Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-pentedrone
Abstract
1. Introduction
2. Results
2.1. Participants’ Characteristics
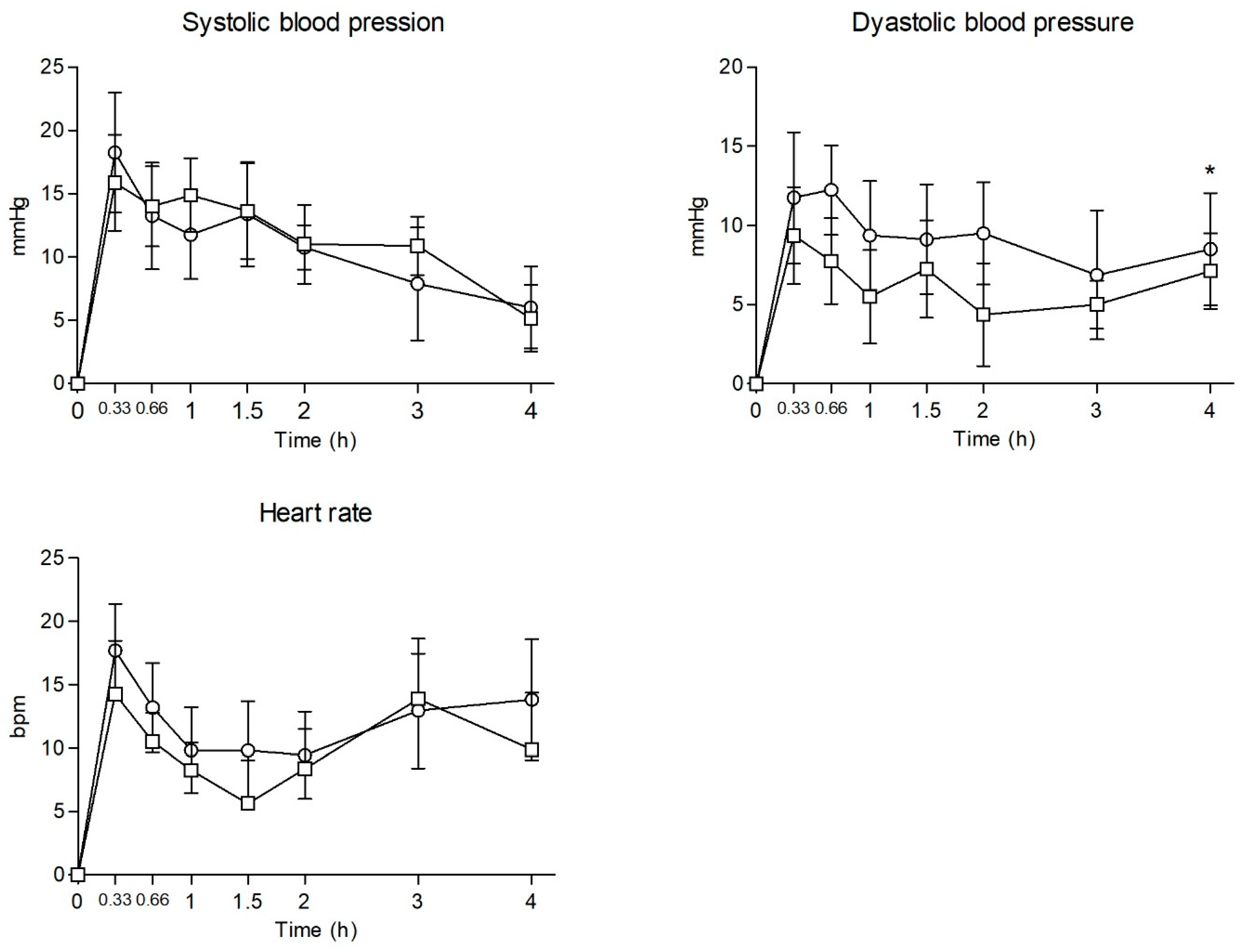
2.2. Physiological Effects
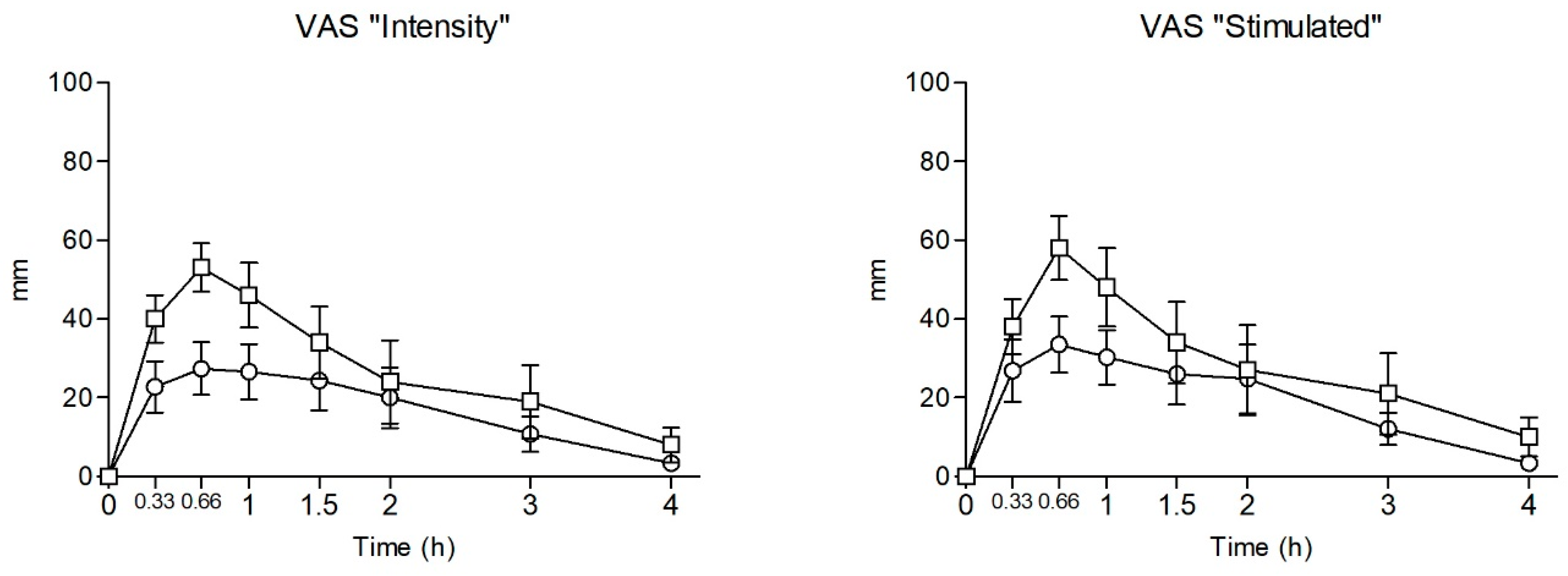
