Molecular Imaging of Fibroblast Activation Protein in Response to Cardiac Injury Using [68Ga]Ga-DATA5m.SA.FAPi
Abstract
1. Introduction
2. Results
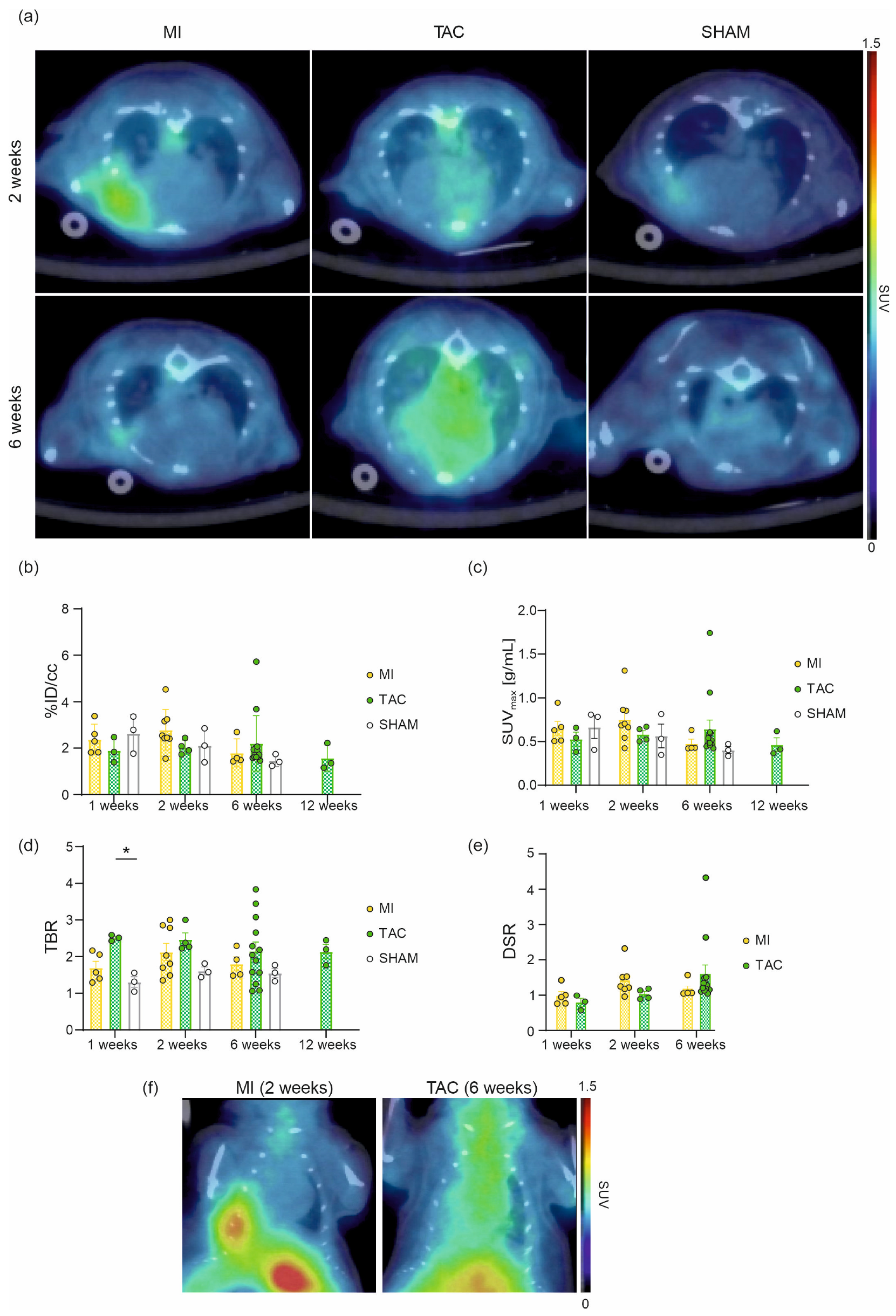
2.1. In Vivo Cardiac Imaging of [68Ga]Ga-DATA5m.SA.FAPi
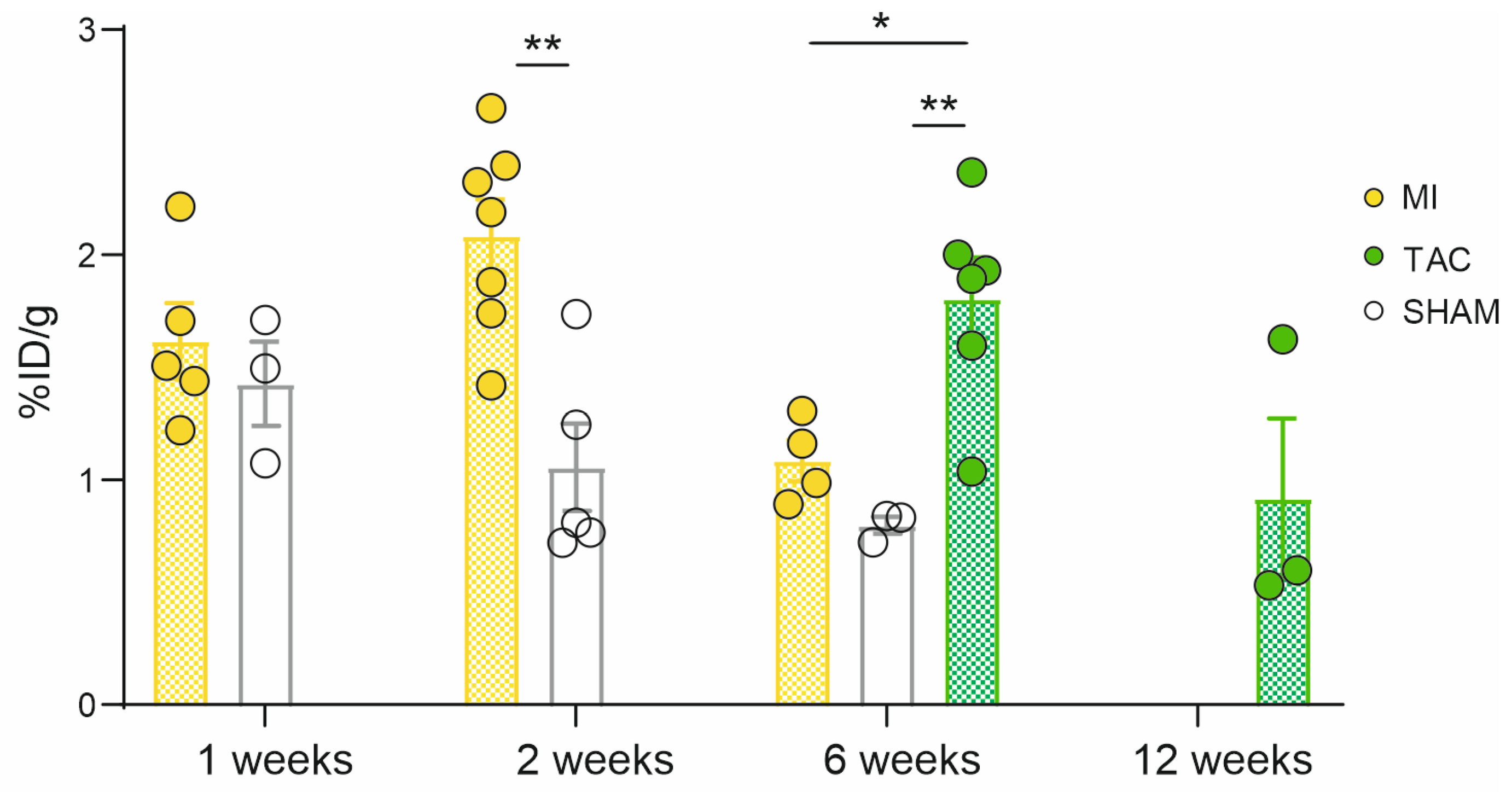
2.2. Ex Vivo Cardiac Uptake of [68Ga]Ga-DATA5m.SA.FAPi, Autoradiography and IHC
2.3. Disease Characteristics
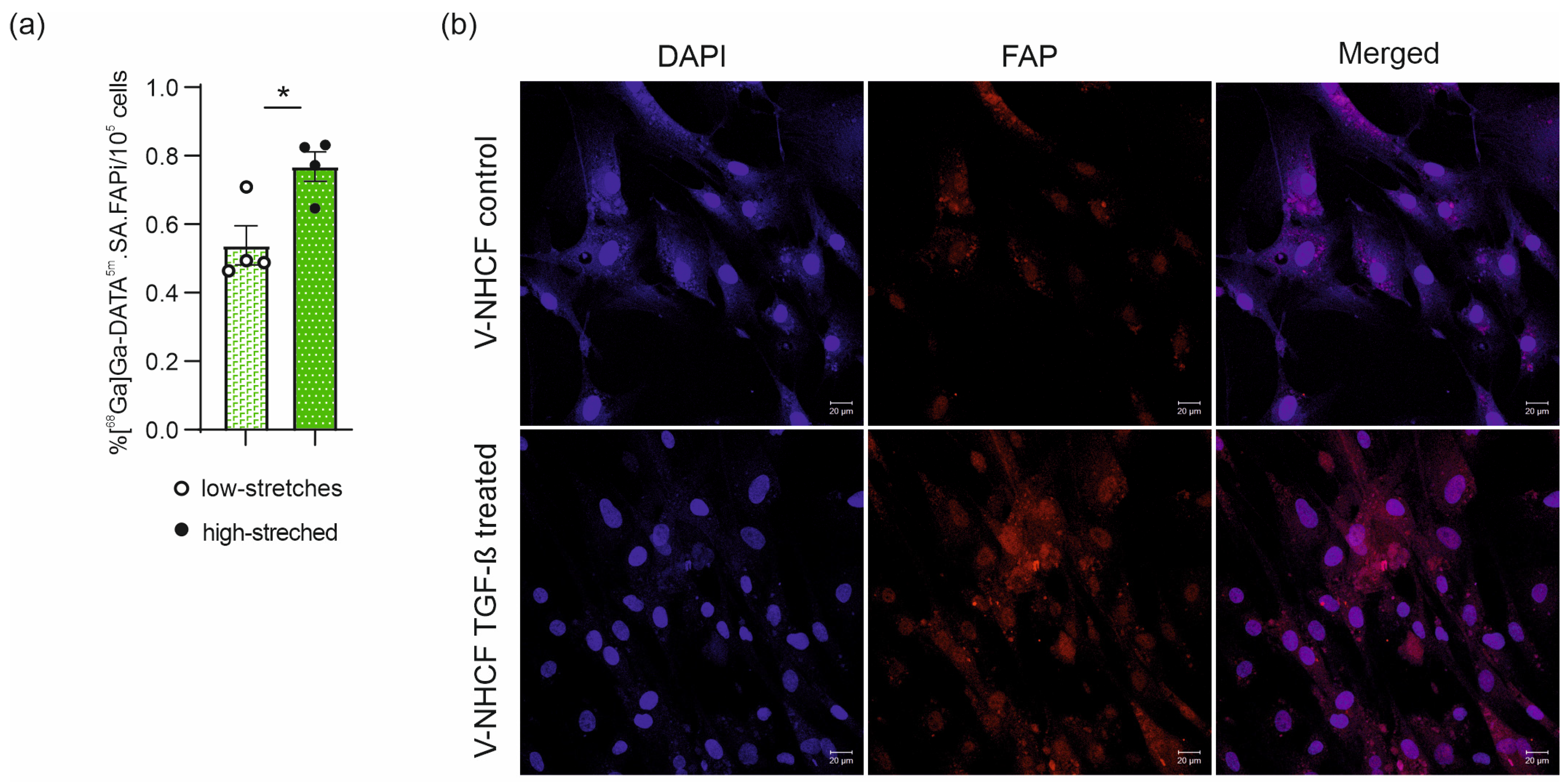
2.4. In Vivo [68Ga]Ga-DATA5m.SA.FAPi Cell Uptake
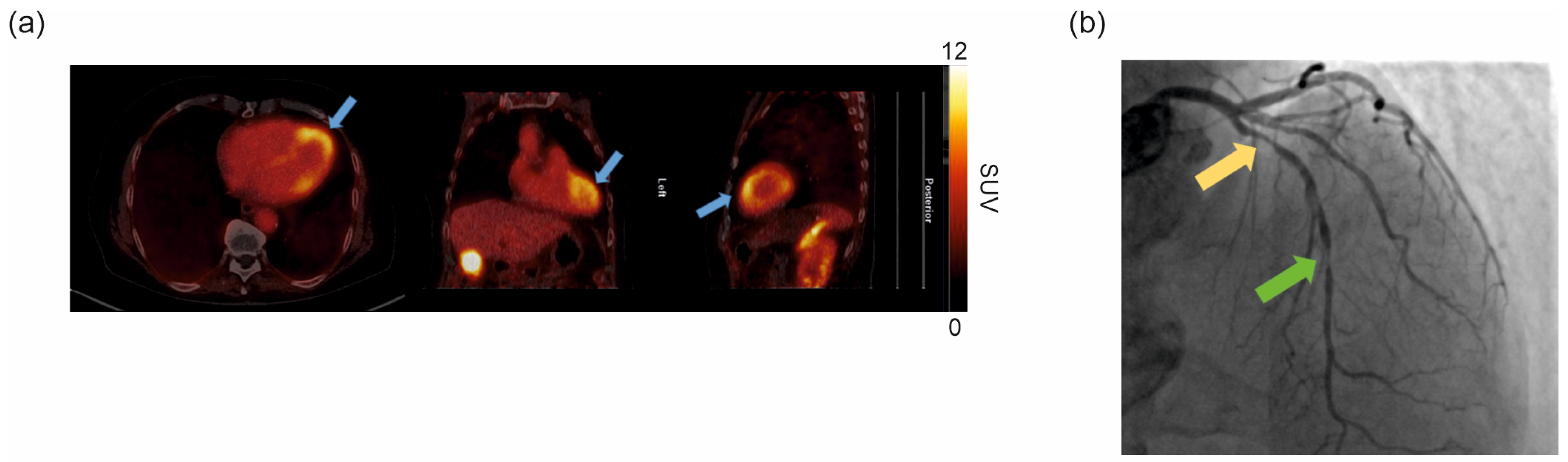
2.5. Clinical [68Ga]Ga-DATA5m.SA.FAPi PET/CT
3. Discussion
4. Materials and Methods
4.1. Radiosynthesis of [68Ga]Ga-DATA5m.SA.FAPi
4.2. Surgical Mouse Models
4.3. General Anesthesia, Analgesia, and Preparation
4.4. Mouse Model of Myocardial Infarction
4.5. Pressure Overload Induced LV Hypertrophy Model
4.6. Postoperative Care
4.7. Preclinical In Vivo Imaging of [68Ga]Ga-DATA5m.SA.FAPi and Data Analysis
