; Maor, E. Investigation of cardiac arrhythmia events in patients treated with lamotrigine: FDA adverse event reporing system analysis. Epilepsia 2023, 64, 2322–2329. [Google Scholar] [CrossRef]
- Chavez, P.; Casso Dominguez, A.; Herzog, E. Evolving Electrocardiographic Changes in Lamotrigine Overdose: A Case Report and Literature Review. Cardiovasc. Toxicol. 2015, 15, 394–398. [Google Scholar] [CrossRef]
- Omer, H.; Omer, M.H.; Alyousef, A.R.; Alzammam, A.M.; Ahmad, O.; Alanazi, H.A. Unmasking of Brugada syndrome by lamotrigine in a patient with pre-existing epilepsy: A case report with review of the literature. Front. Cardiovasc. Med. 2022, 9, 1005952. [Google Scholar] [CrossRef]
- Leong, K.M.; Seligman, H.; Varnava, A.M. Proarrhythmogenic effects of lamotrigine during ajmaline testing for Brugada syndrome. HeartRhythm Case Rep. 2017, 3, 167–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Curia, G.; Biagini, G.; Perucca, E.; Avoli, M. Lacosamide: A new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs 2009, 23, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Ingleby-Talecki, L.; van Dijkman, S.C.; Oosterholt, S.P.; Della Pasqua, O.; Winter, C.; Cunnington, M.; Rebar, L.; Forero-Schwanhaeuser, S.; Patel, V.; Cooper, J.A.; et al. Cardiac sodium channel inhibition by lamotrigine: In vitro characterization and clinical implications. Clin. Transl. Sci. 2022, 15, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- Vilin, Y.Y.; Ruben, P.C. Slow inactivation in voltage-gated sodium channels: Molecular substrates and contributions to channelopathies. Cell Biochem. Biophys. 2001, 35, 171–190. [Google Scholar] [CrossRef]
- Eaholtz, G.; Scheuer, T.; Catterall, W.A. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron 1994, 12, 1041–1048. [Google Scholar] [CrossRef]
- West, J.W.; Patton, D.E.; Scheuer, T.; Wang, Y.; Goldin, A.L.; Catterall, W.A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Natl. Acad. Sci. USA 1992, 89, 10910–10914. [Google Scholar] [CrossRef]
- Chahine, M.; Deschenes, I.; Trottier, E.; Chen, L.Q.; Kallen, R.G. Restoration of fast inactivation in an inactivation-defective human heart sodium channel by the cysteine modifying reagent benzyl-MTS: Analysis of IFM-ICM mutation. Biochem. Biophys. Res. Commun. 1997, 233, 606–610. [Google Scholar] [CrossRef]
- Kellenberger, S.; Scheuer, T.; Catterall, W.A. Movement of the Na+ channel inactivation gate during inactivation. J. Biol. Chem. 1996, 271, 30971–30979. [Google Scholar] [CrossRef]
- Goodchild, S.J.; Ahern, C.A. Conformational photo-trapping in Na(V)1.5: Inferring local motions at the “inactivation gate”. Biophys. J. 2024, 123, 2167–2175. [Google Scholar] [CrossRef]
- Liu, Y.; Bassetto, C.A.Z., Jr.; Pinto, B.I.; Bezanilla, F. A mechanistic reinterpretation of fast inactivation in voltage-gated Na(+) channels. Nat. Commun. 2023, 14, 5072. [Google Scholar] [CrossRef]
- O’Reilly, J.P.; Wang, S.Y.; Wang, G.K. Residue-specific effects on slow inactivation at V787 in D2-S6 of Na(v)1.4 sodium channels. Biophys. J. 2001, 81, 2100–2111. [Google Scholar] [CrossRef]
- Balser, J.R.; Nuss, H.B.; Chiamvimonvat, N.; Perez-Garcia, M.T.; Marban, E.; Tomaselli, G.F. External pore residue mediates slow inactivation in mu 1 rat skeletal muscle sodium channels. J. Physiol. 1996, 494 Pt 2, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Q.; Yan, N. A structural atlas of druggable sites on Na(v) channels. Channels 2024, 18, 2287832. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Huang, J.; Fan, X.; Wang, K.; Jin, X.; Huang, G.; Li, J.; Pan, X.; Yan, N. Structural mapping of Na(v)1.7 antagonists. Nat. Commun. 2023, 14, 3224. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fan, X.; Jin, X.; Teng, L.; Yan, N. Dual-pocket inhibition of Na(v) channels by the antiepileptic drug lamotrigine. Proc. Natl. Acad. Sci. USA 2023, 120, e2309773120. [Google Scholar] [CrossRef]
