Identification and Characterization of Antiviral Activity of Synthetic Compounds Against Mayaro Virus
Abstract
1. Introduction
2. Results
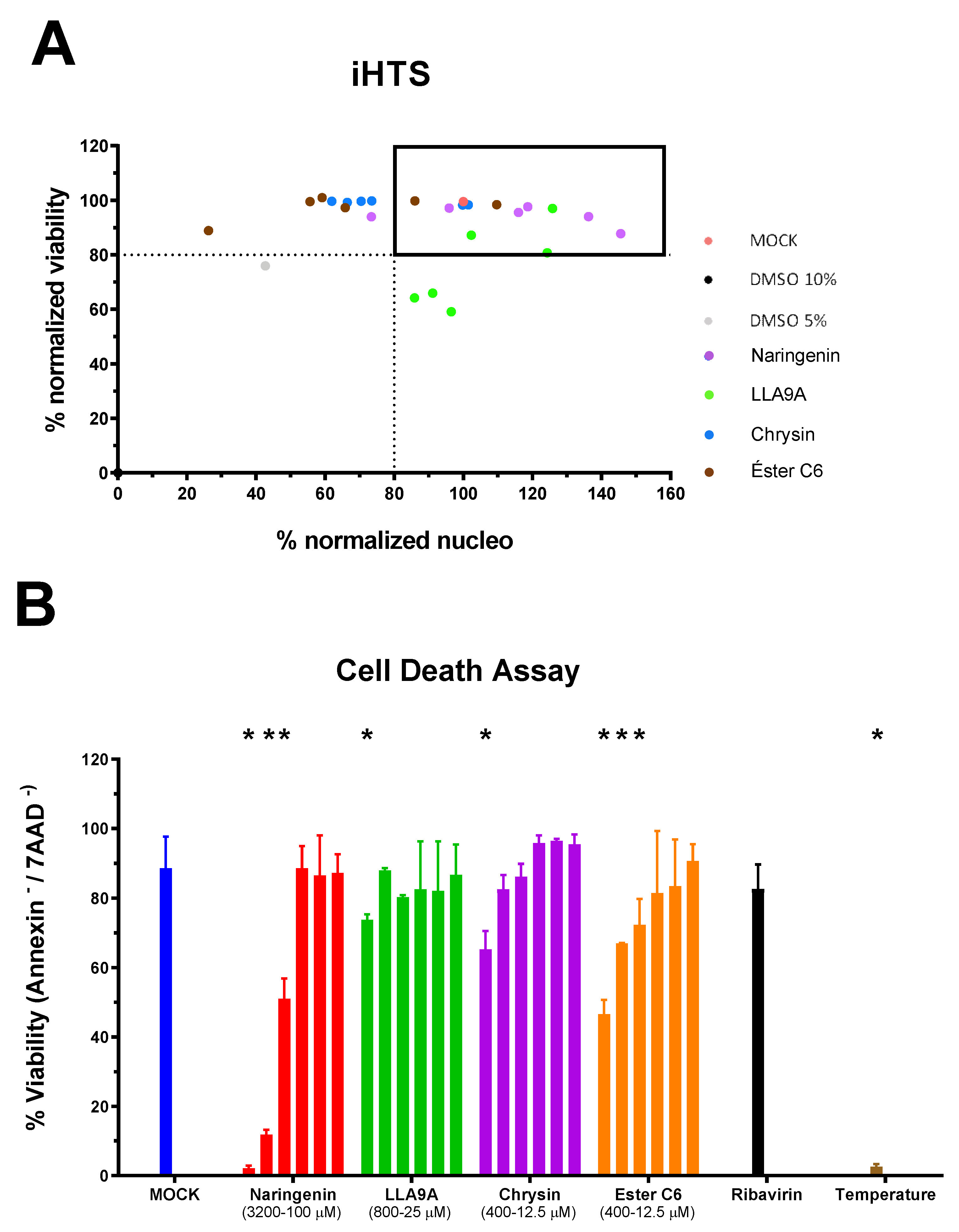
2.1. Standardization of Antiviral Screening Test for MAYV in iHTS
2.2. Standardization of MAYV Infection in Huh7.5 Cells by Flow Cytometry
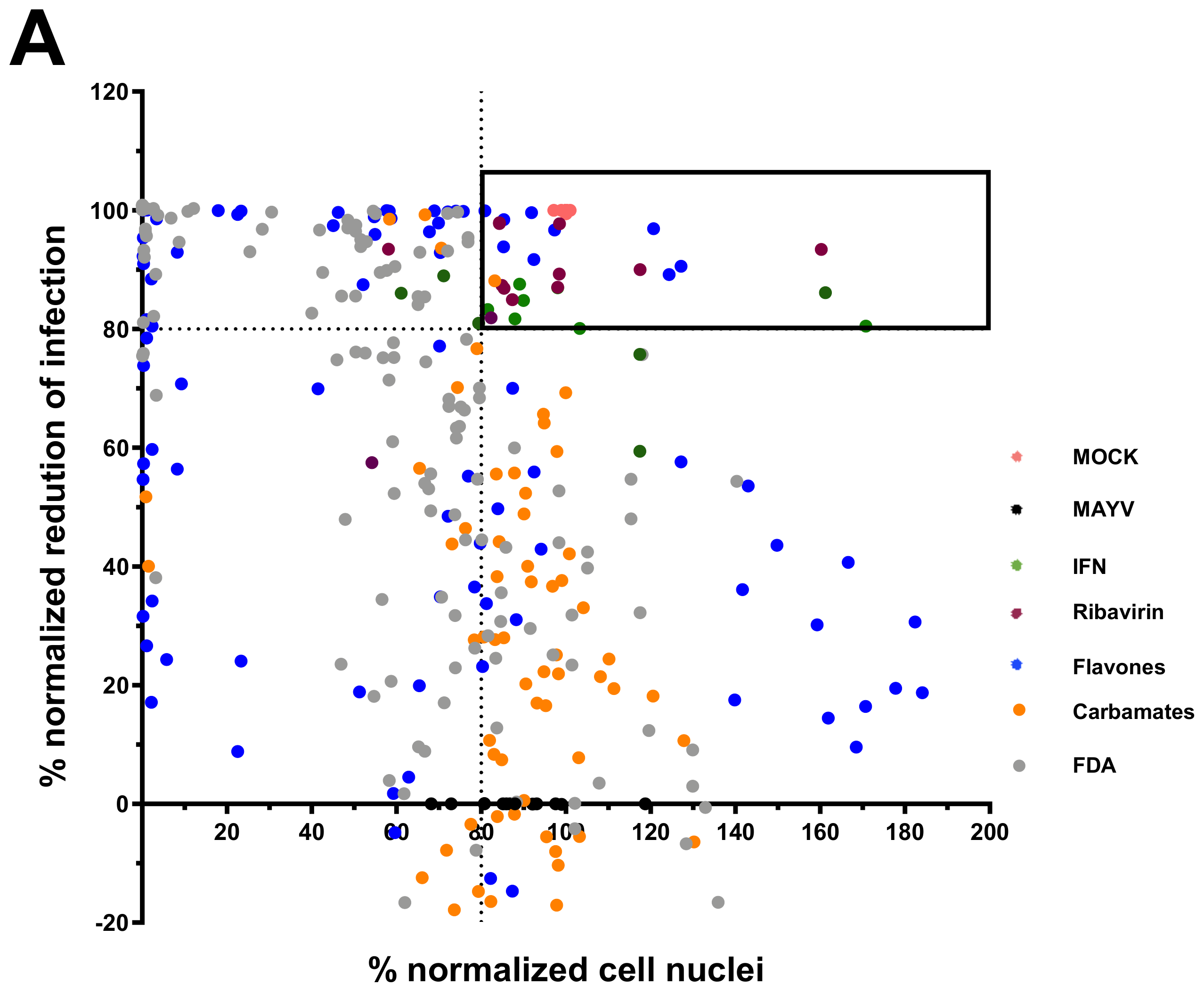
2.3. Screening of Anti-MAYV Antiviral Compounds
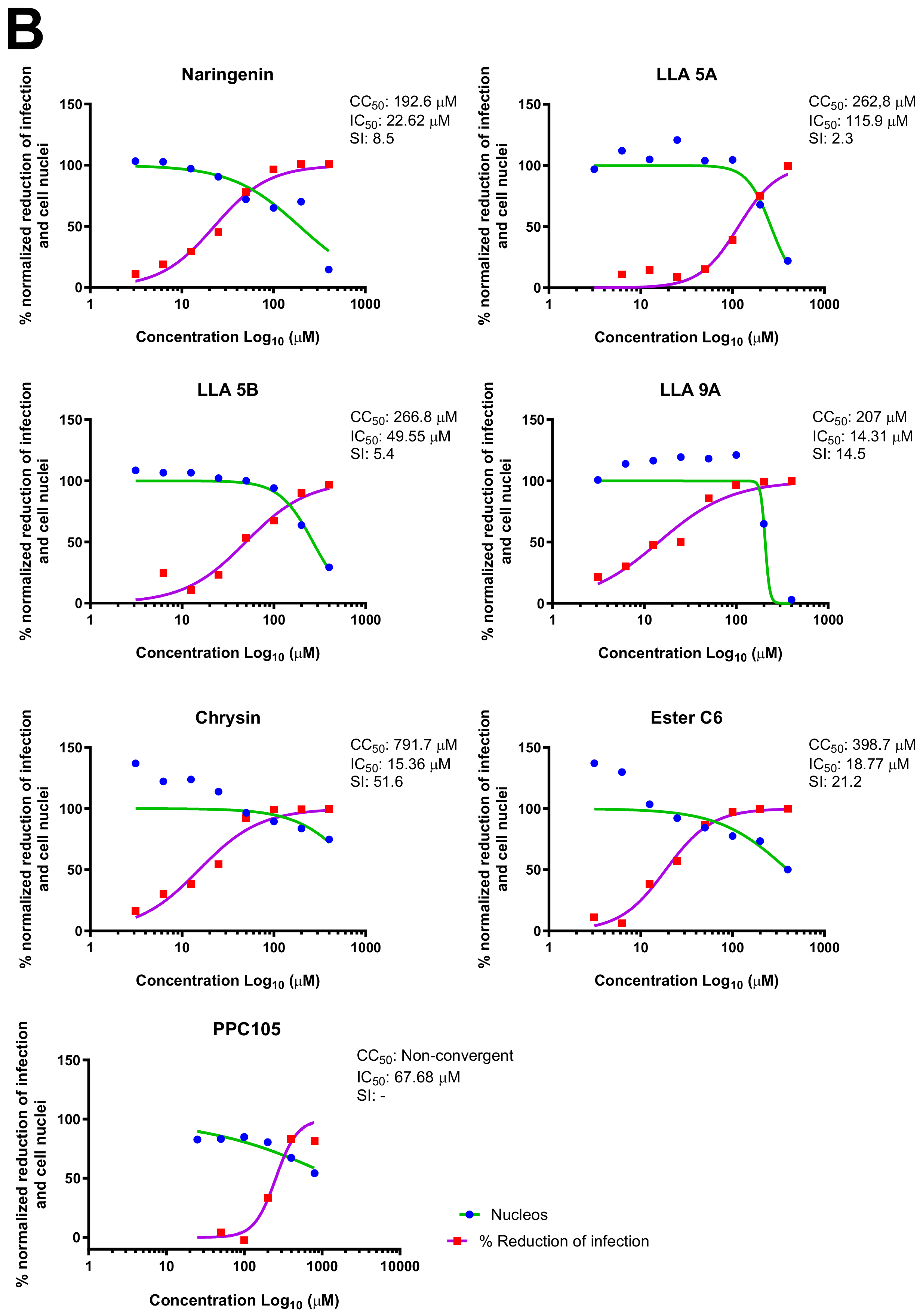
2.4. MNTC Determination
2.5. Virucidal Assay
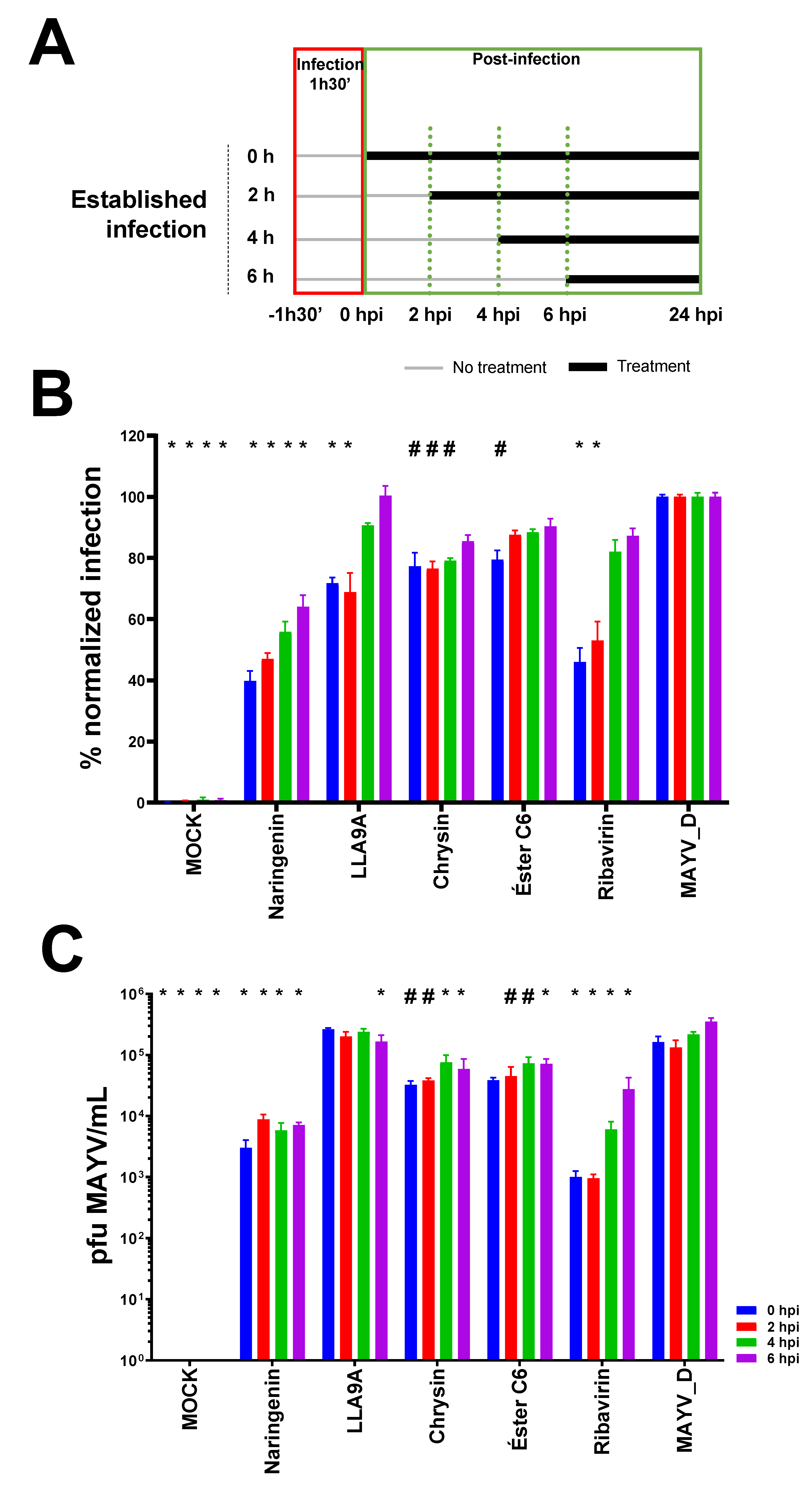
2.6. Time Course Drug Administration Assay
2.7. Adsorption and Internalization Viral Assays
2.8. Antiviral Effectiveness of the Compounds in Established MAYV Infection in Cell Culture
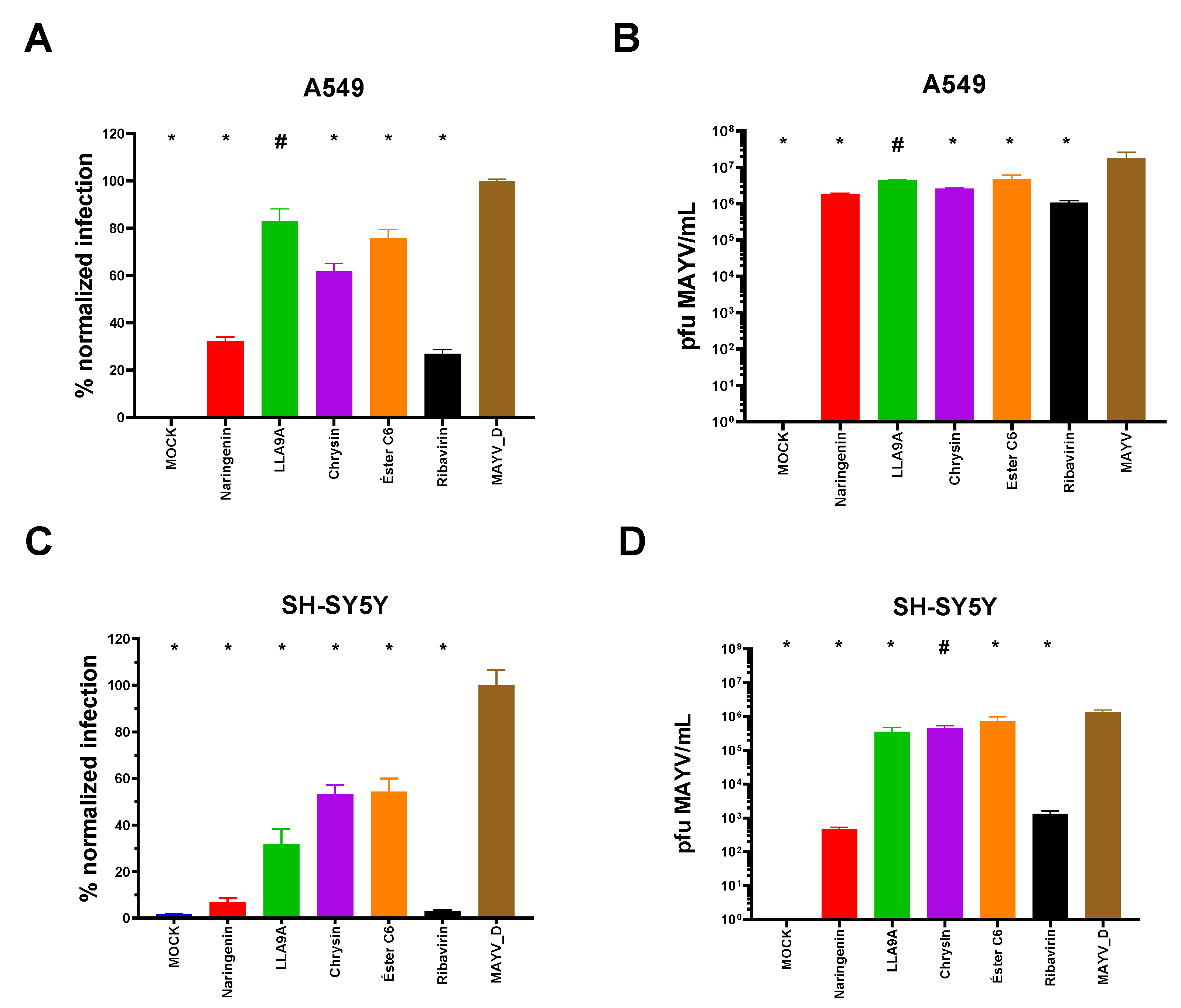
2.9. Antiviral Effectiveness Test for Different Cell Lineages
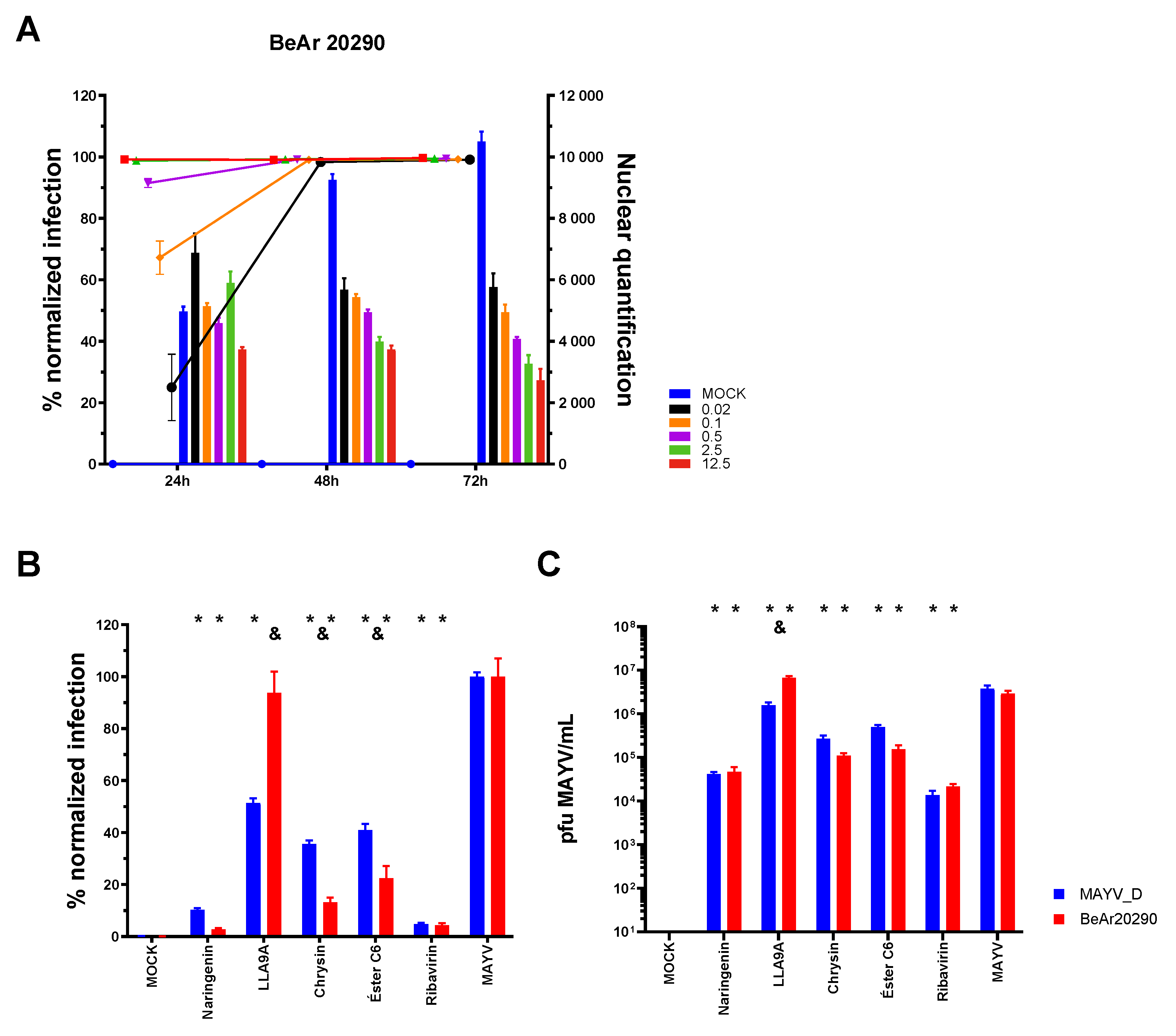
2.10. Activity of Compounds Against Infection by Different MAYV Genotypes
3. Discussion
4. Methods
4.1. Cell Line and Viruses
4.2. Library of Compounds
4.3. Indirect Immunofluorescence Assay (IFA) for iHTS
4.4. Screening for the Anti-MAYV Activity of the Compounds
4.5. Determination of the Maximum Non-Toxic Concentration (MNTC) of Anti-MAYV Selected Compounds
4.6. Time Course Drug Administration Assay
4.7. Antiviral Effectiveness Test in Established Infection
4.8. Anti-MAYV Activity in Different Human Cell Lines
4.9. Anti-MAYV Activity Against Different Viral Strains
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar-Luis, M.A.; del Valle-Mendoza, J.; Sandoval, I.; Silva-Caso, W.; Mazulis, F.; Carrillo-Ng, H.; Tarazona-Castro, Y.; Martins-Luna, J.; Aquino-Ortega, R.; Peña-Tuesta, I.; et al. A Silent Public Health Threat: Emergence of Mayaro Virus and Co-Infection with Dengue in Peru. BMC Res. Notes 2021, 14, 29. [Google Scholar] [CrossRef]
- de Figueiredo, M.L.G.; Figueiredo, L.T.M. Emerging Alphaviruses in the Americas: Chikungunya and Mayaro. Rev. Soc. Bras. Med. Trop. 2014, 47, 677–683. [Google Scholar] [CrossRef]
- Lednicky, J.; Beau De Rochars, V.M.; Elbadry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Okech, B.; et al. Mayaro Virus in Child with Acute Febrile Illness, Haiti, 2015. Emerg. Infect. Dis. 2016, 22, 2000–2002. [Google Scholar] [CrossRef]
- Llagonne-Barets, M.; Icard, V.; Leparc-Goffart, I.; Prat, C.; Perpoint, T.; André, P.; Ramière, C. A Case of Mayaro Virus Infection Imported from French Guiana. J. Clin. Virol. 2016, 77, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Mutricy, R.; Matheus, S.; Mosnier, É.; Martinez-Lorenzi, E.; De Laval, F.; Nacher, M.; Niemetzky, F.; Naudion, P.; Djossou, F.; Rousset, D.; et al. Mayaro Virus Infection in French Guiana, a Cross Sectional Study. Infect. Genet. Evol. 2022, 99, 2003–2019. [Google Scholar] [CrossRef] [PubMed]
- da Brasil, M.S. Boletim de Vigilância Laboratorial Dos Arbovírus. Bol. Epidemiológico 2021, 52, 7–18. [Google Scholar]
- Casseb, A.D.R.; Casseb, L.M.N.; da Silva, S.P.; da Costa Vasconcelos, P.F. Arbovírus: Importante Zoonose Na Amazônia Brasileira. Veterinária E Zootec. 2013, 20, 9–21. [Google Scholar]
- da Costa, V.G.; de Rezende Féres, V.C.; Saivish, M.V.; de Lima Gimaque, J.B.; Moreli, M.L. Silent Emergence of Mayaro and Oropouche Viruses in Humans in Central Brazil. Int. J. Infect. Dis. 2017, 62, 84–85. [Google Scholar] [CrossRef]
- Lima, W.G.; Pereira, R.S.; da Cruz Nizer, W.S.; Brito, J.C.M.; Godói, I.P.; Cardoso, V.N.; Fernandes, S.O.A.; Ferreira, J.M.S. Rate of Exposure to Mayaro Virus (MAYV) in Brazil between 1955 and 2018: A Systematic Review and Meta-Analysis. Arch. Virol. 2021, 166, 347–361. [Google Scholar] [CrossRef]
- Saatkamp, C.J.; Rodrigues, L.R.R.; Pereira, A.M.N.; Coelho, J.A.; Marques, R.G.B.; de Souza, V.C.; do Nascimento, V.A.; Saatkamp, J.G.D.S.; Naveca, F.G.; de Figueiredo, R.M.P. Mayaro Virus Detection in the Western Region of Pará State, Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e0055-2020. [Google Scholar] [CrossRef]