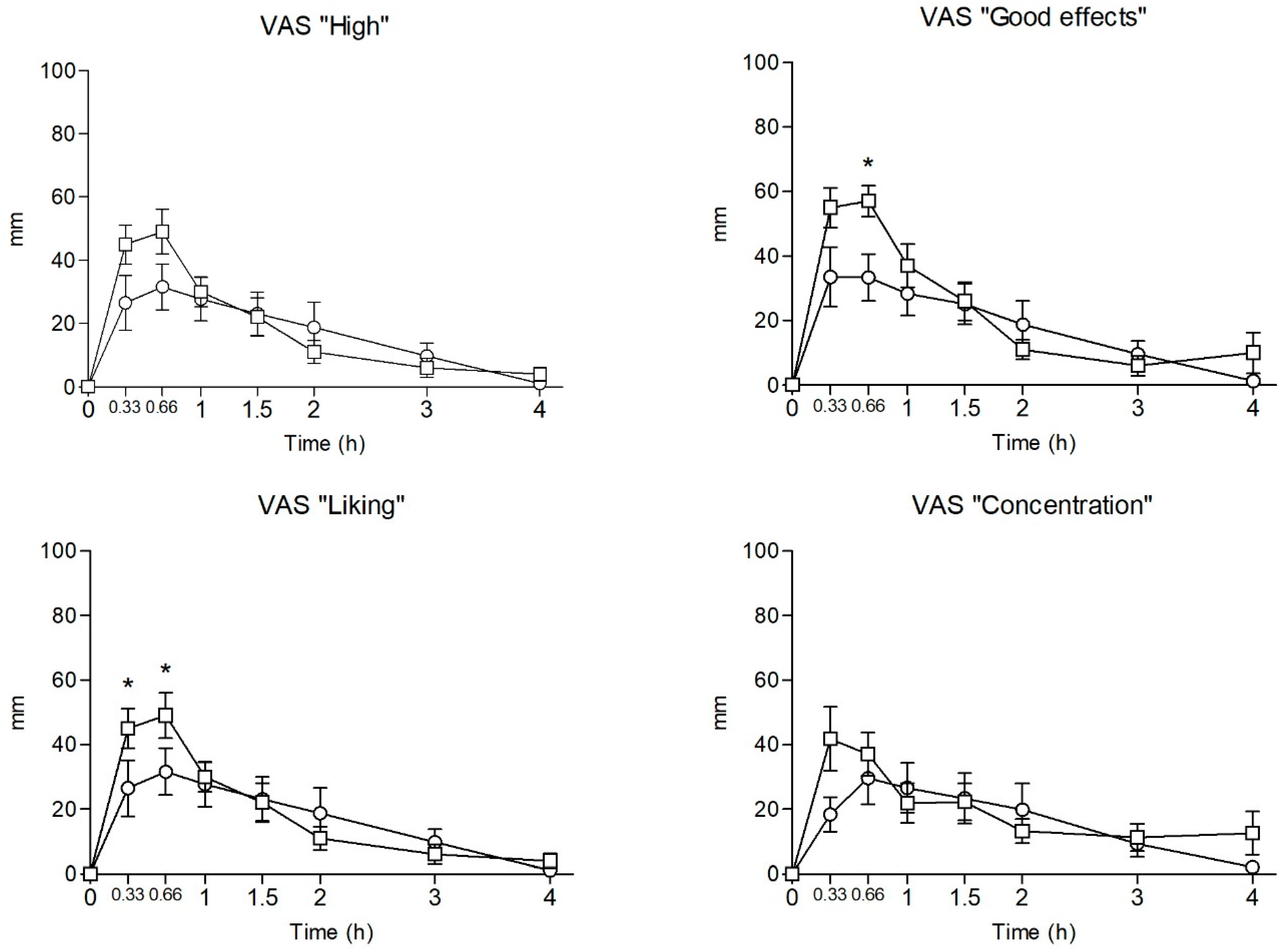
2.3. Subjective Effects
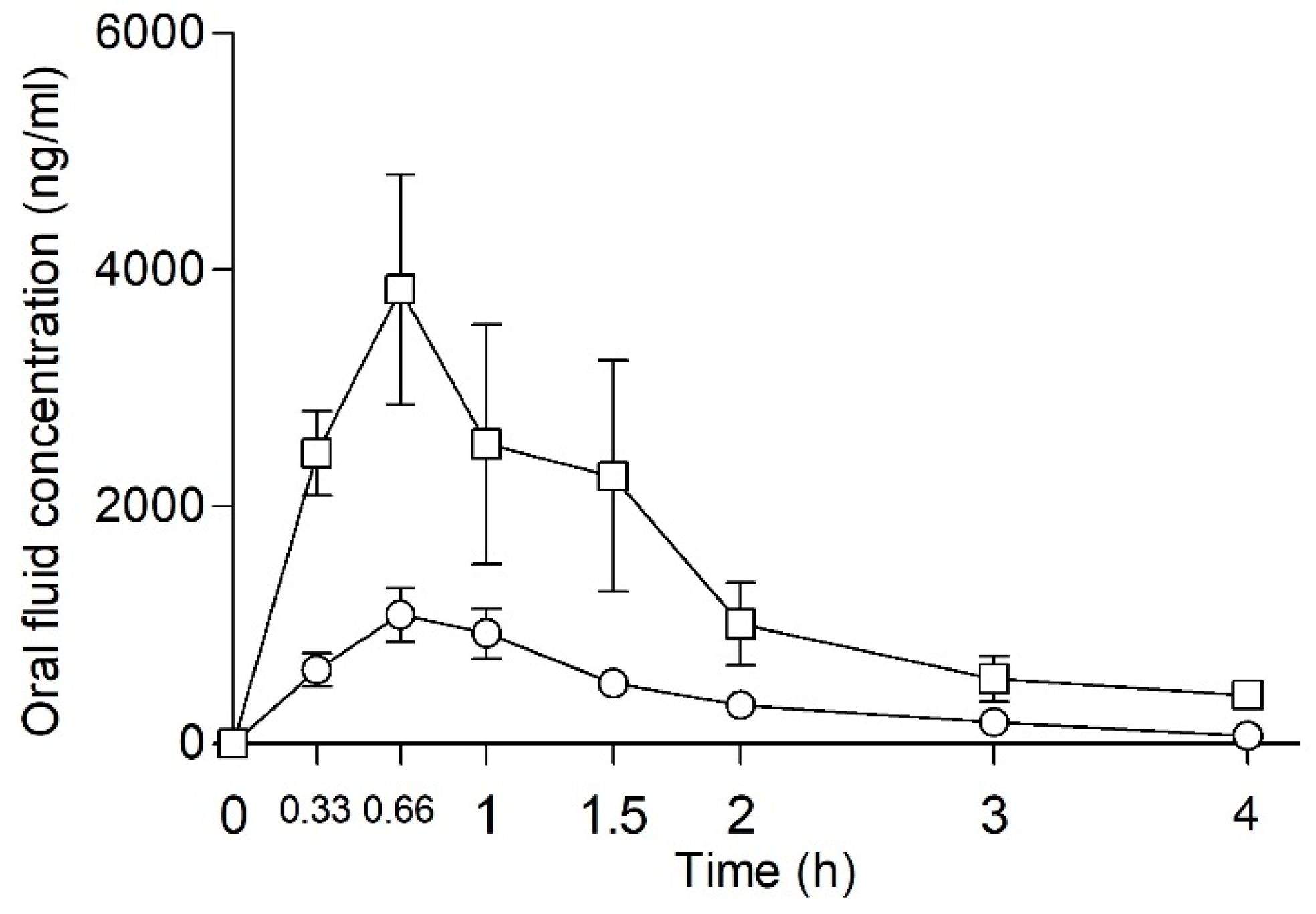
2.4. Oral Fluid Concentration of NEP and NEH
3. Discussion
4. Materials and Methods
4.1. Procedures
4.2. Physiological Effects
4.3. Subjective Effects
4.4. Oral Fluid Concentrations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Drug Report 2024—Drug Market Patterns and Trends. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2024-drug-market-trends.html (accessed on 21 March 2025).
