Exploring Ginseng Bioactive Compound’s Role in Hypertension Remedy: An In Silico Approach
Abstract
1. Introduction
2. Results
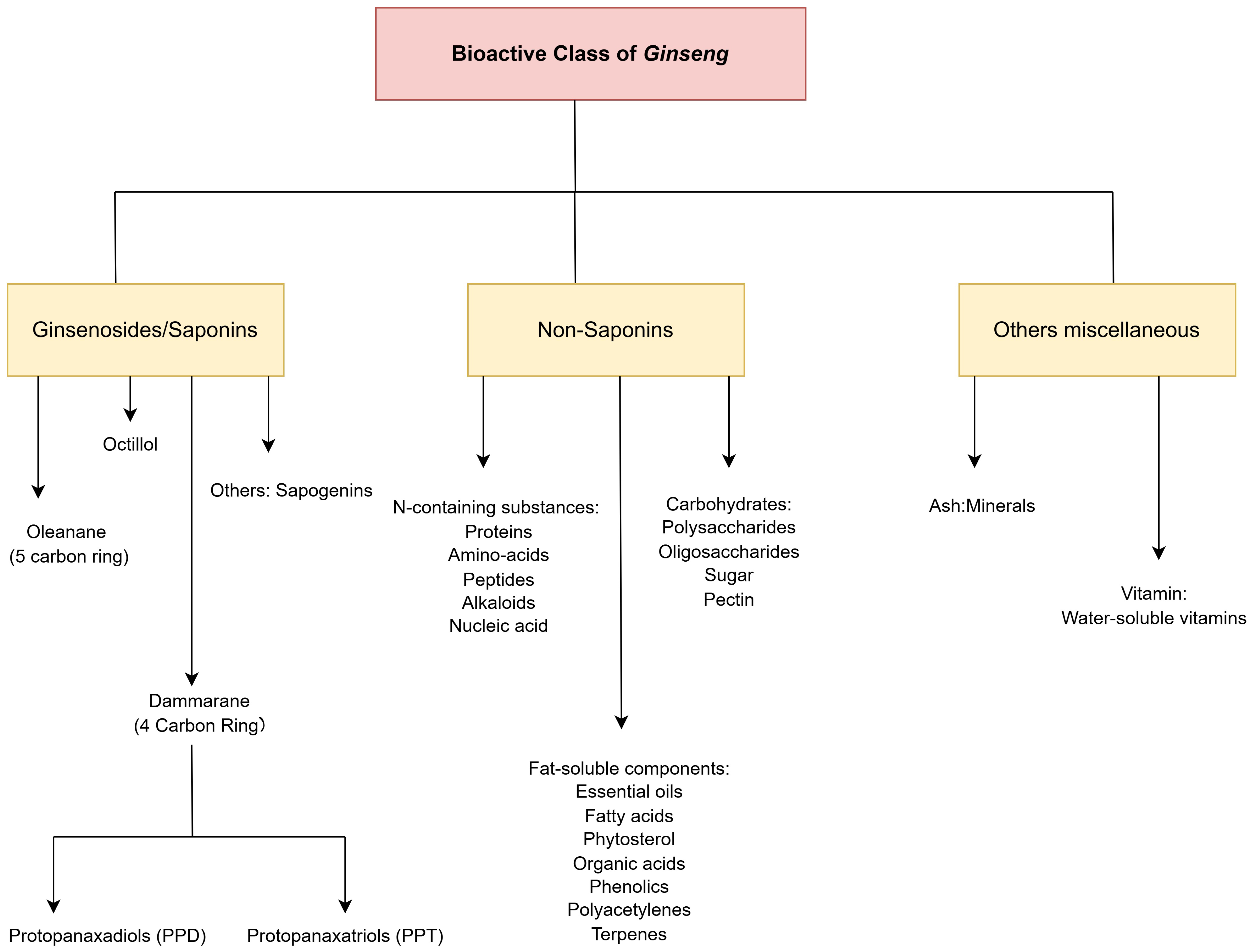
2.1. Collection of Phytochemicals, Target Proteins, and Genes
2.2. Intersection Gene Analysis
2.3. GO and KEGG Enrichment Pathway Examination
2.4. Molecular Docking Study
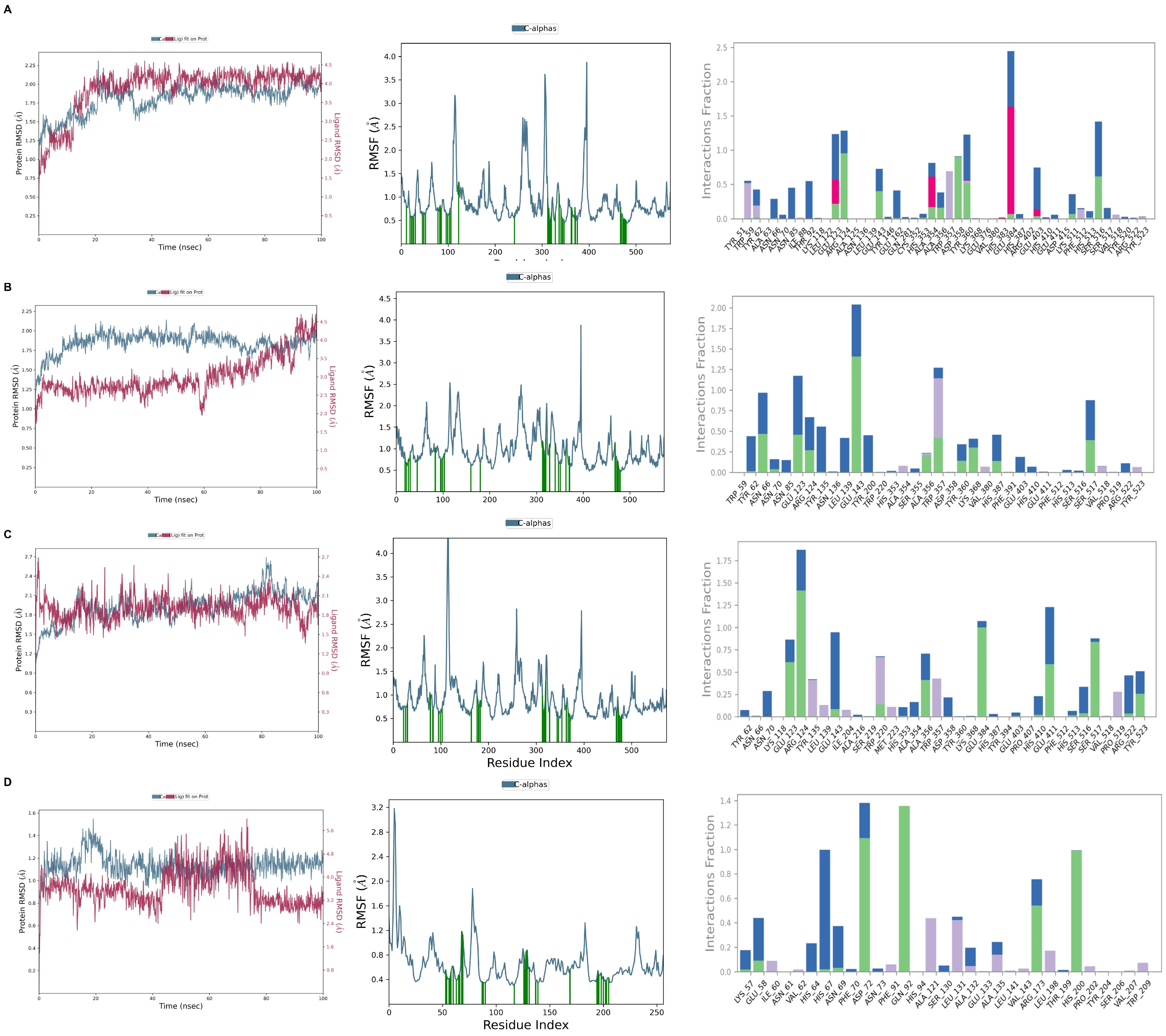
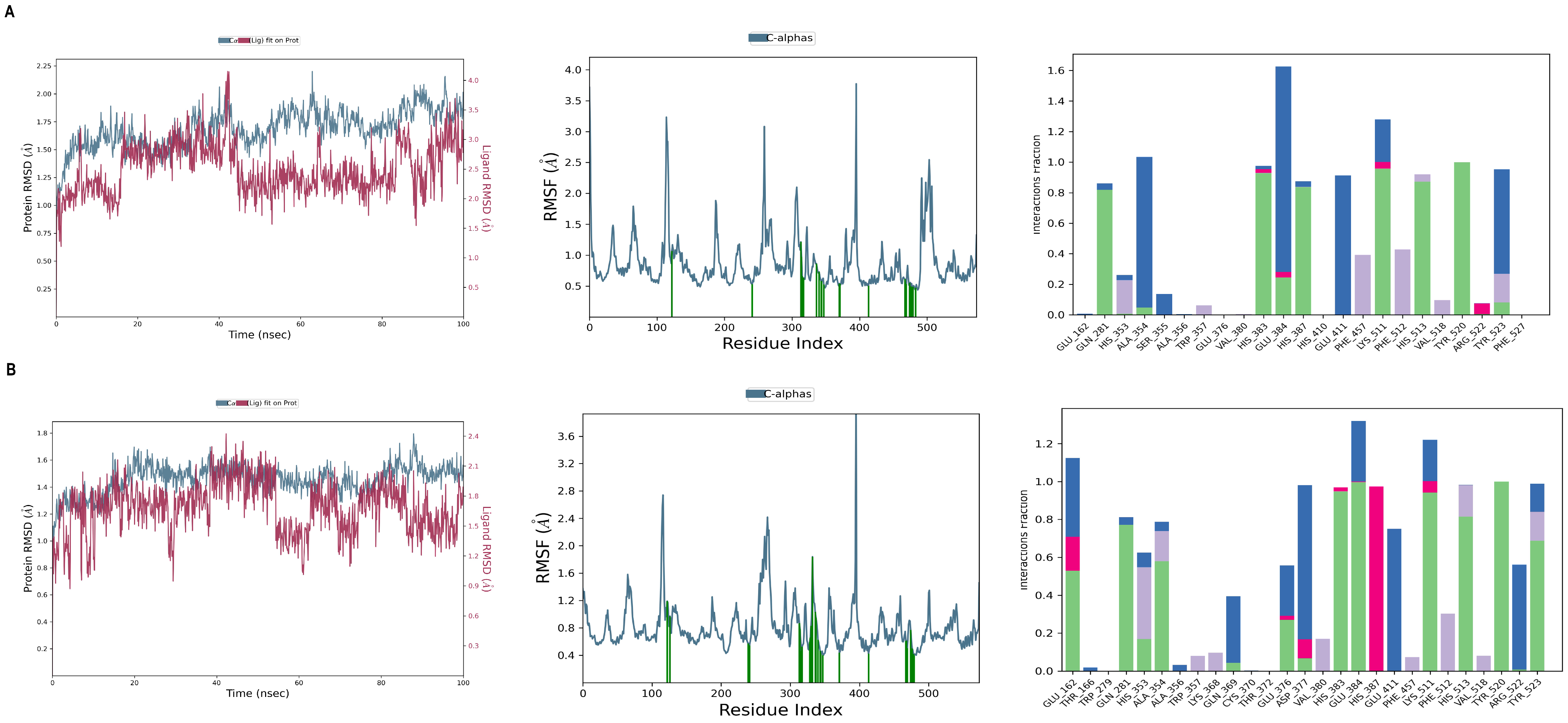
2.5. Molecular Dynamics Simulation
2.6. In Silico ADME and Toxicity Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of Compound and Target Proteins
4.2. Determination of Intersection Genes
4.3. Building Protein–Protein Interactions
4.4. Major Pathway and Gene Function Analysis Through Bioinformatics Tools
4.5. Molecular Docking of Ligands with Their Receptors
4.6. Validation of Protein–Ligand Score with Molecular Dynamic Simulation
4.7. In Silico ADME and Toxicity Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HBP | High blood pressure |
| ACE | Angiotensin-converting enzyme |
| CA-I | Carbonic anhydrase-I |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| FDA | Food and drug administration |
| DL | Drug-likeness |
| PPI | Protein-protein interaction |
| STRING | Search tool for retrieval of interacting genes/proteins |
| DC | Degree of connectivity |
| CC | Closeness centrality |
| BP | Biological processes |
| MF | Molecular function |
| DAVID | Database for annotation, visualization, and integrated discovery |
