Abstract
Background: This study investigates the effects of cadmium chloride (CdCl2) on hippocampal peroxisome proliferator-activated receptor gamma (PPARγ) expression and examines whether PPARγ activation mediates the neuroprotective effects of quercetin (QUR). Methods: Sixty adult male rats were included in this study, separated into 12 rats per group as follows: control, CdCl2 (0.5 mg/kg), CdCl2 + PPARγ agonist (Pioglitazone, 10 mg/kg), CdCl2 + QUR (25 mg/kg), and CdCl2 + QUR + PPARγ antagonist (GW9662, 1 mg/kg). Treatments were administered orally for 30 days. At the end of the experiment, behavioral memory tests, hippocampal histology, markers of cholinergic function, neuroplasticity, oxidative stress, inflammation, and apoptosis, as well as transcription levels of some genes were carried out. Results: CdCl2 exposure significantly reduced hippocampal PPARγ mRNA and DNA binding potential and nuclear levels. Additionally, CdCl2 impaired spatial, short-term, and recognition memory, decreased granular cell density in the dentate gyrus (DG), and reduced levels of neuroprotective factors, including Nrf2, brain-derived neurotrophic factor (BDNF), acetylcholine (ACh), and several antioxidant enzymes including heme-oxygenase-1 (HO-1) and superoxide dismutase (SOD), as well as reduced glutathione (GSH). Conversely, CdCl2 elevated levels of oxidative stress, inflammation, and apoptosis markers such as interleukin-6 (IL-6), malondialdehyde (MDA), Bax, tumor necrosis factor-α (TNF-α), and cleaved caspase-3. QUR and Pioglitazone reversed these effects, restoring expression and PPARγ activation, improving memory, and modulating antioxidant and anti-inflammatory pathways. In contrast, blocking PPARγ with GW9662 negated the neuroprotective effects of QUR, exacerbating oxidative stress and inflammation by reversing all their beneficial effects. Conclusions: Activation of PPARγ by QUR or Pioglitazone offers a promising therapeutic strategy for mitigating CdCl2-induced neurotoxicity.
1. Introduction
A wide range of neurological diseases involve the gradual degeneration and death of neurons within the central nervous system. Neurological diseases result in profound impairments in cognitive functions such as memory, learning, concentration, and overall intellectual ability [1]. Prominent examples include spinocerebellar ataxia, Huntington’s disease, dementia, Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), prion disease, and spinal muscular atrophy [1,2,3]. The etiology of these disorders is multifactorial, with both genetic predispositions and environmental factors—such as diet, infections, pharmaceuticals, and environmental pollutants—playing significant roles in their onset and progression [2]. Over recent decades, cadmium (Cd) has emerged as one of the most prominent environmental pollutants implicated in neurodegeneration, particularly in AD, PD, and ALS [3,4,5,6,7,8].
Cd, a ubiquitous heavy metal, is readily absorbed into the body through various routes, including ingestion, dermal exposure, and inhalation, ultimately accumulating in the brain. This metal has increased exposure due to mining activities in the land, tobacco smoking, and its wide use in plastics, paints, and batteries [3]. Cd’s long biological half-life, averaging 23 years, coupled with its minimal urinary excretion, facilitates its accumulation and results in significant multi-organ toxicity [4]. In the central nervous system, Cd disrupts neuronal function by penetrating the blood–brain barrier (BBB), where it impairs neurogenesis, cholinergic function, synaptic plasticity, and neurotransmitter synthesis while simultaneously activating apoptosis pathways [3,5]. The precise mechanisms underlying Cd-induced neurotoxicity are still not fully elucidated but are thought to involve DNA damage, oxidative stress, neuroinflammation, and apoptotic signaling cascades [3,5,6].
Recent studies have highlighted the critical neuroprotective role of peroxisome proliferator-activated receptor gamma (PPARγ) in mitigating neurodegeneration [7,8]. The nuclear hormone receptor transcription factor, PPARγ, is distributed in the majority of the tissues and is highly expressed in the brain. This receptor regulates a wide range of genes involved in metabolic processes, immune responses, redox balance, inflammation, and apoptosis [7]. Importantly, dysregulation of PPARγ expression and activity has been observed in several neurological disorders, including AD, PD, stroke, ALS, and multiple sclerosis [7,8]. Activation of PPARγ has been shown to alleviate oxidative stress, modulate inflammation, prevent neuronal apoptosis, and enhance synaptic function, thereby offering a promising therapeutic strategy for various neurodegenerative conditions [7].
In recent years, plant-based compounds have gained considerable attention as potential treatments for neurological disorders due to their natural antioxidant and anti-inflammatory properties. Quercetin (QUR) is abundant in nuts commonly isolated from diverse vegetables and fruits and has demonstrated significant neuroprotective effects across a variety of preclinical models [9,10,11,12,13,14,15,16,17]. QUR has been shown to exert its protective effects through its potent antioxidant and anti-inflammatory actions, mediated via the regulation of key intracellular signaling pathways, including those involved in oxidative stress and inflammation [9,10]. Notably, QUR has been found to protect against Cd-induced hippocampal and cortical damage by scavenging free radicals, enhancing antioxidant capacity, activating Nrf2, inhibiting NF-κB, and improving synaptic transmission. A full review of the antioxidant and anti-inflammatory neuroprotective effects of QUR against CdCl2-induced toxicity is discussed in excellent studies and reviews [11,12,13,14,15,16,17].
While the molecular mechanisms underlying CdCl2-induced neurotoxicity have been well-established, the specific role of PPARγ in this process has never been systematically examined. This hypothesis is further supported by studies demonstrating that Cd exposure leads to the degradation of PPARγ in pulmonary macrophages [18], impairs PPARγ-mediated metabolic regulation in the liver [19], and induces nephrotoxicity by suppressing PPARγ activity in renal cells [20]. In the same manner, despite the growing body of evidence supporting QUR’s neuroprotective potential, the effects of CdCl2 on PPARγ expression and activation remain poorly understood, particularly in the context of neurodegeneration. Given QUR’s known antioxidant and anti-inflammatory properties, it is plausible that CdCl2 exposure may suppress PPARγ activity, thereby exacerbating oxidative stress and inflammation in the brain. We built this hypothesis based on the reported neuroprotective antioxidant and anti-inflammatory effects of QUR in the lung, liver, and heart of various diseased models due to PPARγ activation [21,22,23,24,25,26,27].
In this study, we aim to investigate the role of PPARγ in mediating CdCl2-induced memory dysfunction and hippocampal damage in rate and if the neuroprotective effects of QUR against such neurotoxicity are mediated by activating this transcription factor. The findings of this study demonstrate that Cd-induced neurotoxicity is associated with downregulation and reduced nuclear activity of PPARγ. Furthermore, we show that the full protective antioxidant and anti-inflammatory effect of QUR is mediated through the activation of PPARγ, highlighting its crucial role in regulating cholinergic function, synaptic activity, NF-κB, and the Nrf2/antioxidant axis. Importantly, these neuroprotective effects are completely abolished by GW9662, a selective PPARγ antagonist, providing compelling evidence of the pivotal role of PPARγ in modulating neuroinflammation and oxidative stress in Cd-induced neurotoxicity.
2. Results
2.1. Effects of QUR and Pioglitazone on Neurological Scores and Memory Function in Rats
The total neurological score was significantly increased in CdCl2-treated rats compared to the control group (Figure 1A). CdCl2-intoxicated rats took less time to explore the novel object or enter the dark room during the NORT and PALT (Figure 1B and 1C, respectively). There were no significant variations in the time spent by the CdCl2-treated rats finding the hidden platform as measured on days 1, 2, 3, and 4 during the MWM test (Figure 1D). However, CdCl2-treated rats showed a significantly reduced area under the curve (AUC) for the corresponding swimming time taken to find the hidden platform on these days (Figure 1E). In addition, the CdCl2-intoxicated rats showed a significant reduction during the trial phase in the amount of time needed to cross over the platform (Figure 1F). The treatment with either QUR or Pioglitazone significantly lowered the total neuro-logical score, and these rats spent a longer time exploring the novel object or entering the dark area in the NORT and PALT (Figure 1A–C). They also exhibited a progressive significant decrease in the swimming times taken to locate the hidden platform during days 1–4, as well as a reduction in their AUC scores as compared to the CdCl2-treated rats (Figure 1D,E). The rats treated with CdCl2 + QUR and CdCl2 + Pioglitazone also undertook significantly more crossing trials to find the hidden platform (Figure 1F). No significant variations were shown between these parameters when the CdCl2 + QUR-treated rats were compared with the CdCl2 rats which were administered Pioglitazone. On the other hand, when the comparison was made against CdCl2 + QUR model rats, the neurological scores and the time needed to find the hidden platform were significantly increased, and the number of crossings over the hidden platform, the time needed to explore the novel subject, and the time taken to enter the dark area were significantly reduced in CdCl2 + QUR + GW9662 rats (Figure 1A–F). Notably, the mean values of all these measures were not significantly varied between CdCl2- and CdCl2 + QUR + GW9662 rats.