- UFRJ. UFRJ Detecta Vírus Mayaro No Estado Do Rio de Janeiro. Available online: https://g1.globo.com/rj/rio-de-janeiro/noticia/2019/05/16/pesquisadores-da-ufrj-anunciar-que-descobriram-virus-mayaro-no-estado-rio.ghtml (accessed on 1 April 2025).
- Marques, R.E.; Shimizu, J.F.; Nogueira, M.L.; Vasilakis, N. Current Challenges in the Discovery of Treatments against Mayaro Fever. Expert. Opin. Ther. Targets 2024, 28, 345–356. [Google Scholar] [CrossRef]
- Lorenz, C.; Ribeiro, A.F.; Chiaravalloti-neto, F. Mayaro Virus Distribution in South America. Acta Trop. 2019, 198, 105093. [Google Scholar] [CrossRef] [PubMed]
- Mavian, C.; Rife, B.D.; Dollar, J.J.; Cella, E.; Ciccozzi, M.; Prosperi, M.C.F.; Lednicky, J.; Morris, J.G.; Capua, I.; Salemi, M. Emergence of Recombinant Mayaro Virus Strains from the Amazon Basin. Sci. Rep. 2017, 7, 8718. [Google Scholar] [CrossRef]
- Brunini, S.; França, D.D.S.; Silva, J.B.; Silva, L.N.; Silva, F.P.A.; Spadoni, M.; Rezza, G. High Frequency of Mayaro Virus IgM among Febrile Patients, Central Brazil. Emerg. Infect. Dis. 2017, 23, 1025–1026. [Google Scholar] [CrossRef]
- Coimbra, T.L.M.; Santos, C.L.S.; Suzuki, A.; Petrella, S.M.C.; Bisordi, I.; Nagamori, A.H.; Marti, A.T.; Santos, R.N.; Fialho, D.M.; Lavigne, S.; et al. Mayaro Virus: Imported Cases of Human Infection in São Paulo State, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2007, 49, 221–224. [Google Scholar] [CrossRef] [PubMed]
- de Paula Silveira-Lacerda, E.; Laschuk Herlinger, A.; Tanuri, A.; Rezza, G.; Anunciação, C.E.; Ribeiro, J.P.; Tannous, I.P.; Abrantes, G.R.; da Silva, E.G.; Arruda, K.F.; et al. Molecular Epidemiological Investigation of Mayaro Virus in Febrile Patients from Goiania City. Infect. Genet. Evol. 2021, 95, 2017–2018. [Google Scholar] [CrossRef]
- Lopes, N.; Nozawa, C.; Linhares, R.E.C. Características Gerais e Epidemiologia Dos Arbovírus Emergentes No Brasil. Rev. Pan-Amaz. Saúde 2014, 5, 55–64. [Google Scholar] [CrossRef]
- Romeiro, M.F.; Fumagalli, M.J.; dos Anjos, A.B.; Figueiredo, L.T.M. Serological Evidence of Mayaro Virus Infection in Blood Donors from São Carlos, São Paulo, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 693–696. [Google Scholar] [CrossRef]
- Pereira, T.N.; Carvalho, F.D.; De Mendonça, S.F.; Rocha, M.N.; Moreira, L.A. Vector Competence of Aedes Aegypti, Aedes Albopictus, and Culex Quinquefasciatus Mosquitoes for Mayaro Virus. PLoS Negl. Trop. Dis. 2020, 14, e0007518. [Google Scholar] [CrossRef]
- Mezencio, J.M.; de Souza, W.; Fonseca, M.E.; Rebello, M.A. Replication of Mayaro Virus in Aedes Albopictus Cells: An Electron Microscopic Study. Arch. Virol. 1989, 104, 299–308. [Google Scholar] [CrossRef]
- Fumagalli, M.J.; de Souza, W.M.; de Castro-Jorge, L.A.; de Carvalho, R.V.H.; Castro, Í.A.; de Almeida, L.G.N.; Consonni, S.R.; Zamboni, D.S.; Figueiredo, L.T.M. Chikungunya Virus Exposure Partially Cross-Protects against Mayaro Virus Infection in Mice. J. Virol. 2021, 95, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Earnest, J.T.; Kim, A.S.; Angeliadis, M.; Holtsberg, F.W.; Javad Aman, M.; Jangra, R.K.; Chandran, K.; Daily, J.P.; Diamond, M.S.; et al. Near-Germline Human Monoclonal Antibodies Neutralize and Protect against Multiple Arthritogenic Alphaviruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2100104118. [Google Scholar] [CrossRef]
- Webb, E.M.; Azar, S.R.; Haller, S.L.; Langsjoen, R.M.; Cuthbert, C.E.; Ramjag, A.T.; Luo, H.; Plante, K.; Wang, T.; Simmons, G.; et al. Effects of Chikungunya Virus Immunity on Mayaro Virus Disease and Epidemic Potential. Sci. Rep. 2019, 9, 20399. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. [Google Scholar] [CrossRef]
- Anderson, C.R.; Downs, W.G.; Wattley, G.H.; Ahin, N.W.; Reese, A.A. Mayaro Virus: A New Human Disease Agent: II. Isolation from Blood of Patients in Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- da Brasil, M.S. Epidemias Simultâneas de Mayaro e Febre Amarela Em Belterra, Pará. Bol. Epidemiológico 1978, 10, 146–152. [Google Scholar]