| RCSB | Research collaboratory for structural bioinformatics |
| PDB | Protein data bank |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuation |
| MAPK | Mitogen-activated protein kinase |
| GPCR | G protein-coupled receptor |
References
- Ma, J.; Li, Y.; Yang, X.; Kai, L.; Zhang, X.; Zuo, X.; Ye, R.; Wang, Z.; Shi, R.; Meng, Q.; et al. Signaling pathways in vascular function and hypertension: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Adugna, M.; Chanie, G.S.; Mohammed, A. Prevalence and associated factors of hypertension complications among hypertensive patients at University of Gondar Comprehensive Specialized Referral Hospital. Clin. Epidemiol. Glob. Health 2022, 13, 100951. [Google Scholar] [CrossRef]
- Mizher, H.A.A.; Noor, M.I.H.M.; Zaini, S. Natural remedies for hypertension: A systematic review. Pharmacol. Res. Nat. Prod. 2025, 6, 100145. [Google Scholar] [CrossRef]
- Meher, M.; Pradhan, S.; Pradhan, S.R. Risk Factors Associated With Hypertension in Young Adults: A Systematic Review. Cureus 2023, 15, e37467. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Angeli, F. Advances in the Treatment Strategies in Hypertension: Present and Future. JCDD 2022, 9, 72. [Google Scholar] [CrossRef]