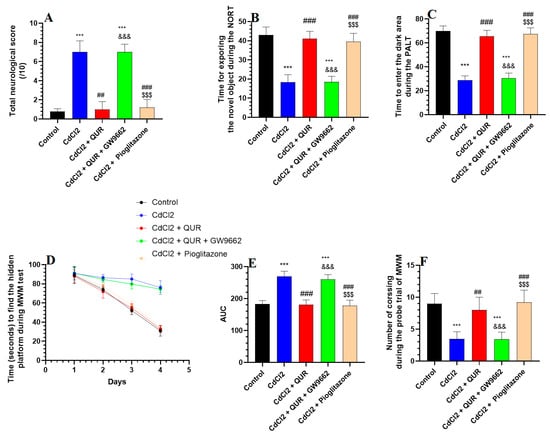
Figure 1.
The total neurological score (A) alongside the outcomes of the novel object recognition test (NORT) (B), passive avoidance learning test (PALT) (C), and Morris Water Maze (MWM) (D–F) across all experimental rat groups. Data are expressed as mean ± SD (n = 8 per group). Statistical significance was determined at p < 0.05. ***: Compared to the control group (p < 0.001); ##, ###: Compared to CdCl2-treated rats (p < 0.001); &&&: Compared to CdCl2 + QUR-treated rats (p < 0.001); and $$$: Compared to CdCl2 + QUR + GW9662 (p < 0.001). QUR: Quercetin; GW9662: PPARγ inhibitor.
2.2. Effect on Hippocampal Levels of Markers of Inflammation and Oxidative Stress
As shown in Table 1, CdCl2 exposure led to significant elevation in MDA, 8-OHdG, TNF-α, IL-6, and RAGE levels, along with increased nuclear NF-κB in the hippocampus compared to control rats. Conversely, antioxidant markers such as GSH, SOD, and HO-1, as well as nuclear Nrf2, were markedly diminished in CdCl2-treated rats relative to the control group (Table 1). Co-treatment with either QUR or Pioglitazone effectively mitigated these effects, as demonstrated by lower MDA, 8-OHdG, TNF-α, IL-6, and RAGE levels, as well as reduced nuclear NF-κB, compared to the CdCl2-intoxicated group (Table 1). Simultaneously, hippocampal GSH, SOD, HO-1, and nuclear Nrf2 levels were significantly elevated in these treatment groups relative to CdCl2-exposed rats (Table 1). Interestingly, in QUR-treated rats, the levels of GSH, SOD, HO-1, and 8-OHdG, as well as nuclear Nrf2, were comparable to those observed in control animals (Table 1). However, introducing GW9662 alongside QUR reversed these protective effects, leading to a resurgence of MDA, 8-OHdG, TNF-α, IL-6, RAGE, and nuclear NF-κB levels, while simultaneously reducing GSH, SOD, HO-1, and nuclear Nrf2 when compared to CdCl2 + QUR- or CdCl2 + Pioglitazone-treated groups (Table 1). Notably, no significant differences were observed between the CdCl2-only group and the CdCl2 + QUR + GW9662-treated rats, indicating that GW9662 negated the beneficial impact of QUR (Table 1).

Table 1.
Hippocampal oxidative stress and inflammation profiles in different rat groups.
2.3. Effect on Hippocampal Levels of BDNF and Other Cholinergic Markers
As presented in Table 2, CdCl2 exposure led to a significant decline in hippocampal levels of ACh, BDNF, and ChAT, while AchE levels were markedly elevated compared to the control group. Treatment with either QUR or Pioglitazone effectively counteracted these alterations, resulting in significantly higher levels of ACh, BDNF, and ChAT, along with a notable reduction in AchE levels relative to CdCl2-exposed rats (Table 2). Notably, no significant differences were observed between the CdCl2 + QUR- and CdCl2 + Pioglitazone-treated groups. However, co-administration of GW9662 with QUR reversed these beneficial effects, as evidenced by a significant reduction in ACh, BDNF, and ChAT levels, along with a concomitant rise in AchE, compared to rats treated with CdCl2 + QUR alone (Table 2). Importantly, these values did not significantly differ from those observed in the CdCl2 model group, suggesting that GW9662 negated the protective effects of QUR (Table 2)

Table 2.
The neurological scores and levels of BDNF and cholinergic neurological markers in the hippocampi of all groups of rats.
2.4. Effect on Apoptotic and Anti-Apoptotic Markers
As illustrated in Figure 2A–D, CdCl2 exposure resulted in a significant decrease in hippocampal Bcl-2 levels, accompanied by an increase in caspase-3 and Bax expression, as well as an elevated Bax/Bcl-2 ratio, when compared to the control group. However, treatment with either QUR or Pioglitazone effectively counteracted these changes, leading to significantly higher Bcl-2 levels and lower caspase-3 and Bax levels, along with a reduced Bax/Bcl-2 ratio relative to CdCl2-exposed rats (Figure 2A–D). No statistically significant differences were observed among these treatment groups or in comparison to control rats. In contrast, co-administration of GW9662 with QUR led to a notable decline in Bcl-2 levels, paralleled by reductions in Bax, caspase-3, and the Bax/Bcl-2 ratio, compared to CdCl2 + QUR-treated rats (Figure 2A–D). Importantly, these apoptotic markers in the CdCl2 + QUR + GW9662 group were not significantly different from those observed in CdCl2-treated rats, indicating that GW9662 abolished the protective effects of QUR (Figure 2A–D).
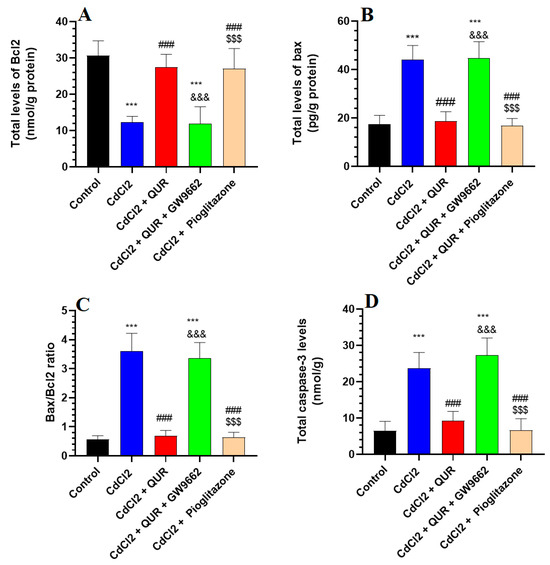
Figure 2.
Hippocampal analysis of the levels of Bax, Bcl-2, Bax/Bcl-2 ratio (A–C), and caspase-3 (D) across experimental groups. The values were significantly different at p < 0.05. ***: Compared to control (p < 0.05, 0.01, & 0.001 Statistical significance was determined at p < 0.05. ***: Compared to the control group (p < 0.001); ###: Compared to CdCl2-treated rats (p < 0.001); &&&: Compared to CdCl2 + QUR-treated rats (p < 0.001); and $$$: Compared to CdCl2 + QUR + GW9662 (p < 0.001).