- Esposito, D.L.A.; da Fonseca, B.A.L. Will Mayaro Virus Be Responsible for the next Outbreak of an Arthropod-Borne Virus in Brazil? Braz. J. Infect. Dis. 2017, 21, 540–544. [Google Scholar] [CrossRef]
- McGill, P.E. Viral Infections: α-Viral Arthropathy. Baillieres. Clin. Rheumatol. 1995, 9, 145–150. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Freitas, R.B.; Travassos da Rosa, J.F.; Gabbay, Y.B.; Mello, W.A.; LeDuc, J.W. An Outbreak of Mayaro Virus Disease in Belterra, Brazil. I. Clin. Virol. Findings. Am. J. Trop. Med. Hyg. 1981, 30, 674–681. [Google Scholar] [CrossRef]
- Theilacker, C.; Held, J.; Allering, L.; Emmerich, P.; Schmidt-Chanasit, J.; Kern, W.V.; Panning, M. Prolonged Polyarthralgia in a German Traveller with Mayaro Virus Infection without Inflammatory Correlates. BMC Infect. Dis. 2013, 13, 2011–2014. [Google Scholar] [CrossRef]
- da Silva Pessoa Vieira, C.J.; da Silva, D.J.F.; Barreto, E.S.; Siqueira, C.E.H.; Colombo, T.E.; Ozanic, K.; Schmidt, D.J.; Drumond, B.P.; Mondini, A.; Nogueira, M.L.; et al. Detection of Mayaro Virus Infections during a Dengue Outbreak in Mato Grosso, Brazil. Acta Trop. 2015, 147, 12–16. [Google Scholar] [CrossRef]
- Pan American Health Organization; World Health Organization. Epidemiological Alert: Mayaro Fever; PAHO/WHO: Washington, DC, USA, 2019. [Google Scholar]
- Andreolla, A.P.; Borges, A.A.; Bordignon, J.; Duarte dos Santos, C.N. Mayaro Virus: The State-of-the-Art for Antiviral Drug Development. Viruses 2022, 14, 1787. [Google Scholar] [CrossRef]
- Mello, M.V.P.; Domingos, T.F.S.; Ferreira, D.F.; Ribeiro, M.M.J.; Ribeiro, T.P.; Rodrigues, C.R.; Souza, A.M.T. Antiviral Drug Discovery and Development for Mayaro Fever—What Do We Have so Far? Mini-Rev. Med. Chem. 2020, 20, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; de Meneses, M.D.F.; Borges, J.C.; da Silva Pinheiro, L.C.; Caldas, L.A.; Cirne-Santos, C.C.; de Mello, M.V.P.; de Souza, A.M.T.; Castro, H.C.; de Palmer Paixão, I.C.N.; et al. Thieno[2,3-b]Pyridine Derivatives: A New Class of Antiviral Drugs against Mayaro Virus. Arch. Virol. 2017, 162, 1577–1587. [Google Scholar] [CrossRef]
- Dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; De Meneses, M.D.F.; Soares, M.R.; Ferreira, D. Quercetin and Quercetin 3-O-Glycosides from Bauhinia longifolia (Bong.) Steud. Show Anti-Mayaro Virus Activity. Parasites Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lima-Camara, T.N. Arboviroses Emergentes e Novos Desafios Para a Saúde Pública No Brasil. Rev. Saude Publica 2016, 50, 36. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.D.; Soares, R.O.; Santos-junior, N.N.; Trabuco, A.C.; Cintra, A.C.; Figueiredo, L.T.; Caliri, A.; Sampaio, S.V.; Aquino, V.H. Phospholipase A 2 Isolated from the Venom of Crotalus Durissus Terrificus Inactivates Dengue Virus and Other Enveloped Viruses by Disrupting the Viral Envelope. PLoS ONE 2014, 9, e112351. [Google Scholar] [CrossRef]
- Spindola, K.C.W.; Simas, N.K.; Salles, T.S.; De Meneses, M.D.F.; Sato, A.; Ferreira, D.; Romão, W.; Kuster, R.M. Anti-Mayaro Virus Activity of Cassia Australis Extracts (Fabaceae, Leguminosae). Parasites Vectors 2014, 7, 537. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef]
- Daar, E.S.; Tierney, C.; Fischl, M.A.; Sax, P.E.; Mollan, K.; Budhathoki, C.; Godfrey, C.; Jahed, N.C.; Myers, L.; Katzenstein, D.; et al. Atazanavir plus Ritonavir or Efavirenz as Part of a 3-Drug Regimen for Initial Treatment of HIV-1: A Randomized Trial. Ann. Intern. Med. 2011, 154, 445–456. [Google Scholar] [CrossRef]
- Mittal, S.; Bandaranayake, R.M.; King, N.M.; Prabu-Jeyabalan, M.; Nalam, M.N.L.; Nalivaika, E.A.; Kurt Yilmaz, N.; Schiffer, C.A. Structural and Thermodynamic Basis of Amprenavir/Darunavir and Atazanavir Resistance in HIV-1 Protease with Mutations at Residue 50. J. Virol. 2013, 87, 4176–4184. [Google Scholar] [CrossRef] [PubMed]
