Unveiling the Antioxidant Power and Secondary Metabolites of Tabebuia chrysantha (Jacq.) Leaves and Flowers from Ecuador
Abstract
1. Introduction
2. Results
2.1. Bioactive Compound Determination
2.2. Antioxidant Capacity Determination
2.3. Correlation Between Bioactive Compounds and Antioxidant Capacity
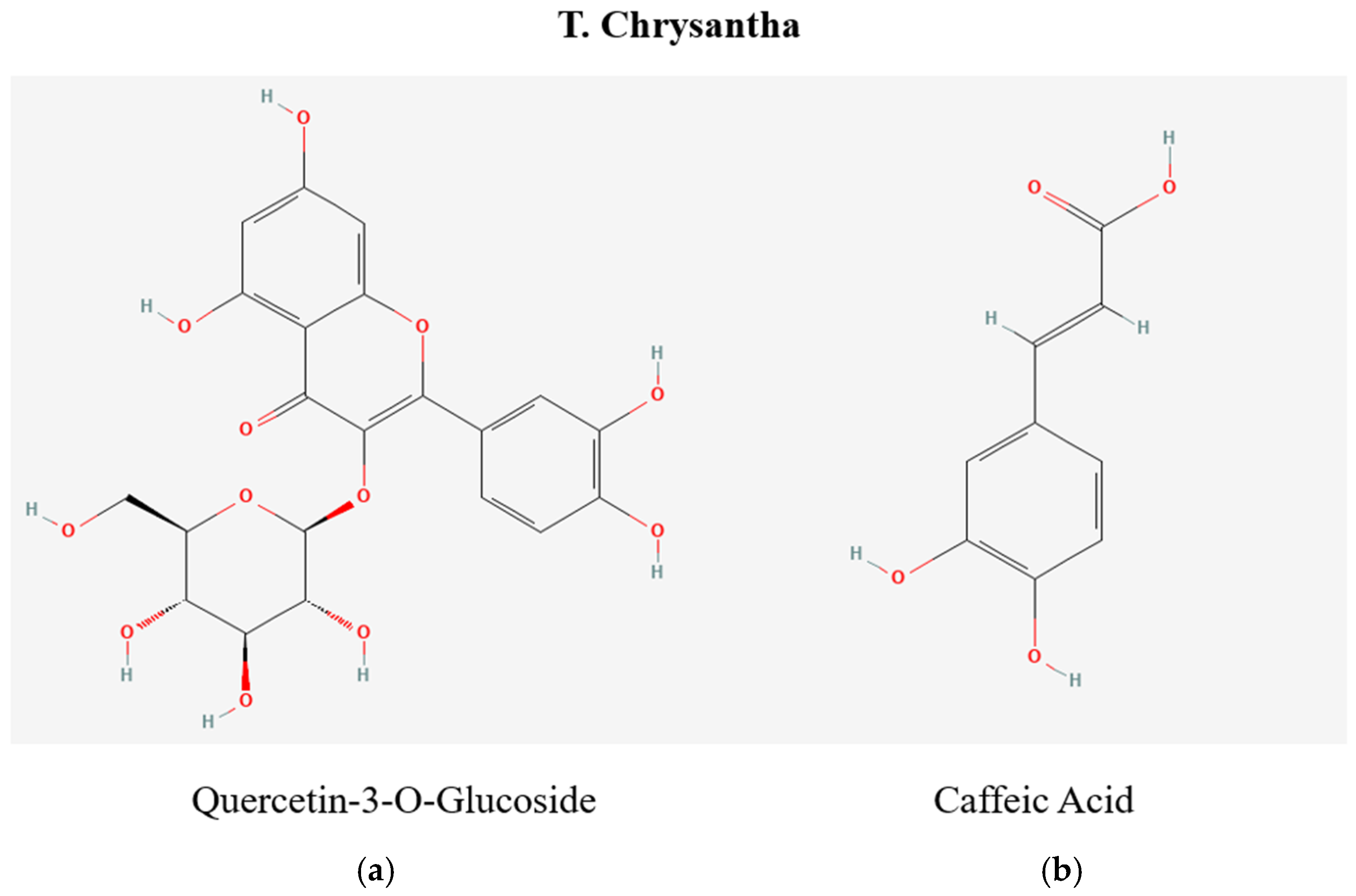
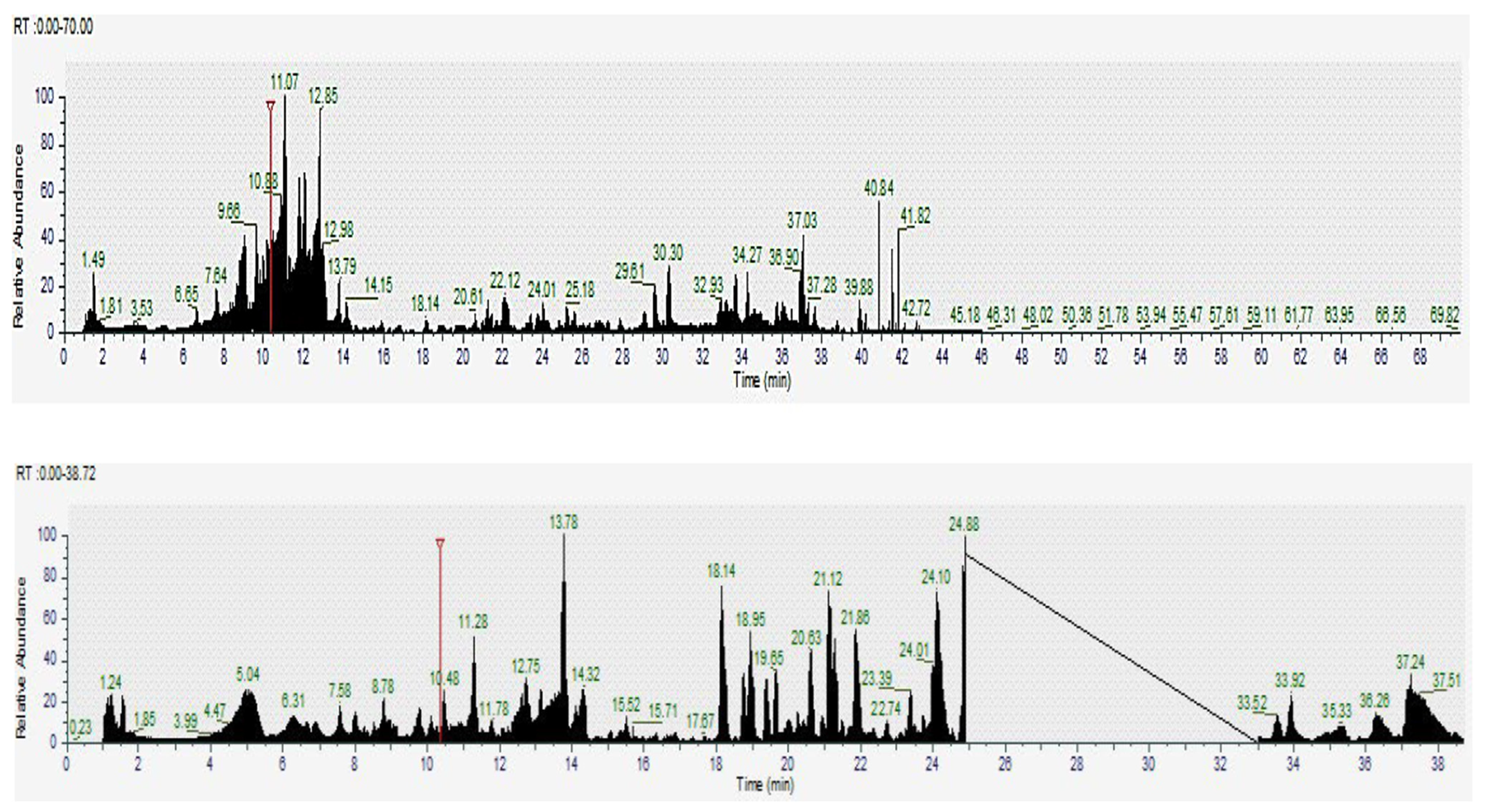
