Diosmin Administration Slightly Counteracted the Changes in Bone Mechanical Properties Induced by Experimental Type 1 Diabetes in Rats
Abstract
1. Introduction
2. Results
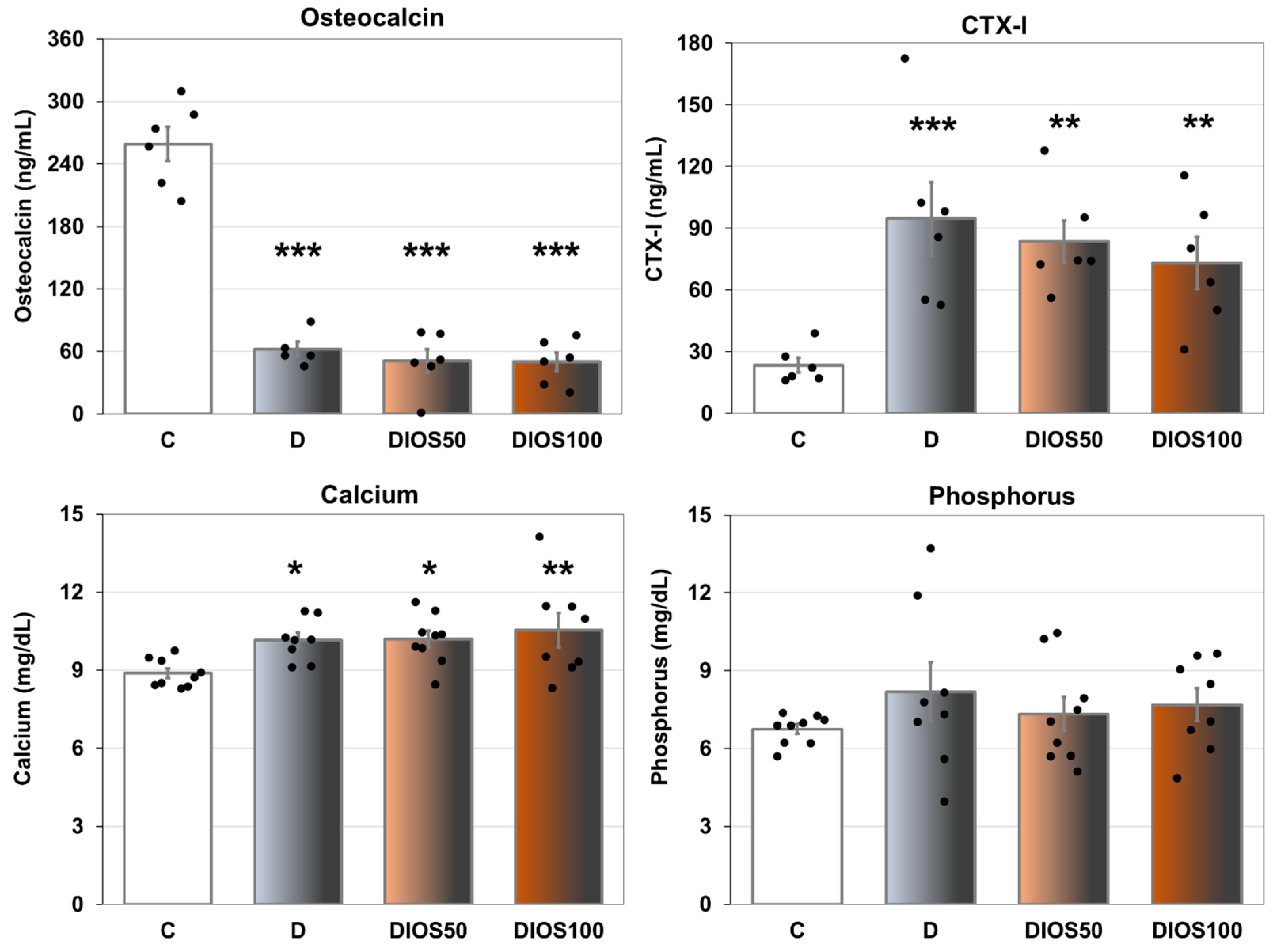
2.1. Effect of Diosmin on Serum Markers of Bone Turnover in T1D Rats
2.2. Effect of Diosmin on Bone Macrometric Parameters, Mass, Density and Mineralization in Femur and Tibia of T1D Rats
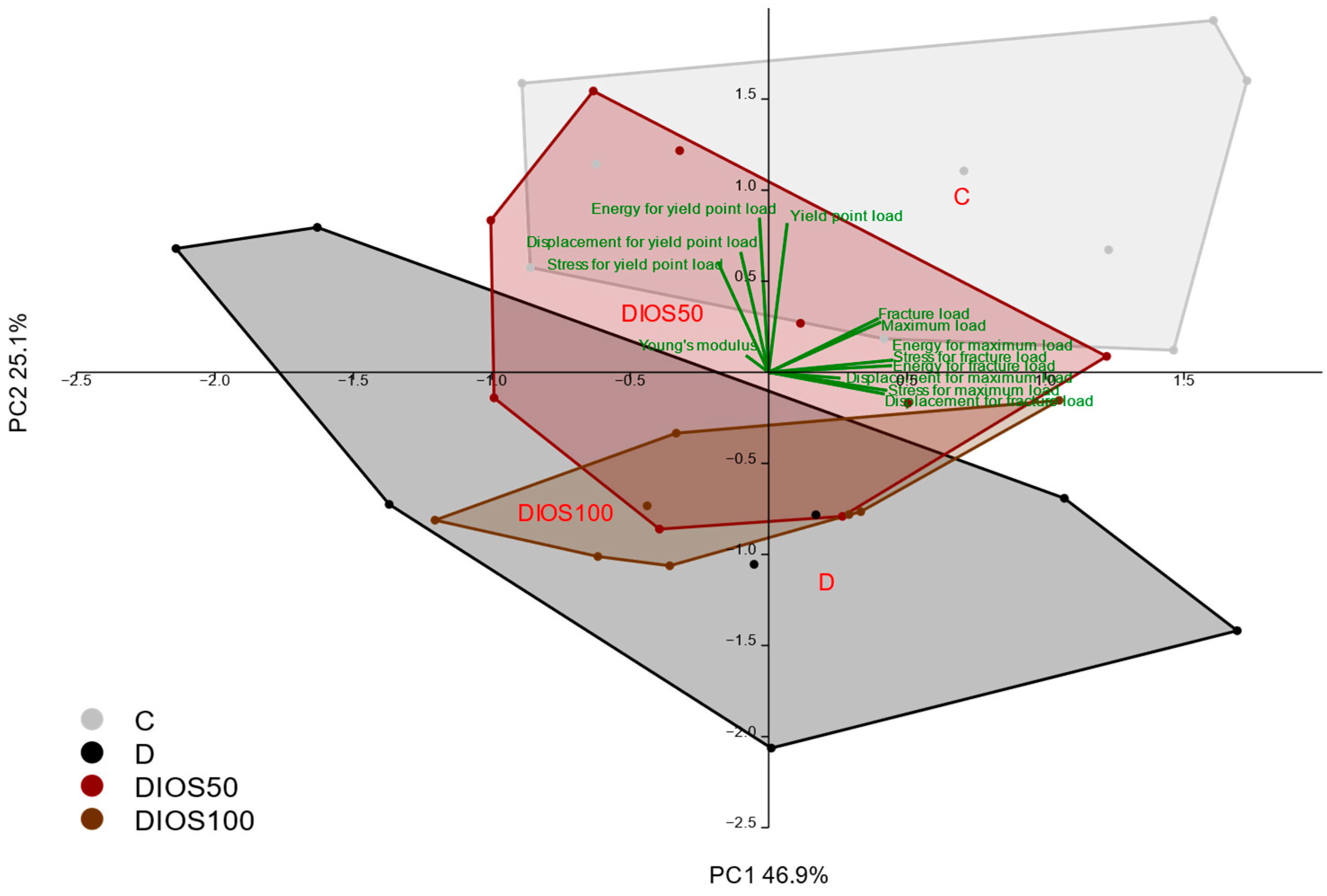
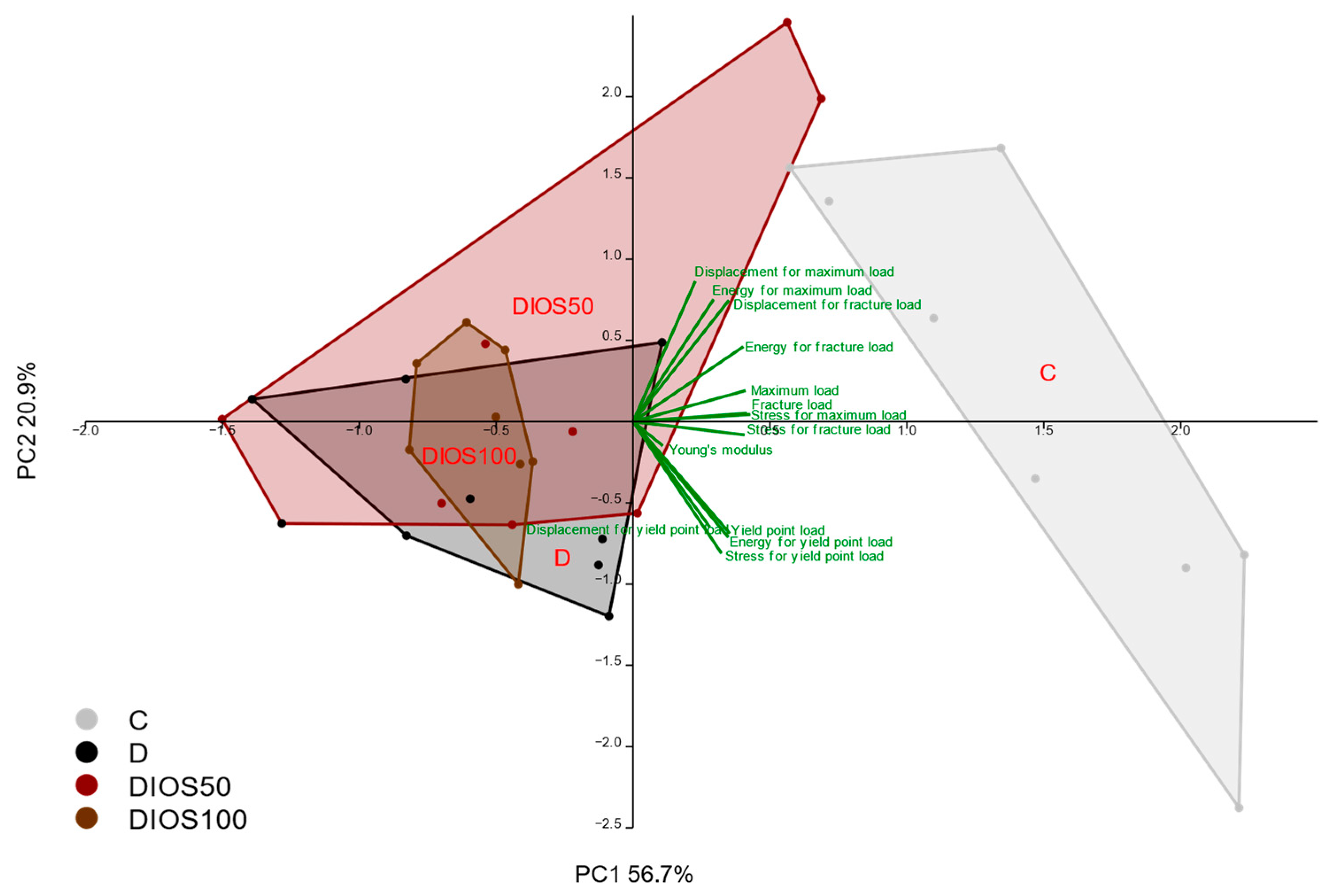
2.3. Effect of Diosmin on Mechanical Properties of Proximal Tibial Metaphysis in T1D Rats
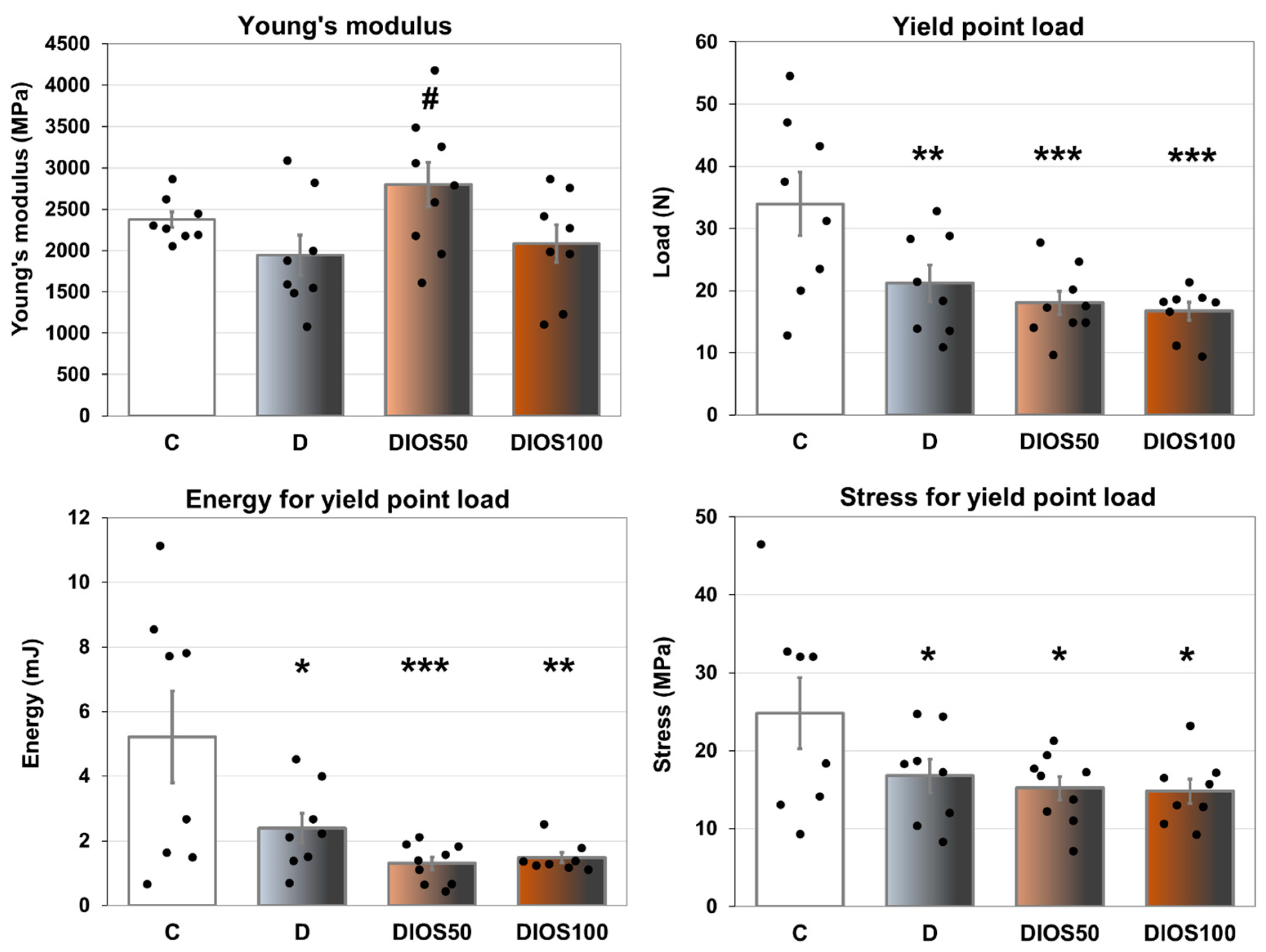
2.4. Effect of Diosmin on Mechanical Properties of Femoral Diaphysis and Femoral Neck in T1D Rats
| Parameter/Group | C | D | DIOS50 | DIOS100 | ANOVA | |
|---|---|---|---|---|---|---|
| Young’s modulus (MPa) | 7093 ± 320 | 7458 ± 529 | 7552 ± 293 | 7125 ± 433 | F3,30 = 0.352 | p = 0.788 |
| Yield point load (N) | 97.9 ± 2.9 | 73.8 ± 2.9 *** | 82.7 ± 3.2 ***,# | 72.3 ± 1.3 *** | F3,30 = 18.600 | p < 0.001 |