- Skwarecki, A.S.; Nowak, M.G.; Milewska, M.J. Amino Acid and Peptide-Based Antiviral Agents. Chem. Med. Chem. 2021, 16, 3106–3135. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque de Oliveira Mendes, L.; Ponciano, C.S.; Depieri Cataneo, A.H.; Wowk, P.F.; Bordignon, J.; Silva, H.; Vieira de Almeida, M.; Ávila, E.P. The Anti-Zika Virus and Anti-Tumoral Activity of the Citrus Flavanone Lipophilic Naringenin-Based Compounds. Chem. Biol. Interact. 2020, 331, 109218. [Google Scholar] [CrossRef]
- Geris, R.; Ribeiro, P.R.; Da Silva Brandão, M.; Da Silva, H.H.G.; Da Silva, I.G. Bioactive Natural Products as Potential Candidates to Control Aedes Aegypti, the Vector of Dengue. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 37, pp. 277–376. [Google Scholar]
- Qadir, M.I.; Abbas, K.; Tahir, M.; Irfan, M.; Fiza, S.; Bukhari, R.; Ahmed, B.; Hanif, M.; Rasul, A.; Ali, M. Dengue Fever: Natural Management. Pak. J. Pharm. Sci. 2015, 28, 647–655. [Google Scholar] [PubMed]
- Ponciano, C.S.; Ávila, E.P.; Grazul, R.M.; de Oliveira Mendes, L.A.; de Almeida, M.V. Natural Products and Their Derivatives as Anti-Flavivirus Drug Candidates. Med. Chem. Res. 2021, 30, 1056–1073. [Google Scholar] [CrossRef]
- Sánchez, I.; Gómez-Garibay, F.; Taboada, J.; Ruiz, B.H. Antiviral Effect of Flavonoids on the Dengue Virus. Phyther. Res. 2000, 14, 89–92. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Duarte dos Santos, C.N.; Bordignon, J. The Citrus Flavanone Naringenin Impairs Dengue Virus Replication in Human Cells. Sci. Rep. 2017, 7, 43976. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Mizokami, S.S.; Borghi, S.M.; Bordignon, J.; Silva, R.L.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; et al. The Citrus Flavonone Naringenin Reduces Lipopolysaccharide-Induced Inflammatory Pain and Leukocyte Recruitment by Inhibiting NF-ΚB Activation. J. Nutr. Biochem. 2016, 33, 8–14. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Ávila, E.P.; Mendes, L.A.O.; de Oliveira, V.G.; Ferraz, C.R.; de Almeida, M.V.; Frabasile, S.; Duarte Dos Santos, C.N.; Verri, W.A., Jr.; Bordignon, J. Flavonoids as Molecules With Anti-Zika Virus Activity. Front. Microbiol. 2021, 12, 710359. [Google Scholar] [CrossRef]
- Ávila, E.P.; de Oliveira, L.A.; Neto, B.A.D.; de Almeida, M.V.; Pliego, J.R., Jr. Flavanone-Enabled CuAAC Reaction: Noninnocent Reagents Driving a Mononuclear Mechanism Over the Dinuclear Paradigm. Chem.—A Eur. J. 2025, 31, e202500121. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jeong, M.S.; Mun, S.H.; Cho, J.; Seo, M.D.; Kim, H.; Lee, J.; Song, J.H.; Ko, H.J. Antiviral Activity of Chrysin against Influenza Virus Replication via Inhibition of Autophagy. Viruses 2021, 13, 1350. [Google Scholar] [CrossRef]
- Bhat, S.A.; Hasan, S.K.; Parray, Z.A.; Siddiqui, Z.I.; Ansari, S.; Anwer, A.; Khan, S.; Amir, F.; Mehmankhah, M.; Islam, A.; et al. Potential Antiviral Activities of Chrysin against Hepatitis B Virus. Gut Pathog. 2023, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- da Brasil, M.S. Monitoramento de Casos de Febre Do Mayaro e Febre Do Oropouche Até a Semana Epidemiológica 35, 2019. Bol. Epidemiológico 2019, 50, 14–16. [Google Scholar]
- Ahmadi, A.; Hassandarvish, P.; Lani, R.; Yadollahi, P.; Jokar, A.; Bakar, S.A.; Zandi, K. Inhibition of Chikungunya Virus Replication by Hesperetin and Naringenin. RSC Adv. 2016, 6, 69421–69430. [Google Scholar] [CrossRef]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of Alphavirus Entry and Replication Identified with a Stable Chikungunya Replicon Cell Line and Virus-Based Assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef]
- Suroengrit, A.; Yuttithamnon, W.; Srivarangkul, P.; Pankaew, S.; Kingkaew, K.; Chavasiri, W.; Boonyasuppayakorn, S. Halogenated Chrysins Inhibit Dengue and Zika Virus Infectivity. Sci. Rep. 2017, 7, 13696. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; de Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A.; et al. The Citrus Flavonoid Naringenin Impairs the in Vitro Infection of Human Cells by Zika Virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Lin, W.; Kitsberg, D.; Polyak, S.J.; Chung, R.T.; Yarmush, M.L. Naringenin Inhibits the Assembly and Long-Term Production of Infectious Hepatitis C Virus Particles through a PPAR-Mediated Mechanism. J. Hepatol. 2011, 55, 963–971. [Google Scholar] [CrossRef]