- Asiamah, I.; Obiri, S.A.; Tamekloeb, W.; Armah, F.A.; Borquaye, L.S. Applications of molecular docking in natural products-based drug discovery. Sci. Afr. 2023, 20, e01593. [Google Scholar]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The antihypertensive potential of flavonoids from Chinese Herbal Medicine: A review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
- Verma, T.; Sinha, M.; Bansal, N.; Yadav, S.R.; Shah, K.; Chauhan, N.S. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2021, 11, 155–184. [Google Scholar] [CrossRef]
- Jung, J.; Kim, K.H.; Yang, K.; Bang, K.-H.; Yang, T.-J. Practical application of DNA markers for high-throughput authentication of Panax ginseng and Panax quinquefolius from commercial ginseng products. J. Ginseng Res. 2014, 38, 123–129. [Google Scholar] [CrossRef]
- Li, F.; Lv, C.; Li, Q.; Wang, J.; Song, D.; Liu, P.; Zhang, D.; Lu, J. Chemical and bioactive comparison of flowers of Panax ginseng Meyer, Panax quinquefolius L., Panax notoginseng Burk. J. Ginseng Res. 2017, 41, 487–495. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, H.-Y. Ginseng-derived compounds as potential anticancer agents targeting cancer stem cells. J. Ginseng Res. 2024, 48, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.-O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, Y.; Zhang, Z.; Lian, X.-Y. The necessity of eliminating the interference of panaxatriol saponins to maximize the preventive effect of panaxadiol saponins against Parkinson’s disease in rats. J. Ginseng Res. 2024, 48, 464–473. [Google Scholar] [CrossRef]