| Displacement for yield point load (mm) | 0.275 ± 0.004 | 0.247 ± 0.017 | 0.273 ± 0.010 | 0.254 ± 0.009 | F3,30 = 1.722 | p = 0.184 |
| Energy for yield point load (mJ) | 12.6 ± 0.5 | 8.9 ± 0.9 *** | 10.9 ± 0.6 # | 8.9 ± 0.3 *** | F3,30 = 8.106 | p < 0.001 |
| Stress for yield point load (MPa) | 108.2 ± 3.5 | 98.3 ± 6.0 | 112.8 ± 5.6 | 99.3 ± 2.2 | F3,30 = 2.318 | p = 0.096 |
| Maximum load (N) | 139.2 ± 5.8 | 113.7 ± 6.4 ** | 116.7 ± 4.0 ** | 111.5 ± 3.5 *** | F3,30 = 6.623 | p = 0.001 |
| Displacement for maximum load (mm) | 0.543 ± 0.034 | 0.497 ± 0.041 | 0.517 ± 0.031 | 0.530 ± 0.031 | F3,30 = 0.327 | p = 0.806 |
| Energy for maximum load (mJ) | 45.2 ± 4.9 | 34.0 ± 5.6 | 35.4 ± 3.1 | 34.5 ± 3.3 | F3,30 = 1.544 | p = 0.224 |
| Stress for maximum load (MPa) | 153.3 ± 4.4 | 150.0 ± 7.9 | 157.7 ± 2.3 | 153.1 ± 5.0 | F3,30 = 0.390 | p = 0.761 |
| Fracture load (N) | 139.1 ± 5.7 | 113.1 ± 6.1 *** | 116.5 ± 4.0 ** | 109.8 ± 3.1 *** | F3,30 = 7.507 | p < 0.001 |
| Displacement for fracture load (mm) | 0.548 ± 0.035 | 0.504 ± 0.045 | 0.519 ± 0.031 | 0.549 ± 0.041 | F3,30 = 0.333 | p = 0.801 |