- Sajitha Lulu, S.; Thabitha, A.; Vino, S.; Mohana Priya, A.; Rout, M. Naringenin and Quercetin—Potential Anti-HCV Agents for NS2 Protease Targets. Nat. Prod. Res. 2016, 30, 464–468. [Google Scholar] [CrossRef]
- Guler, H.I.; Ay Sal, F.; Can, Z.; Kara, Y.; Yildiz, O.; Belduz, A.O.; Canakci, S.; Kolayli, S. Targeting CoV-2 Spike RBD and ACE-2 Interaction with Flavonoids of Anatolian Propolis by In Silico and In Vitro Studies in Terms of Possible COVID-19 Therapeutics. Turk. J. Biol. 2021, 45, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a Flavanone with Antiviral and Anti-Inflammatory Effects: A Promising Treatment Strategy against COVID-19. Phyther. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Zanello, P.R.; Koishi, A.C.; Rezende Júnior Cde, O.; Oliveira, L.A.; Pereira, A.A.; de Almeida, M.V.; Duarte dos Santos, C.N.; Bordignon, J. Quinic Acid Derivatives Inhibit Dengue Virus Replication In Vitro. Virol. J. 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Dörfelt, P.; Chatterjee, N.; Helle, J.; Bader, M.I.; Vollmer, G.; Kretzschmar, G.; Kuhlee, F.; Thieme, D.; Zierau, O. Assessment of the Effects of Naringenin-Type Flavanones in Uterus and Vagina. J. Steroid Biochem. Mol. Biol. 2015, 145, 49–57. [Google Scholar] [CrossRef]
- Zandi, K.; Lani, R.; Wong, P.F.; Teoh, B.T.; Sam, S.S.; Johari, J.; Mustafa, M.R.; Abu Bakar, S. Flavone Enhances Dengue Virus Type-2 (NGC Strain) Infectivity and Replication in Vero Cells. Molecules 2012, 17, 2437–2445. [Google Scholar] [CrossRef]
- Paredes, A.; Alzuru, M.; Mendez, J.; Rodríguez-Ortega, M. Anti-Sindbis Activity of Flavanones Hesperetin and Naringenin. Biol. Pharm. Bull. 2003, 26, 108–109. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Delang, L. Antiviral Strategies against Arthritogenic Alphaviruses. Microorganisms 2020, 8, 1365. [Google Scholar] [CrossRef]
- Sugasti-Salazar, M.; Campos, D.; Valdés-Torres, P.; González-Santamaría, J. Targeting Host PIM Protein Kinases Reduces MayaroVirus Replication. Viruses 2022, 14, 422. [Google Scholar] [CrossRef]
- Amaya, M.; Keck, F.; Lindquist, M.; Voss, K.; Scavone, L.; Kehn-Hall, K.; Roberts, B.; Bailey, C.; Schmaljohn, C.; Narayanan, A. The Ubiquitin Proteasome System Plays a Role in Venezuelan Equine Encephalitis Virus Infection. PLoS ONE 2015, 10, e0124792. [Google Scholar] [CrossRef]
- Cruz, D.J.M.; Koishi, A.C.; Taniguchi, J.B.; Li, X.; Milan Bonotto, R.; No, J.H.; Kim, K.H.; Baek, S.; Kim, H.Y.; Windisch, M.P.; et al. High Content Screening of a Kinase-Focused Library Reveals Compounds Broadly-Active against Dengue Viruses. PLoS Negl. Trop. Dis. 2013, 7, e2073. [Google Scholar] [CrossRef]
- Koishi, A.C.; Zanello, P.R.; Bianco, É.M.; Bordignon, J.; dos Santos, C.N.D. Screening of Dengue Virus Antiviral Activity of Marine Seaweeds by an In Situ Enzyme-Linked Immunosorbent Assay. PLoS ONE 2012, 7, e51089. [Google Scholar] [CrossRef] [PubMed]
- Espósito, D.L.A.; da Fonseca, B.A.L. Complete Genome Sequence of Mayaro Virus (Togaviridae, Alphavirus) Strain BeAr 20290 from Brazil. Genome Announc. 2015, 3, 141660. [Google Scholar] [CrossRef] [PubMed]
- Radigan, K.A.; Misharin, A.V.; Chi, M.; Budinger, G.R.S. Modeling Human Influenza Infection in the Laboratory. Infect. Drug Resist. 2015, 8, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zeng, W.; Zhang, F.; Zhang, C.; Liang, W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. 2017, 199, 3466–3477. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the No-CGMP-PKG-KATP Channel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A. Naringenin Reduces Inflammatory Pain in Mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef]