4.8. Ex Vivo Biodistribution and Autoradiography
4.9. Histology and Immunohistochemistry
4.10. Human Cardiac Fibroblasts Cell Culture
4.11. In Vivo [68Ga]Ga-DATA5m.SA.FAPi Cell Uptake and FAP Expression
4.12. Clinical [68Ga]Ga-DATA5m.SA.FAPi PET/CT Study
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | amyloid light chain |
| CAF | cancer associated fibroblast |
| CMR | cardiovascular magnetic resonance |
| CT | computed tomography |
| DATA | 2,2′-(6-((carboxymethyl)amino)-1,4-diazepane-1,4-diyl) diacetic acid |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DSR | disease-to-sham ratio |
| EDTA | ethylenediaminetetraacetic acid |
| ECM | extracellular matrix |
| FAP | fibroblast activation protein |
| FAPi | FAP inhibitor |
| FBS | fetal bovine serum |
| HBR | heart-to-bodyweight ratio |
| HCl | hydrochloric acid |
| HE | hematoxylin/eosin |
| HPLC | high-performance liquid chromatography HU: Hounsfield Units |
| IHC | immunohistochemistry |
| i.p. | intraperitoneal |
| i.v. | intravenous |
| LAD | left anterior descending coronary artery |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| MI | myocardial infarction |
| MeCN | acetonitrile |
| MRI | magnetic resonance imaging |
| NH4OAc | ammonium acetate |
| PBS | phosphate buffered saline |
| PET | positron emission tomography |
| SA | squaric acid |
| s.c. | subcutaneous |
| TAC | Transverse aortic constriction |
| TBR | tumor-to-background ratio |
| TLC | thin layer chromatography |
| TME | tumor microenvironment |
| V-NHCF | ventricular normal human cardiac fibroblasts |
References
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jäger, D.; Mier, W.; et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef]