- Chen, S.; Zhou, W.; Lai, M. Synthetic Cathinones: Epidemiology, Toxicity, Potential for Abuse, and Current Public Health Perspective. Brain Sci. 2024, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New Psychoactive Substances: Challenges for Drug Surveillance, Control, and Public Health Responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- Kuropka, P.; Zawadzki, M.; Szpot, P. A Review of Synthetic Cathinones Emerging in Recent Years (2019–2022). Forensic Toxicol. 2023, 41, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, L.C.; Wood, D.; Archer, J.; Hudson, S.; Dargan, P. Severe Toxicity to the New Psychoactive Substances 3-Hydroxyphencyclidine and N-Ethylhexedrone: An Analytically Confirmed Case Report. J. Med. Toxicol. 2020, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, E.; Banaszkiewicz, L.; Woźniak, M.K.; Kata, M.; Szpiech, B.; Kaliszan, M. Fatal N-Ethylhexedrone Intoxication. J. Anal. Toxicol. 2021, 45, e1–e6. [Google Scholar] [CrossRef]
- Adamowicz, P.; Jurczyk, A.; Gil, D.; Szustowski, S. A Case of Intoxication with a New Cathinone Derivative α-PiHP—A Presentation of Concentrations in Biological Specimens. Leg. Med. 2020, 42, 101626. [Google Scholar] [CrossRef]
- Lajtai, A.; Mayer, M.; Lakatos, Á.; Kuzma, M.; Miseta, A. New Psychoactive versus Conventional Stimulants—A Ten-Year Review of Casework in Hungary. Leg. Med. 2020, 47, 101780. [Google Scholar] [CrossRef]
- La Maida, N.; Di Trana, A.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. A Review of Synthetic Cathinone–Related Fatalities From 2017 to 2020. Ther. Drug Monit. 2021, 43, 52–68. [Google Scholar] [CrossRef]
- Persson, M.; Vikingsson, S.; Kronstrand, R.; Green, H. Characterization of Neurotransmitter Inhibition for Seven Cathinones by a Proprietary Fluorescent Dye Method. Drug Test. Anal. 2024, 16, 339–347. [Google Scholar] [CrossRef]
- Nadal-Gratacós, N.; Ríos-Rodríguez, E.; Pubill, D.; Batllori, X.; Camarasa, J.; Escubedo, E.; Berzosa, X.; López-Arnau, R. Structure–Activity Relationship of N-Ethyl-Hexedrone Analogues: Role of the α-Carbon Side-Chain Length in the Mechanism of Action, Cytotoxicity, and Behavioral Effects in Mice. ACS Chem. Neurosci. 2023, 14, 787–799. [Google Scholar] [CrossRef]
- Carrola, J.; Duarte, N.; Florindo, P.; Henriques, S.; Da Silva, G.; Bijlsma, L.; Moreira, R.; Correia, C.; Perry, M.D.J.; Lopes, Á.; et al. Metabolism of N-Ethylhexedrone and Buphedrone: An in Vivo Study in Mice Using HPLC-MS/MS. J. Chromatogr. B 2020, 1159, 122340. [Google Scholar] [CrossRef] [PubMed]
- De Mello-Sampayo, C.; Vaz, A.R.; Henriques, S.C.; Fernandes, A.; Paradinha, F.; Florindo, P.; Faria, P.; Moreira, R.; Brites, D.; Lopes, A. Designer Cathinones N-Ethylhexedrone and Buphedrone Show Different In Vitro Neurotoxicity and Mice Behaviour Impairment. Neurotox. Res. 2021, 39, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Gatch, M.B.; Shetty, R.A.; Sumien, N.; Forster, M.J. Behavioral Effects of Four Novel Synthetic Cathinone Analogs in Rodents. Addict. Biol. 2021, 26, e12987. [Google Scholar] [CrossRef]
- Massano, M.; Nuñez-Montero, M.; Papaseit, E.; Hladun, O.; Pérez-Maña, C.; Ventura, M.; Marchei, E.; Alladio, E.; Gerace, E.; Pichini, S.; et al. Metabolic Profile of N-Ethylhexedrone, N-Ethylpentedrone, and 4-Chloromethcathinone in Urine Samples by UHPLC-QTOF-HRMS. J. Pharm. Biomed. Anal. 2024, 241, 115994. [Google Scholar] [CrossRef]
- Lefeuvre, S.; Richeval, C.; Lelong, J.; Venisse, N.; Humbert, L.; Brunet, B. N-Ethylhexedrone: A Very Long and Bad Trip! A Case Series. J. Anal. Toxicol. 2024, 48, 507–513. [Google Scholar] [CrossRef]
- Pieprzyca, E.; Skowronek, R.; Czekaj, P. Toxicological Analysis of Cases of Mixed Poisonings with Synthetic Cathinones and Other Drugs of Abuse. J. Anal. Toxicol. 2023, 46, 1008–1015. [Google Scholar] [CrossRef]
- Kovács, K.; Kereszty, É.; Berkecz, R.; Tiszlavicz, L.; Sija, É.; Körmöczi, T.; Jenei, N.; Révész-Schmehl, H.; Institóris, L. Fatal Intoxication of a Regular Drug User Following N-Ethyl-Hexedrone and ADB-FUBINACA Consumption. J. Forensic Leg. Med. 2019, 65, 92–100. [Google Scholar] [CrossRef]