- Mielczarek, C. Acid–Base Properties of Selected Flavonoid Glycosides. Eur. J. Pharm. Sci. 2005, 25, 273–279. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Vasantha Rupasinghe, H.P. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Kozłowska, J.; Grela, E.; Baczynska, D.; Grabowiecka, A.; Anioł, M. Novel O-Alkyl Derivatives of Naringenin and Their Oximes with Antimicrobial and Anticancer Activity. Molecules 2019, 24, 679. [Google Scholar] [CrossRef]
- Stompor-gorący, M.; Bajek-bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham-Ul-Haq; IqraYasmin; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and Therapeutic Properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; De Gomes, M.G.; Goes, A.T.R.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid Chrysin Prevents Age-Related Cognitive Decline via Attenuation of Oxidative Stress and Modulation of BDNF Levels in Aged Mouse Brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef]
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing Nutritional Component Chrysin as a Therapeutic Agent: Bioavailability and Pharmacokinetics Consideration, and ADME Mechanisms. Biomed. Pharmacother. 2021, 142, 112080. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H.; Shope, R.E.; Walton, T.E. Cell Cultures for Diagnosis of Arbovirus Infections in Livestock and Wildlife. J. Tissue Cult. Methods 1988, 11, 157–163. [Google Scholar] [CrossRef]
- Koishi, A.C.; Suzukawa, A.A.; Zanluca, C.; Camacho, D.E.; Comach, G.; Duarte dos Santos, C.N. Development and Evaluation of a Novel High-Throughput Image-Based Fluorescent Neutralization Test for Detection of Zika Virus Infection. PLoS Negl. Trop. Dis. 2018, 12, e0006342. [Google Scholar] [CrossRef]
- Ávila, E.P.; Mendes, L.A.O.; De Almeida, W.B.; Santos, H.F.D.; De Almeida, M.V. Conformational Analysis and Reactivity of Naringenin. J. Mol. Struct. 2021, 1245, 131027. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Wang, W.X.; Wang, Z.Q.; Chen, L.J.; Zhang, J.Y.; Liu, X.C.; Wu, S.P.; Zhang, Y.M. Synthesis and Antitumor Activity Evaluation of Chrysin Derivatives. Eur. J. Med. Chem. 2014, 75, 297–300. [Google Scholar] [CrossRef]
- Cheng, N.; Yi, W.-B.; Wang, Q.-Q.; Peng, S.-M.; Zou, X.-Q. Synthesis and α-Glucosidase Inhibitory Activity of Chrysin, Diosmetin, Apigenin, and Luteolin Derivatives. Chinese Chem. Lett. 2014, 25, 1094–1098. [Google Scholar] [CrossRef]
- De Castro, P.P.; Rimulo, I.M.R.; De Almeida, A.M.; Diniz, R.; Amarante, G.W. Brønsted Acid-Catalyzed Epimerization-Free Preparation of Dual-Protected Amino Acid Derivatives. ACS Omega 2017, 2, 2967–2976. [Google Scholar] [CrossRef]
- De Castro, P.P.; Batista, G.M.F.; Dos Santos, H.F.; Amarante, G.W. Theoretical Study on the Epimerization of Azlactone Rings: Keto-Enol Tautomerism or Base-Mediated Racemization? ACS Omega 2018, 3, 3507–3512. [Google Scholar] [CrossRef]
- de Castro, P.P.; Campos, D.L.; Pavan, F.R.; Amarante, G.W. Dual-Protected Amino Acid Derivatives as New Antitubercular Agents. Chem. Biol. Drug Des. 2018, 92, 1576–1580. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Structure of Compounds | Molar Mass |
|---|---|---|
| Naringenin |  | 272.25 g/mol |
| CSP5B | 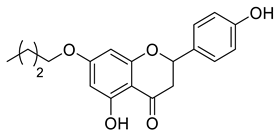 | 328.36 g/mol |
| CSP4B | 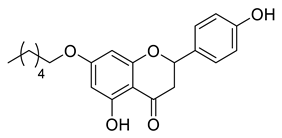 | 356.41 g/mol |
| CSP3B | 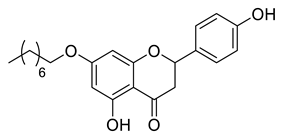 | 384.47 g/mol |
| CSP2B | 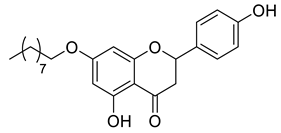 | 398.49 g/mol |
| LLA8B | 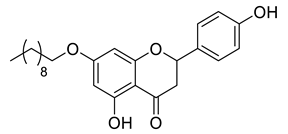 | 412.52 g/mol |
| LLA7B | 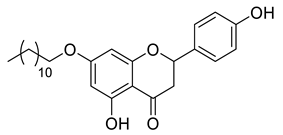 | 440.57 g/mol |
| LLA9A | 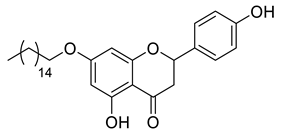 | 496.68 g/mol |
| CSP7A | 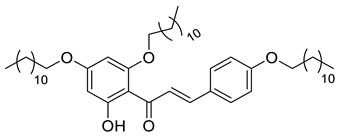 | 777.21 g/mol |
| CSP1A | 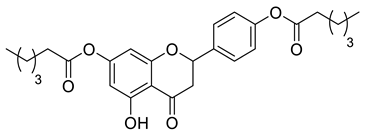 | 468.54 g/mol |
| CSP1B | 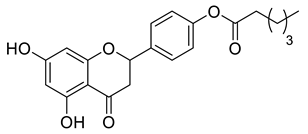 | 370.39 g/mol |
| LLA11A | 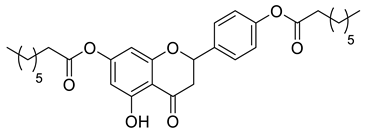 | 524.65 g/mol |
| LLA11B |  | 398.45 g/mol |
| LLA5A | 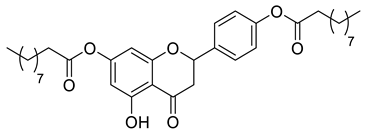 | 580.75 g/mol |
| LLA5B | 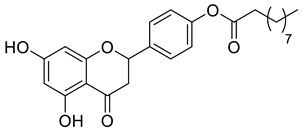 | 426.51 g/mol |
| LLA4A | 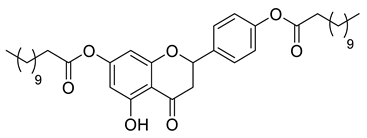 | 636.86 g/mol |
