A Comparative Study of N-Acetyl Cysteine, Rosuvastatin, and Vitamin E in the Management of Patients with Non-Alcoholic Steatohepatitis: A Randomized Controlled Trial
Abstract
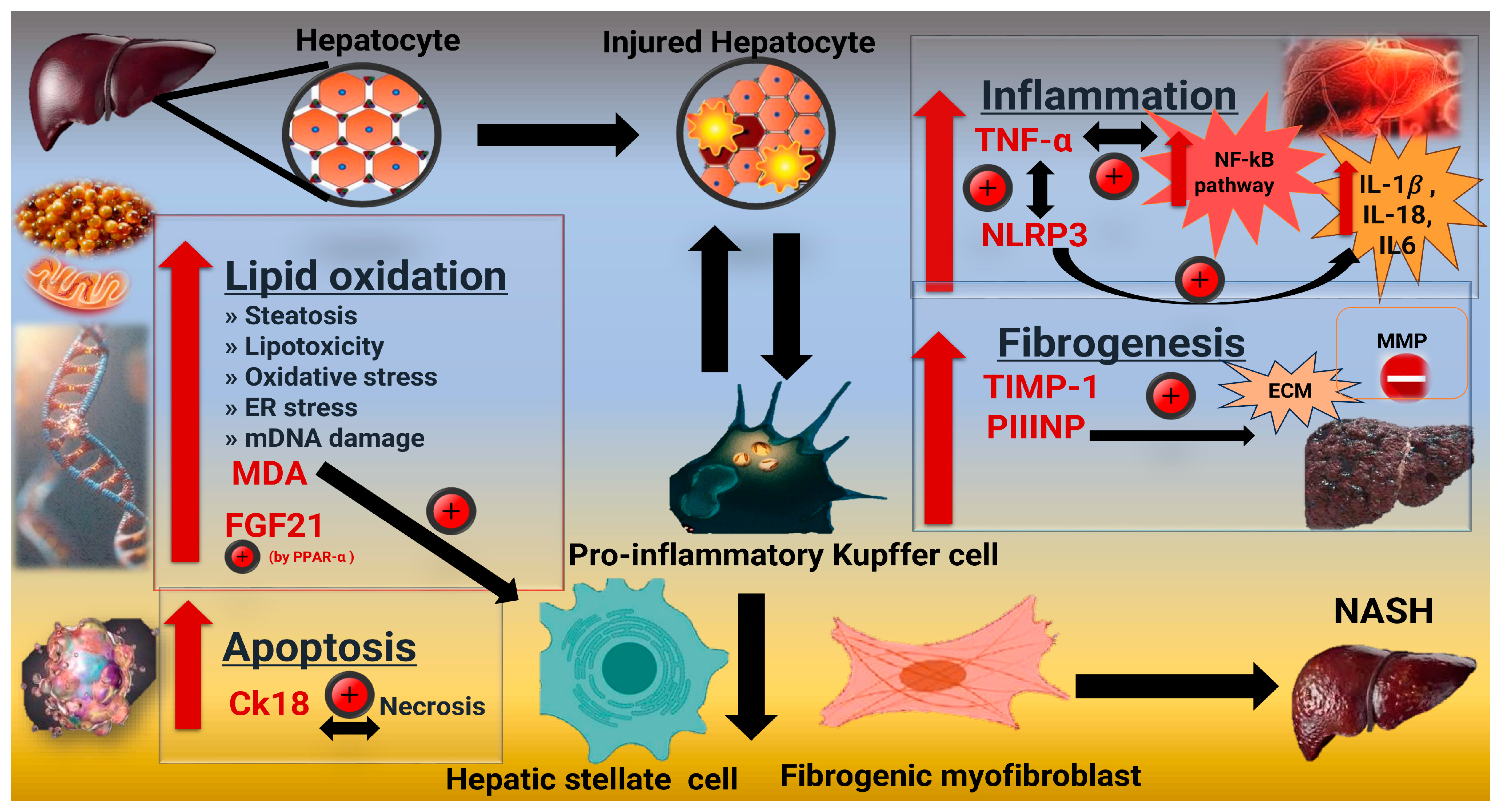
1. Introduction
2. Results
2.1. Socio-Demographic, Anthropometric Measurements Assessments Among the Studied Groups Before and After Treatment
2.2. Steatosis and Fibrosis Degree and Studied Markers Among the Studied Groups Before and After Treatment
2.2.1. Steatosis and Fibrosis
2.2.2. Scoring System