2.5. Effect on the mRNA and Nuclear Activity of PPARα
As depicted in Figure 3A,B, CdCl2 exposure led to a pronounced decline in both the mRNA expression and nuclear DNA binding activity of PPARα in the hippocampus compared to control rats. Conversely, a substantial upregulation in PPARγ transcript levels and its DNA binding activity was evident in the hippocampi of rats receiving either CdCl2 + QUR or CdCl2 + Pioglitazone treatment (Figure 3A,B). Notably, no significant discrepancies in PPARγ expression or nuclear binding activity were detected between these two treatment groups or in comparison to control animals. However, the introduction of GW9662 alongside QUR reversed these molecular alterations, resulting in a marked suppression of PPARγ mRNA expression and nuclear DNA binding activity relative to the CdCl2 + QUR-treated group (Figure 3A,B). Importantly, PPARγ transcript levels and nuclear activity in the CdCl2 + QUR + GW9662-treated rats were statistically indistinguishable from those observed in the CdCl2-only group, indicating a complete abrogation of QUR’s modulatory effects by GW9662 (Figure 3A,B).
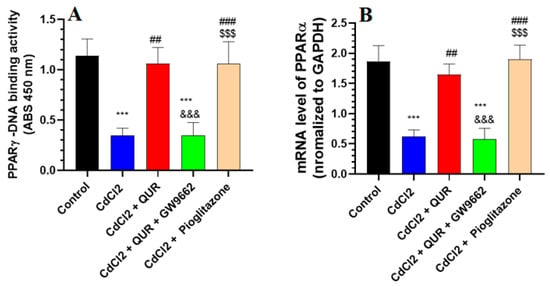
Figure 3.
Nuclear DNA binding activity (A) and mRNA levels (B) of PPARα in the hippocampi of all groups of rats. The values were significantly different at p < 0.05. ***: Compared to control (p < 0.05, 0.01, & 0.001 Statistical significance was determined at p < 0.05. ***: Compared to the control group (p < 0.001); ##, ###: Compared to CdCl2-treated rats (p < 0.001); &&&: Compared to CdCl2 + QUR-treated rats (p < 0.001); and $$$: Compared to CdCl2 + QUR + GW9662 (p < 0.001).
2.6. Effect on Dental Gyrus Histopathology
In the hippocampal dentate gyrus (DG) of untreated control rats, the structural integrity remained intact, exhibiting its characteristic tri-layered organization comprising the polymorphic, granular, and molecular layers. The granular layer comprises intact round cells of five to eight layers (Figure 4A). In the DG of CdCl2-exposed rats, a marked decline in the cellular density of the granular layer was observed, with the layer thinning to only two to three cell layers. Additionally, a majority of the cells exhibited pyknosis, indicative of nuclear condensation and cellular degeneration (Figure 4B). On the other hand, almost normal DGs with six to eight granular layers of healthy intact cells were observed in the hippocampi of CdCl2 + QUR- (Figure 4C,D) and CdCl2 + Pioglitazone-treated rats (Figure 4E). Similarly, the DG of the hippocampi from CdCl2 + QUR + GW9662-treated rats exhibited comparable damage, characterized by an increased presence of pyknotic cells, further exacerbating the deterioration of the granular layer (Figure 4F).
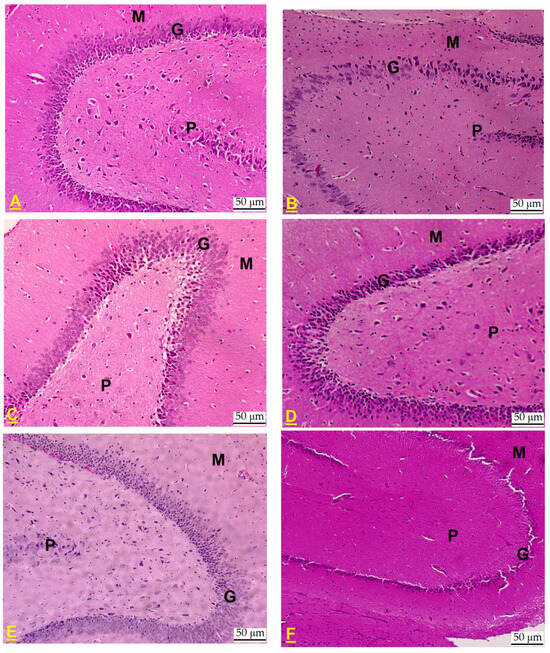
Figure 4.
Histological alterations in the dentate gyrus (DG) of the hippocampus across experimental rat groups. (A) The DG from a control rat displayed normal architecture, with distinct polymorphic (P), granular (G), and molecular (M) layers. The granular layer consisted of intact, round cells organized in five to eight layers. (B) In contrast, the DG from a CdCl2-treated rat exhibited a notable reduction in the number of cells within the granular layer, now comprising only two to three layers. A majority of the cells displayed pyknosis, indicative of cellular degeneration. (C,D): taken from a CdCl2 + QUR-treated rat, which exhibited a normal DG with six to eight granular layers of healthy intact cells. (E) Taken from a CdCl2 + Pioglitazone-treated rat, it showed an almost normal structure with a normal number of cells forming the granular layer. (F) Taken from a CdCl2 + QUR + GW9662-treated rat, showing similar damage in the granular layer with an increased number of pyknotic cells. Hematoxylin and Eosin, 200×.
3. Discussion
This study presents significant findings regarding the neural toxicity induced by CdCl2 and the protective effects conferred by quercetin (QUR). The results demonstrate that CdCl2-induced hippocampal damage and associated memory deficits are linked to a marked impairment in hippocampal levels of peroxisome proliferator-activated receptor gamma (PPARγ), a key regulator of oxidative stress, inflammation, and apoptosis within neural tissues. Notably, activation of PPARγ by QUR fully mitigated the oxidative and inflammatory damage caused by Cd ions in the hippocampus, effectively restoring short-term and recognition memory functions. These effects were comparable to those observed with Pioglitazone, a selective PPARγ agonist. Importantly, this study provides new insight into the upstream mechanisms underlying the neuroprotective actions of QUR. These data also suggest that QUR’s antioxidant, anti-inflammatory, and anti-apoptotic effects in the CdCl2-treated brain are primarily mediated through the upregulation and nuclear activation of PPARγ. This conclusion is further substantiated by the observation that inhibition of PPARγ using GW9662, a selective antagonist, abolished the neuroprotective effects of QUR and reversed its beneficial impact on hippocampal morphology and memory (Figure 5).
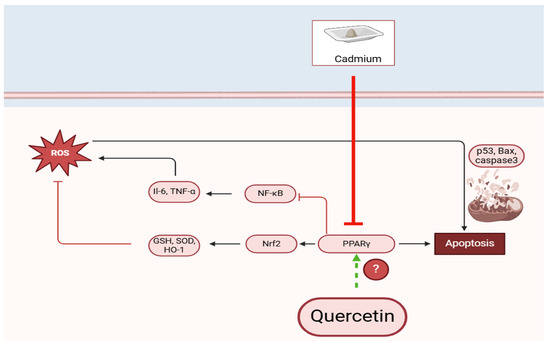
Figure 5.
A graphic abstract demonstrates the precise neuroprotective effect of quercetin against cadmium-induced hippocampal damage in rats. (?) We are still not sure if this quercetin activates this or not.
Oxidative stress and inflammation are well-established contributors to neurodegeneration in numerous neurological disorders. The detrimental role of these factors in synaptic plasticity, long-term potentiation (LTP), memory impairment, and neurodegeneration has been extensively documented [5,6]. In this context, Cd ions have been shown to disrupt mitochondrial electron transport, replace essential zinc ions, deplete antioxidants, and increase reactive oxygen species (ROS) production, thereby impeding neural energy metabolism and calcium signaling [28,29]. Furthermore, CdCl2 exposure leads to diminished nuclear activation of Nrf2 and enhances the activation of NF-κB, both of which exacerbate oxidative stress and inflammation. The resulting accumulation of ROS and inflammatory cytokines contributes to intrinsic cell apoptosis by promoting DNA damage, increasing p53 expression, and upregulating Bax, which translocates to the nucleus and induces the release of cytochrome c, triggering apoptotic cascades [30]. Moreover, Cd ions disrupt synaptic plasticity and memory function by reducing brain-derived neurotrophic factor (BDNF) levels and impairing cholinergic function, either by promoting acetylcholine (Ach) degradation through acetylcholinesterase (AchE) upregulation or by inhibiting Ach synthesis via suppression of choline acetyltransferase (ChAT) [17,31,32].