| LLA4B | 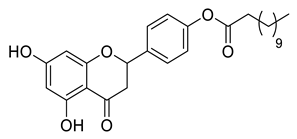 | 454.55 g/mol |
| LLA10A | 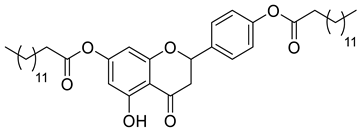 | 692.96 g/mol |
| LLA10B | 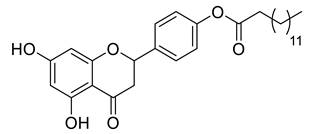 | 482.61 g/mol |
| LLA12A | 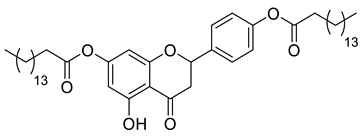 | 749.07 g/mol |
| LLA12B | 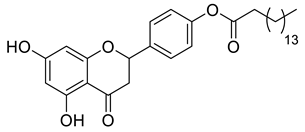 | 510.66 g/mol |
| Compounds | Structure of Compounds | Molar Mass |
|---|---|---|
| PPC140 | 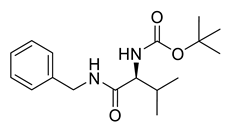 | 306.40 g/mol |
| PPC81 | 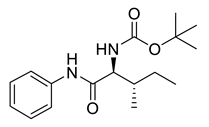 | 306.41 g/mol |
| PPC97 | 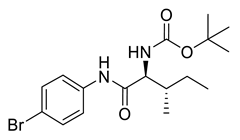 | 385.30 g/mol |
| PPC96 | 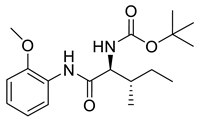 | 336.43 g/mol |
| PPC105 | 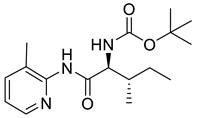 | 321.42 g/mol |
| PPC88 | 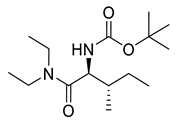 | 286.42 g/mol |
| PPC111 | 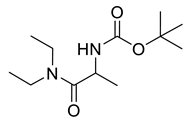 | 244.34 g/mol |
| PPC138 | 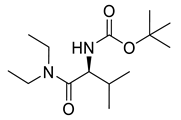 | 272.39 g/mol |
| PPC141 | 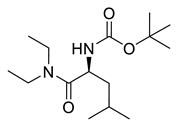 | 286.42 g/mol |
| PPC145 | 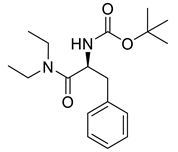 | 320.43 g/mol |
| PPC87 | 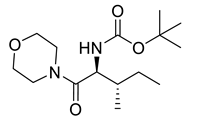 | 300.40 g/mol |
| PPC107 | 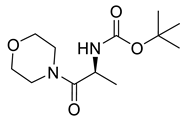 | 258.32 g/mol |
| PPC110 | 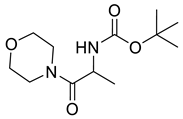 | 258.32 g/mol |
| PPC139 | 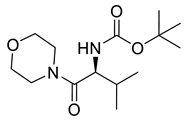 | 286.37 g/mol |
| PPC142 | 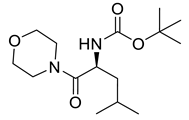 | 300.43 g/mol |
| PPC123 | 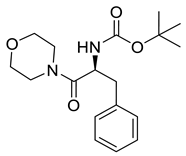 | 334.42 g/mol |
| PPC116 | 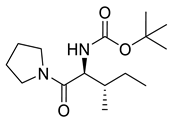 | 284.40 g/mol |
| PPC117 | 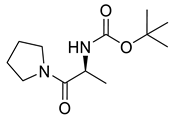 | 242.32 g/mol |
| PPC146 | 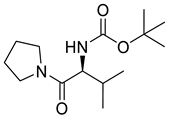 | 270.37 g/mol |
| PPC120 | 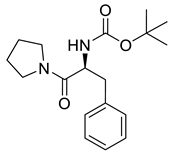 | 318.42 g/mol |
| Compounds | Structure Compounds | Molar Mass |
|---|---|---|
| Chrysin | 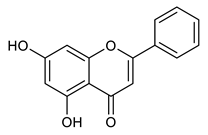 | 254.24 g/mol |
| Chrysin acetate | 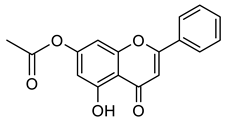 | 296.28 g/mol |
| Chrysin ester C6 | 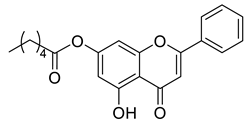 | 352.39 g/mol |
| Chrysin ester C10 | 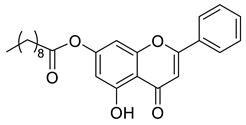 | 408.49 g/mol |
| Chrysin ester C14 | 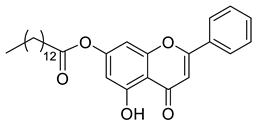 | 464,60 g/mol |
| Chrysin ester C6 | 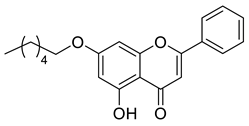 | 338.40 g/mol |
| Chrysin ester C12 | 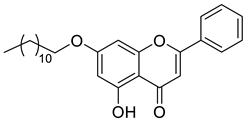 | 422.56 g/mol |
| Chrysin ester C16 |  | 478.67 g/mol |
| Cat. No | Product Name * | Brief Description | Product Data Sheet |
|---|---|---|---|
| 0425 | UK 14,304 | Alpha2 agonist | Datasheet_0425 |
| 0427 | Bromocriptine mesylate | Selective D2-like agonist | Datasheet_0427 |
| 0475 | Dihydroergotamine mesylate | Partial alpha agonist; nonselective | Datasheet_0475 |
| 0515 | Dobutamine hydrochloride | Alpha1, beta1, and beta2 agonist | Datasheet_0515 |
| 0685 | Diltiazem hydrochloride | Ca2+ channel blocker (L-type) | Datasheet_0685 |
| 0694 | Pilocarpine hydrochloride | Muscarinic agonist | Datasheet_0694 |
| 0869 | Felbamate | NMDA antagonist; acts on the glycine site | Datasheet_0869 |
| 0902 | Cimetidine | H2 antagonist, I1 agonist | Datasheet_0902 |
| 0909 | Tropicamide | Selective M4 muscarinic antagonist | Datasheet_0909 |
| 0911 | Glibenclamide | Kir6 (KATP) channel blocker | Datasheet_0911 |
| 0999 | Tamoxifen citrate | Estrogen receptor partial agonist/antagonist | Datasheet_0999 |
| 1097 | Taxol | Promotes assembly and inhibits disassembly | Datasheet_1097 |
| 1226 | Etoposide | Topoisomerase II inhibitor | Datasheet_1226 |
| 1328 | Flumazenil | Benzodiazepine antagonist | Datasheet_1328 |
| 1453 | Clemastine fumarate | H1 antagonist | Datasheet_1453 |
| 1470 | Flecainide acetate | Cardiac Na+ channel blocker; Antiarrhythmic | Datasheet_1470 |
| 1530 | Lovastatin | Potent HMG-CoA reductase inhibitor | Datasheet_1530 |
| 1692 | Cilostazol | PDE3A inhibitor; Additionally, adenosine uptake inhibitor | Datasheet_1692 |
| 1706 | Acetaminophen | Cyclooxygenase inhibitor; may be selective for CO | Datasheet_1706 |