2.3. Laboratory Values of the Studied Groups Before and After Treatment
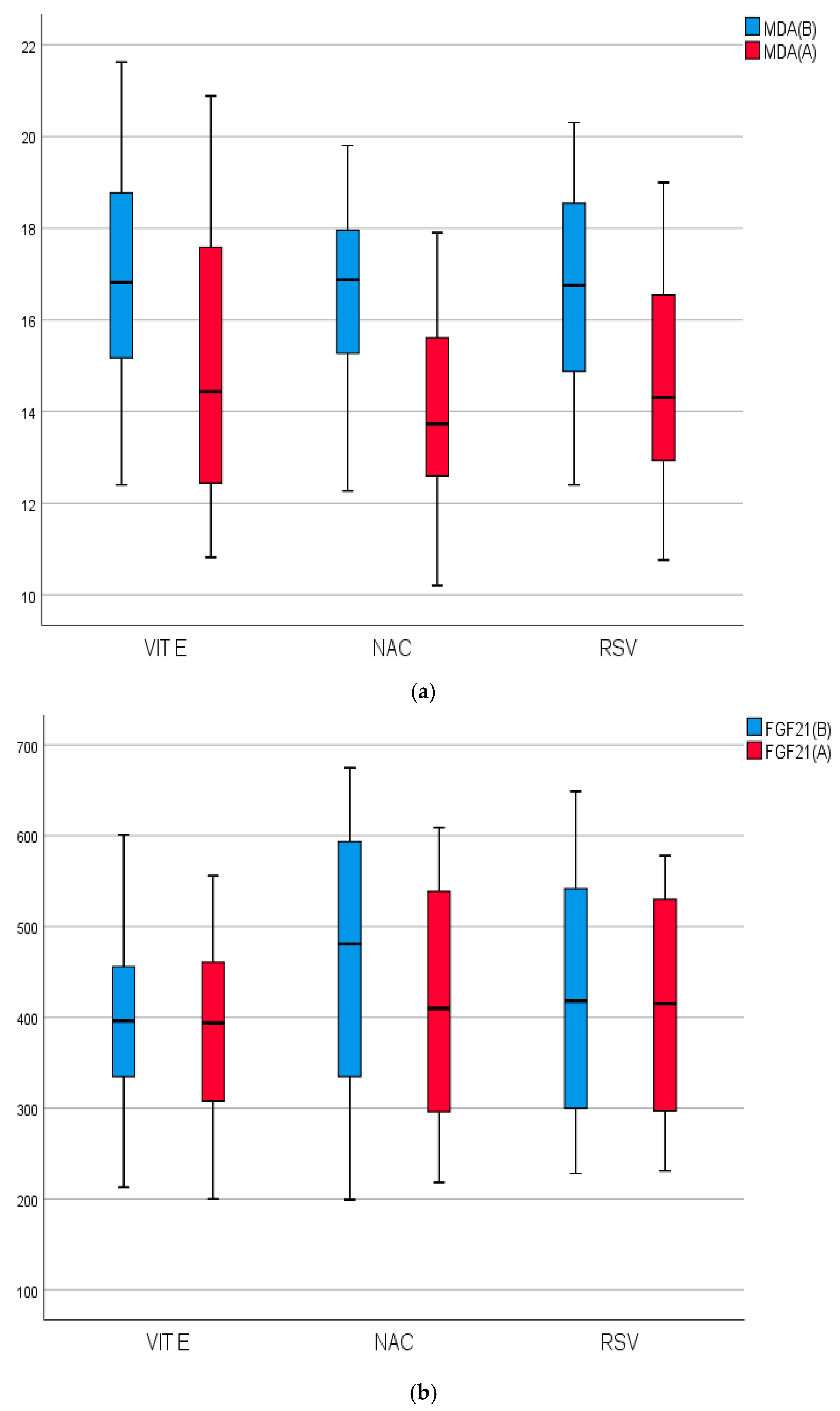
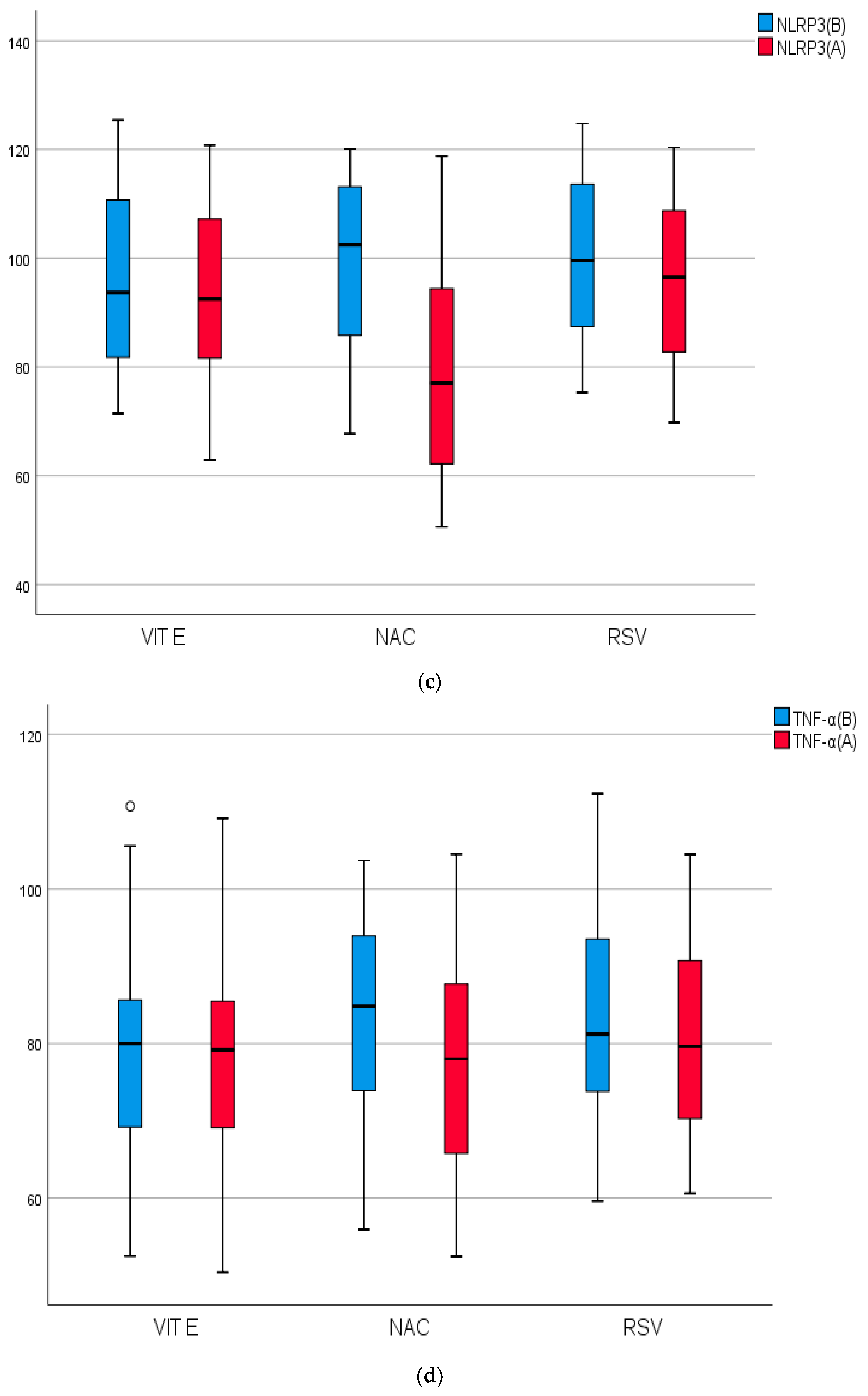
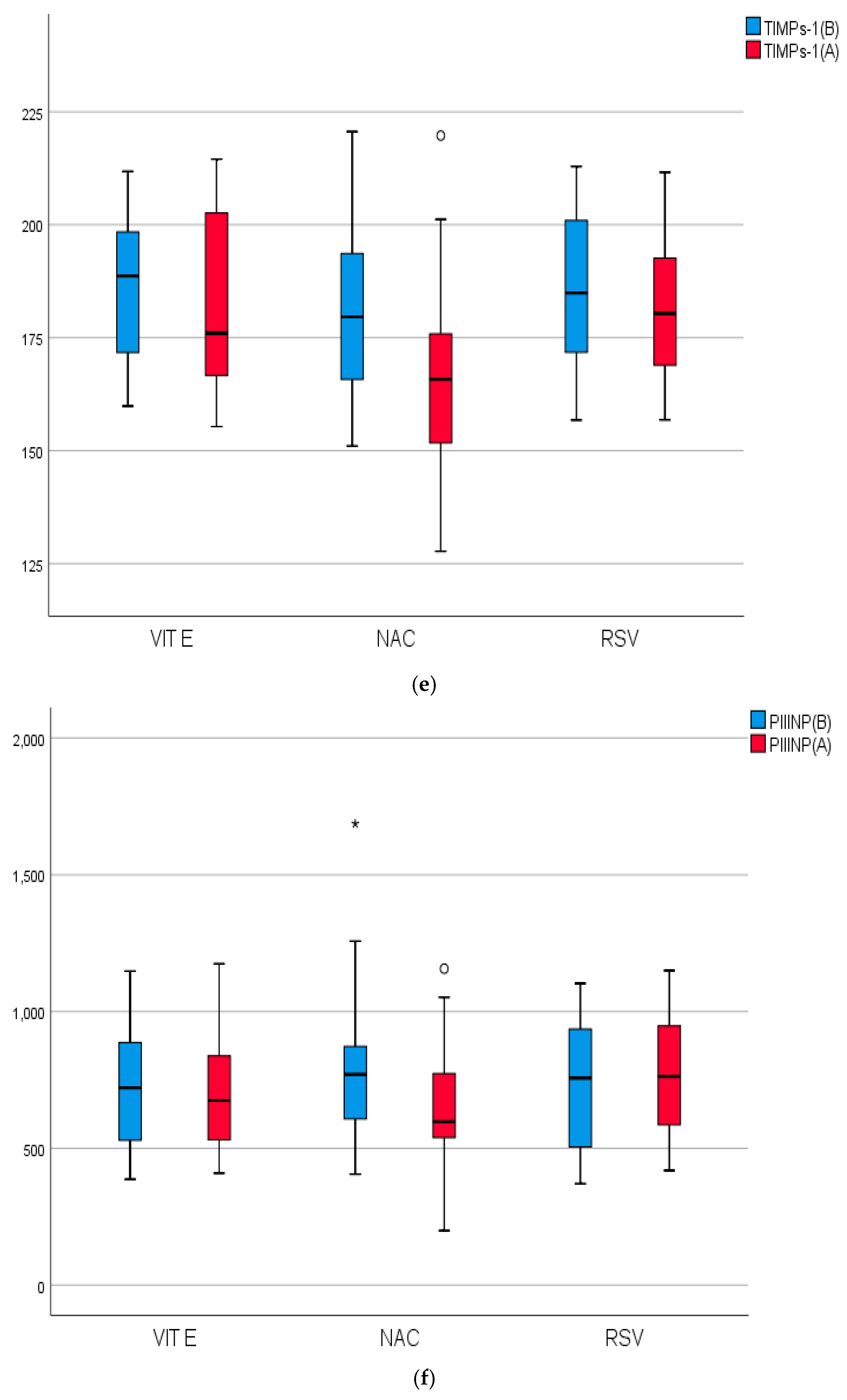
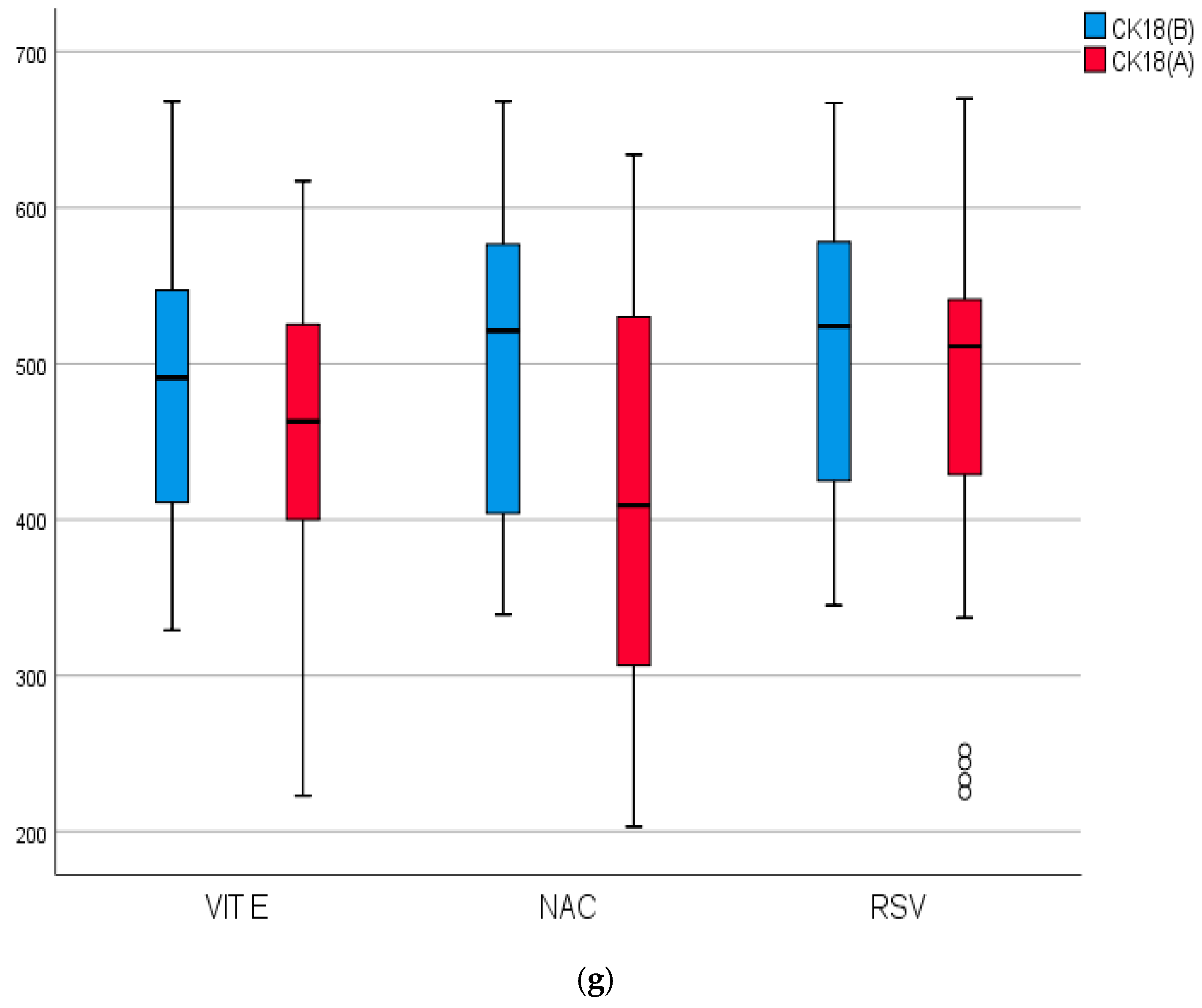
2.4. Biomarkers of Inflammation, Fibrosis, and Apoptosis of the Studied Groups Before and After Treatment
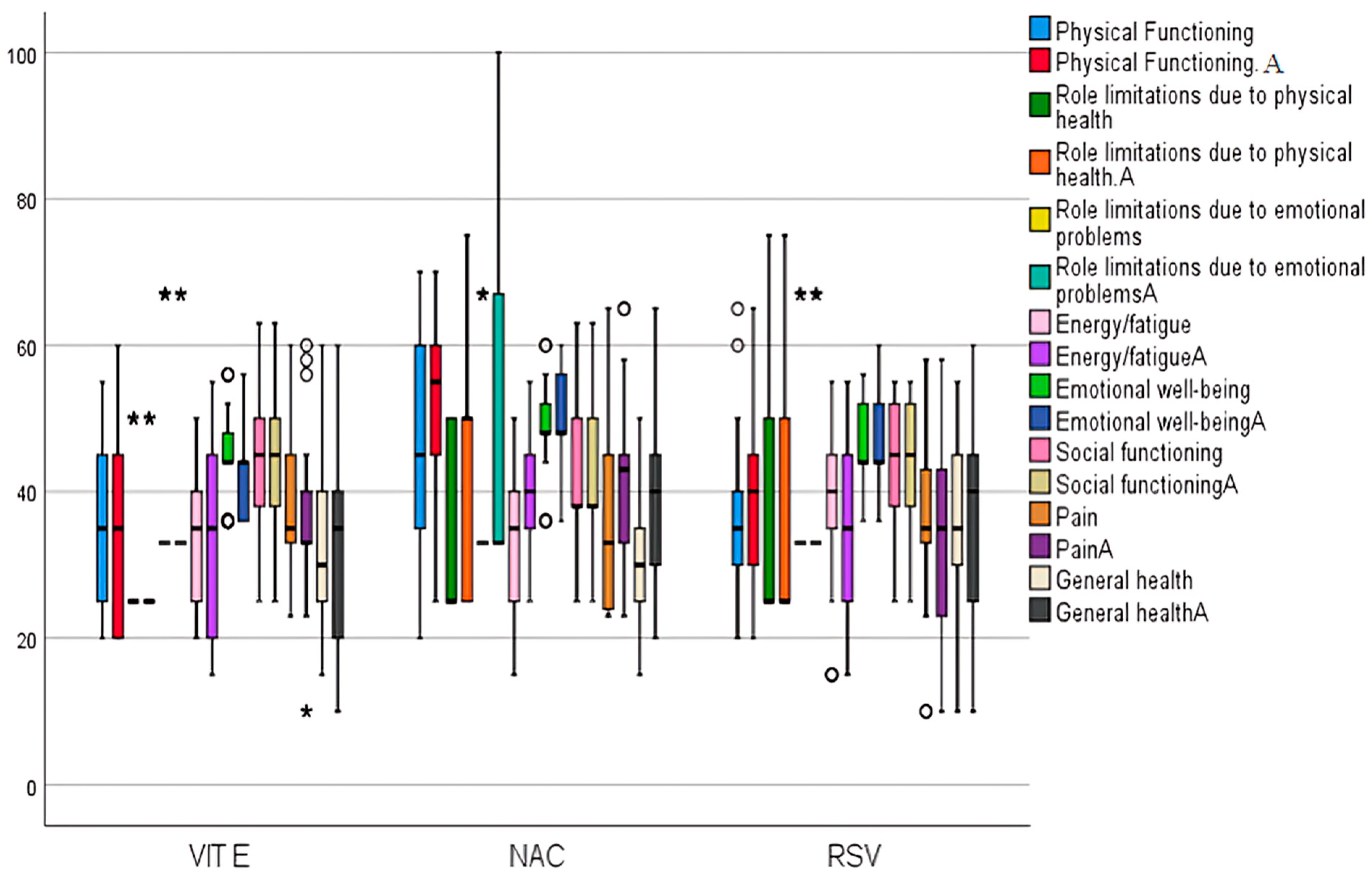
2.5. Assessment of Quality of Life Short-Form 36 (SF-36) Domains and Adverse Events Version 5.00 (CTCAE) Among Studied Groups Before and After Treatment
3. Discussion
The Limitations
4. Materials and Methods
4.1. Patients
4.2. Methods
4.2.1. Study Design
- The control grp, grp 1, NASH patients, perceived regular treatment (VE 400 IU®; PHARCO-pharmaceuticals), twice daily, for 6 months [95];
- In the treated grp, grp 2, patients received a high dose of NAC, Gemacysteine 300 mg®; GEMA-Pharma 1200 mg twice daily, for 6 months;
- In the treated grp, grp 3, patients received RSV; Crestor 20 mg®; AstraZeneca: 20 mg/day orally, for 6 months.