In alignment with previous studies, this study established an animal model of hippocampal damage induced by chronic CdCl2 administration over 30 days. The observed deficits in memory, evidenced by reductions in BDNF, ACh, AChE, and ChAT levels, along with altered performance in cognitive assessments such as the Morris Water Maze (MWM), Novel Object Recognition Test (NORT), and Passive Avoidance Learning Test (PALT), substantiate previous research employing similar methodologies [17,27,33,34]. Furthermore, the elevated concentrations of malondialdehyde (MDA), 8-hydroxy-2′-deoxyguanosine (8-OHdG), TNF-α, IL-6, Bax, and caspase-3, in conjunction with diminished levels of Bcl-2, glutathione (GSH), superoxide dismutase (SOD), and heme oxygenase-1 (HO-1) in the hippocampi of CdCl2-treated rats, strongly implicate the activation of oxidative stress, inflammatory, and apoptotic pathways in the neurotoxic effects of CdCl2. Moreover, these alterations were linked to suppressed Nrf2 activation and enhanced NF-κB activation, corroborating earlier studies that described similar molecular changes in response to Cd exposure [3,35,36,37,38,39,40,41].
In contrast, this study also reveals the potential of QUR to attenuate CdCl2-induced hippocampal damage and prevent memory decline in rats. QUR achieved this by mitigating oxidative stress, inflammation, and apoptosis, particularly by suppressing lipid peroxidation, activating Nrf2, inhibiting NF-κB, upregulating BDNF, and restoring normal cholinergic function through modulation of Ach and its associated enzymes. The neuroprotective effects observed align with prior studies demonstrating that quercetin (QUR) mitigates hippocampal and cortical degeneration in a range of experimental models of neurodegenerative disorders, including aging, Alzheimer’s disease (AD), stroke, Parkinson’s disease (PD), as well as in cases of intoxication with heavy metals and other neurotoxic agents [11,12,13,14,15,16,17]. The neuroprotective mechanisms of QUR are multifaceted and not entirely understood, but they are largely attributed to the attenuation of oxidative stress and inflammation. Notably, QUR’s antioxidant activity is facilitated by its unique chemical structure, which contains multiple phenolic hydroxyl groups and a conjugated double bond, enabling it to scavenge ROS efficiently [9]. Additionally, its anti-inflammatory effects are likely mediated through the suppression of immune cell infiltration, inflammatory cytokine production, and activation of key signaling pathways, including NF-κB, NLRP3 inflammasomes, and TLR2/MyD88/NF-κB signaling, while simultaneously activating SIRT1, Nrf2, and AMPK [11,12,13,14,15,16,17,42,43,44,45,46,47,48]. Furthermore, QUR acts as a natural inhibitor of AchE, enhancing cell survival, synaptic plasticity, and memory function through modulation of acetylcholine levels and signaling pathways, including the BDNF, PI3K/Akt, and MAPK/ERK pathways [49,50,51,52].
PPARγ agonists, including thiazolidinedione derivatives like Pioglitazone, are widely used in clinical practice. PPARγ is abundantly expressed in various brain regions, including the hippocampus, thalamus, and basal ganglia, and is present in neurons, microglia, and astrocytes [7,42]. Beyond its role in metabolic regulation, PPARγ serves as a neuroprotective agent capable of attenuating cognitive deficits and neural damage in a variety of animal models of neurodegenerative diseases [10,42,43,44]. A novel finding of this study is the significant reduction in the expression, translation, and nuclear activity of PPARγ in the hippocampi of CdCl2-treated rats. The findings of this investigation also demonstrated that activation of PPARγ with Pioglitazone not only attenuated hippocampal damage by reducing oxidative stress and inflammation but also improved spatial and recognition memory. This effect was accompanied by restored levels of BDNF and Ach, as well as enhanced synaptic plasticity and mitochondrial function through increased Nrf2 activation and reduced NF-κB levels. These findings suggest that PPARγ activation exerts antioxidant, anti-inflammatory, and anti-apoptotic effects in the brain by modulating key transcription factors involved in these processes.
This study is the first to demonstrate that CdCl2-induced toxicity is associated with downregulating the transcription and nuclear activity of PPARγ’s in the hippocampus, consistent with previous reports showing depletion of PPARγ in the cortices and hippocampi of various animal models of neurodegeneration, including ischemia, AD, PD, diabetes mellitus, and ALS [7,53,54,55,56,57]. Furthermore, treatment with Rosiglitazone has been shown to improve memory function, synaptic plasticity, and long-term potentiation (LTP) in aged rats and prevent hippocampal damage in AD models [55,56,57,58,59,60]. Similarly, Pioglitazone has been demonstrated to attenuate cognitive deficits and reduce oxidative stress and inflammation in AD, dementia, and diabetes mellitus models, as well as in models of LPS-induced neuroinflammation [28,54,55,58,59]. Clinical studies have confirmed the efficacy of Pioglitazone in improving memory and cognitive function in patients with AD and multiple sclerosis [60,61,62].
Additionally, PPARγ neuroprotection is linked to its regulation of glucose receptors, upregulation of BDNF, enhancement of mitochondrial biogenesis, and activation of antioxidant and anti-apoptotic pathways such as Nrf2 and Wnt/β-catenin [7,63,64,65,66,67,68]. Building upon prior work and others’ findings, this study further elucidates the role of PPARγ in mediating QUR’s neuroprotective effects in CdCl2-intoxicated rats. The ameliorative effects of QUR on hippocampal damage, inflammatory markers, Nrf2/antioxidant axis, BDNF levels, apoptosis, and cholinergic markers were found to be predominantly mediated by PPARγ activation. These findings were corroborated by the blockade of PPARγ activation using GW9662, which eliminated the neuroprotective benefits of QUR.
However, the mechanism by which QUR activates PPARγ remains unexplored and may require further investigation. Previously, our team has shown that treatment with QUR attenuates CdCl2-mediated hippocampal damage in rats by activating SIRT1 [17]. The relationship between SIRT1 and PPARγ is complex and somewhat paradoxical. On one hand, SIRT1 can directly inhibit the transcription and activation of PPARγ [69]. On the other hand, SIRT1 can deacetylate PGC1α, which subsequently enhances the transcriptional activity of PPARγ [69]. Moreover, PPARγ itself can bind directly to the SIRT1 promoter and inhibit its transcription [70]. Concurrently, AMPK serves as a potent activator of SIRT1 [71,72]. In both clinical and experimental contexts, PPARγ agonists like Troglitazone and Pioglitazone have been shown to phosphorylate and activate AMPK independently of PPARγ activity modulation [72]. These agonists can improve insulin signaling, regulate glucose and lipid metabolism, and offer protection against Type 2 diabetes mellitus (T2DM) by stimulating AMPK activity in adipose tissue, liver, and muscle, irrespective of PPARγ activation [72,73,74]. Given these observations, it remains unclear whether SIRT1 or PPARγ is the primary upstream target of QUR. It is plausible that QUR activates SIRT1 in the hippocampus through the PPARγ/AMPK axis, or alternatively, QUR may stimulate PPARγ via the SIRT1/PGC1α pathway. Consequently, further studies are essential to clarify these interactions and to determine the primary neural regulator through which QUR exerts its effects.
4. Materials and Methods
4.1. Animals
Adult male Wistar rats (220 ± 20 g, 7–8 weeks of age) were collected from the animal center at King Saud University, KSA. The animals were housed in a separate room at 22 ± 2 °C. The light was always controlled on a 12 h-cycle. Throughout the experiments, all rats were supplied with a sterile standard diet and tap water with 24-h free access. Drug administration, anesthesia, euthanasia, and blood and tissue collections were performed following the international principles of laboratory animal care published by NIH (number 82-23, 1985) after being approved by the institutional ethical committee.