| 1944 | Loratidine | Peripheral H1 antagonist; antiallergic agent | Datasheet_1944 |
| 1965 | Simvastatin | HMG-CoA reductase inhibitor | Datasheet_1965 |
| 2004 | Isradipine | Ca2+ channel blocker (L-type) | Datasheet_2004 |
| 2175 | Tetrabenazine | Potent inhibitor of vesicular monoamine transporter | Datasheet_2175 |
| 2280 | Raloxifene hydrochloride | Selective estrogen receptor modulator (SERM) | Datasheet_2280 |
| 2429 | Fexofenadine hydrochloride | H1 receptor antagonist; nonsedating antiallergic | Datasheet_2429 |
| 2513 | Acyclovir | Inhibits viral DNA polymerase; antiherpetic agent | Datasheet_2513 |
| 2571 | Amlodipine besylate | Ca2+ channel blocker (L-type) | Datasheet_2571 |
| 2578 | Benazepril hydrochloride | Angiotensin-converting enzyme (ACE) inhibitor | Datasheet_2578 |
| 2624 | Decitabine | DNA methyltransferase inhibitor | Datasheet_2624 |
| 2664 | Cabergoline | Selective D2-like agonist | Datasheet_2664 |
| 2673 | Acarbose | Glucosidase alpha inhibitor (intestinal) | Datasheet_2673 |
| 2682 | Sodium 4-Phenylbutyrate | Histone deacetylase inhibitor | Datasheet_2682 |
| 2685 | Carvedilol | Beta-adrenoceptor and alpha1-adrenoceptor antagonist | Datasheet_2685 |
| 2688 | CPT 11 | DNA topoisomerase I inhibitor; antitumor | Datasheet_2688 |
| 2796 | (S)-(+)-Ibuprofen | Cyclooxygenase inhibitor (COX-1 > COX-2) | Datasheet_2796 |
| 2839 | Levetiracetam | Antiepileptic; binds SV2A | Datasheet_2839 |
| 2864 | Metformin hydrochloride | Activator of LKB1/AMPK; antidiabetic agent | Datasheet_2864 |
| 2917 | Venlafaxine hydrochloride | Dual serotonin/noradrenalin reuptake inhibitor | Datasheet_2917 |
| 2960 | Felodipine | Ca2+ channel blocker (L-type) | Datasheet_2960 |
| 2964 | Doxazosin mesylate | Alpha1 antagonist | Datasheet_2964 |
| 3016 | Miconazole nitrate | Antifungal agent | Datasheet_3016 |
| 3108 | Bumetanide | Na+/2Cl-/K+ (NKCC) symporter inhibitor | Datasheet_3108 |
| 3118 | Ranolazine dihydrochloride | Antianginal; activates pyruvate dehydrogenase | Datasheet_3118 |
| 3256 | Metoprolol tartrate | Selective beta1 antagonist | Datasheet_3256 |
| 3259 | Gemcitabine hydrochloride | DNA synthesis inhibitor | Datasheet_3259 |
| 3287 | Prochlorperazine dimaleate | D2 receptor antagonist; additionally, 5-HT3 and nAChR | Datasheet_3287 |
| 3309 | Fluvastatin sodium | Potent HMG-CoA reductase inhibitor | Datasheet_3309 |
| 3388 | Anastrozole | Potent aromatase (CYP19) inhibitor | Datasheet_3388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreolla, A.P.; Koishi, A.C.; Borges, A.A.; Oliveira, L.A.d.; Oliveira, V.G.d.; Lima, N.M.; Ávila, E.P.; de Castro, P.P.; Amarante, G.W.; de Almeida, M.V.; et al. Identification and Characterization of Antiviral Activity of Synthetic Compounds Against Mayaro Virus. Pharmaceuticals 2025, 18, 717. https://doi.org/10.3390/ph18050717
Andreolla AP, Koishi AC, Borges AA, Oliveira LAd, Oliveira VGd, Lima NM, Ávila EP, de Castro PP, Amarante GW, de Almeida MV, et al. Identification and Characterization of Antiviral Activity of Synthetic Compounds Against Mayaro Virus. Pharmaceuticals. 2025; 18(5):717. https://doi.org/10.3390/ph18050717
Chicago/Turabian StyleAndreolla, Ana Paula, Andrea Cristine Koishi, Alessandra Abel Borges, Larissa Albuquerque de Oliveira, Viviane Guedes de Oliveira, Nerilson Marques Lima, Eloah Pereira Ávila, Pedro Pôssa de Castro, Giovanni Wilson Amarante, Mauro Vieira de Almeida, and et al. 2025. "Identification and Characterization of Antiviral Activity of Synthetic Compounds Against Mayaro Virus" Pharmaceuticals 18, no. 5: 717. https://doi.org/10.3390/ph18050717
APA StyleAndreolla, A. P., Koishi, A. C., Borges, A. A., Oliveira, L. A. d., Oliveira, V. G. d., Lima, N. M., Ávila, E. P., de Castro, P. P., Amarante, G. W., de Almeida, M. V., Bordignon, J., & Duarte dos Santos, C. N. (2025). Identification and Characterization of Antiviral Activity of Synthetic Compounds Against Mayaro Virus. Pharmaceuticals, 18(5), 717. https://doi.org/10.3390/ph18050717