4.2.2. Ethical Approval
4.2.3. Anthropometric Measurements
4.2.4. FibroScan® Examination of the Liver Tissue and Fibrosis Scores
4.2.5. Biochemical Assays
4.2.6. Evaluation of Study Participants’ Adverse Events, and Health-Related Quality of Life
4.2.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.; Abdel-Hamed, A.; Abo-Elmatty, D.; Khedr, N.; Ghattas, M. Pentoxifylline and its association with kaempferol improve NASH-associated manifestation in mice through anti-apoptotic, anti-necroptotic, antioxidant, and anti-inflammatory mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8644–8659. [Google Scholar]
- Petrelli, F.; Manara, M.; Colombo, S.; De Santi, G.; Ghidini, M.; Mariani, M.; Iaculli, A.; Rausa, E.; Rampulla, V.; Arru, M.; et al. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis: HCC and Steatosis or Steatohepatitis. Neoplasia 2022, 30, 100809. [Google Scholar] [CrossRef]
- Tomah, S.; Hamdy, O.; Abuelmagd, M.M.; Hassan, A.H.; Alkhouri, N.; Al-Badri, M.R.; Gardner, H.; Eldib, A.H.; Eid, E.A. Prevalence of and risk factors for non-alcoholic fatty liver disease (NAFLD) and fibrosis among young adults in Egypt. BMJ Open Gastroenterol. 2021, 8, e000780. [Google Scholar] [CrossRef]
- Hassan, A.M.; Elhaw, M.H.; Ahmed, A.A.-E.; Mansour, T.M.; Abd-Elaziz, T.M.; Shoaeir, M.Z. Value of screening for nonalcoholic fatty liver disease in hyperuricemic patients with normal body mass index by two-dimensional ultrasound: Upper Egypt experience. Al-Azhar Assiut Med. J. 2020, 18, 104–109. [Google Scholar] [CrossRef]
- Allen, A.M.; Lazarus, J.V.; Younossi, Z.M. Healthcare and socioeconomic costs of NAFLD: A global framework to navigate the uncertainties. J. Hepatol. 2023, 79, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Younossi, Z.; Aggarwal, P.; Shrestha, I.; Fernandes, J.; Johansen, P.; Augusto, M.; Nair, S. The burden of non-alcoholic steatohepatitis: A systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. Innov. Hepatol. 2022, 4, 100525. [Google Scholar] [CrossRef] [PubMed]
- Filozof, C.; Goldstein, B.J.; Williams, R.N.; Sanyal, A. Non-Alcoholic Steatohepatitis: Limited Available Treatment Options but Promising Drugs in Development and Recent Progress Towards a Regulatory Approval Pathway. Drugs 2015, 75, 1373–1392. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bezsonov, E.E.; Baig, M.S.; Popkova, T.V.; Nedosugova, L.V.; Starodubova, A.V.; Orekhov, A.N. Mitochondrial Mutations and Genetic Factors Determining NAFLD Risk. Int. J. Mol. Sci. 2021, 22, 4459. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Hahn, M.; Webb, M.; Shibolet, O.; Kariv, R.; Tirosh, O. Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants 2020, 9, 578. [Google Scholar] [CrossRef]
- Merino de Paz, N.; Quevedo-Abeledo, J.C.; Gómez-Bernal, F.; de Vera-González, A.; Abreu-González, P.; Martín-González, C.; González-Gay, M.; Ferraz-Amaro, I. Malondialdehyde Serum Levels in a Full Characterized Series of 430 Rheumatoid Arthritis Patients. J. Clin. Med. 2024, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hong, W.; Lu, S.; Li, Y.; Guan, Y.; Weng, X.; Feng, Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front. Pharmacol. 2022, 13, 780496. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Xu, C.; Yu, C.; Li, Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Can. J Gastroenterol. Hepatol. 2016, 2016, 6489012. [Google Scholar] [CrossRef]
- Calcagno, D.M.; Chu, A.; Gaul, S.; Taghdiri, N.; Toomu, A.; Leszczynska, A.; Kaufmann, B.; Papouchado, B.; Wree, A.; Geisler, L. NOD-like receptor protein 3 activation causes spontaneous inflammation and fibrosis that mimics human NASH. Hepatology 2022, 76, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, J.; Tacke, F. Controversies and Opportunities in the Use of Inflammatory Markers for Diagnosis or Risk Prediction in Fatty Liver Disease. Front. Immunol. 2020, 11, 634409. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.E.; Zakaria, A.Y. Targeting HSP47 and HSP70: Promising therapeutic approaches in liver fibrosis management. J. Transl. Med. 2022, 20, 544. [Google Scholar] [CrossRef]
- de Oliveira dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef]
- Falamarzi, K.; Malekpour, M.; Tafti, M.F.; Azarpira, N.; Behboodi, M.; Zarei, M. The role of FGF21 and its analogs on liver associated diseases. Front. Med. 2022, 9, 967375. [Google Scholar] [CrossRef]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Daniels, S.J.; Holm Nielsen, S.; Bager, C.; Rasmussen, D.G.K.; Loomba, R.; Surabattula, R.; Villesen, I.F.; Luo, Y.; Shevell, D.; et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 736–750. [Google Scholar] [CrossRef]
- Shan, L.; Wang, F.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis. Biomed. Pharmacother. 2023, 161, 114472. [Google Scholar] [CrossRef] [PubMed]
- Heyens, L.J.M.; Busschots, D.; Koek, G.H.; Robaeys, G.; Francque, S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front. Med. 2021, 8, 615978. [Google Scholar] [CrossRef]
- Sugimoto, M.; Saiki, H.; Tamai, A.; Seki, M.; Inuzuka, R.; Masutani, S.; Senzaki, H. Ventricular fibrogenesis activity assessed by serum levels of procollagen type III N-terminal amino peptide during the staged Fontan procedure. J. Thorac. Cardiovasc. Surg. 2016, 151, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Deng, L.; Zhang, Q.; Guo, J.; Zhou, J.; Song, W.; Yuan, F. Diagnostic Value of CK-18, FGF-21, and Related Biomarker Panel in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017, 2017, 9729107. [Google Scholar] [CrossRef]
- Intke, C.; Korpelainen, S.; Lappalainen, M.; Vänskä, M.; Hämäläinen, S.; Pulkki, K.; Jantunen, E.; Juutilainen, A.; Purhonen, A.K. Serum caspase-cleaved cytokeratin-18 fragment as a prognostic biomarker in hematological patients with febrile neutropenia. Clin. Exp. Med. 2022, 22, 83–93. [Google Scholar] [CrossRef]
- Wong, V.W.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH-current progress and future promise. Nature reviews. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef]
- Keating, S.E.; Sabag, A.; Hallsworth, K.; Hickman, I.J.; Macdonald, G.A.; Stine, J.G.; George, J.; Johnson, N.A. Exercise in the Management of Metabolic-Associated Fatty Liver Disease (MAFLD) in Adults: A Position Statement from Exercise and Sport Science Australia. Sports Med. 2023, 53, 2347–2371. [Google Scholar] [CrossRef]
- Li, C.-Y.; Lin, W.-C.; Moonmanee, T.; Chan, J.P.-W.; Wang, C.-K. The Protective Role of Vitamin E against Oxidative Stress and Immunosuppression Induced by Non-Esterified Fatty Acids in Bovine Peripheral Blood Leukocytes. Animals 2024, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Chee, N.M.-Z.; Sinnanaidu, R.P.; Chan, W.-K. Vitamin E improves serum markers and histology in adults with metabolic dysfunction-associated steatotic liver disease: Systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2024, 39, 2545–2554. [Google Scholar] [CrossRef]