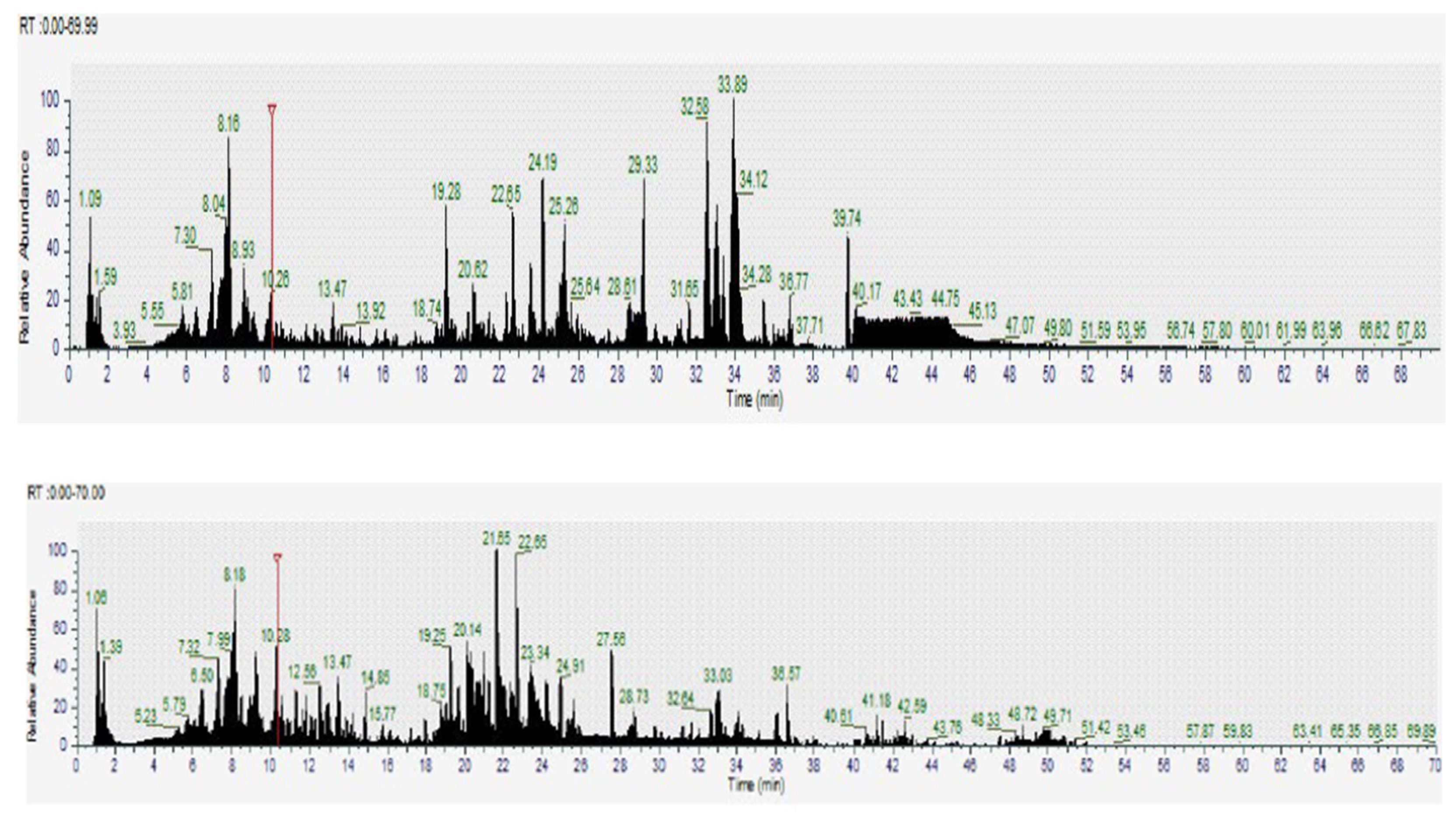
2.4. Screening of Bioactive Compounds by Liquid Chromatography Coupled with Mass Spectrometry LC-MS
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Extraction of Bioactive Compounds
4.3. Determination of Active Ingredients
4.4. Evaluation of Antioxidant Capacity Using FRAP, DPPH, and ABTS Assays
4.5. Determination of Bioactive Compounds by LC-MS
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panda, S.P.; Panigrahy, U.P.; Panda, S.; Jena, B.R. Stem extract of Tabebuia chrysantha induces apoptosis by targeting sEGFR in Ehrlich Ascites Carcinoma. J. Ethnopharmacol. 2019, 235, 219–226. [Google Scholar] [CrossRef]
- Döring, M. GBIF Backbone Patch. GBIF Secretariat. Checklist Dataset. 2023. Available online: https://doi.org/10.15468/l07d61 (accessed on 4 March 2025).