- Woźniak, M.K.; Banaszkiewicz, L.; Wiergowski, M.; Tomczak, E.; Kata, M.; Szpiech, B.; Namieśnik, J.; Biziuk, M. Development and Validation of a GC–MS/MS Method for the Determination of 11 Amphetamines and 34 Synthetic Cathinones in Whole Blood. Forensic Toxicol. 2020, 38, 42–58. [Google Scholar] [CrossRef]
- Mikołajczyk, A.; Adamowicz, P.; Tokarczyk, B.; Sekuła, K.; Gieroń, J.; Wrzesień, W.; Stanaszek, R. Determination of n-ethylhexedrone, a new cathinone derivative, in blood collected from drivers—Analysis of three cases. Probl. Forensic Sci. 2017, 109, 53–63. [Google Scholar]
- Duart-Castells, L.; Nadal-Gratacós, N.; Muralter, M.; Puster, B.; Berzosa, X.; Estrada-Tejedor, R.; Niello, M.; Bhat, S.; Pubill, D.; Camarasa, J.; et al. Role of Amino Terminal Substitutions in the Pharmacological, Rewarding and Psychostimulant Profiles of Novel Synthetic Cathinones. Neuropharmacology 2021, 186, 108475. [Google Scholar] [CrossRef]
- Hagan, K.S.; Reidy, L. Detection of Synthetic Cathinones in Victims of Sexual Assault. Forensic Sci. Int. 2015, 257, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Drevin, G.; Gaulier, J.-M.; Hakim, F.; Gish, A.; Férec, S.; Renard, L.; Malbranque, S.; Briet, M.; Abbara, C. Synthetic Cathinones in Drug-Facilitated Sexual Assault: A Case Report Involving the Novel Generation Substituted Cathinone N-Ethylpentedrone and a Review of the Literature. Forensic Sci. Int. 2024, 359, 112030. [Google Scholar] [CrossRef] [PubMed]
- Deville, M.; Fedorowicz, R.; Grandjean, F.; Simon, M.; Charlier, C. Synthetic Cathinones in Belgium: Two Case Reports with Different Outcomes Observed in the Emergency Room. J. Anal. Toxicol. 2023, 46, e291–e295. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.-A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; De La Torre, R.; Farré, M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacol 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; De Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Torrens, M.; Fonseca, F.; Grifell, M.; Ventura, M.; De La Torre, R.; Farré, M. Acute Pharmacological Effects of Oral and Intranasal Mephedrone: An Observational Study in Humans. Pharmaceuticals 2021, 14, 100. [Google Scholar] [CrossRef]
- Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; De La Torre, R.; et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology 2021, 10, 788. [Google Scholar] [CrossRef]
- Poyatos, L.; Lo Faro, A.F.; Berardinelli, D.; Sprega, G.; Malaca, S.; Pichini, S.; Huestis, M.A.; Papaseit, E.; Pérez-Mañá, C.; Busardò, F.P.; et al. Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans. Int. J. Mol. Sci. 2022, 23, 14636. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; De La Rosa, G.; Martín, S.; Barriocanal, A.M.; Carabias, L.; Kelmendi, B.; Taoussi, O.; et al. Pharmacological Effects of Methylone and MDMA in Humans. Front. Pharmacol. 2023, 14, 1122861. [Google Scholar] [CrossRef] [PubMed]
- Di Trana, A.; La Maida, N.; De La Rosa, G.; Di Giorgi, A.; Graziano, S.; Aldhaehri, K.; Papaseit, E.; Hladun, O.; Farré, M.; Pérez, C.; et al. Early and Mid-Term Disposition of α-PVP and Its Unknown Metabolites in Urine and Oral Fluid Through a Multi-Analytical Hyphenated Approach Following a Single Non-Controlled Administration to Healthy Volunteers. AAPS J. 2025, 27, 25. [Google Scholar] [CrossRef]
- Simmons, S.J.; Leyrer-Jackson, J.M.; Oliver, C.F.; Hicks, C.; Muschamp, J.W.; Rawls, S.M.; Olive, M.F. DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants. ACS Chem. Neurosci. 2018, 9, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Ellefsen, K.N.; Concheiro, M.; Huestis, M.A. Synthetic Cathinone Pharmacokinetics, Analytical Methods, and Toxicological Findings from Human Performance and Postmortem Cases. Drug Metab. Rev. 2016, 48, 237–265. [Google Scholar] [CrossRef]
- Ramaekers, J.G.; Reckweg, J.T.; Mason, N.L.; Kuypers, K.P.C.; Toennes, S.W.; Theunissen, E.L. Safety and Cognitive Pharmacodynamics Following Dose Escalations with 3-Methylmethcathinone (3-MMC): A First in Human, Designer Drug Study. Neuropsychopharmacology 2024. [Google Scholar] [CrossRef] [PubMed]
- Roseman, L.; Leech, R.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front. Hum. Neurosci. 2014, 8, 204. [Google Scholar] [CrossRef]