- McTaggart, F.; Buckett, L.; Davidson, R.; Holdgate, G.; McCormick, A.; Schneck, D.; Smith, G.; Warwick, M. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am. J. Cardiol. 2001, 87, 28b–32b. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, F. Comparative pharmacology of rosuvastatin. Atheroscler. Suppl. 2003, 4, 9–14. [Google Scholar] [CrossRef]
- Mahalwar, R.; Khanna, D. Pleiotropic antioxidant potential of rosuvastatin in preventing cardiovascular disorders. Eur. J. Pharmacol. 2013, 711, 57–62. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, X.; Zhang, B.; Lan, T.; Liu, B. A Systematic Review of Statins for the Treatment of Nonalcoholic Steatohepatitis: Safety, Efficacy, and Mechanism of Action. Molecules 2024, 29, 1859. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Kuo, C.T.; Chang, G.J.; Chen, Y.H.; Lai, Y.J.; Cheng, M.L.; Chen, W.J. Rosuvastatin suppresses atrial tachycardia-induced cellular remodeling via Akt/Nrf2/heme oxygenase-1 pathway. J. Mol. Cell. Cardiol. 2015, 82, 84–92. [Google Scholar] [CrossRef]
- Tenório, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Shabalala, S.C.; Johnson, R.; Louw, J.; et al. N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature. Antioxidants 2020, 9, 1283. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Complexity of NAC Action as an Antidiabetic Agent: Opposing Effects of Oxidative and Reductive Stress on Insulin Secretion and Insulin Signaling. Int. J. Mol. Sci. 2022, 23, 2965. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.-K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.-H.; Wong, V.W.-S. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Abdelaziz, H.; Yossif, T.; Ossama, M. Cytokeratin 18 as a non invasive marker in diagnosis of NASH and its usefulness in correlation with disease severity in Egyptian patients. QJM Int. J. Med. 2020, 113, hcaa052-010. [Google Scholar] [CrossRef]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 2021, 13, 1688–1698. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Role of Vitamin E in the Treatment of NAFLD. Diseases 2018, 6, 86. [Google Scholar] [CrossRef]
- jawad Fairooz, A.; yawuz Jamal, M.; Mehdi Alkhalidi, N. Therapeutic Effects of Vitamin E in Non-alcoholic Fatty Liver Disease: An Open-Labeled Clinical Trial. Iraqi J. Pharm. Sci. 2022, 31, 135–143. [Google Scholar] [CrossRef]
- Khoshbaten, M.; Aliasgarzadeh, A.; Masnadi, K.; Tarzamani, M.K.; Farhang, S.; Babaei, H.; Kiani, J.; Zaare, M.; Najafipoor, F. N-acetylcysteine improves liver function in patients with non-alcoholic Fatty liver disease. Hepat. Mon. 2010, 10, 12–16. [Google Scholar]
- Tsai, C.-C.; Chen, Y.-J.; Yu, H.-R.; Huang, L.-T.; Tain, Y.-L.; Lin, I.-C.; Sheen, J.-M.; Wang, P.-W.; Tiao, M.-M. Long term N-acetylcysteine administration rescues liver steatosis via endoplasmic reticulum stress with unfolded protein response in mice. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Kargiotis, K.; Athyros, V.G.; Giouleme, O.; Katsiki, N.; Katsiki, E.; Anagnostis, P.; Boutari, C.; Doumas, M.; Karagiannis, A.; Mikhailidis, D.P. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J. Gastroenterol. 2015, 21, 7860. [Google Scholar] [CrossRef]
- Marinho, T.d.S.; Kawasaki, A.; Bryntesson, M.; Souza-Mello, V.; Barbosa-da-Silva, S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Rosuvastatin limits the activation of hepatic stellate cells in diet-induced obese mice. Hepatol. Res. 2017, 47, 928–940. [Google Scholar] [CrossRef]
- Yakaryilmaz, F.; Guliter, S.; Savas, B.; Erdem, O.; Ersoy, R.; Erden, E.; Akyol, G.; Bozkaya, H.; Ozenirler, S. Effects of vitamin E treatment on peroxisome proliferator-activated receptor-α expression and insulin resistance in patients with non-alcoholic steatohepatitis: Results of a pilot study. Intern. Med. J. 2007, 37, 229–235. [Google Scholar] [CrossRef]
- Cheng, J.; Joyce, A.; Yates, K.; Aouizerat, B.; Sanyal, A.J. Metabolomic profiling to identify predictors of response to vitamin E for non-alcoholic steatohepatitis (NASH). PLoS ONE 2012, 7, e44106. [Google Scholar] [CrossRef]
- Mazo, D.F.; de Oliveira, M.G.; Pereira, I.V.; Cogliati, B.; Stefano, J.T.; de Souza, G.F.; Rabelo, F.; Lima, F.R.; Alves, V.A.F.; Carrilho, F.J. S-nitroso-N-acetylcysteine attenuates liver fibrosis in experimental nonalcoholic steatohepatitis. Drug Des. Dev. Ther. 2013, 7, 553–563. [Google Scholar]
- Vargas, J.I.; Arrese, M.; Shah, V.H.; Arab, J.P. Use of Statins in Patients with Chronic Liver Disease and Cirrhosis: Current Views and Prospects. Curr. Gastroenterol. Rep. 2017, 19, 43. [Google Scholar] [CrossRef]
- Zheng, S.-H.; Chen, X.-X.; Chen, Y.; Wu, Z.-C.; Chen, X.-Q.; Li, X.-L. Antioxidant vitamins supplementation reduce endometriosis related pelvic pain in humans: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 79. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-acetylcysteine Can Induce Massive Oxidative Stress, Resulting in Cell Death with Apoptotic Features in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef]
- Xu, C.; Yang, S.; Zhu, L.; Cai, X.; Sheng, Y.; Zhu, S.; Xu, J. Regulation of N-acetyl cysteine on gut redox status and major microbiota in weaned piglets. J. Anim. Sci. 2014, 92, 1504–1511. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A review on various uses of N-acetyl cysteine. Cell J. 2017, 19, 11. [Google Scholar]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef]
- Niture, S.K.; Jaiswal, A.K. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free. Radic. Biol. Med. 2013, 57, 119–131. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, M.; Zhu, P.; Huang, H.; Zhuang, Q.; Shen, J.; Cai, Y.; Zhao, M.; He, Q. New insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3214196. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M.; Abtahi, S.R.; Karimi, G. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: A review. Food Chem. Toxicol. 2018, 120, 261–276. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, X.; Sho, T.; Luo, W.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. Effects of n-acetyl-cysteine supplementation in late gestational diet on maternal-placental redox status, placental NLRP3 inflammasome, and fecal microbiota in sows. J. Anim. Sci. 2019, 97, 1757–1771. [Google Scholar]
- Liu, Y.; Yao, W.; Xu, J.; Qiu, Y.; Cao, F.; Li, S.; Yang, S.; Yang, H.; Wu, Z.; Hou, Y. The anti-inflammatory effects of acetaminophen and N-acetylcysteine through suppression of the NLRP3 inflammasome pathway in LPS-challenged piglet mononuclear phagocytes. Innate Immunity 2015, 21, 587–597. [Google Scholar] [CrossRef]
- Ding, K.; Song, C.; Hu, H.; Yin, K.; Huang, H.; Tang, H. The Role of NLRP3 Inflammasome in Diabetic Cardiomyopathy and Its Therapeutic Implications. Oxid. Med. Cell Longev. 2022, 2022, 3790721. [Google Scholar] [CrossRef]
- de Andrade, K.Q.; Moura, F.A.; dos Santos, J.M.; de Araújo, O.R.; de Farias Santos, J.C.; Goulart, M.O. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef]