- Rosenkrans, Z.T.; Massey, C.F.; Bernau, K.; Ferreira, C.A.; Jeffery, J.J.; Schulte, J.J.; Moore, M.; Valla, F.; Batterton, J.M.; Drake, C.R.; et al. [68 Ga]Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3705–3716. [Google Scholar] [CrossRef]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F]FAPI-42 PET imaging in cancer patients: Optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2833–2843. [Google Scholar] [CrossRef]
- Sun, F.; Wang, C.; Feng, H.; Yu, F.; Zhang, X.; Zhang, P.; Du, X. Visualization of Activated Fibroblasts in Heart Failure with Preserved Ejection Fraction with [18F]AlF-NOTA-FAPI-04 PET/CT Imaging. Mol. Pharm. 2023, 20, 2634–2641. [Google Scholar] [CrossRef]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3651–3667. [Google Scholar] [CrossRef]
- Lindner, T.; Altmann, A.; Krämer, S.; Kleist, C.; Loktev, A.; Kratochwil, C.; Giesel, F.; Mier, W.; Marme, F.; Debus, J.; et al. Design and development of 99mTc-labeled FAPI tracers for SPECT imaging and 188re therapy. J. Nucl. Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Kuwert, T.; Atzinger, A.; Cordes, M.; Schett, G.; Ramming, A.; Götz, T. Fibroblast Activation Protein Inhibitor Imaging in Nonmalignant Diseases: A New Perspective for Molecular Imaging. J. Nucl. Med. 2022, 63, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Notohamiprodjo, S.; Nekolla, S.G.; Robu, S.; Asiares, A.V.; Kupatt, C.; Ibrahim, T.; Laugwitz, K.-L.; Makowski, M.R.; Schwaiger, M.; Weber, W.A.; et al. Imaging of cardiac fibroblast activation in a patient after acute myocardial infarction using 68Ga-FAPI-04. J. Nucl. Cardiol. 2022, 29, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Sewald, K.; Kafert-Kasting, S. Fraunhofer Projekt FibroPaths. 2022. Available online: http://www.item.fraunhofer.de (accessed on 29 June 2022).