4.2. Experimental Design
In this study, a cohort of 60 male rats was randomly allocated into five distinct experimental groups, each consisting of 12 animals. The groups were categorized as follows: (1) Control Group: This group served as the baseline and received a daily oral administration of the vehicle (0.5% carboxymethylcellulose) without any active treatment. (2) CdCl2-Treated Group: Rats in this group were subjected to chronic oral administration of cadmium chloride (CdCl2) at a dose of 0.5 mg/kg per day to induce neurotoxic effects. (3) CdCl2 + Pioglitazone Group: In addition to receiving CdCl2 (0.5 mg/kg), this group was administered Pioglitazone, a selective peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, at a dose of 10 mg/kg per day, to assess its potential neuroprotective properties. (4) CdCl2 + Quercetin (QUR)-Treated Group: This group received both CdCl2 (0.5 mg/kg) and quercetin (QUR) at a dose of 25 mg/kg per day to evaluate the protective effects of QUR against CdCl2-induced neurotoxicity. (5) CdCl2 + QUR + GW9662 Group: Rats in this group were treated with CdCl2 (0.5 mg/kg) and QUR (25 mg/kg), and also received GW9662, a PPAR-γ antagonist, administered intraperitoneally at a dose of 1 mg/kg per day, to determine the role of PPAR-γ inhibition in modulating the effects of QUR.
For all oral treatments (QUR, Pioglitazone, and CdCl2), an intragastric tube was used for precise dosing. GW9662 was given 1 h before treatment with QUR, which was also given 2 h before treatment with Cd. All treatments, including QUR, Pioglitazone, and GW9662, were administered for 30 days on a daily basis according to the above sequence. The treatments were administered for a predetermined duration, with all groups being monitored for physiological and behavioral changes.
4.3. Dose Selection and Protocol Regimen
As per the results from our labs and the results of other reports, treatment with CdCl2 solution (0.5 mg/kg) for 30 consecutive days impairs memory and learning function and induces hippocampal degeneration in rats [17,27,33]. Oral treatment with QUR at a dose of 25 mg/kg was fully safe in control rodents and attenuated the functional and structural neurological changes in these CdCl2-treated rats [17]. The administration of Pioglitazone (10 mg/kg) via repeated oral dosing was employed to enhance the activation of PPARγ across the brain and other tissues. This regimen has demonstrated protective effects on renal, hepatic, ocular, and neurological systems in various experimental models [75,76,77,78,79,80]. The repeated in vivo intraperitoneal administration of GW9662 at 1 mg/kg has been used in several studies to completely inhibit PPARγ in the brains, kidneys, and retinas of rodents [76,77,79,81]. We have also confirmed this in preliminary data where the activity of PPARγ was almost inhibited by 90% in the brain tissue using this protocol of administration (i.e., at least 2 h before treatment with QUR).
4.4. Assessment of Neurological Score
The assessment of the neurological score was performed as described by others [82]. This test assesses the ability of each rat to perform 10 tasks that reflect its awareness, alertness, balance, motor function, and general behavior. A score of 0 was given if the rat performed the task, and a score of 1 was given if it failed to perform the task. Therefore, the maximum score was 10, and a higher score indicates a worse neurological assessment. These tasks are well described in [82] and include exiting a circle 30 cm in diameter, mono-/hemiparesis of the legs, walking in a straight line, exhibiting a startled reflex to a loud hand clap, showing seeking behavior in the environment, balancing on a beam 7 mm in width for 10 s, balancing a round stick for 10 s, and 3 other independent tasks involving crossing 3 different beams (30 cm × 3 mm, 30 cm × 2 cm, and 30 cm × 1 cm). The test was conducted over the last three days of the experiment, and average readings were taken for each rat.
4.5. Assessment of Memory Function
Spatial and short-term memory in rats were evaluated using the Morris Water Maze (MWM) and the Passive Avoidance Learning Test (PALT), respectively, while the ability to recognize objects was assessed through the Novel Object Recognition Test (NORT). These tests are well described in our laboratories and have been used to test memory function by other researchers [82,83].
4.6. The Morris Water Maze (MWM)
The MWM was performed every morning in a circular swimming pool (1 m height × 60 cm depth), which was hypothetically divided into 4 quadrants and camouflaged with milk; a hidden rescue platform was embedded in one of the quadrants (1.8 cm below the water level). During the test, the rat is released from one quadrant and swims to find the hidden platform within 100 s. If the rat finds it, it is allowed to stay there for 15 s before returning it to its cage. If the rat does not find it, it is directed towards it. This process was repeated over 4 days (days 26–29), each comprising 3 trials. On day 30, a probe trial was performed in which the rats were given an opportunity to retake the test without the platform. The number of crossings over the platform’s original location was recorded for each rat.
4.7. Passive Learning Avoidance Test (PALT)
At midday on day 29, the PALT was conducted in a wooden box (50 × 50 × 35 cm) with an electrical mesh floor; the box was comprised of two rooms, one illuminated and the other dark, separated by a door. Initially, each rat underwent an exploration of 3 trials, each of 5 min (separated by 10 min). In the course of these trials, the rat was initially placed in the lit section with the door open and allowed to freely enter the dark compartment to explore. Following this, a fourth trial was conducted in which a similar procedure was used. However, in this trial, once the rat entered the dark area, the door was closed, and the rat was subjected to a 2-s electrical foot shock at 50 Hz. Afterward, the rat was returned to its cage, and the test was repeated after 3 h. Rats with functional short-term memory typically avoid entering the dark area. The apparatus was cleaned between each trial.
4.8. The Novel Object Recognition Test (NORT)
The NORT was also conducted on day 30 in a wooden box with an open roof (50 × 50 × 50 cm). The test comprises two trials; in the first trial, each rat was placed in the box and allowed to freely explore two identical objects (red plastic cubes) for 15 min. Two hours later, the same rat was reintroduced into the wooden box, where it was given the opportunity to explore two objects for a duration of 5 min—one familiar (a red plastic cup) and the other novel (a glass cube). The time each rat spent exploring the novel object was recorded. Rats with intact recognition memory spent more time exploring the new object in the second trial.
4.9. Collection of Brains and Hippocampi
All rats from the experimental groups were euthanized using ketamine (80 mg/kg body weight) and decapitated. The skulls were opened, and the brains were carefully extracted while kept on ice. The brains of the first four rats were promptly preserved in 10% buffered formalin and sent to the pathology lab for routine histological analysis. The hippocampi of the remaining eight rats were quickly harvested by a trained anatomist using a dissecting microscope (INFITEK INC., Jinan, Shandong, China) on ice, snap-frozen in liquid nitrogen, and stored at −80 °C for further analysis.
4.10. Enzyme-Linked Immunosorbent Assays (ELISA)
Parts of the collected hippocampi (40 mg) of each rat were homogenized using a Q-Sonica homogenizer (Newtown, CT, USA) for 20 s in ice at 30 Hz in a neural cell lysis buffer (cat # 87792, Thermo Scientific, Waltham, MA, USA). Other fractions of the hippocampi were used for the extraction of the nuclear and cytoplasmic proteins (NE-Per) (cat # 78833, Thermo Scientific, Waltham, MA, USA). All samples were centrifuged for 15 min at 11,600× g to collect the supernatants. Protein concentrations in the samples were determined using a Pierce BCA assay kit (Cat # 23225, Thermo Scientific, Waltham, MA, USA). The supernatants of the total hippocampal tissue extract were subjected to ELISA to measure the levels of tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), total glutathione (GSH), acetylcholine (Ach), acetylcholine esterase (AchE), acetylcholine acetyltransferase (AchT) superoxide dismutase (SOD), Heme oxygenase-1 (HO-1), brain-derived neurotrophic factor (BDNF), Bax, Bcl2, and caspapse-3. The ELISA also measured the levels of Nrf2, PPARα, and NF-κB in the nuclear extracts. All kits were specific to rats and were purchased from MyBioSource (San Diego, CA, USA). All samples were analyzed in duplicate for n = 8 rats/group using an ELx800 microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
4.11. Real-Time PCR (qPCR)
For the amplification of PPARγ (NM_013124.3), the primers used were (F: 5′-CATGACCAGGGAGTTCCTCAA-3′; R: 5′-AGCAAACTCAAACTTAGGCTCCAT-3′, producing a 73 bp fragment), and for GAPDH (NM_017008.3), the primers were (F: 5′-CCATCAACGACCCCTTCATT-3′; R: 5′-CACGACATACTCAGCACCAGC-3′, producing a 193 kD product). Briefly, total RNA was extracted from 30 mg of frozen hippocampal tissue using the TRIZOL reagent. The first cDNA strand was synthesized using a commercially available kit (Catalog # ab 286905). Primers for Nrf2, NF-κB, and β-actin (the reference gene) were sourced from ThermoFisher. Real-time PCR amplification was performed using a CFX96 real-time PCR machine (BioRad, Hercules, CA, USA) with the Ssofast Evergreen Supermix kit, following the manufacturer’s guidelines. Expression levels of Nrf2 and NF-κB were normalized to β-actin mRNA levels, and data were analyzed using the 2ΔΔCT method. Six samples were analyzed per group.