| Energy for fracture load (mJ) | 45.8 ± 5.0 | 35.0 ± 6.2 | 35.7 ± 3.1 | 36.8 ± 4.6 | F3,30 = 1.162 | p = 0.341 |
| Stress for fracture load (MPa) | 153.1 ± 4.3 | 149.3 ± 7.7 | 157.5 ± 2.3 | 150.8 ± 4.9 | F3,30 = 0.525 | p = 0.669 |
| Maximum load in femoral neck (N) | 96.8 ± 4.2 | 84.8 ± 5.9 | 80.2 ± 5.1 | 80.5 ± 5.3 | F3,29 = 2.220 | p = 0.107 |
2.5. Effect of Diosmin on Histomorphometric Parameters of Femur in T1D Rats
3. Discussion
4. Materials and Methods
4.1. Animals and In Vivo Experiments
- –
- Group C—healthy (non-diabetic) control rats (n = 9; non-fasting glucose levels at the beginning of vehicle administration in the range of 97–145 mg/dL, and at the end in the range of 101–131 mg/dL).
- –
- Group D—diabetic control rats (n = 8; non-fasting glucose levels at the beginning of vehicle administration ranging from 444 mg/dL to above the upper limit, and at the end ranging from 508 mg/dL to above the upper limit).
- –
- Group DIOS50—diabetic rats receiving diosmin at a dose of 50 mg/kg (n = 9; non-fasting glucose levels at the beginning of diosmin administration ranging from 492 mg/dL to above the upper limit, and at the end ranging from 484 mg/dL to above the upper limit).
- –
- Group DIOS100—diabetic rats receiving diosmin at a dose of 100 mg/kg (n = 8; non-fasting glucose levels at the beginning of diosmin administration ranging from 510 mg/dL to above the upper limit, and at the end ranging from 486 mg/dL to above the upper limit).
4.2. Measurements of Serum Biochemical Parameters
4.3. Bone Composition Measurements
4.4. Bone Mechanical Properties Measurements
4.5. Bone Histomorphometric Measurements
4.6. Statistical Analysis
- -
- The mechanical properties of cancellous bone (the proximal tibial metaphysis): yield point load in the tibial metaphysis; displacement, energy and stress for yield point load in the tibial metaphysis; maximum load in the tibial metaphysis; displacement, energy and stress for maximum load in the tibial metaphysis; fracture load in the tibial metaphysis; displacement, energy and stress for fracture load in the tibial metaphysis; Young’s modulus in the tibial metaphysis.