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Rauber, S.; Atzinger, A.; Agarwal, R.; Götz, T.I.; Soare, A.; Cordes, M.; Prante, O.; Bergmann, C.; Kleyer, A.; et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann. Rheum. Dis. 2020, 79, 1485–1491. [Google Scholar] [CrossRef]
- Durant, F.; Whited, J.L. Finding Solutions for Fibrosis: Understanding the Innate Mechanisms Used by Super-Regenerator Vertebrates to Combat Scarring. Adv. Sci. 2021, 8, 2100407. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Q.; Wu, S.; Xu, X.; Li, X.; Liang, S.; Pan, G.; Zuo, C.; Zhao, X.; Cheng, C.; et al. Molecular imaging of fibroblast activity in pressure overload heart failure using [68 Ga]Ga-FAPI-04 PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 465–474. [Google Scholar] [CrossRef]
- Tillmanns, J.; Weiglein, J.; Neuser, J.; Fraccarollo, D.; Galuppo, P.; König, T.; Diekmann, J.; Ross, T.; Bengel, F.; Bauersachs, J.; et al. Circulating soluble fibroblast activation protein (FAP) levels are independent of cardiac and extra-cardiac FAP expression determined by targeted molecular imaging in patients with myocardial FAP activation. Int. J. Cardiol. 2024, 406, 132044. [Google Scholar] [CrossRef]
- Lin, K.; Chen, X.; Xue, Q.; Yao, S.; Miao, W. Diffuse uptake of [68Ga]Ga-FAPI in the left heart in a patient with hypertensive heart disease by PET/CT. J. Nucl. Cardiol. 2022, 29, 3596–3598. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Shi, L.; Wang, J.; Xu, Q.; Yu, B.; Qu, A. A time-series minimally invasive transverse aortic constriction mouse model for pressure overload-induced cardiac remodeling and heart failure. Front. Cardiovasc. Med. 2023, 10, 1110032. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.; He, S.; Gai, Y.; Qin, C.; Hu, F.; Wang, Y.; Wang, Z.; Bai, P.; Wang, J.; et al. 68Ga-FAPI PET visualize heart failure: From mechanism to clinic. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 475–485. [Google Scholar] [CrossRef]
- Glasenapp, A.; Derlin, K.; Gutberlet, M.; Hess, A.; Ross, T.L.; Wester, H.-J.; Bengel, F.M.; Thackeray, J.T. Molecular Imaging of Inflammation and Fibrosis in Pressure Overload Heart Failure. Circ. Res. 2021, 129, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Wang, Y.; Zhu, K.; Zheng, D.; Song, Y.; Jiang, D.; Qin, C.; Lan, X. Noninvasive Monitoring of Reparative Fibrosis after Myocardial Infarction in Rats Using 68Ga-FAPI-04 PET/CT. Mol. Pharm. 2022, 19, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zan, C.; Liang, Z.; Chen, X.; Li, J.; Ren, N.; Shi, Y.; Zhang, M.; Lan, L.; Li, H.; et al. Potential Value of FAPI PET/CT in the Detection and Treatment of Fibrosing Mediastinitis: Preclinical and Pilot Clinical Investigation. Mol. Pharm. 2023, 20, 4307–4318. [Google Scholar] [CrossRef] [PubMed]
- TTreutlein, C.; Distler, J.H.W.; Tascilar, K.; Fakhouri, S.C.; Györfi, A.-H.; Atzinger, A.; Matei, A.-E.; Dees, C.; Büttner-Herold, M.; Kuwert, T.; et al. Assessment of myocardial fibrosis in patients with systemic sclerosis using [68Ga]Ga-FAPI-04-PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1629–1635. [Google Scholar] [CrossRef]