- Vinueza, M. Tabebuia chrysantha (Jacq.) Nicholson. 201). Available online: https://ecuadorforestal.org/fichas-tecnicas-de-especies-forestales/ficha-tecnica-no-6-guayacan (accessed on 5 March 2025).
- Rojas-Rodríguez, F.; Torres-Córdoba, G. Árboles del Valle Central de Costa Rica: Reproducción cortés amarillo Tabebuia chrysantha (Jacq.) Nichols. Revista Forestal Mesoamericana Kurú 2016, 13, 66–68. [Google Scholar] [CrossRef][Green Version]
- Block, G.; Dietrich, M.; Norkus, E.P.; Morrow, J.D.; Hudes, M.; Caan, B.; Packer, L. Factors Associated with Oxidative Stress in Human Populations. Am. J. Epidemiol. 2022, 156, 274–285. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Sierra, J.; Arrubla, R.; Martínez, P. Metabolitos secundarios en el guayacán amarillo y en el guayacán. Scientia et Technica 2011, 1, 297–301. [Google Scholar]
- Cardona-Trujillo, M.C.; Jiménez-González, F.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. In Vitro Anti-Toxoplasma Activity of Extracts Obtained from Tabebuia rosea and Tabebuia chrysantha: The Role of β-Amyrin. Molecules 2024, 29, 920. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Taher, M.A.; Saleh, E.; AbouZid, S.F.; Mohammed, R. Genus Tabebuia: A comprehensive review journey from past achievements to future perspectives. Arabian J. Chem. 2021, 14, 103046. [Google Scholar] [CrossRef]
- Jiménez, F.V.L.; Sepúlveda, J. Anti-infectious activity in plants of the genus Tabebuia. Scientiarum 2013, 18, 257–267. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Sign Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia leaves during Mediterranean summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Johnson, S.D. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zargoosh, Z.; Ghavam, M.; Tavili, A. Environmental factors affecting the phenolic content and antioxidant activity of medicinal plants: A review. Ind. Crops Product. 2023, 198, 116684. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids sensors of environmental changes? A review on their biosynthesis and functions in plants under abiotic stress. Plant Cell Environ. 2020, 43, 2357–2375. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Martins, F.O.; Esteves, P.F.; Mendes, G.S.; Barbi, N.S.; Menezes, F.S. Phytochemical and pharmacological overview of Bignoniaceae family. J. Pharm. Pharmacol. 2018, 70, 1293–1308. [Google Scholar] [CrossRef][Green Version]
- Ferraz-Filha, Z.S.; Araújo, M.C.D.P.M.; Ferrari, F.C.; Dutra, I.P.A.R. Tabebuia roseoalba: In vivo hypouricemic and anti-inflammatory effects of its ethanolic extract and constituents. Planta Med. 2016, 82, 1395–1402. [Google Scholar] [CrossRef]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2019, 65, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Claros, P. Evaluacion de la Capacidad Antioxidante Total y Contenido de Polifenoles Totales del Phaseolus vulgaris “Frijol”; Universidad Nacional José Faustino Sánchez Carrión: Huacho, Peru, 2021; Available online: https://repositorio.unjfsc.edu.pe/handle/20.500.14067/5297 (accessed on 5 March 2025).
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Met. 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Evaluation of aluminum complexation reaction for flavonoid content assay. Food Anal. Met. 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional. Indian. J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Sachett, A.; Gallas-Lopes, M.; Conterato, G.M.M.; Herrmann, A.; Piato, A. Antioxidant Activity by DPPH Assay: In Vitro Protocol. Protocols Io. 2021. Available online: https://www.protocols.io/view/antioxidant-activity-by-dpph-assay-in-vitro-protocbtbpnimn (accessed on 3 September 2024).