- Kirkpatrick, M.G.; Baggott, M.J.; Mendelson, J.E.; Galloway, G.P.; Liechti, M.E.; Hysek, C.M.; de Wit, H. MDMA effects consistent across laboratories. Psychopharmacology 2014, 231, 3899–3905. [Google Scholar] [CrossRef]
- Studerus, E.; Vizeli, P.; Harder, S.; Ley, L.; Liechti, M.E. Prediction of MDMA response in healthy humans: A pooled analysis of placebo-controlled studies. J. Psychopharmacol. 2021, 35, 556–565. [Google Scholar] [CrossRef]
- Vizeli, P.; Liechti, M.E. Safety pharmacology of acute MDMA administration in healthy subjects. J. Psychopharmacol. 2017, 31, 576–588. [Google Scholar] [CrossRef]
- Schmid, Y.; Rickli, A.; Schaffner, A.; Duthaler, U.; Grouzmann, E.; Hysek, C.M.; Liechti, M.E. Interactions between bupropion and 3,4-methylenedioxymethamphetamine in healthy subjects. J. Pharmacol. Exp. Ther. 2015, 353, 102–111. [Google Scholar] [CrossRef]
- van Wel, J.H.; Kuypers, K.P.; Theunissen, E.L.; Bosker, W.M.; Bakker, K.; Ramaekers, J.G. Effects of acute MDMA intoxicationon mood and impulsivity: Role of the 5-HT2 and 5-HT1 receptors. PLoS ONE 2012, 7, e40187. [Google Scholar] [CrossRef]
- Reynolds, A.R.; Strickland, J.C.; Stoops, W.W.; Lile, J.A.; Rush, C.R. Buspirone Maintenance Does Not Alter the Reinforcing, Subjective, and Cardiovascular Effects of Intranasal Methamphetamine. Drug Alcohol Depend. 2017, 181, 25–29. [Google Scholar] [CrossRef]
- van Wel, J.H.; Kuypers, K.P.; Theunissen, E.L.; Toennes, S.W.; Spronk, D.B.; Verkes, R.J.; Ramaekers, J.G. Single doses of THC and cocaine decrease proficiency of impulse control in heavy cannabis users. Br. J. Pharmacol. 2013, 170, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Regnier, S.D.; Stoops, W.W.; Lile, J.A.; Alcorn, J.L.; Bolin, B.L.; Reynolds, A.R.; Hays, L.R.; Rayapati, A.O.; Rush, C.R. Naltrexone-bupropion combinations do not affect cocaine self-administration in humans. Pharmacol. Biochem. Behav. 2023, 224, 173526. [Google Scholar] [CrossRef] [PubMed]
- Stoops, W.W.; Strickland, J.C.; Alcorn, J.L.; Hays, L.R.; Rayapati, A.O.; Lile, J.A.; Rush, C.R. Influence of phendimetrazine maintenance on the reinforcing, subjective, performance, and physiological effects of intranasal cocaine. Psychopharmacology 2019, 236, 2569–2577. [Google Scholar] [CrossRef]
- Pike, E.; Stoops, W.W.; Rush, C.R. Acute buspirone dosing enhances abuse-related subjective effects of oral methamphetamine. Pharmacol. Biochem. Behav. 2016, 150–151, 87–93. [Google Scholar] [CrossRef]
- Marks, K.R.; Lile, J.A.; Stoops, W.W.; Rush, C.R. Separate and combined impact of acute naltrexone and alprazolam on subjective and physiological effects of oral d-amphetamine in stimulant users. Psychopharmacology 2014, 231, 2741–2750. [Google Scholar] [CrossRef]
- Axelsson, M.A.B.; Lövgren, H.; Kronstrand, R.; Green, H.; Bergström, M.A. Retrospective Identification of New Psychoactive Substances in Patient Samples Submitted for Clinical Drug Analysis. Basic Clin. Pharma. Toxicol. 2022, 131, 420–434. [Google Scholar] [CrossRef]
- Angerer, V.; Schmid, Y.; Franz, F.; Gnann, H.; Speer, J.M.; Gnann, A.; Helmecke, S.; Buchwald, A.; Brandt, S.D.; Passie, T.; et al. Acute psychotropic, autonomic, and endocrine effects of 5,6-methylenedioxy-2-aminoindane (MDAI) compared with 3,4-methylenedioxymethamphetamine (MDMA) in human volunteers: A self-administration study. Drug Test. Anal. 2024, 16, 1002–1011. [Google Scholar] [CrossRef]
- Irvine, R.J.; Keane, M.; Felgate, P.; McCann, U.D.; Callaghan, P.D.; White, J.M. Plasma drug concentrations and physiological measures in ‘dance party’ participants. Neuropsychopharmacology 2006, 31, 424–430. [Google Scholar] [CrossRef]
- Morefield, K.M.; Keane, M.; Felgate, P.; White, J.M.; Irvine, R.J. Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational ‘ecstasy’ users. Addiction 2011, 106, 1293–1300. [Google Scholar] [CrossRef]
- Freeman, T.P.; Morgan, C.J.; Vaughn-Jones, J.; Hussain, N.; Karimi, K.; Curran, H.V. Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high’. Addiction 2012, 107, 792–800. [Google Scholar] [CrossRef]