- -
- The mechanical properties of compact bone (the femoral diaphysis): yield point load in the femoral diaphysis; displacement, energy and stress for yield point load in the femoral diaphysis; maximum load in the femoral diaphysis; displacement, energy and stress for maximum load in the femoral diaphysis; fracture load in the femoral diaphysis; displacement, energy and stress for fracture load in the femoral diaphysis; Young’s modulus in the femoral diaphysis.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BV/TV | bone volume/tissue volume ratio |
| Ct.Ar | transverse cross-sectional area of the cortical bone |
| CTX-I | C-terminal telopeptide fragments of type I collagen |
| LSD | least significant difference |
| Ma.Ar | transverse cross-sectional area of the marrow cavity |
| Ma.Ar/Tt.Ar | transverse cross-sectional area of the marrow cavity/total diaphysis area ratio |
| MANOVA | multivariate analysis of variance |
| PCA | principal component analysis |
| p.o. | per os |
| STZ | streptozotocin |
| T1D | type 1 diabetes |
| Tb.N | trabecular number |
| Tb.Sp | trabecular separation |
| Tb.Th | trabecular thickness |
| Tt.Ar | transverse cross-sectional area of the whole diaphysis |
References
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Sala, D.; Zorzano, A. Differential Control of Muscle Mass in Type 1 and Type 2 Diabetes Mellitus. Cell Mol. Life Sci. 2015, 72, 3803–3817. [Google Scholar] [CrossRef]
- Weber, D.R.; Long, F.; Zemel, B.S.; Kindler, J.M. Glycemic Control and Bone in Diabetes. Curr. Osteoporos. Rep. 2022, 20, 379–388. [Google Scholar] [CrossRef]
- Khosla, S.; Samakkarnthai, P.; Monroe, D.G.; Farr, J.N. Update on the Pathogenesis and Treatment of Skeletal Fragility in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Kupai, K.; Kang, H.L.; Pósa, A.; Csonka, Á.; Várkonyi, T.; Valkusz, Z. Bone Loss in Diabetes Mellitus: Diaporosis. Int. J. Mol. Sci. 2024, 25, 7269. [Google Scholar] [CrossRef]
- Costantini, S.; Conte, C. Bone Health in Diabetes and Prediabetes. World J. Diabetes 2019, 10, 421–445. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.E.; Coleman, C.M. Impact of Diabetes Mellitus on Bone Health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, R.J. Is Diabetic Skeletal Fragility Associated with Microvascular Complications in Bone? Curr. Osteoporos. Rep. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Sheng, N.; Xing, F.; Wang, J.; Zhang, Q.Y.; Nie, R.; Li-Ling, J.; Duan, X.; Xie, H.Q. Recent Progress in Bone-Repair Strategies in Diabetic Conditions. Mater. Today Bio 2023, 23, 100835. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic Vascular Diseases: Molecular Mechanisms and Therapeutic Strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Almutlaq, N.; Neyman, A.; DiMeglio, L.A. Are Diabetes Microvascular Complications Risk Factors for Fragility Fracture? Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, V.V.; Hansen, S.; Frost, M.; Brixen, K.; Hermann, A.P. Bone Disease in Diabetes: Another Manifestation of Microvascular Disease? Lancet Diabetes Endocrinol. 2017, 5, 827–838. [Google Scholar] [CrossRef]
- Draghici, A.E.; Zahedi, B.; Taylor, J.A.; Bouxsein, M.L.; Yu, E.W. Vascular Deficits Contributing to Skeletal Fragility in Type 1 Diabetes. Front. Clin. Diabetes Healthc. 2023, 4, 1272804. [Google Scholar] [CrossRef]
- Le, T.; Salas Sanchez, A.; Nashawi, D.; Kulkarni, S.; Prisby, R.D. Diabetes and the Microvasculature of the Bone and Marrow. Curr. Osteoporos. Rep. 2024, 22, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Giacchetti, G.; Micucci, M. Antioxidant, Anti-Inflammatory, Anti-Diabetic, and pro-Osteogenic Activities of Polyphenols for the Treatment of Two Different Chronic Diseases: Type 2 Diabetes Mellitus and Osteoporosis. Biomolecules 2024, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Fajardo, C.M.; Soto-Rodriguez, S.; Kajander, E.R.U.; Lupu, R.I.; Pozo-Rodriguez, M.; Boira-Nacher, B.; Font-Alberich, M.; Gambini-Castell, M.; Olaso-Gonzalez, G.; et al. Osteoporosis: Causes, Mechanisms, Treatment and Prevention: Role of Dietary Compounds. Pharmaceuticals 2024, 17, 1697. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, M.A.; Siddiqui, S.; Misra, A.; Yadav, K.; Srivastava, A.; Trivedi, A.; Husain, I.; Ahmad, R. Phytoestrogens as Potential Anti-Osteoporosis Nutraceuticals: Major Sources and Mechanism(s) of Action. J. Steroid Biochem. Mol. Biol. 2025, 251, 106740. [Google Scholar] [CrossRef]