- Roden, D.M. Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing Clin. Electrophysiol. 1998, 21, 1029–1034. [Google Scholar] [CrossRef]
- Milberg, P.; Ramtin, S.; Monnig, G.; Osada, N.; Wasmer, K.; Breithardt, G.; Haverkamp, W.; Eckardt, L. Comparison of the in vitro electrophysiologic and proarrhythmic effects of amiodarone and sotalol in a rabbit model of acute atrioventricular block. J. Cardiovasc. Pharmacol. 2004, 44, 278–286. [Google Scholar] [CrossRef]
- Hornyik, T.; Castiglione, A.; Franke, G.; Perez-Feliz, S.; Major, P.; Hiripi, L.; Koren, G.; Bosze, Z.; Varro, A.; Zehender, M.; et al. Transgenic LQT2, LQT5, and LQT2-5 rabbit models with decreased repolarisation reserve for prediction of drug-induced ventricular arrhythmias. Br. J. Pharmacol. 2020, 177, 3744–3759. [Google Scholar] [CrossRef]
- Roden, D.M. Predicting drug-induced QT prolongation and torsades de pointes. J. Physiol. 2016, 594, 2459–2468. [Google Scholar] [CrossRef]
- Shah, R.R. Drug-induced QT interval prolongation: Does ethnicity of the thorough QT study population matter? Br. J. Clin. Pharmacol. 2013, 75, 347–358. [Google Scholar] [CrossRef]
- Holzem, K.M.; Gomez, J.F.; Glukhov, A.V.; Madden, E.J.; Koppel, A.C.; Ewald, G.A.; Trenor, B.; Efimov, I.R. Reduced response to IKr blockade and altered hERG1a/1b stoichiometry in human heart failure. J. Mol. Cell Cardiol. 2016, 96, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Errington, A.C.; Stohr, T.; Heers, C.; Lees, G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol. Pharmacol. 2008, 73, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, V.A.; Sabbah, H.N.; Higgins, R.S.; Silverman, N.; Lesch, M.; Undrovinas, A.I. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 1998, 98, 2545–2552. [Google Scholar] [CrossRef]
- Horvath, B.; Bers, D.M. The late sodium current in heart failure: Pathophysiology and clinical relevance. ESC Heart Fail. 2014, 1, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Shlobin, N.A.; Li, J.; Sander, J.W.; Keezer, M.R.; Thijs, R.D. Cardiac Conduction Delay for Sodium Channel Antagonist Antiseizure Medications: An Analysis of the Canadian Longitudinal Study on Aging. Neurology 2025, 104, e210302. [Google Scholar] [CrossRef]
- Frommeyer, G.; Weller, J.; Ellermann, C.; Bogeholz, N.; Leitz, P.; Dechering, D.G.; Kochhauser, S.; Wasmer, K.; Eckardt, L. Ivabradine Reduces Digitalis-induced Ventricular Arrhythmias. Basic. Clin. Pharmacol. Toxicol. 2017, 121, 526–530. [Google Scholar] [CrossRef]
- Milberg, P.; Frommeyer, G.; Ghezelbash, S.; Rajamani, S.; Osada, N.; Razvan, R.; Belardinelli, L.; Breithardt, G.; Eckardt, L. Sodium channel block by ranolazine in an experimental model of stretch-related atrial fibrillation: Prolongation of interatrial conduction time and increase in post-repolarization refractoriness. Europace 2013, 15, 761–769. [Google Scholar] [CrossRef]
- Franz, M.R.; Gray, R.A.; Karasik, P.; Moore, H.J.; Singh, S.N. Drug-induced post-repolarization refractoriness as an antiarrhythmic principle and its underlying mechanism. Europace 2014, 16 (Suppl. 4), iv39–iv45. [Google Scholar] [CrossRef]