- Mallaroni, P.; Mason, N.L.; Reckweg, J.T.; Paci, R.; Ritscher, S.; Toennes, S.W.; Theunissen, E.L.; Kuypers, K.P.C.; Ramaekers, J.G. Assessment of the Acute Effects of 2C-B vs. Psilocybin on Subjective Experience, Mood, and Cognition. Clin. Pharmacol. Ther. 2023, 114, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Farré, M.; Pérez-Mañá, C.; Torrens, M.; Ventura, M.; Pujadas, M.; de la Torre, R.; González, D. Acute Pharmacological Effects of 2C-B in Humans: An Observational Study. Front. Pharmacol. 2018, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.; Sanders, J.W.; Reydellet, D.; Barba, T.; Luan, L.X.; Angona, Ó.S.; Ona, G.; Allocca, G.; Smith, C.H.; Daily, Z.G.; et al. Exploring 5-MeO-DMT as a pharmacological model for deconstructed consciousness. Neurosci. Conscious. 2025, 2025, niaf007. [Google Scholar] [CrossRef]
- Nuñez-Montero, M.; Lombroni, C.; Maida, N.L.; Rotolo, M.C.; Pichini, S.; Papaseit, E.; Hladun, O.; Ventura, M.; Poyatos, L.; Pérez-Mañá, C.; et al. GC-MS/MS Determination of Synthetic Cathinones: 4-Chloromethcathinone, N-Ethyl Pentedrone, and N-Ethyl Hexedrone in Oral Fluid and Sweat of Consumers under Controlled Administration: Pilot Study. Int. J. Mol. Sci. 2023, 24, 9387. [Google Scholar] [CrossRef]
| NEH (n = 8) | NEP (n = 8) | t-Student | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Parameter | Mean ± SD | Dunnett’s Test | Mean ± SD | Dunnett’s Test | T | p-Value | F | p-Value | T-C |
| SBP | Emax | 22.13 ± 12.56 | 20.63 ± 10.63 | 0.258 | 0.800 | |||||
| AUC0–4 | 41.02 ± 33.55 | 44.68 ± 26.03 | −0.244 | 0.811 | ||||||
| T-C | a, b, c, d, e | a, b, c, d, e | 0.325 | 0.941 | -- | |||||
| DBP | Emax | 16.75 ± 9.50 | 11.88 ± 10.30 | 0.985 | 0.341 | |||||
| AUC0–4 | 34.73 ± 33.94 | 23.48 ± 23.74 | 0.769 | 0.455 | ||||||
| T-C | a, b, c, d, e | a, b, d, g | 4.590 | <0.001 | h | |||||
| HR | Emax | 21.63 ± 10.05 | 22.31 ± 11.11 | −0.130 | 0.899 | |||||
| AUC0–4 | 39.60 ± 30.41 | 46.21 ± 37.67 | −0.386 | 0.705 | ||||||
| T-C | a, f | a, b, c, d, e, f, g | 0.253 | 0.970 | -- | |||||
| NEH | NEP | t-Student | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Parameter | Mean ± SD | Dunnett’s Test | Mean ± SD | Dunnett’s Test | T | p-Value | F | p-Value | T-C |
| Intensity | Emax | 32.88 ± 20.50 | 55.50 ± 19.57 | −2.258 | 0.040 | |||||
| AUC0–4 | 67.50 ± 55.71 | 108.55 ± 81.58 | −1.176 | 0.259 | ||||||
| T-C | a, b, c, d, e | a, b, c, d, e | 2.055 | 0.056 | -- | |||||
| Stimulated | Emax | 37.13 ± 22.39 | 59.50 ± 23.69 | −1.941 | 0.073 | |||||
| AUC0–4 | 78.17 ± 58.51 | 115.16 ± 94.36 | −0.650 | 0.526 | ||||||
| T-C | a, b, c, d, e | a, b, c, d, e, f | ||||||||
| High | Emax | 34.63 ± 21.60 | 51.13 ± 17.25 | −1.688 | 0.113 | |||||
| AUC0–4 | 66.93 ± 56.96 | 70.65 ± 30.84 | −0.162 | 0.874 | ||||||
| T-C | a, b, c, d, e | a, b, c, d | 2.893 | 0.009 | NS | |||||
| Good effects | Emax | 36.38 ± 23.23 | 61.00 ± 14.85 | −2.526 | 0.024 | |||||
| AUC0–4 | 71.03 ± 60.00 | 85.40 ± 22.49 | −0.706 | 0.492 | ||||||
| T-C | a, b, c, d, e | a, b, c, d | 3.226 | 0.004 | c | |||||
| Bad effects | Emax | 3.88 ± 4.83 | 40.13 ± 36.88 | −2.756 | 0.015 | |||||
| AUC0–4 | 4.06 ± 5.06 | 71.29 ± 101.56 | −1.870 | 0.083 | ||||||
| T-C | NS | c, d | 2.289 | 0.033 | d | |||||
| Liking | Emax | 38.63 ± 21.76 | 72.38 ± 16.95 | −3.461 | 0.004 | |||||
| AUC0–4 | 71.81 ± 50.34 | 94.28 ± 21.78 | −1.159 | 0.266 | ||||||
| T-C | a, b, c, d, e | a, b, c, d | 7.074 | <0.001 | a, b | |||||
| Drowsiness | Emax | 4.00 ± 7.87 | 29.00 ± 34.62 | −1.992 | 0.066 | |||||
| AUC0–4 | 4.26 ± 8.37 | 52.53 ± 88.29 | −1.540 | 0.146 | ||||||
| T-C | NS | e | 2.250 | 0.036 | NS | |||||
| Concentration | Emax | 31.63 ± 25.48 | 48.63 ± 23.10 | −1.398 | 0.184 | |||||
| AUC0–4 | 64.08 ± 56.58 | 73.99 ± 31.01 | −0.434 | 0.671 | ||||||
| T-C | a, b, c, d, e | a, b, c, d | 1.953 | 0.069 | -- | |||||
| Dizziness | Emax | 2.88 ± 5.00 | 5.50 ± 14.35 | −0.489 | 0.633 | |||||
| AUC0–4 | 3.28 ± 5.15 | 12.14 ± 32.85 | −0.753 | 0.464 | ||||||
| T-C | NS | NS | 0.887 | 0.520 | -- | |||||
| Different body feeling | Emax | 27.88 ± 24.63 | 34.25 ± 21.71 | −0.549 | 0.592 | |||||
| AUC0–4 | 54.69 ± 51.36 | 63.00 ± 55.44 | 0.715 | 0.486 | ||||||
| T-C | c, e | c | 1.138 | 0.348 | -- | |||||
| Open | Emax | 40.00 ± 26.55 | 65.88 ± 18.43 | −2.264 | 0.040 | |||||
| AUC0–4 | 76.75 ± 63.71 | 114.01 ± 46.17 | −1.340 | 0.202 | ||||||
| T-C | a, b, c, d, e | a, b, c, d, f | 2.314 | 0.032 | h | |||||
| Trust | Emax | 38.75 ± 28.74 | 64.38 ± 20.04 | −2.069 | 0.058 | |||||