4.12. Determination of PPARα Nuclear Activity
The binding activity of PPARγ with DNA was measured as previously described by others, using a rat-specific non-radioactive colorimetric transcription activity kit (Cat. # 10006855 Cayman, Ann Arbor, MI, USA). The principle of the test is based on the primary antibody detection of PPARγ in the nuclear extract that binds to the specific double-stranded DNA that is immobilized in the wells. The detection is achieved by adding an HRP-conjugated secondary antibody and reading the signal at 450 nm. The protocol was conducted for 8 samples for each group, following the manufacturer’s instructions.
4.13. Histological Assessment
The rat brains were immersed in 10% buffered formalin for 20 h to fix them, followed by deparaffinization using pure xylene. Tissue rehydration was performed by immersing the brain tissues in descending alcohol concentrations (100–70%). Small sections of 4–5 μm were obtained using the rotatory microtome; these sections were then placed on glass slides and routinely stained with Harris hematoxylin and Eosin. Mounting media and coverslips were added, and all slides were examined and photographed using a light microscope (Leica DM6, Leia microsystem, Wetzlar, Germany).
4.14. Statistical Analysis
The data for all parameters were gathered and processed using GraphPad Prism software (version 8, GraphPad Software Inc., La Jolla, CA, USA). Statistical significance was assessed via one-way ANOVA, with Tukey’s test applied as a post hoc analysis (p < 0.05). All results are presented as the mean ± standard deviation (SD).
5. Conclusions
In summary, this study provides compelling evidence that CdCl2-induced hippocampal damage and cognitive impairments are closely associated with the downregulation of PPARγ, a critical regulator of oxidative stress, inflammation, and apoptosis in the brain. Activation of PPARγ by quercetin (QUR) effectively attenuates these neurotoxic effects, restoring normal hippocampal function and memory performance. The neuroprotective effects of QUR were shown to be primarily mediated through the upregulation and nuclear activation of PPARγ, highlighting the pivotal role of this receptor in mitigating oxidative damage and inflammation in the brain. Furthermore, these findings suggest that QUR’s ability to activate PPARγ represents a novel and crucial upstream mechanism underlying its neuroprotective actions. These results not only contribute to our understanding of the molecular mechanisms driving CdCl2-induced neurotoxicity but also suggest that PPARγ modulation may represent a promising therapeutic target for mitigating neurodegenerative processes associated with heavy metal toxicity. Given the complexity of the interactions between PPARγ, SIRT1, and AMPK, further investigations are necessary to elucidate the precise pathways through which QUR activates PPARγ and to determine its potential therapeutic applications in neurodegenerative diseases.
Study Limitations
While this study provides valuable insights into the neuroprotective role of PPARγ activation by quercetin (QUR) in alleviating CdCl2-induced hippocampal damage, several limitations should be considered. First, this study was conducted in an animal model, and further research is needed to validate the findings in human subjects or more complex in vitro models, which could provide a better understanding of the translational potential of these results. Second, while we demonstrated that PPARγ activation mediates the neuroprotective effects of QUR, the exact molecular interactions between PPARγ, SIRT1, and AMPK, as well as their contribution to the observed effects, remain to be fully elucidated. Future studies should focus on identifying the specific signaling pathways involved in QUR’s modulation of PPARγ, SIRT1, and AMPK and whether these pathways could be targeted for therapeutic interventions. Additionally, long-term studies examining the chronic effects of QUR on neurodegeneration and cognitive function are needed to determine its sustained efficacy and potential therapeutic relevance in treating neurodegenerative diseases. Lastly, research exploring the potential synergistic effects of QUR in combination with other PPARγ modulators or neuroprotective agents may open new avenues for developing more effective treatment strategies for metal-induced neurotoxicity and other neurodegenerative conditions.
Funding
This research was funded by the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia (grant number: RSPD2025R805).
Institutional Review Board Statement
All the experiments and protocols used in this study were approved by the Research Ethics Committee (REC) at King Saud University (No: KSU-SE-20-41; Approval date: 05 Oct 2020), Riyadh, Saudi Arabia.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The author extends thanks to the Researchers Supporting Project number (RSPD2025R805), King Saud University, Riyadh, Saudi Arabia. Also, the author extends thanks to Ghedeir Alshammari’s research team for their contribution and assistance during the stages of this experiment, as well as thanks to the technicians working in the Animals Unit at King Saud University.
Conflicts of Interest
The author declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| QUR | Quercetin |
| CdCl2 | Cadmium chloride |
References
- Lamptey, R.N.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Gülöksüz, S. Shedding light on the etiology of neurodegenerative diseases and dementia: The exposome paradigm. npj Ment. Health Res. 2022, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.-A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar]
- Ji, Y.; Wang, H.; Zhao, X.; Wang, Q.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.; Meng, X.; Xu, D. Crosstalk between endoplasmic reticulum stress and mitochondrial pathway mediates cadmium-induced germ cell apoptosis in testes. Toxicol. Sci. 2011, 124, 446–459. [Google Scholar] [CrossRef]
- Wang, B.; Du, Y. Cadmium and its neurotoxic effects. Oxidative Med. Cell. Longev. 2013, 2013, 898034. [Google Scholar] [CrossRef]
- Kumar, B.P.; Kumar, A.P.; Jose, J.A.; Prabitha, P.; Yuvaraj, S.; Chipurupalli, S.; Jeyarani, V.; Manisha, C.; Banerjee, S.; Jeyabalan, J.B. Minutes of PPAR-γ agonism and neuroprotection. Neurochem. Int. 2020, 140, 104814. [Google Scholar] [CrossRef]
- Risner, M.; Saunders, A.; Altman, J.; Ormandy, G.; Craft, S.; Foley, I.; Zvartau-Hind, M.; Hosford, D.; Roses, A. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenom. J. 2006, 6, 246–254. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological activity of quercetin: An updated review. Evid. Based Complement. Altern. Med. 2022, 2022, 3997190. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a therapeutic product: Evaluation of its pharmacological action and clinical applications—A review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Nunes, M.A.G.; Rubin, M.A.; da Cruz, I.B. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+, K+-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Unsal, C.; Kanter, M.; Aktas, C.; Erboga, M. Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol. Ind. Health 2015, 31, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Unsal, C.; Aktas, C.; Erboga, M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol. Ind. Health 2016, 32, 541–550. [Google Scholar] [CrossRef]