- Shah, R.R.; Hondeghem, L.M. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005, 2, 758–772. [Google Scholar] [CrossRef]
- Milberg, P.; Hilker, E.; Ramtin, S.; Cakir, Y.; Stypmann, J.; Engelen, M.A.; Monnig, G.; Osada, N.; Breithardt, G.; Haverkamp, W.; et al. Proarrhythmia as a class effect of quinolones: Increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. J. Cardiovasc. Electrophysiol. 2007, 18, 647–654. [Google Scholar] [CrossRef]
- Christensen, J.; Trabjerg, B.B.; Dreier, J.W. Cardiac morbidity and mortality associated with the use of lamotrigine. Epilepsia 2022, 63, 2371–2380. [Google Scholar] [CrossRef]
- Kim, S.; Welch, L.; De Los Santos, B.; Radwanski, P.B.; Munger, M.A.; Kim, K. Association of Ventricular Arrhythmias with Lamotrigine: An Observational Cohort Study. medRxiv 2024. [Google Scholar] [CrossRef]
- Dias, P.; Meng, X.; Selimi, Z.; Struckman, H.; Veeraraghavan, R.; Radwanski, P.B. Lamotrigine promotes reentrant ventricular tachycardia in murine hearts. Epilepsia 2025. [Google Scholar] [CrossRef]
- Roden, D.M. Pharmacology and Toxicology of Nav1.5-Class 1 anti-arrhythmic drugs. Card. Electrophysiol. Clin. 2014, 6, 695–704. [Google Scholar] [CrossRef]
- Kim, S.; Welch, L.; Santos, B.L.; Radwanski, P.B.; Munger, M.A.; Kim, K. Association of atrial fibrillation with lamotrigine: An observational cohort study. Pharmacotherapy 2025, 45, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, C.; Wolfes, J.; Eckardt, L.; Frommeyer, G. Role of the rabbit whole-heart model for electrophysiologic safety pharmacology of non-cardiovascular drugs. Europace 2021, 23, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.E.; Palmer, K.L.; Krasowski, M.D. Correlation of elevated lamotrigine and levetiracetam serum/plasma levels with toxicity: A long-term retrospective review at an academic medical center. Toxicol. Rep. 2021, 8, 1592–1598. [Google Scholar] [CrossRef]
- Hentschel, M.; Stoffel-Wagner, B.; Surges, R.; von Wrede, R.; Dolscheid-Pommerich, R.C. Value of drug level concentrations of brivaracetam, lacosamide, and perampanel in care of people with epilepsy. Epilepsia 2024, 65, 620–629. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar]
- Detry, M.A.; Ma, Y. Analyzing Repeated Measurements Using Mixed Models. JAMA 2016, 315, 407–408. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfes, J.; Achenbach, P.; Wegner, F.K.; Rath, B.; Eckardt, L.; Frommeyer, G.; Ellermann, C. Cardiac Electrophysiological Effects of the Sodium Channel-Blocking Antiepileptic Drugs Lamotrigine and Lacosamide. Pharmaceuticals 2025, 18, 726. https://doi.org/10.3390/ph18050726
Wolfes J, Achenbach P, Wegner FK, Rath B, Eckardt L, Frommeyer G, Ellermann C. Cardiac Electrophysiological Effects of the Sodium Channel-Blocking Antiepileptic Drugs Lamotrigine and Lacosamide. Pharmaceuticals. 2025; 18(5):726. https://doi.org/10.3390/ph18050726
Chicago/Turabian StyleWolfes, Julian, Philipp Achenbach, Felix K. Wegner, Benjamin Rath, Lars Eckardt, Gerrit Frommeyer, and Christian Ellermann. 2025. "Cardiac Electrophysiological Effects of the Sodium Channel-Blocking Antiepileptic Drugs Lamotrigine and Lacosamide" Pharmaceuticals 18, no. 5: 726. https://doi.org/10.3390/ph18050726
APA StyleWolfes, J., Achenbach, P., Wegner, F. K., Rath, B., Eckardt, L., Frommeyer, G., & Ellermann, C. (2025). Cardiac Electrophysiological Effects of the Sodium Channel-Blocking Antiepileptic Drugs Lamotrigine and Lacosamide. Pharmaceuticals, 18(5), 726. https://doi.org/10.3390/ph18050726