| AUC0–4 | 75.54 ± 63.94 | 126.47 ± 57.88 | −1.671 | 0.117 | ||||||
| T-C | a, b, c, d, e | a, b, c, d, e, f | 2.042 | 0.057 | -- | |||||
| Feeling close to others | Emax | 42.75 ± 28.08 | 65.25 ± 23.49 | −1.738 | 0.104 | |||||
| AUC0–4 | 82.22 ± 64.49 | 119.35 ± 59.51 | −1.187 | 0.255 | ||||||
| T-C | a, b, c, d, e | a, b, c, d, f | 1.822 | 0.091 | -- | |||||
| I want to be with other people | Emax | 27.75 ± 30.21 | 67.50 ± 34.98 | −2.432 | 0.029 | |||||
| AUC0–4 | 58.64 ± 70.59 | 122.95 ± 75.39 | −1.761 | 0.100 | ||||||
| T-C | a, b, c, d, e | NS | 3.026 | 0.006 | b, c | |||||
| I want to hug someone | Emax | 27.25 ± 29.47 | 34.38 ± 28.73 | −0.490 | 0.632 | |||||
| AUC0–4 | 55.27 ± 66.29 | 78.64 ± 77.79 | −0.647 | 0.528 | ||||||
| T-C | b, c, d | a, b, c, d, e | 0.887 | 0.520 | -- | |||||
| ARCI-PCAG | Emax | −2.25 ± 1.16 | 4.25 ± 4.06 | −4.351 | 0.001 | |||||
| AUC0–4 | −4.31 ± 3.92 | 5.88 ± 9.26 | −2.864 | 0.012 | ||||||
| T-C | c | -- | 2.814 | 0.034 | f, g | |||||
| ARCI-MBG | Emax | 4.88 ± 3.94 | 10.75 ± 9.47 | −0.204 | 0.841 | |||||
| AUC0–4 | 10.88 ± 10.88 | 1.50 ± 4.00 | 0.025 | 0.981 | ||||||
| T-C (df=) | c, e | c, e | 1.002 | 0.414 | -- | |||||
| ARCI-LSD | Emax | 0.88 ± 2.10 | 1.75 ± 3.01 | −0.6.74 | 0.511 | |||||
| AUC0–4 | 1.13 ± 4.32 | 4.56 ± 7.47 | −1.127 | 0.279 | ||||||
| T-C | -- | c | 1.053 | 0.388 | -- | |||||
| ARCI-BG | Emax | 3.50 ± 2.33 | 1.75 ± 4.17 | 1.037 | 0.317 | |||||
| AUC0–4 | 7.88 ± 5.93 | 4.06 ± 9.71 | 0.948 | 0.359 | ||||||
| T-C | c, e,f | -- | 1.218 | 0.313 | -- | |||||
| ARCI-A | Emax | 4.13 ± 2.85 | 4.88 ± 2.75 | −0.536 | 0.601 | |||||
| AUC0–4 | 10.56 ± 7.48 | 11.00 ± 7.60 | −0.116 | 0.909 | ||||||
| T-C | c, e, f | c, e, f, g | 0.980 | 0.426 | -- | |||||
| VESSPA-S | Emax | 0.34 ± 0.40 | 1.04 ± 0.93 | −1.964 | 0.070 | |||||
| AUC0–4 | 0.44 ± 0.50 | 2.31 ± 2.53 | −2.049 | 0.060 | ||||||
| T-C | NS | e, f, g | 2.237 | 0.077 | ||||||
| VESSPA-ANX | Emax | 1.46 ± 1.14 | 1.67 ± 0.93 | −0.398 | 0.697 | |||||
| AUC0–4 | 3.46 ± 3.09 | 4.29 ± 3.42 | −0.511 | 0.617 | ||||||
| T-C | c, e, f | c, e, f, g | 0.572 | 0.684 | -- | |||||
| VESSPA-CP | Emax | 0.00 ± 0.00 | 0.06 ± 0.12 | −1.433 | 0.174 | |||||
| AUC0–4 | 0.00 ± 0.00 | 0.06 ± 0.12 | −1.433 | 0.174 | ||||||
| T-C | NS | NS | 2.053 | 0.099 | -- | |||||
| VESSPA-SOC | Emax | 1.31 ± 1.11 | 1.42 ± 0.87 | −0.213 | 0.834 | |||||
| AUC0–4 | 2.54 ± 2.55 | 2.60 ± 2.03 | −0.055 | 0.957 | ||||||
| T-C | c | c, g | 1.394 | 0.248 | -- | |||||
| VESSPA-ACT | Emax | 1.79 ± 0.99 | 2.04 ± 0.73 | −0.578 | 0.573 | |||||
| AUC0–4 | 3.75 ± 2.20 | 3.79 ± 1.45 | −0.044 | 0.965 | ||||||
| T-C | c, e | c, e | 0.978 | 0.427 | -- | |||||
| VESSPA-PS | Emax | 0.29 ± 0.35 | 0.63 ± 0.71 | −1.185 | 0.256 | |||||
| AUC0–4 | 0.58 ± 0.65 | 1.42 ± 1.69 | −1.294 | 0.217 | ||||||
| T-C | c | NS | 0.636 | 0.639 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Montero, M.; Pérez-Mañá, C.; Hladun, O.; Poyatos, L.; Caicedo, D.A.; De la Rosa, G.; Argote, M.C.; Martín, S.; Ventura, M.; Maida, N.L.; et al. Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-pentedrone. Pharmaceuticals 2025, 18, 721. https://doi.org/10.3390/ph18050721
Núñez-Montero M, Pérez-Mañá C, Hladun O, Poyatos L, Caicedo DA, De la Rosa G, Argote MC, Martín S, Ventura M, Maida NL, et al. Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-pentedrone. Pharmaceuticals. 2025; 18(5):721. https://doi.org/10.3390/ph18050721
Chicago/Turabian StyleNúñez-Montero, Melani, Clara Pérez-Mañá, Olga Hladun, Lourdes Poyatos, Dolly Andrea Caicedo, Georgina De la Rosa, Martha Catalina Argote, Soraya Martín, Mireia Ventura, Nunzia La Maida, and et al. 2025. "Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-pentedrone" Pharmaceuticals 18, no. 5: 721. https://doi.org/10.3390/ph18050721
APA StyleNúñez-Montero, M., Pérez-Mañá, C., Hladun, O., Poyatos, L., Caicedo, D. A., De la Rosa, G., Argote, M. C., Martín, S., Ventura, M., Maida, N. L., Di Trana, A., Graziano, S., Pichini, S., Farré, M., & Papaseit, E. (2025). Acute Pharmacological Effects of Two Synthetic Cathinones in Humans: An Observational Study of N-Ethylhexedrone and N-Ethyl-nor-pentedrone. Pharmaceuticals, 18(5), 721. https://doi.org/10.3390/ph18050721