- Thaweesang, S. Antioxidant activity and total phenolic compounds of fresh and blanching banana blossom (Musa ABB CV. Kluai “Namwa”) in Thailand. IOP Conf. Series Mater. Sci. Eng. 2019, 639, 012047. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversosmétodos químicos para determiner actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Tohma, H.; Koksal, E.; Kılıc, O.; Alan, Y.; Yılmaz, M.A.; Gulcin, I.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants 2016, 5, 5040038. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar]
- Cellier, G.; Moreau, A.; Chabirand, A.; Hostachy, B.; Ailloud, F.; Prior, P. A Duplex PCR Assay for the Detection of Ralstonia solanacearum Phylotype II Strains in Musa spp. PLoS ONE 2015, 10, e0122182. [Google Scholar] [CrossRef] [PubMed]
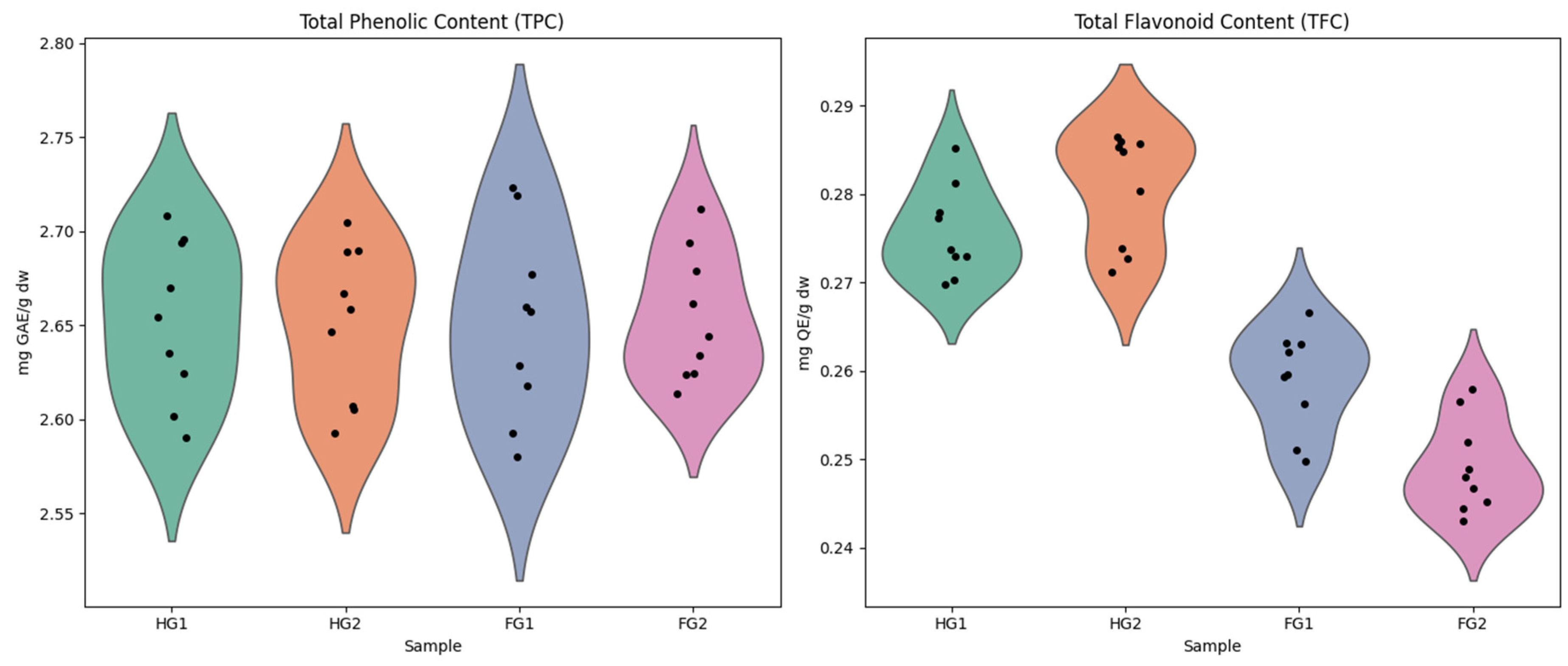
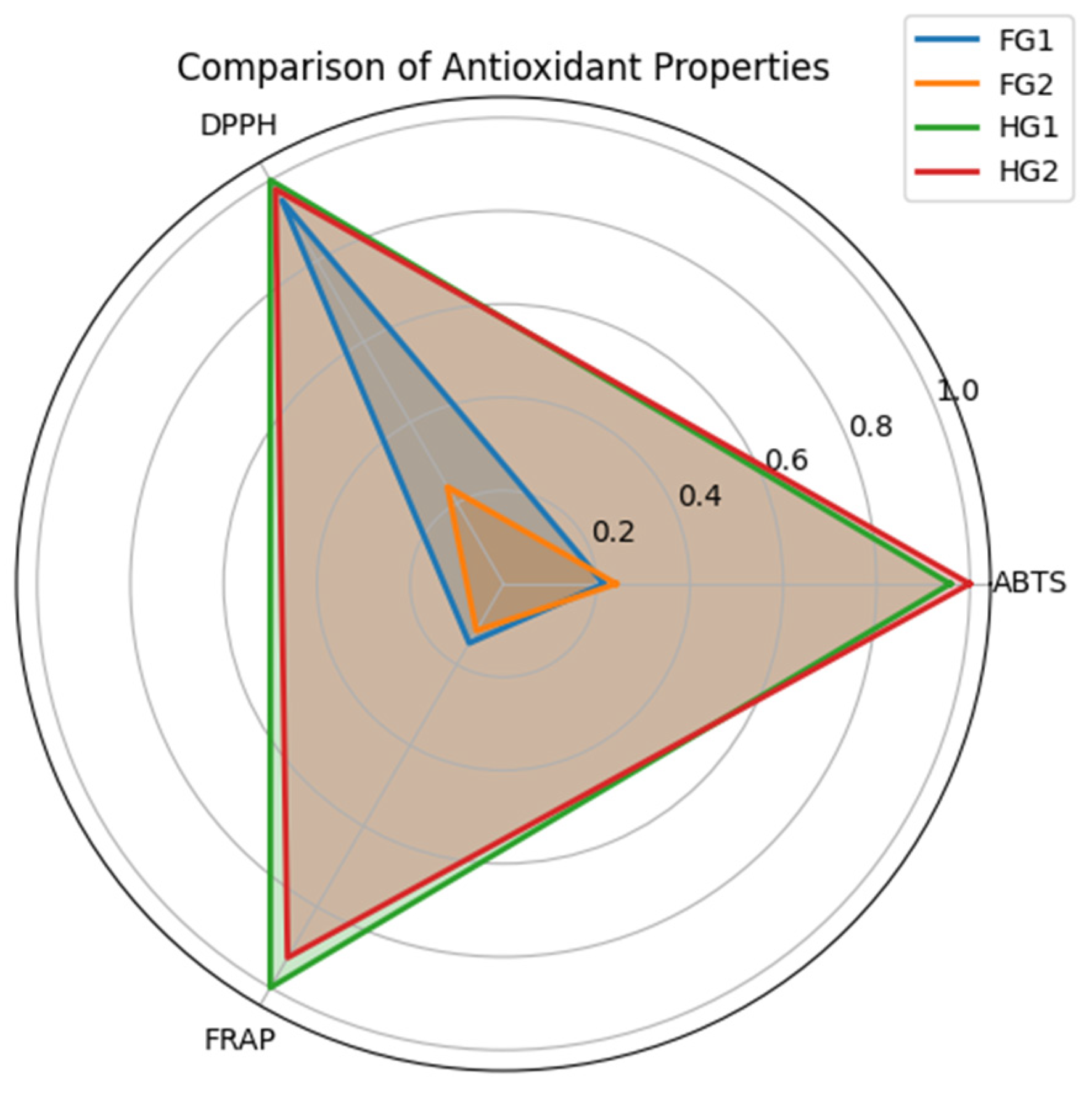

| Samples | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | ABTS (µmol Trolox/g DW) | DPPH (µmol Trolox/g DW) | FRAP (µmol Fe2+/g DW) |
|---|---|---|---|---|---|
| HG1 | 2.645 ± 0.041 | 0.275 ± 0.005 | 10.57 ± 0.76 | 37.24 ± 0.89 | 22.67 ± 0.43 |
| HG2 | 2.646 ± 0.042 | 0.280 ± 0.005 | 10.84 ± 0.51 | 37.04 ± 1.23 | 20.88 ± 0.68 |
| FG1 | 2.653 ± 0.048 | 0.260 ± 0.006 | 2.32 ± 0.53 | 35.93 ± 1.36 | 3.33 ± 0.14 |
| FG2 | 2.646 ± 0.042 | 0.249 ± 0.006 | 2.61 ± 0.06 | 9.07 ± 0.21 | 2.65 ± 0.10 |
| ID | m/z | Retention Time | Proposed Compound Identity | Molecular Ion | Molecular Formula | Plant Source | Ionization Mode |
|---|---|---|---|---|---|---|---|
| 2057 | 465.307 | 1511 | Quercetin-3-O-glucoside (Hyperoside) | M+H | C21H20O12 | Flower and Leaf | Positive |
| 3915 | 449.276 | 7392 | Luteolin-8-C-glucoside | M+H | C21H20O11 | Flower and Leaf | Positive |