- Martiniakova, M.; Sarocka, A.; Penzes, N.; Biro, R.; Kovacova, V.; Mondockova, V.; Sevcikova, A.; Ciernikova, S.; Omelka, R. Protective Role of Dietary Polyphenols in the Management and Treatment of Type 2 Diabetes Mellitus. Nutrients 2025, 17, 275. [Google Scholar] [CrossRef]
- Karimi, S.M.; Bayat, M.; Rahimi, R. Plant-Derived Natural Medicines for the Management of Osteoporosis: A Comprehensive Review of Clinical Trials. J. Tradit. Complement. Med. 2024, 14, 1–18. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of Diosmin, a Citrus Flavone Glycoside: An Updated Review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 1–18. [Google Scholar] [CrossRef]
- Mustafa, S.; Akbar, M.; Khan, M.A.; Sunita, K.; Parveen, S.; Pawar, J.S.; Massey, S.; Agarwal, N.R.; Husain, S.A. Plant Metabolite Diosmin as the Therapeutic Agent in Human Diseases. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100122. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Woźniak, M.; Feldo, M.; Kocki, J.; Szewczyk, K. Diosmin-Isolation Techniques, Determination in Plant Material and Pharmaceutical Formulations, and Clinical Use. Nat. Prod. Commun. 2013, 8, 545–550. [Google Scholar] [CrossRef]
- Rahman, L.; Talha Khalil, A.; Ahsan Shahid, S.; Shinwari, Z.K.; Almarhoon, Z.M.; Alalmaie, A.; Sharifi-Rad, J.; Calina, D. Diosmin: A Promising Phytochemical for Functional Foods, Nutraceuticals and Cancer Therapy. Food Sci. Nutr. 2024, 12, 6070–6092. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of in Vitro and in Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, M.H.; Cheng, J.T.; Wu, M.C. Antihyperglycaemic Action of Diosmin, a Citrus Flavonoid, Is Induced through Endogenous β-Endorphin in Type I-like Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2017, 44, 549–555. [Google Scholar] [CrossRef]
- Ahmed, S.; Mundhe, N.; Borgohain, M.; Chowdhury, L.; Kwatra, M.; Bolshette, N.; Ahmed, A.; Lahkar, M. Diosmin Modulates the NF-kB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation 2016, 39, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.M.; Abo-Salem, O.M.; El Esawy, B.H.; El Askary, A. The Potential Protective Effects of Diosmin on Streptozotocin-Induced Diabetic Cardiomyopathy in Rats. Am. J. Med. Sci. 2020, 359, 32–41. [Google Scholar] [CrossRef]
- Wojnar, W.; Kaczmarczyk-Sedlak, I.; Zych, M. Diosmin Ameliorates the Effects of Oxidative Stress in Lenses of Streptozotocin-Induced Type 1 Diabetic Rats. Pharmacol. Rep. 2017, 69, 995–1000. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant Potential of Diosmin and Diosmetin against Oxidative Stress in Endothelial Cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef]
- Arafa, E.S.A.; Elgendy, N.O.; Elhemely, M.A.; Abdelaleem, E.A.; Mohamed, W.R. Diosmin Mitigates Dexamethasone-Induced Osteoporosis in Vivo: Role of Runx2, RANKL/OPG, and Oxidative Stress. Biomed. Pharmacother. 2023, 161, 114461. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Pal, S.; Mohamed, R.; Singh, P.; Chattopadhyay, S.; Pal China, S.; Porwal, K.; Sanyal, S.; Gayen, J.R.; Chattopadhyay, N. A Nutraceutical Composition Containing Diosmin and Hesperidin Has Osteogenic and Anti-Resorptive Effects and Expands the Anabolic Window of Teriparatide. Biomed. Pharmacother. 2019, 118, 109207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Porwal, K.; Kulkarni, C.; Pal, S.; Sihota, P.; Kumar, S.; Tiwari, M.C.; Katekar, R.; Kumar, A.; Singh, P.; et al. Diosmin, a Citrus Fruit-Derived Phlebotonic Bioflavonoid Protects Rats from Chronic Kidney Disease-Induced Loss of Bone Mass and Strength without Deteriorating the Renal Function. Food Funct. 2022, 13, 2184–2199. [Google Scholar] [CrossRef]
- Chandran, S.V.; Vairamani, M.; Selvamurugan, N. Osteostimulatory Effect of Biocomposite Scaffold Containing Phytomolecule Diosmin by Integrin/FAK/ERK Signaling Pathway in Mouse Mesenchymal Stem Cells. Sci. Rep. 2019, 9, 11900. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Ielapi, N.; Bitonti, A.; Candido, S.; Fregola, S.; Gallo, A.; Loria, A.; Muraca, L.; Raimondo, L.; Velcean, L.; et al. Efficacy of a Low-Dose Diosmin Therapy on Improving Symptoms and Quality of Life in Patients with Chronic Venous Disease: Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 999. [Google Scholar] [CrossRef]