- Abdelhafez, D.; Aboelkomsan, E.; El Sadik, A.; Lasheen, N.; Ashur, S.; Elshimy, A.; Morcos, G.N.B. The Role of Mesenchymal Stem Cells with Ascorbic Acid and N-Acetylcysteine on TNF-α, IL 1β, and NF-κβ Expressions in Acute Pancreatitis in Albino Rats. J. Diabetes Res. 2021, 2021, 6229460. [Google Scholar] [CrossRef]
- Karmaus, P.W.; Shi, M.; Perl, S.; Biancotto, A.; Candia, J.; Cheung, F.; Kotliarov, Y.; Young, N.; Fessler, M.B. Effects of rosuvastatin on the immune system in healthy volunteers with normal serum cholesterol. JCI Insight 2019, 4, e131530. [Google Scholar] [CrossRef]
- Chen, W.; Deng, Z.; Zhu, J.; Yuan, L.; Li, S.; Zhang, Y.; Wu, J.; Huang, Z.; Qin, T.; Ye, W. Rosuvastatin suppresses TNF-α-induced matrix catabolism, pyroptosis and senescence via the HMGB1/NF-κB signaling pathway in nucleus pulposus cells: Role of rosuvastatin in alleviating intervertebral disc degeneration. Acta Biochim. Biophys. Sin. 2023, 55, 795. [Google Scholar]
- Murali, C.N.; Soler-Alfonso, C.; Loomes, K.M.; Shah, A.A.; Monteil, D.; Padilla, C.D.; Scaglia, F.; Ganetzky, R. TRMU deficiency: A broad clinical spectrum responsive to cysteine supplementation. Mol. Genet. Metab. 2021, 132, 146–153. [Google Scholar] [CrossRef]
- Bogani, P.; Canavesi, M.; Hagen, T.M.; Visioli, F.; Bellosta, S. Thiol supplementation inhibits metalloproteinase activity independent of glutathione status. Biochem. Biophys. Res. Commun. 2007, 363, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Crinelli, R.; Zara, C.; Galluzzi, L.; Buffi, G.; Ceccarini, C.; Smietana, M.; Mari, M.; Magnani, M.; Fraternale, A. Activation of NRF2 and ATF4 Signaling by the Pro-Glutathione Molecule I-152, a Co-Drug of N-Acetyl-Cysteine and Cysteamine. Antioxidants 2021, 10, 175. [Google Scholar] [CrossRef]
- Aslantaş, E.E.; Aksoy, Y.; Akkaya Ulum, Y.Z.; Ceyhan, D.; Peynircioglu, B.; Buzoğlu, H.D. Effects of calcium hydroxide and N-acetylcysteine on MMP-2, MMP-9, TIMP-1 and TIMP-2 in LPS-stimulated macrophage cell lines. Turk. J. Biochem. 2018, 43, 571–577. [Google Scholar] [CrossRef]
- Tang, M.; Yang, Z.; Liu, J.; Zhang, X.; Guan, L.; Liu, X.; Zeng, M. Combined intervention with N-acetylcysteine and desipramine alleviated silicosis development by regulating the Nrf2/HO-1 and ASMase/ceramide signaling pathways. Ecotoxicol. Environ. Saf. 2022, 242, 113914. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Lee, K.-C.; Huang, Y.-T.; Wang, Y.-W.; Hou, M.-C.; Lee, F.-Y.; Lin, H.-C.; Lee, S.-D. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J. Hepatol. 2008, 49, 25–33. [Google Scholar] [CrossRef]
- Gonsebatt, M.; Del Razo, L.; Cerbon, M.; Zúñiga, O.; Sanchez-Peña, L.; Ramírez, P. Arsenite induced oxidative damage in mouse liver is associated with increased cytokeratin 18 expression. Arch. Toxicol. 2007, 81, 619–626. [Google Scholar] [CrossRef]
- Ntamo, Y.; Ziqubu, K.; Chellan, N.; Nkambule, B.B.; Nyambuya, T.M.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Marcheggiani, F.; Tiano, L.; Dludla, P.V. Drug-induced liver injury: Clinical evidence of N-acetyl cysteine protective effects. Oxidative Med. Cell. Longev. 2021, 2021, 3320325. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Ittiwat, W.; Stremmel, W.; Chamulitrat, W. Redox regulation of cytokeratin 18 protein by NADPH oxidase 1 in preneoplastic human epithelial cells. J. Cancer Res. Clin. Oncol. 2011, 137, 1669–1678. [Google Scholar] [CrossRef]
- Aleksandrovich, M.I. Dynamics of IL-6 and CK-18 Concentration in Blood Plasma in Patients with Familial hypercholesterinemia with Non-Alcoholic Steatohepatitis Against the Background of Statin Therapy and Hepatoprotector. Therapy 2018, 2, 50–55. [Google Scholar]
- Kravchenko, L.; Appelhans, O.; Poliakov, A.; Borysiuk, Y.; Ivanova, N.; Neskoromna, N.; Rosumenko, M. Quercetin effectiveness in the complex hypolipidemic therapy of patients with nonalcoholic fatty liver disease with metabolic syndrome. World Med. Biol. 2022, 82, 76–82. [Google Scholar] [CrossRef]
- Hatami, B.; Abdi, S.; Pourhoseingholi, M.A.; Eghlimi, H.; Rabbani, A.H.; Masoumi, M.; Hajimohammadebrahim-Ketabforoush, M. The effects of N-acetylcysteine on hepatic, hematologic, and renal parameters in cirrhotic patients: A randomized controlled trial. Gastroenterol. Hepatol. Bed Bench 2023, 16, 432–440. [Google Scholar] [CrossRef]
- Nikbaf-Shandiz, M.; Adeli, S.; Faghfouri, A.H.; Khademi, F.; Jamilian, P.; Zarezadeh, M.; Ebrahimi-Mamaghani, M. The efficacy of N-acetylcysteine in improving liver function: A systematic review and meta-analysis of controlled clinical trials. PharmaNutrition 2023, 24, 100343. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Eslamian, G.; Malekpour Alamdari, N.; Abbasi, M.; Fazeli Taherian, S.; Behtaj, D.; Zand, H. Effects of N-acetylcysteine on aging cell and obesity complications in obese adults: A randomized, double-blind clinical trial. Front. Nutr. 2023, 10, 1237869. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, H.; Jin, X.; Wang, L.; Huang, J. The effects of N-acetylcysteine supplement on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1209614. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; Escobar, C. Rosuvastatin-Based Lipid-Lowering Therapy for the Control of LDL Cholesterol in Patients at High Vascular Risk. J. Clin. Med. 2024, 13, 1894. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; Chang, L.-H.; Chen, H.-S. The effect of statin treatment on glucose homeostasis in prediabetic individuals: A prospective, randomized, controlled trial. J. Chin. Med. Assoc. 2024, 87, 664–669. [Google Scholar] [CrossRef]
- de Denus, S.; Spinler, S.A.; Miller, K.; Peterson, A.M. Statins and liver toxicity: A meta-analysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 584–591. [Google Scholar] [CrossRef]
- Famularo, G.; Miele, L.; Minisola, G.; Grieco, A. Liver toxicity of rosuvastatin therapy. World J. Gastroenterol. 2007, 13, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e152. [Google Scholar] [CrossRef]
- Chin, R.; Lee, B.Y. Chapter 16–Data Interpretation and Conclusions. In Principles and Practice of Clinical Trial Medicine; Chin, R., Lee, B.Y., Eds.; Academic Press: New York, NY, USA, 2008; pp. 361–388. [Google Scholar]
- Cuschieri, S. The CONSORT statement. Saudi J. Anaesth. 2019, 13, S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Pandyarajan, V.; Gish, R.G.; Alkhouri, N.; Noureddin, M. Screening for Nonalcoholic Fatty Liver Disease in the Primary Care Clinic. Gastroenterol Hepatol 2019, 15, 357–365. [Google Scholar]
- Flegal, K.M.; Graubard, B.I. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am. J. Clin. Nutr. 2009, 89, 1213–1219. [Google Scholar] [CrossRef]
- Ho, S.Y.; Lam, T.H.; Janus, E.D. Waist to stature ratio is more strongly associated with cardiovascular risk factors than other simple anthropometric indices. Ann. Epidemiol. 2003, 13, 683–691. [Google Scholar] [CrossRef]