- Hess, A.; Renko, A.; Schäfer, A.; Jung, M.; Fraccarollo, D.; Schmitto, J.D.; Diekmann, J.; Thum, T.; Bengel, F.M.; Bauersachs, J.; et al. Spatial FAP Expression as Detected by 68 Ga-FAPI-46 Identifies Myofibroblasts Beyond the Infarct Scar After Reperfusion. Mol. Imaging Biol. 2025. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Wang, S.; Du, B.; Li, X.; Li, Y. Highlighting Fibroblasts Activation in Fibrosis: The State-of-The-Art Fibroblast Activation Protein Inhibitor PET Imaging in Cardiovascular Diseases. J. Clin. Med. 2023, 12, 6033. [Google Scholar] [CrossRef]
- Greifenstein, L.; Engelbogen, N.; Lahnif, H.; Sinnes, J.; Bergmann, R.; Bachmann, M.; Rösch, F. Synthesis, Labeling and Preclinical Evaluation of a Squaric Acid Containing PSMA Inhibitor Labeled with 68Ga: A Comparison with PSMA-11 and PSMA-617. ChemMedChem 2020, 15, 695–704. [Google Scholar] [CrossRef]
- Cui, J.; Li, Z.; Jin, C.; Jin, Z. Knockdown of fibronectin extra domain B suppresses TGF-β1-mediated cell proliferation and collagen deposition in keloid fibroblasts via AKT/ERK signaling pathway. Biochem. Biophys. Res. Commun. 2020, 526, 1131–1137. [Google Scholar] [CrossRef]
- Rai, V.; Sharma, P.; Agrawal, S.; Agrawal, D.K. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol. Cell Biochem. 2017, 424, 123–145. [Google Scholar] [CrossRef]
- González, A.; López, B.; Ravassa, S.; San José, G.; Díez, J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1421–1432. [Google Scholar] [CrossRef]
- Lin, K.; Shi, D.; Wang, A.; Ge, J.; Cheng, D.; Yan, Y. Noninvasive Monitoring of Early Cardiac Fibrosis in Diabetic Mice by [68Ga]Ga-DOTA-FAPI-04 PET/CT Imaging. ACS Omega 2023, 9, 17195–17203. [Google Scholar] [CrossRef] [PubMed]
- Kupusovic, J.; Kessler, L.; Kazek, S.; Chodyla, M.K.; Umutlu, L.; Zarrad, F.; Nader, M.; Fendler, W.P.; Varasteh, Z.; Hermann, K.; et al. Delayed 68Ga-FAPI-46 PET/MR imaging confirms ongoing fibroblast activation in patients after acute myocardial infarction. IJC Heart Vasc. 2024, 50, 101340. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.K.; Craig, N.J.; Loganath, K.; Joshi, S.; Tsampasian, V.; Mahendran, M.; Lenell, J.; Tzolos, E.; Singh, T.; Whittington, B.; et al. Myocardial Fibroblast Activation After Acute Myocardial Infarction: A Positron Emission Tomography and Magnetic Resonance Study. J. Am. Coll. Cardiol. 2025, 85, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, J.; Zhang, Y.; Tao, D.; Zhao, K.; Shi, Q.; Zhang, G.; Wang, H. Simultaneous 18F-labeled AlF-FAPI PET/MR images targeting the myocardial fibrosis in coronary artery disease and degenerative mitral valve regurgitant participants with left ventricular mechanical dyssynchrony. Clinics 2025, 80, 100624. [Google Scholar] [CrossRef]
- Santer, D.; Nagel, F.; Gonçalves, I.F.; Kaun, C.; Wojta, J.; Fagyas, M.; Krššák, M.; Balogh, Á.; Papp, Z.; Tóth, A.; et al. Tenascin-C aggravates ventricular dilatation and angiotensin-converting enzyme activity after myocardial infarction in mice. ESC Heart Fail. 2020, 7, 2113–2122. [Google Scholar] [CrossRef]
- Thessaloniki, P.I. Association of Left Ventricular Mass with Dilatation of Ascending Aorta in Patients with Hypertension: A Retrospective, Case Control Study. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2020. [Google Scholar]