- Bozkurt, A.K.; van Rijn, M.J.; Bouskela, E.; Gastaldi, G.; Glauser, F.; Haller, H.; Rosas-Saucedo, J.; Zingg, D.; Calabrese, A.; Rabe, E.; et al. Enhancing Identification and Treatment of Patients with Concomitant Chronic Venous Insufficiency and Diabetes Mellitus. A Modified Delphi Study from the CODAC (ChrOnic Venous Disease and Diabetes Advisory Council) Group. Int. Angiol. 2023, 42, 427–435. [Google Scholar] [CrossRef]
- Jarošíková, R.; Roztočil, K.; Husáková, J.; Dubský, M.; Bém, R.; Wosková, V.; Fejfarová, V. Chronic Venous Disease and Its Intersections with Diabetes Mellitus. Physiol. Res. 2023, 72, 280–286. [Google Scholar] [CrossRef]
- Klasik-Ciszewska, S.; Londzin, P.; Grzywnowicz, K.; Borymska, W.; Zych, M.; Kaczmarczyk-Żebrowska, I.; Folwarczna, J. Effect of Chrysin, a Flavonoid Present in Food, on the Skeletal System in Rats with Experimental Type 1 Diabetes. Nutrients 2025, 17, 316. [Google Scholar] [CrossRef]
- Londzin, P.; Kisiel-Nawrot, E.; Kocik, S.; Janas, A.; Trawczyński, M.; Cegieła, U.; Folwarczna, J. Effects of Diosgenin on the Skeletal System in Rats with Experimental Type 1 Diabetes. Biomed. Pharmacother. 2020, 129, 110342. [Google Scholar] [CrossRef]
- Folwarczna, J.; Janas, A.; Pytlik, M.; Cegieła, U.; Śliwinśki, L.; Krivošíková, Z.; Štefíková, K.; Gajdoš, M. Effects of Trigonelline, an Alkaloid Present in Coffee, on Diabetes-Induced Disorders in the Rat Skeletal System. Nutrients 2016, 8, 8030133. [Google Scholar] [CrossRef]
- Janas, A.; Kruczek, E.; Londzin, P.; Borymski, S.; Czuba, Z.P.; Folwarczna, J. Negligible Effect of Estrogen Deficiency on Development of Skeletal Changes Induced by Type 1 Diabetes in Experimental Rat Models. Mediators Inflamm. 2020, 2020, 2793804. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Althagafy, H.S.; Baraka, M.A.; Amin, H. Hepatoprotective Effects of Diosmin: A Narrative Review. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 279–295. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Folwarczna, J.; Zych, M.; Nowińska, B.; Pytlik, M.; Bialik, M.; Jagusiak, A.; Lipecka-Karcz, M.; Matysiak, M. Effect of Diosgenin, a Steroidal Sapogenin, on the Rat Skeletal System. Acta Biochim. Pol. 2016, 63, 287–295. [Google Scholar] [CrossRef]
- Folwarczna, J.; Pytlik, M.; Zych, M.; Cegieła, U.; Kaczmarczyk-Sedlak, I.; Nowińska, B.; Śliwiński, L. Favorable Effect of Moderate Dose Caffeine on the Skeletal System in Ovariectomized Rats. Mol. Nutr. Food Res. 2013, 57, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.P.; Specker, B.; Korach, K.S. Recent Experimental and Clinical Findings in the Skeleton Associated with Loss of Estrogen Hormone or Estrogen Receptor Activity. J. Steroid Biochem. Mol. Biol. 2010, 118, 264–272. [Google Scholar] [CrossRef]
- Siwak, J.; Lewinska, A.; Wnuk, M.; Bartosz, G. Protection of Flavonoids against Hypochlorite-Induced Protein Modifications. Food Chem. 2013, 141, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Hegsted, M.; Jones, K.L.; Delany, J.P.; Kime, J.C.; Melancon, L.E.; Tulley, R.T.; Hong, K.D. Comparison of Bone Density Measurement Techniques: DXA and Archimedes’ Principle. J. Bone Miner. Res. 1997, 12, 1903–1907. [Google Scholar] [CrossRef]
- Stürmer, E.K.; Seidlová-Wuttke, D.; Sehmisch, S.; Rack, T.; Wille, J.; Frosch, K.H.; Wuttke, W.; Stürmer, K.M. Standardized Bending and Breaking Test for the Normal and Osteoporotic Metaphyseal Tibias of the Rat: Effect of Estradiol, Testosterone, and Raloxifene. J. Bone Miner. Res. 2006, 21, 89–96. [Google Scholar] [CrossRef]
- Turner, C.H.; Burr, D.B. Basic Biomechanical Measurements of Bone: A Tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
| Parameter/Group | C | D | DIOS50 | DIOS100 | ANOVA | |
|---|---|---|---|---|---|---|
| Bone length (mm) | 36.40 ± 0.34 | 34.28 ± 0.31 *** | 34.68 ± 0.23 *** | 34.67 ± 0.24 *** | F3,30 = 11.241 | p < 0.001 |
| Bone diameter (mm) | 3.60 ± 0.05 | 3.33 ± 0.09 ** | 3.35 ± 0.05 ** | 3.37 ± 0.07 * | F3,30 = 3.978 | p = 0.017 |
| Bone mass (g) | 0.855 ± 0.022 | 0.702 ± 0.024 *** | 0.713 ± 0.014 *** | 0.697 ± 0.020 *** | F3,30 = 15.027 | p < 0.001 |
| Bone density (g/cm3) | 1.626 ± 0.005 | 1.551 ± 0.012 *** | 1.576 ± 0.013 ** | 1.562 ± 0.010 *** | F3,30 = 10.705 | p < 0.001 |
| Bone mineral density (g/cm3) | 0.753 ± 0.006 | 0.695 ± 0.010 *** | 0.712 ± 0.010 ** | 0.695 ± 0.011 *** | F3,30 = 8.374 | p < 0.001 |
| Bone mineral mass (g) | 0.396 ± 0.009 | 0.314 ± 0.010 *** | 0.322 ± 0.006 *** | 0.310 ± 0.009 *** | F3,30 = 23.629 | p < 0.001 |
| Bone water mass (g) | 0.259 ± 0.007 | 0.218 ± 0.010 ** | 0.221 ± 0.007 ** | 0.218 ± 0.009 ** | F3,30 = 5.919 | p = 0.003 |