- Whitfield, K.C.; Wozniak, R.; Pradinuk, M.; Karakochuk, C.D.; Anabwani, G.; Daly, Z.; MacLeod, S.M.; Larson, C.P.; Green, T.J. Anthropometric measures are simple and accurate paediatric weight-prediction proxies in resource-poor settings with a high HIV prevalence. Arch. Dis. Child. 2017, 102, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Colditz, G.A. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern. Med. 2015, 175, 1412–1413. [Google Scholar] [CrossRef]
- Ali, A.H.; Al Juboori, A.; Petroski, G.F.; Diaz-Arias, A.A.; Syed-Abdul, M.M.; Wheeler, A.A.; Ganga, R.R.; Pitt, J.B.; Spencer, N.M.; Hammoud, G.M.; et al. The Utility and Diagnostic Accuracy of Transient Elastography in Adults with Morbid Obesity: A Prospective Study. J. Clin. Med. 2022, 11, 1201. [Google Scholar] [CrossRef]
- De, A.; Keisham, A.; Mishra, S.; Mehta, M.; Verma, N.; Premkumar, M.; Taneja, S.; Das, A.; Singh, V.; Duseja, A. FibroScan-AST (FAST) Score for Nonalcoholic Steatohepatitis–Validation in an Indian Cohort. J. Clin. Exp. Hepatol. 2022, 12, 440–447. [Google Scholar] [CrossRef]
- John, K.; Franck, M.; Al Aoua, S.; Rau, M.; Huber, Y.; Schattenberg, J.M.; Geier, A.; Bahr, M.J.; Wedemeyer, H.; Schulze-Osthoff, K. Non-invasive detection of fibrotic NASH in NAFLD patients with low or intermediate FIB-4. J. Clin. Med. 2022, 11, 4394. [Google Scholar] [CrossRef] [PubMed]
- Tavaglione, F.; Jamialahmadi, O.; De Vincentis, A.; Qadri, S.; Mowlaei, M.E.; Mancina, R.M.; Ciociola, E.; Carotti, S.; Perrone, G.; Bruni, V. Development and validation of a score for fibrotic nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2023, 21, 1523–1532.e1521. [Google Scholar] [CrossRef]
- Boursier, J.; Anty, R.; Vonghia, L.; Moal, V.; Vanwolleghem, T.; Canivet, C.M.; Michalak, S.; Bonnafous, S.; Michielsen, P.; Oberti, F.; et al. Screening for therapeutic trials and treatment indication in clinical practice: MACK-3, a new blood test for the diagnosis of fibrotic NASH. Aliment. Pharmacol. Ther. 2018, 47, 1387–1396. [Google Scholar] [CrossRef]
- Abu, O.; Orobator, O.; Momodu, I. Evaluation of the effect of total saponins and tannins isolated from Dialium guineense stem bark on CCl4-Induced hepatotoxicity in wistar rats. Glob. J. Med. Clin. Case Rep. 2022, 9, 35–38. [Google Scholar] [CrossRef]
- Belfield, A.; Goldberg, D. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme 1971, 12, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Shyamkrishnan, R.; Saharia, G.K.; Panda, S.; Mangaraj, M. Evaluation of Homocysteine and Gamma-Glutamyl Transferase Concentrations As Markers of Chronic Kidney Disease: An Indian Perspective. Cureus 2022, 14, e22959. [Google Scholar] [CrossRef]
- Ahmed Mobasher, M.; Galal El-Tantawi, H.; Samy El-Said, K. Metformin ameliorates oxidative stress induced by diabetes mellitus and hepatocellular carcinoma in rats. Rep. Biochem. Mol. Biol. 2020, 9, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.D.; Wong, E.T. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin. Chem. 1980, 26, 555–561. [Google Scholar] [CrossRef]
- Talke, H.; Schubert, G. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin. Wochenschr. 1965, 43, 174–175. [Google Scholar] [CrossRef]
- Tiffany, T.; Jansen, J.; Burtis, C.; Overton, J.; Scott, C. Enzymatic kinetic rate and end-point analyses of substrate, by use of a GeMSAEC fast analyzer. Clin. Chem. 1972, 18, 829–840. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Flier, J.S.; Kahn, C.R.; Roth, J. Receptors, antireceptor antibodies and mechanisms of insulin resistance. N. Engl. J. Med. 1979, 300, 413–419. [Google Scholar] [PubMed]
- Salgado, A.L.; Carvalho, L.; Oliveira, A.C.; Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.; Ekici, M.; Onder Karayigit, M.; Turgut, N.H.; Kara, H.; Arslanbas, E. Zingerone ameliorates oxidative stress and inflammation in bleomycin-induced pulmonary fibrosis: Modulation of the expression of TGF-β1 and iNOS. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1659–1670. [Google Scholar] [CrossRef]
- Osman, W.a.A.; Taher, H.; Darweesh, H.; Abdel Samie, M.; Shaker, O.G.; Labib, D.A.; Ateyya, H. The possible anti-inflammatory effect of extra virgin olive oil with colchicine in treatment of resistant cases of familial Mediterranean fever in a cohort of pediatric Egyptian patients. Future J. Pharm. Sci. 2024, 10, 17. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Lou, F.Y.; Li, X.; Ma, J.H.; Zhu, Z.T.; Li, H.; Zhai, Y.F.; Chen, H.; Zhang, Q.; Liu, Z.; et al. Correlation of CD3+/CD4+, and serum CK-18 fragment levels with glucose and lipid metabolism in elderly type 2 diabetes patients with nonalcoholic fatty liver disease. Am. J. Transl. Res. 2021, 13, 2546–2554. [Google Scholar]
- Esa, S.A.; Rawy, A.M.; EL-Behissy, M.M.; Kamel, M.H.; El-Hwaitty, H.M.M.M. Study of the level of sputum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor metalloproteinase-1 (TIMP-1) in COPD patients. Egypt. J. Chest Dis. Tuberc. 2014, 63, 861–867. [Google Scholar] [CrossRef]
- Barchetta, I.; Del Ben, M.; Angelico, F.; Di Martino, M.; Fraioli, A.; La Torre, G.; Saulle, R.; Perri, L.; Morini, S.; Tiberti, C.; et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. BMC Med. 2016, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Martin, T.; Bae, J.P.; Paczkowski, R.; Freeman, E. Health-related quality of life burden of nonalcoholic steatohepatitis: A robust pragmatic literature review. J. Patient-Rep. Outcomes 2018, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Nader, F.; Loomba, R.; Anstee, Q.M.; Ratziu, V.; Harrison, S.; Sanyal, A.J.; Schattenberg, J.M.; Barritt, A.S.; et al. Obeticholic Acid Impact on Quality of Life in Patients With Nonalcoholic Steatohepatitis: REGENERATE 18-Month Interim Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2050–2058.e2012. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Elliott, R.A.; Petrie, K.; Kuruvilla, L.; George, J. Interventions for improving medication-taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst. Rev. 2020, 5, Cd012419. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Mofrad, P.S.; Contos, M.J.; Sargeant, C.; Luketic, V.A.; Sterling, R.K.; Stravitz, R.T.; Shiffman, M.L.; Clore, J.; Mills, A.S. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2004, 2, 1107–1115. [Google Scholar] [CrossRef]
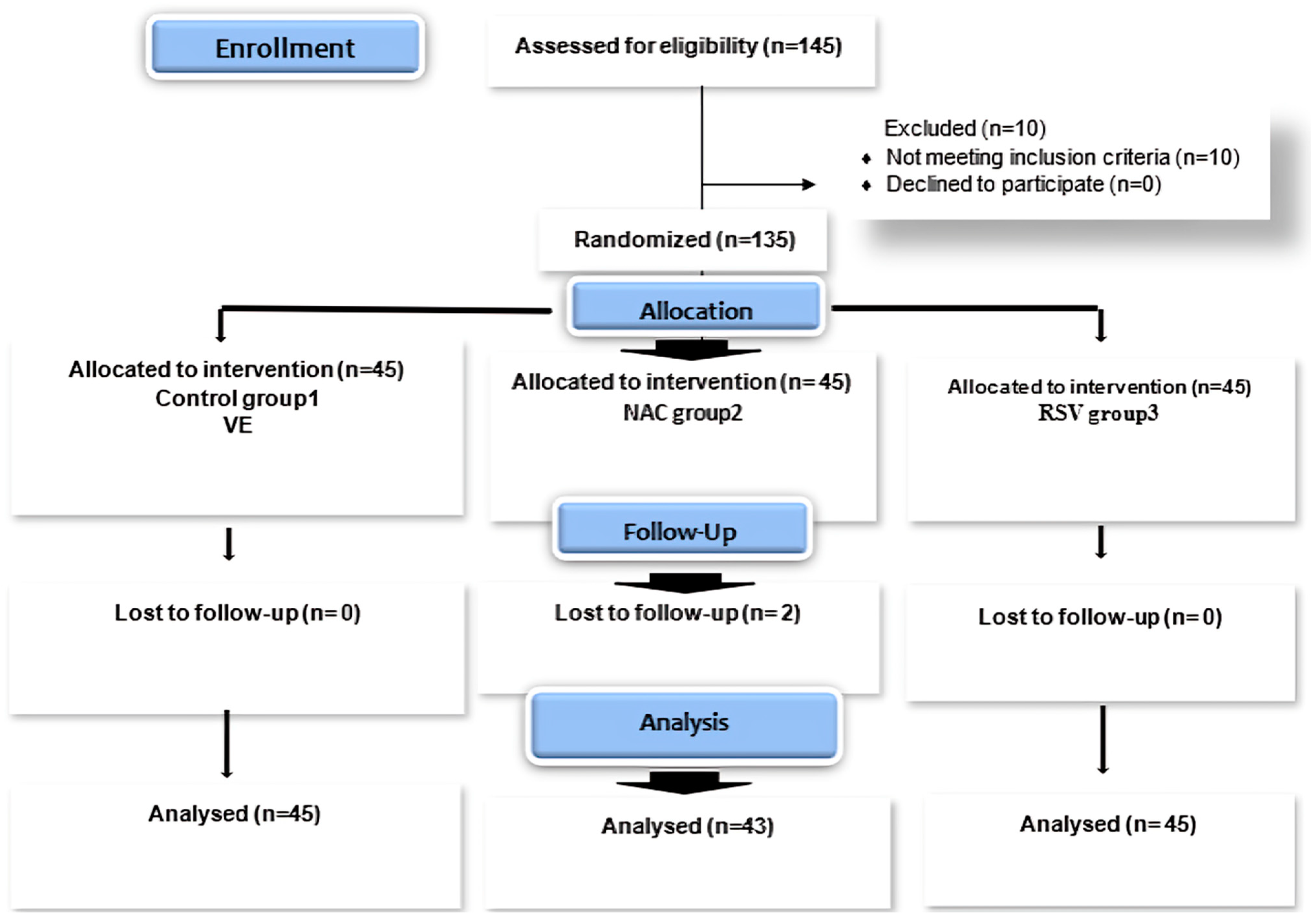
| Control Group 1 (VE) n = 45 | NAC Group 2 n = 43 | RSV Group 3 n = 45 | Test of Significance | Intergroup Significance | ||
|---|---|---|---|---|---|---|
| Age (y) | 47.53 ± 10.44 | 43.26 ± 12.94 | 49.49 ± 10.1 | F = 3.45 p = 0.034 * | P1 = 0.081 P2 = 0.409 P3 = 0.01 * | |
| Sex | P1 = 0.296 | |||||
| Female | 16(35.6) | 20(46.5) | 31(68.9) | Mc = 10.38 | P2 = 0.001 * | |
| Male | 29(64.4) | 23(53.5) | 14(31.1) | p = 0.006 * | P3 = 0.03 * | |
| Smoking | ||||||
| Non smoker | 28(62.2) | 30(69.8) | 31(68.9) | Mc = 2.66 | P1 = 0.515 | |