- Shati, A.A. Concomitant Administration of Quercetin and α-tocopherol Protects Rats from Cadmium Chloride Induced Neural Apoptosis and Cognitive Dysfunction. J. Eng. Appl. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Gupta, R.; Shukla, R.K.; Chandravanshi, L.P.; Srivastava, P.; Dhuriya, Y.K.; Shanker, J.; Singh, M.P.; Pant, A.B.; Khanna, V.K. Protective role of quercetin in cadmium-induced cholinergic dysfunctions in rat brain by modulating mitochondrial integrity and MAP kinase signaling. Mol. Neurobiol. 2017, 54, 4560–4583. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Cheng, X.; Qiao, X. The alleviative effects of quercetin on cadmium-induced necroptosis via inhibition ROS/iNOS/NF-κB pathway in the chicken brain. Biol. Trace Elem. Res. 2021, 199, 1584–1594. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Al-Qahtani, W.H.; Alshuniaber, M.A.; Yagoub, A.E.A.; Al-Khalifah, A.S.; Al-Harbi, L.N.; Alhussain, M.H.; AlSedairy, S.A.; Yahya, M.A. Quercetin improves the impairment in memory function and attenuates hippocampal damage in cadmium chloride-intoxicated male rats by suppressing acetylcholinesterase and concomitant activation of SIRT1 signaling. J. Funct. Foods 2021, 86, 104675. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Liu, S.; Pyles, J.M.; Lapi, S.E.; Saleem, K.; Antony, V.B.; Gonzalez, M.L.; Crossman, D.K.; Carter, A.B. Impaired PPARγ activation by cadmium exacerbates infection-induced lung injury. JCI Insight 2023, 8, e166608. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, J.; Zhang, Y.; Wang, X.; Li, Y.; Song, J.; Shao, J.; Su, P. Paternal cadmium exposure induces glucolipid metabolic reprogramming in offspring mice via PPAR signaling pathway. Chemosphere 2023, 339, 139592. [Google Scholar] [CrossRef]
- Mori, C.; Lee, J.-Y.; Tokumoto, M.; Satoh, M. Cadmium toxicity is regulated by peroxisome proliferator-activated receptor δ in human proximal tubular cells. Int. J. Mol. Sci. 2022, 23, 8652. [Google Scholar] [CrossRef]
- Reiterer, G.; Toborek, M.; Hennig, B. Quercetin protects against linoleic acid-induced porcine endothelial cell dysfunction. J. Nutr. 2004, 134, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Moon, J.; Cho, Y.; Chung, J.H.; Shin, M.-J. Quercetin up-regulates expressions of peroxisome proliferator-activated receptor γ, liver X receptor α, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr. Res. 2013, 33, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Beekmann, K.; Rubió, L.; de Haan, L.H.; Actis-Goretta, L.; van der Burg, B.; van Bladeren, P.J.; Rietjens, I.M. The effect of quercetin and kaempferol aglycones and glucuronides on peroxisome proliferator-activated receptor-gamma (PPAR-γ). Food Funct. 2015, 6, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Yeh, C.-L.; Yeh, S.-L.; Lin, E.-S.; Wang, L.-Y.; Wang, Y.-H. Quercetin metabolites inhibit MMP-2 expression in A549 lung cancer cells by PPAR-γ associated mechanisms. J. Nutr. Biochem. 2016, 33, 45–53. [Google Scholar] [CrossRef]
- Castrejón-Tellez, V.; Rodríguez-Pérez, J.M.; Pérez-Torres, I.; Pérez-Hernández, N.; Cruz-Lagunas, A.; Guarner-Lans, V.; Vargas-Alarcón, G.; Rubio-Ruiz, M.E. The effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int. J. Mol. Sci. 2016, 17, 1069. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef]
- Ballav, S.; Ranjan, A.; Basu, S. Partial Activation of PPAR-γ by Synthesized Quercetin Derivatives Modulates TGF-β1-Induced EMT in Lung Cancer Cells. Adv. Biol. 2023, 7, 2300037. [Google Scholar] [CrossRef]
- Alsaud, M.M.; Alhowail, A.H.; Aldubayan, M.A.; Almami, I.S. The ameliorative effect of pioglitazone against neuroinflammation caused by doxorubicin in rats. Molecules 2023, 28, 4775. [Google Scholar] [CrossRef]
- Branca, J.J.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X. Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef]
- Mimouna, S.B.; Chemek, M.; Boughammoura, S.; Banni, M.; Messaoudi, I. Early-life exposure to cadmium triggers distinct Zn-dependent protein expression patterns and impairs brain development. Biol. Trace Elem. Res. 2018, 184, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Saleem, S.; Naqvi, F.; Hasan, K.A.; Naqvi, F.; Haider, S. Thymol mitigates cadmium-induced behavioral and cognitive deficits by up-regulating hippocampal BDNF levels in rats. Pak. J. Pharm. Sci. 2022, 35. [Google Scholar]
- El-Kott, A.F.; Bin-Meferij, M.M.; Eleawa, S.M.; Alshehri, M.M. Kaempferol protects against cadmium chloride-induced memory loss and hippocampal apoptosis by increased intracellular glutathione stores and activation of PTEN/AMPK induced inhibition of Akt/mTOR signaling. Neurochem. Res. 2020, 45, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol inhibits tau phosphorylation in the brains of control and cadmium chloride-treated rats by activating PP2A and PI3K/Akt induced-inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373. [Google Scholar] [CrossRef]
- Shagirtha, K.; Muthumani, M.; Prabu, S.M. Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1039–1050. [Google Scholar]
- Kim, S.; Cheon, H.-S.; Kim, S.-Y.; Juhnn, Y.-S.; Kim, Y.-Y. Cadmium induces neuronal cell death through reactive oxygen species activated by GADD153. BMC Cell Biol. 2013, 14, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wang, Y.; Wang, C. Effect of cadmium choride on oxidative damage and expression of NF-κB in brain tissue of mice. Wei Sheng Yan Jiu J. Hyg. Res. 2017, 46, 807–812. [Google Scholar]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef]
- Tang, K.-K.; Liu, X.-Y.; Wang, Z.-Y.; Qu, K.-C.; Fan, R.-F. Trehalose alleviates cadmium-induced brain damage by ameliorating oxidative stress, autophagy inhibition, and apoptosis. Metallomics 2019, 11, 2043–2051. [Google Scholar] [CrossRef]
- Shati, A.A.; El-Kott, A.F. Resolvin D1 protects against cadmium chloride-induced memory loss and hippocampal damage in rats: A comparison with docosahexaenoic acid. Hum. Exp. Toxicol. 2021, 40, S215–S232. [Google Scholar] [CrossRef]
- Arab, H.H.; Eid, A.H.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.; Darwish, H.W.; Georgy, G.S. Neuroprotective impact of linagliptin against cadmium-induced cognitive impairment and neuropathological aberrations: Targeting SIRT1/Nrf2 axis, apoptosis, and autophagy. Pharmaceuticals 2023, 16, 1065. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C.; Duchen, M.R. PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem. Res. 2015, 40, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Nicolakakis, N.; Hamel, E. The nuclear receptor PPARγ as a therapeutic target for cerebrovascular and brain dysfunction in Alzheimer’s disease. Front. Aging Neurosci. 2010, 2, 1389. [Google Scholar] [CrossRef]
- D’Angelo, M.; Castelli, V.; Catanesi, M.; Antonosante, A.; Dominguez-Benot, R.; Ippoliti, R.; Benedetti, E.; Cimini, A. PPARγ and cognitive performance. Int. J. Mol. Sci. 2019, 20, 5068. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Chen, C.-M.; Lee, M.-R.; Chen, H.-W.; Chen, H.-M.; Wu, Y.-S.; Hung, C.-H.; Kang, J.-J.; Chang, C.-P.; Chang, C. Modulation of energy deficiency in Huntington’s disease via activation of the peroxisome proliferator-activated receptor gamma. Hum. Mol. Genet. 2010, 19, 4043–4058. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Tsai, T.-Y.; Wang, C.-J. The potential benefits of quercetin for brain health: A review of anti-inflammatory and neuroprotective mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Nassiri-Asl, M. Quercetin in food: Possible mechanisms of its effect on memory. J. Food Sci. 2018, 83, 2280–2287. [Google Scholar] [CrossRef]
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.-Y.; He, F.; Xu, J.; Wang, H.-Q. Quercetin protects against okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS ONE 2016, 11, e0152371. [Google Scholar] [CrossRef]