| Bone organic substances mass (g) | 0.201 ± 0.006 | 0.169 ± 0.007 *** | 0.170 ± 0.003 *** | 0.168 ± 0.004 *** | F3,30 = 9.603 | p < 0.001 |
| Bone mineral mass/bone mass ratio | 0.463 ± 0.003 | 0.448 ± 0.005 * | 0.452 ± 0.004 | 0.445 ± 0.005 ** | F3,30 = 3.696 | p = 0.022 |
| Bone water mass/bone mass ratio | 0.302 ± 0.003 | 0.310 ± 0.006 | 0.309 ± 0.006 | 0.313 ± 0.005 | F3,30 = 0.778 | p = 0.515 |
| Bone organic substances mass/bone mass ratio | 0.234 ± 0.002 | 0.241 ± 0.006 | 0.239 ± 0.004 | 0.242 ± 0.003 | F3,30 = 0.785 | p = 0.512 |
| Calcium content (g/g of bone mineral) | 0.422 ± 0.003 | 0.419 ± 0.003 | 0.421 ± 0.005 | 0.418 ± 0.002 | F3,30 = 0.331 | p = 0.803 |
| Phosphorus content (g/g of bone mineral) | 0.171 ± 0.001 | 0.167 ± 0.001 * | 0.167 ± 0.001 ** | 0.167 ± 0.001 * | F3,30 = 3.549 | p = 0.026 |
| Parameter/Group | C | D | DIOS50 | DIOS100 | ANOVA | |
|---|---|---|---|---|---|---|
| Displacement for yield point load (mm) | 0.285 ± 0.062 | 0.217 ± 0.025 | 0.134 ± 0.016 ** | 0.183 ± 0.017 * | F3,29 = 3.401 | p = 0.031 |
| Maximum load (N) | 65.0 ± 3.2 | 31.9 ± 2.3 *** | 32.8 ± 2.6 *** | 29.6 ± 1.2 *** | F3,29 = 46.270 | p < 0.001 |
| Displacement for maximum load (mm) | 0.903 ± 0.060 | 0.531 ± 0.078 | 0.764 ± 0.220 | 0.623 ± 0.072 | F3,29 = 1.434 | p = 0.253 |
| Energy for maximum load (mJ) | 37.2 ± 3.0 | 10.7 ± 1.9 *** | 18.2 ± 5.7 *** | 12.4 ± 1.4 *** | F3,29 = 10.843 | p < 0.001 |
| Stress for maximum load (MPa) | 46.0 ± 2.7 | 25.6 ± 2.1 *** | 27.6 ± 2.2 *** | 26.2 ± 1.4 *** | F3,29 = 19.847 | p < 0.001 |
| Fracture load (N) | 48.7 ± 3.3 | 24.1 ± 2.5 *** | 25.0 ± 2.7 *** | 24.5 ± 1.8 *** | F3,29 = 20.205 | p < 0.001 |
| Displacement for fracture load (mm) | 1.396 ± 0.065 | 0.911 ± 0.070 * | 1.063 ± 0.199 | 0.945 ± 0.087 * | F3,29 = 2.994 | p = 0.047 |
| Energy for fracture load (mJ) | 65.0 ± 3.7 | 21.4 ± 2.4 *** | 26.5 ± 5.6 *** | 21.1 ± 2.5 *** | F3,29 = 28.331 | p < 0.001 |
| Stress for fracture load (MPa) | 34.8 ± 3.2 | 19.3 ± 2.1 *** | 21.1 ± 2.5 *** | 21.5 ± 1.5 *** | F3,29 = 8.581 | p < 0.001 |
| Parameter/Group | C | D | DIOS50 | DIOS100 | ANOVA | ||
|---|---|---|---|---|---|---|---|
| Femoral metaphysis | BV/TV (%) | 33.05 ± 2.21 | 27.89 ± 4.55 | 28.54 ± 1.68 | 24.27 ± 0.78 | F3,18 = 2.628 | p = 0.082 |
| Tb.Th (μm) | 47.34 ± 2.85 | 38.70 ± 5.40 | 36.76 ± 3.0 * | 34.53 ± 1.40 ** | F3,18 = 3.170 | p = 0.050 | |
| Tb.Sp (μm) | 96.27 ± 4.58 | 102.02 ± 9.80 | 92.24 ± 6.27 | 109.02 ± 7.95 | F3,18 = 1.161 | p = 0.352 | |
| Tb.N (1/mm) | 6.98 ± 0.17 | 7.13 ± 0.25 | 7.09 ± 0.21 | 7.11 ± 0.47 | F3,18 = 0.042 | p = 0.988 | |
| Femoral diaphysis | Tt.Ar (mm2) | 9.543 ± 0.235 | 8.617 ± 0.272 * | 8.547 ± 0.324 * | 8.374 ± 0.192 ** | F3,26 = 4.538 | p = 0.011 |
| Ct.Ar (mm2) | 5.927 ± 0.193 | 5.224 ± 0.116 *** | 5.275 ± 0.101 ** | 5.013 ± 0.061 *** | F3,26 = 9.630 | p < 0.001 | |
| Ma.Ar (mm2) | 3.616 ± 0.199 | 3.392 ± 0.175 | 3.272 ± 0.231 | 3.361 ± 0.147 | F3,26 = 0.606 | p = 0.617 | |
| Ma.Ar/Tt.Ar (%) | 37.8 ± 1.6 | 39.2 ± 0.9 | 38.0 ± 1.4 | 40.0 ± 0.9 | F3,26 = 0.698 | p = 0.562 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzywnowicz, K.; Londzin, P.; Klasik-Ciszewska, S.; Borymska, W.; Zych, M.; Kaczmarczyk-Żebrowska, I.; Folwarczna, J. Diosmin Administration Slightly Counteracted the Changes in Bone Mechanical Properties Induced by Experimental Type 1 Diabetes in Rats. Pharmaceuticals 2025, 18, 715. https://doi.org/10.3390/ph18050715
Grzywnowicz K, Londzin P, Klasik-Ciszewska S, Borymska W, Zych M, Kaczmarczyk-Żebrowska I, Folwarczna J. Diosmin Administration Slightly Counteracted the Changes in Bone Mechanical Properties Induced by Experimental Type 1 Diabetes in Rats. Pharmaceuticals. 2025; 18(5):715. https://doi.org/10.3390/ph18050715
Chicago/Turabian StyleGrzywnowicz, Kacper, Piotr Londzin, Sylwia Klasik-Ciszewska, Weronika Borymska, Maria Zych, Ilona Kaczmarczyk-Żebrowska, and Joanna Folwarczna. 2025. "Diosmin Administration Slightly Counteracted the Changes in Bone Mechanical Properties Induced by Experimental Type 1 Diabetes in Rats" Pharmaceuticals 18, no. 5: 715. https://doi.org/10.3390/ph18050715
APA StyleGrzywnowicz, K., Londzin, P., Klasik-Ciszewska, S., Borymska, W., Zych, M., Kaczmarczyk-Żebrowska, I., & Folwarczna, J. (2025). Diosmin Administration Slightly Counteracted the Changes in Bone Mechanical Properties Induced by Experimental Type 1 Diabetes in Rats. Pharmaceuticals, 18(5), 715. https://doi.org/10.3390/ph18050715