| 8557 | 465.351 | 11,135 | Quercetin-3-O-β-D-galactopyranoside | M+H | C21H20O12 | Flower and Leaf | Positive |
| 11474 | 419.357 | 13,177 | Liquiritin | M+H | C21H22O9 | Flower and Leaf | Positive |
| 13457 | 593.187 | 14,666 | Acacetin-7-O-rhamnosylglucoside (Fortunellin) | M+H | C28H32O14 | Flower and Leaf | Positive |
| 1820 | 179.188 | 19.878 | Caffeic acid * | M-H | C9H8O4 | Flower and Leaf | Negative |
| 2443 | 223.233 | 24.767 | Sinapinic acid | M-H | C11H12O5 | Flower and Leaf | Negative |
| 1966 | 340.046 | 21.087 | Aristolochic acid C | M-H | C17H11NO7 | Flower and Leaf | Negative |
| 1242 | 375.129 | 15.621 | Deoxyloganic acid | M-H | C16H24O10 | Flower and Leaf | Negative |
| 2136 | 206.169 | 1623 | α-Lipoamide | M+H | C8H15NOS2 | Leaf | Positive |
| 2446 | 190.135 | 3799 | Kynurenic acid | M+H | C10H7NO3 | Leaf | Positive |
| 3159 | 419.286 | 5999 | Aloin A | M+H | C21H22O9 | Leaf | Positive |
| 4606 | 463.088 | 8073 | Kaempferol-3-O-glucuronide | M+H | C21H18O12 | Leaf | Positive |
| 6315 | 449.320 | 9576 | Plantaginin | M+H | C21H20O11 | Leaf | Positive |
| 6331 | 481.365 | 9653 | 3,5,7,8,3,4-Hexahydroxyflavone-8-O-glucoside | M+H | C21H20O13 | Leaf | Positive |
| 7746 | 519.389 | 10.51 | 6-O-Malonylcosmosiin | M+H | C24H22O14 | Leaf | Positive |
| 10032 | 479.398 | 12,051 | Isorhamnetin-3-O-glucoside | M+H | C22H22O12 | Leaf | Positive |
| 10052 | 315.316 | 12.09 | Velutin | M+H | C16H12O6 | Leaf | Positive |
| 10463 | 257.284 | 12,389 | Pinocembrin | M+H | C15H12O4 | Leaf | Positive |
| 10896 | 241.265 | 12,693 | 6-Hydroxyflavanone | M+H | C15H12O3 | Leaf | Positive |
| 11006 | 293.368 | 12,932 | (10E,15E)-9,12,13-Trihydroxyoctadeca-10,15-dienoic acid | M-2H2O+H | C18H28O3 | Leaf | Positive |