- Theresa, B.; Cécile, P. Autoradiography. In Imaging Modalities for Biological and Preclinical Research: A Compendium; Walter, A., Mannheim, J.G., Caruana, C.J., Eds.; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 1, pp. 10–18. [Google Scholar]
- Perera-Gonzalez, M.; Kiss, A.; Kaiser, P.; Holzweber, M.; Nagel, F.; Watzinger, S.; Acar, E.; Szabo, P.L.; Gonçalves, I.F.; Weber, L.; et al. The role of tenascin c in cardiac reverse remodeling following banding–debanding of the ascending aorta. Int. J. Mol. Sci. 2021, 22, 2023. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]


| In Vivo | Ex Vivo | ||||||
|---|---|---|---|---|---|---|---|
| Time Point | Mouse Model | SUVmax [g/mL] | n | Cohen’s d | %ID/g | n | Cohen’s d |
| 1 week | MI | 0.6 ± 0.1 | 5 | 0.068 | 1.6 ± 0.17 | 5 | 0.544 |
| TAC | 0.5 ± 0.1 | 3 | 0.730 | ||||
| SHAM | 0.7 ± 0.1 | 3 | 1.4 ± 0.19 | 3 | |||
| 2 weeks | MI | 0.8 ± 0.09 | 7 | 0.983 | 2.1 ± 0.16 | 7 | 2.392 |
| TAC | 0.6 ± 0.04 | 4 | 0.113 | ||||
| SHAM | 0.6 ± 0.1 | 1.1 ± 0.2 | 5 | ||||
| 6 weeks | MI | 0.5 ± 0.05 | 4 | 0.861 | 1.1 ± 0.1 | 4 | 2.087 |
| TAC | 0.6 ± 0.1 | 13 | 0.918 | 1.8 ± 0.2 | 6 | 3.131 | |
| SHAM | 0.4 ± 0.04 | 3 | 0.8 ± 0.04 | 3 | |||
| 12 weeks | MI | ||||||
| TAC | 0.5 ± 0.08 | 3 | 0.9 ± 0.35 | 3 | |||
| SHAM | |||||||
| 1 Week | 2 Weeks | 6 Weeks | 12 Weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Mouse Model | Scan | Biodis | Scan | Biodis | Scan | Biodis | Scan | Biodis |
| MI | 5 | 5 | 7 | 7 | 4 | 4 | ||
| SHAM | 3 | 3 | 5 | 5 | 3 | 3 | ||
| TAC | 3 | 4 | 13 | 6 | 3 | 3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weissenböck, V.; Weber, L.; Schlederer, M.; Silva Sousa, L.; Stampfer, A.; Baydar, S.; Nakuz, T.; Calabretta, R.; Antunes Goncalves, A.I.; Li, X.; et al. Molecular Imaging of Fibroblast Activation Protein in Response to Cardiac Injury Using [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals 2025, 18, 658. https://doi.org/10.3390/ph18050658
Weissenböck V, Weber L, Schlederer M, Silva Sousa L, Stampfer A, Baydar S, Nakuz T, Calabretta R, Antunes Goncalves AI, Li X, et al. Molecular Imaging of Fibroblast Activation Protein in Response to Cardiac Injury Using [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals. 2025; 18(5):658. https://doi.org/10.3390/ph18050658
Chicago/Turabian StyleWeissenböck, Victoria, Lukas Weber, Michaela Schlederer, Laura Silva Sousa, Anna Stampfer, Simge Baydar, Thomas Nakuz, Raffaella Calabretta, Ana Isabel Antunes Goncalves, Xiang Li, and et al. 2025. "Molecular Imaging of Fibroblast Activation Protein in Response to Cardiac Injury Using [68Ga]Ga-DATA5m.SA.FAPi" Pharmaceuticals 18, no. 5: 658. https://doi.org/10.3390/ph18050658
APA StyleWeissenböck, V., Weber, L., Schlederer, M., Silva Sousa, L., Stampfer, A., Baydar, S., Nakuz, T., Calabretta, R., Antunes Goncalves, A. I., Li, X., Rösch, F., Podesser, B. K., Kenner, L., Hacker, M., Kiss, A., & Philippe, C. (2025). Molecular Imaging of Fibroblast Activation Protein in Response to Cardiac Injury Using [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals, 18(5), 658. https://doi.org/10.3390/ph18050658