| Passive | 4(8.9) | 5(11.6) | 2(4.4) | p = 0.616 | P2 = 0.651 | |
| Current smoker | 13(28.9) | 8(18.6) | 12(26.7) | P3 = 0.357 | ||
| Blood pressure among studied cases | ||||||
| Systolic blood pressure (mmHg) | Before | 133.18 ± 14.22 | 133.95 ± 15.29 | 134.8 ± 15.62 | F = 0.131 p = 0.878 | P1 = 0.809 P2 = 0.610 P3 = 0.792 |
| After | 132.13 ± 12.79 | 128.81 ± 13.54 | 132.67 ± 14.26 | F = 1.04 p = 0.357 | P1 = 0.253 P2 = 0.852 P3 = 0.185 | |
| p-value | 0.001 * | 0.001 * | 0.001 * | |||
| Diastolic blood pressure (mmHg) | Before | 84.11 ± 8.21 | 84.37 ± 8.66 | 85.69 ± 9.32 | F = 0.419 p = 0.658 | P1 = 0.889 P2 = 0.394 P3 = 0.481 |
| After | 84.29 ± 7.57 | 82.49 ± 7.17 | 84.8 ± 7.90 | F = 1.13 p = 0.327 | P1 = 0.266 P2 = 0.749 P3 = 0.154 | |
| p-value | 0.749 | 0.001 * | 0.07 | |||
| Anthropometric measurements of the studied groups before and after treatment | ||||||
| Height (cm) | Before | 168.09 ± 8.15 | 168 ± 8.19 | 165.38 ± 9.15 | F = 1.46 p = 0.235 | P1 = 0.961 P2 = 0.133 P3 = 0.151 |
| Weight (kg) | Before | 95.51 ± 15.22 | 91.53 ± 15.80 | 94.36 ± 14.08 | F = 0.809 p = 0.447 | P1 = 0.217 P2 = 0.716 P3 = 0.381 |
| After | 95.2 ± 16.67 | 86.74 ± 16.08 | 91.87 ± 13.06 | F = 3.38 p = 0.037 * | P1 = 0.01 * P2 = 0.304 P3 = 0.120 | |
| p-value | 0.666 | 0.001 * | 0.005 * | |||
| BMI (kg/m2) | Before | 33.78 ± 4.85 | 32.70 ± 6.21 | 34.60 ± 5.23 | F = 1.34 p = 0.265 | P1 = 0.353 P2 = 0.479 P3 = 0.105 |
| After | 33.63 ± 5.17 | 30.92 ± 6.41 | 33.69 ± 4.89 | F = 3.59 p = 0.03 * | P1 = 0.023 * P2 = 0.959 P3 = 0.02 * | |
| p-value | 0.545 | 0.001 * | 0.007 * | |||
| Waist circumference (WC) (cm) | Before | 111.44 ± 11.61 | 108.91 ± 12.85 | 114.38 ± 10.0 | F = 2.48 p = 0.087 | P1 = 0.304 P2 = 0.230 P3 = 0.028 * |
| After | 111.93 ± 13.08 | 103.60 ± 13.95 | 112.98 ± 9.62 | F = 7.57 p = 0.001 * | P1 = 0.002 * P2 = 0.689 P3 = 0.001 * | |
| p-value | 0.357 | 0.001 * | 0.07 | |||
| Hip circumference (HC)(cm) | Before | 119.78 ± 11.19 | 117.33 ± 13.33 | 123.24 ± 9.45 | F = 3.0 p = 0.053 | P1 = 0.315 P2 = 0.152 P3 = 0.016 * |
| After | 120.07 ± 12.08 | 114.28 ± 14.49 | 122.60 ± 9.52 | F = 5.38 p = 0.006 * | P1 = 0.027 * P2 = 0.325 P3 = 0.325 | |
| p-value | 0.569 | 0.001 * | 0.125 | |||
| Waist–hip ratio (WHR) | Before | 0.931 ± 0.05 | 0.928 ± 0.06 | 0.928 ± 0.038 | F = 0.056 p = 0.946 | P1 = 0.812 P2 = 0.749 P3 = 0.937 |
| After | 0.930 ± 0.053 | 0.906 ± 0.06 | 0.921 ± 0.04 | F = 2.66 p = 0.073 | P1 = 0.024 * P2 = 0.41 P3 = 0.145 | |
| p-value | 0.846 | 0.001 * | 0.038 * | |||
| Waist–stature ratio (WSR) | Before | 0.663 ± 0.07 | 0.652 ± 0.09 | 0.696 ± 0.07 | F = 4.01 p = 0.02 * | P1 = 0.495 P2 = 0.04 * P3 = 0.008 * |
| After | 0.666 ± 0.07 | 0.619 ± 0.098 | 0.685 ± 0.07 | F = 7.65 p = 0.001 * | P1 = 0.007 * P2 = 0.277 P3 = 0.001 * | |
| p-value | 0.211 | 0.001 * | 0.019 * | |||
| Midarm circumference (MUAC) | Before | 36.41 ± 3.74 | 35.53 ± 4.06 | 36.54 ± 3.84 | F = 0.874 p = 0.420 | P1 = 0.291 P2 = 0.871 P3 = 0.224 |
| After | 36.42 ± 4.41 | 34.03 ± 4.02 | 35.80 ± 3.62 | F = 4.13 p = 0.018 * | P1 = 0.006 * P2 = 0.465 P3 = 0.042 * | |
| p-value | 0.788 | 0.001 * | 0.002 * | |||
| Control Group (VE) n = 45 | NAC Group n = 43 | RSV Group n = 45 | Test of Significance | Intergroup Significance | ||
|---|---|---|---|---|---|---|
| Steatosis | Before | 302.96 ± 44.82 | 309.23 ± 48.96 | 315.31 ± 44.57 | F = 0.807 p = 0.448 | P1 = 0.525 P2 = 0.206 P3 = 0.538 |
| S1 S2 S3 | 8(17.8) 12(26.7) 25(55.6) | 8(18.6) 11(25.6) 24(55.8) | 6(13.3) 9(20.0) 30(66.7) | |||
| After | 284.62 ± 52.84 | 265.33 ± 65.16 | 291.60 ± 47.99 | F = 2.62 p = 0.08 | P1 = 0.106 P2 = 0.553 P3 = 0.029 * | |
| S0 S1 S2 S3 | 4(8.9) 5(11.1) 12(26.7) 24(53.3) | 12(27.9) 6(14) 7(16.3) 18(41.9) | 5(11.1) 1(2.2) 13(28.9) 26(57.8) | |||
| p-value | 0.017 * | 0.001 * | 0.004 * | |||
| Fibrosis | Before | 5.9(4.4–8.85) | 5.6(4.9–6.4) | 5.5(4.05–7.35) | KW = 0.571 p = 0.752 | P1 = 0.679 P2 = 0.413 P3 = 0.881 |
| F0 F1 F2 F3 F4 | 19(42.2) 8(17.8) 11(24.4) 2(4.4) 5(11.1) | 20(46.5) 16(37.2) 3(7) 3(7) 1(2.3) | 21(46.7) 12(26.7) 6(13.3) 1(2.2) 5(11.1) | |||
| After | 5.6(4.35–8.20) | 4.9(4.4–5.9) | 6(4.55–7.6) | KW = 5.81 p = 0.06 | P1 = 0.051 P2 = 0.987 P3 = 0.028 * | |
| F0 F1 F2 F3 F4 | 22(48.9) 9(20.0) 7(15.6) 2(4.4) 5(11.1) | 31(72.1) 7(16.3) 4(9.3) 0 1(2.3) | 21(46.7) 12(26.7) 5(11.1) 2(4.4) 5(11.1) | |||
| p-value | 0.977 | 0.001 * | 0.218 | |||
| FAST score | Before | 0.370(0.325–0.54) | 0.38(0.3–0.5) | 0.37(0.265–0.495) | KW = 1.30 p = 0.522 | P1 = 0.520 P2 = 0.276 P3 = 0.562 |
| After | 0.26(0.18–0.425) | 0.14(0.09–0.22) | 0.26(0.12–0.36) | KW = 17.56 p = 0.001 * | P1 = 0.001 * P2 = 0.188 P3 = 0.007 * | |
| p-value | 0.001 * | 0.001 * | 0.001 * | |||
| FNI | Before | 0.510(0.315–0.605) | 0.520(0.36–0.59) | 0.43(0.315–0.56) | KW = 2.43 p = 0.297 | P1 = 0.884 P2 = 0.196 P3 = 0.164 |
| After | 0.25(0.18–0.425) | 0.17(0.12–0.29) | 0.24(0.09–0.34) | KW = 7.90 p = 0.019 * | P1 = 0.007 * P2 = 0.039 * P3 = 0.622 | |
| p-value | 0.001 * | 0.001 * | 0.001 * | |||
| FIB-4 | Before | 1.4(1.06–2.26) | 1.11(0.73–1.37) | 1.37(1.09–1.92) | KW = 10.79 p = 0.005 * | P1 = 0.004 * P2 = 0.693 P3 = 0.001 * |
| After | 1.41(0.875–1.85) | 0.81(0.60–0.98) | 1.26(0.975–1.58) | KW = 23.50 p = 0.001 * | P1 = 0.001 * P2 = 0.196 P3 = 0.164 | |
| p-value | 0.413 | 0.001 * | 0.04 * | |||
| MACK-3 | Before | 0.358(0.273–0.421) | 0.332(0.267–0.445) | 0.263(0.182–0.364) | KW = 8.71 p = 0.013 * | P1 = 0.780 P2 = 0.007 * P3 = 0.018 * |
| After | 0.329(0.265–0.433) | 0.253(0.222–0.380) | 0.280(0.197–0.377) | KW = 4.38 p = 0.112 | P1 = 0.043 * P2 = 0.117 P3 = 0.838 | |
| p-value | 0.059 | 0.001 * | 0.046 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, A.Y.; Badawi, R.; Osama, H.; Abdelrahman, M.A.; El-Kalaawy, A.M. A Comparative Study of N-Acetyl Cysteine, Rosuvastatin, and Vitamin E in the Management of Patients with Non-Alcoholic Steatohepatitis: A Randomized Controlled Trial. Pharmaceuticals 2025, 18, 650. https://doi.org/10.3390/ph18050650
Zakaria AY, Badawi R, Osama H, Abdelrahman MA, El-Kalaawy AM. A Comparative Study of N-Acetyl Cysteine, Rosuvastatin, and Vitamin E in the Management of Patients with Non-Alcoholic Steatohepatitis: A Randomized Controlled Trial. Pharmaceuticals. 2025; 18(5):650. https://doi.org/10.3390/ph18050650
Chicago/Turabian StyleZakaria, Amr Y., Rehab Badawi, Hasnaa Osama, Mona A. Abdelrahman, and Asmaa M. El-Kalaawy. 2025. "A Comparative Study of N-Acetyl Cysteine, Rosuvastatin, and Vitamin E in the Management of Patients with Non-Alcoholic Steatohepatitis: A Randomized Controlled Trial" Pharmaceuticals 18, no. 5: 650. https://doi.org/10.3390/ph18050650
APA StyleZakaria, A. Y., Badawi, R., Osama, H., Abdelrahman, M. A., & El-Kalaawy, A. M. (2025). A Comparative Study of N-Acetyl Cysteine, Rosuvastatin, and Vitamin E in the Management of Patients with Non-Alcoholic Steatohepatitis: A Randomized Controlled Trial. Pharmaceuticals, 18(5), 650. https://doi.org/10.3390/ph18050650