| 12854 | 623.430 | 14.12 | Pectolinarin | M+H | C29H34O15 | Leaf | Positive |
| 19210 | 449.108 | 24,978 | Luteolin-6-C-glucoside | M+ | C21H20O11 | Leaf | Positive |
| 1996 | 205.097 | 1472 | L-Tryptophan | M+H | C11H12N2O2 | Leaf | Positive |
| 3409 | 225.184 | 6465 | Phenazine-1-carboxylic acid | M+H | C13H8N2O2 | Leaf | Positive |
| 18477 | 329.309 | 22,338 | Labetalol | M+H | C19H24N2O3 | Leaf | Positive |
| 18850 | 255.316 | 23,717 | 10,11-Dihydro-10-hydroxycarbamazepine | M+H | C15H14N2O2 | Leaf | Positive |
| 321 | 181.254 | 5.192 | Sorbitol | M-H | C6H14O6 | Leaf | Negative |
| 566 | 179.231 | 5.365 | Hexose (e.g., glucose, fructose, mannose, galactose) | M-H | C6H12O6 | Leaf | Negative |
| 1961 | 190.281 | 21.053 | 5-Hydroxyindoleacetic acid (5-HIAA) | M-H | C10H9NO3 | Leaf | Negative |
| 9962 | 451.404 | 12,045 | Eriodictyol-7-O-glucoside | M+H | C21H22O11 | Flower | Positive |
| 10032 | 479.398 | 12,051 | Isorhamnetin-3-O-glucoside | M+H | C22H22O12 | Flower | Positive |
| 17819 | 415.396 | 2051 | Chafuroside A | M+H | C21H20O11 | Flower | Positive |
| 18383 | 427.292 | 21,962 | Leupeptin | M+H | C20H38N6O4 | Flower | Positive |
| 18391 | 291.311 | 21,978 | Catechin | M+H | C15H14O6 | Flower | Positive |
| 18569 | 299.313 | 22,705 | Enterolactone | M+H | C18H18O4 | Flower | Positive |
| 18887 | 471.447 | 23,737 | Glycyrrhetinic acid | M+ | C30H46O4 | Flower | Positive |
| 19210 | 281.303 | 24,938 | Aspartylphenylalanine | M+H | C13H16N2O5 | Flower | Positive |
| 19406 | 299.295 | 25,884 | Enterolactone | M+H | C18H18O4 | Flower | Positive |
| 19422 | 365.417 | 25,948 | Xanthosine 5′-monophosphate (XMP) | M+H | C10H13N4O9P | Flower | Positive |
| 19630 | 427.472 | 2698 | Leupeptin | M+ | C20H38N6O4 | Flower | Positive |