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jembrek, M.J. PI3K/Akt and ERK1/2 Signalling Are Involved in Quercetin-Mediated Neuroprotection against Copper-Induced Injury. Oxidative Med. Cell. Longev. 2020, 2020, 9834742. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Sasi, A.K.; Taheri, M.; Ayatollahi, S.A. The impact of the phytotherapeutic agent quercetin on expression of genes and activity of signaling pathways. Biomed. Pharmacother. 2021, 141, 111847. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.; Florio, T.M.; Giacomo, E.D.; Benedetti, E.; Cristiano, L.; Antonosante, A.; Fidoamore, A.; Massimi, M.; Alecci, M.; Ippoliti, R. PPARβ/δ and γ in a rat model of Parkinson’s disease: Possible involvement in PD symptoms. J. Cell. Biochem. 2015, 116, 844–855. [Google Scholar] [CrossRef]
- Gao, F.; Zang, L.; Wu, D.; Li, Y.; Zhang, Q.; Wang, H.; Tian, G.; Mu, Y. Pioglitazone improves the ability of learning and memory via activating ERK1/2 signaling pathway in the hippocampus of T2DM rats. Neurosci. Lett. 2017, 651, 165–170. [Google Scholar] [CrossRef]
- Beheshti, F.; Hosseini, M.; Hashemzehi, M.; Soukhtanloo, M.; Khazaei, M.; Shafei, M.N. The effects of PPAR-γ agonist pioglitazone on hippocampal cytokines, brain-derived neurotrophic factor, memory impairment, and oxidative stress status in lipopolysaccharide-treated rats. Iran. J. Basic Med. Sci. 2019, 22, 940. [Google Scholar]
- Huang, R.; Zhang, C.; Wang, X.; Hu, H. PPARγ in ischemia-reperfusion injury: Overview of the biology and therapy. Front. Pharmacol. 2021, 12, 600618. [Google Scholar] [CrossRef]
- Alhowail, A.; Alsikhan, R.; Alsaud, M.; Aldubayan, M.; Rabbani, S.I. Protective effects of pioglitazone on cognitive impairment and the underlying mechanisms: A review of literature. Drug Des. Dev. Ther. 2022, 16, 2919–2931. [Google Scholar] [CrossRef]
- Fernandez-Martos, C.M.; Atkinson, R.A.; Chuah, M.I.; King, A.E.; Vickers, J.C. Combination treatment with leptin and pioglitazone in a mouse model of Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 92–106. [Google Scholar] [CrossRef]
- Seok, H.; Lee, M.; Shin, E.; Yun, M.R.; Lee, Y.-H.; Moon, J.H.; Kim, E.; Lee, P.H.; Lee, B.-W.; Kang, E.S. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci. Rep. 2019, 9, 4414. [Google Scholar] [CrossRef]
- Victor, N.; Wanderi, E.; Gamboa, J.; Zhao, X.; Aronowski, J.; Deininger, K.; Lust, W.; Landreth, G.; Sundararajan, S. Altered PPARγ expression and activation after transient focal ischemia in rats. Eur. J. Neurosci. 2006, 24, 1653–1663. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Sauerbeck, A.; Popovich, P.G.; McTigue, D.M. PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro 2013, 5, AN20130030. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Gamboa, J.; Victor, N.; Wanderi, E.; Lust, W.; Landreth, G. Peroxisome proliferator-activated receptor-γ ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience 2005, 130, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Godoy, J.A.; Quintanilla, R.A.; Koenig, C.S.; Bronfman, M. Peroxisome proliferator-activated receptor γ is expressed in hippocampal neurons and its activation prevents β-amyloid neurodegeneration: Role of Wnt signaling. Exp. Cell Res. 2005, 304, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wu, J.-S.; Tsai, H.-D.; Huang, C.-Y.; Chen, J.-J.; Sun, G.Y.; Lin, T.-N. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol. Neurobiol. 2012, 46, 114–124. [Google Scholar] [CrossRef]
- Zolezzi, J.M.; Silva-Alvarez, C.; Ordenes, D.; Godoy, J.A.; Carvajal, F.J.; Santos, M.J.; Inestrosa, N.C. Peroxisome proliferator-activated receptor (PPAR) γ and PPARα agonists modulate mitochondrial fusion-fission dynamics: Relevance to reactive oxygen species (ROS)-related neurodegenerative disorders? PLoS ONE 2013, 8, e64019. [Google Scholar] [CrossRef]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.-J.; Gao, Y.; Bennett, M.V. Peroxisome proliferator-activated receptor γ (PPARγ): A master gatekeeper in CNS injury and repair. Prog. Neurobiol. 2018, 163, 27–58. [Google Scholar] [CrossRef]
- Wang, L.; Botchway, B.O.; Liu, X. The repression of the HMGB1-TLR4-NF-κB signaling pathway by safflower yellow may improve spinal cord injury. Front. Neurosci. 2021, 15, 803885. [Google Scholar] [CrossRef]
- Duan, C.; Jiao, D.; Wang, H.; Wu, Q.; Men, W.; Yan, H.; Li, C. Activation of the PPARγ prevents ferroptosis-induced neuronal loss in response to intracerebral hemorrhage through synergistic actions with the Nrf2. Front. Pharmacol. 2022, 13, 869300. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; de Oliveira, R.M.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Han, L.; Zhou, R.; Niu, J.; McNutt, M.A.; Wang, P.; Tong, T. SIRT1 is regulated by a PPARγ–SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010, 38, 7458–7471. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Ying, N.; Lai, J.; Feng, L.; Zheng, S.; Jiang, F.; Song, Q.; Chai, H.; Dou, X. Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front. Pharmacol. 2020, 11, 560905. [Google Scholar]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Avilucea, P.R.; Ye, J.-M.; Assifi, M.M.; Kraegen, E.W.; Ruderman, N.B. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem. Biophys. Res. Commun. 2004, 314, 580–585. [Google Scholar] [CrossRef]
- Lan, L.F.; Zheng, L.; Yang, X.; Ji, X.T.; Fan, Y.H.; Zeng, J.S. Peroxisome Proliferator-activated Receptor-γ Agonist Pioglitazone Ameliorates White Matter Lesion and Cognitive Impairment in Hypertensive Rats. CNS Neurosci. Ther. 2015, 21, 410–416. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, Q.; Xu, T.; Yao, L.; Feng, J.; Ma, J.; Wang, L.; Lu, C.; Wang, D. Pioglitazone; a peroxisome proliferator-activated receptor γ agonist, ameliorates chronic kidney disease by enhancing antioxidative capacity and attenuating angiogenesis in the kidney of a 5/6 nephrectomized rat model. Cell. Physiol. Biochem. 2016, 38, 1831–1840. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Dai, W.; Hua, B.; Li, H.; Li, W. Pioglitazone downregulates Twist-1 expression in the kidney and protects renal function of Zucker diabetic fatty rats. Biomed. Pharmacother. 2019, 118, 109346. [Google Scholar] [CrossRef]
- Liu, M.; Bachstetter, A.D.; Cass, W.A.; Lifshitz, J.; Bing, G. Pioglitazone attenuates neuroinflammation and promotes dopaminergic neuronal survival in the nigrostriatal system of rats after diffuse brain injury. J. Neurotrauma 2017, 34, 414–422. [Google Scholar] [CrossRef]
- Sun, M.-H.; Chen, K.-J.; Sun, C.-C.; Tsai, R.-K. Protective Effect of Pioglitazone on Retinal Ganglion Cells in an Experimental Mouse Model of Ischemic Optic Neuropathy. Int. J. Mol. Sci. 2022, 24, 411. [Google Scholar] [CrossRef]
- Vázquez-González, D.; Corona, J.C. Pioglitazone enhances brain mitochondrial biogenesis and phase II detoxification capacity in neonatal rats with 6-OHDA-induced unilateral striatal lesions. Front. Neurosci. 2023, 17, 1186520. [Google Scholar] [CrossRef]
- Baumann, A.; Burger, K.; Brandt, A.; Staltner, R.; Jung, F.; Rajcic, D.; Pisarello, M.J.L.; Bergheim, I. GW9662, a peroxisome proliferator-activated receptor gamma antagonist, attenuates the development of non-alcoholic fatty liver disease. Metabolism 2022, 133, 155233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-S.; Li, H.; Zhang, D.-D.; Yan, H.-Y.; Zhang, Z.-H.; Zhou, C.-H.; Ye, Z.-N.; Chen, Q.; Jiang, T.-W.; Liu, J.-P. Inhibition of myeloid differentiation factor 88 (MyD88) by ST2825 provides neuroprotection after experimental traumatic brain injury in mice. Brain Res. 2016, 1643, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Alfaris, N.; Alshammari, G.; Altamimi, J.; Aljabryn, D.; Alagal, R.; Aldera, H.; Alkhateeb, M.; Yahya, M. Ellagic acid prevents streptozotocin-induced hippocampal damage and memory loss in rats by stimulating Nrf2 and nuclear factor-κB, and activating insulin receptor substrate/PI3K/Akt axis. J. Physiol. Pharmacol. 2021, 72, 503–515. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).