| 20879 | 265.297 | 30,936 | Abscisic acid | M+H | C15H20O4 | Flower | Positive |
| 20907 | 323.352 | 30,936 | Chloramphenicol | M+ | C11H12Cl2N2O5 | Flower | Positive |
| 21401 | 277.338 | 32,298 | Glutamylglutamic acid | M+H | C10H16N2O7 | Flower | Positive |
| 21903 | 415.476 | 33,398 | Podophyllotoxin | M+H | C22H22O8 | Flower | Positive |
| 23063 | 609.531 | 35,284 | 3,10S-Dihydroxypheophorbide | M+H | C33H34N4O6 | Flower | Positive |
| 23713 | 457.513 | 36,385 | Oleanolic acid | M+H | C30H48O3 | Flower | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, R.A.; Vivanco Gonzaga, R.F.; Cubi Insuaste, N.S.; Maza Morocho, N.R.; Catana, R.D. Unveiling the Antioxidant Power and Secondary Metabolites of Tabebuia chrysantha (Jacq.) Leaves and Flowers from Ecuador. Pharmaceuticals 2025, 18, 649. https://doi.org/10.3390/ph18050649
Mihai RA, Vivanco Gonzaga RF, Cubi Insuaste NS, Maza Morocho NR, Catana RD. Unveiling the Antioxidant Power and Secondary Metabolites of Tabebuia chrysantha (Jacq.) Leaves and Flowers from Ecuador. Pharmaceuticals. 2025; 18(5):649. https://doi.org/10.3390/ph18050649
Chicago/Turabian StyleMihai, Raluca A., Ramiro Fernando Vivanco Gonzaga, Nelson Santiago Cubi Insuaste, Nilo Rigoberto Maza Morocho, and Rodica D. Catana. 2025. "Unveiling the Antioxidant Power and Secondary Metabolites of Tabebuia chrysantha (Jacq.) Leaves and Flowers from Ecuador" Pharmaceuticals 18, no. 5: 649. https://doi.org/10.3390/ph18050649
APA StyleMihai, R. A., Vivanco Gonzaga, R. F., Cubi Insuaste, N. S., Maza Morocho, N. R., & Catana, R. D. (2025). Unveiling the Antioxidant Power and Secondary Metabolites of Tabebuia chrysantha (Jacq.) Leaves and Flowers from Ecuador. Pharmaceuticals, 18(5), 649. https://doi.org/10.3390/ph18050649