- Kim, Y.W.; Bak, S.B.; Song, Y.R.; Kim, C.-E.; Lee, W.-Y. Systematic exploration of therapeutic effects and key mechanisms of Panax ginseng Using Network-Based Approaches. J. Ginseng Res. 2024, 48, 373–383. [Google Scholar] [CrossRef]
- Balasubramaniam, M.; Sapuan, S.; Hashim, I.F.; Ismail, N.I.; Yaakop, A.S.; Kamaruzaman, N.A.; Mokhtar, A.M.A. The properties and mechanism of action of plant immunomodulators in regulation of immune response—A narrative review focusing on Curcuma longa L., Panax ginseng C. A. Meyer and Moringa oleifera Lam. Heliyon 2024, 10, e28261. [Google Scholar] [CrossRef]
- Shahzadi, Z.; Yousaf, Z.; Anjum, I.; Bilal, M.; Yasin, H.; Aftab, A.; Booker, A.; Ullah, R.; Bari, A. Network pharmacology and molecular docking: Combined computational approaches to explore the antihypertensive potential of Fabaceae species. Bioresour. Bioprocess. 2024, 11, 53. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Xun, Y. Molecular Mechanism of Salvia miltiorrhiza in the Treatment of Colorectal Cancer Based on Network Pharmacology and Molecular Docking Technology. Drug Des. Dev. Ther. 2024, 18, 425–441. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Keng, Y.Y.; Kwa, K.H.; Kurunathan, K. Centrality analysis in a drug network and its application to drug repositioning. Appl. Math. Comput. 2021, 395, 125870. [Google Scholar] [CrossRef]
- Pokhrel, A.; Chong, K.T.; Tayara, H. Therapeutic potential of curcuminoids in type 2 diabetes mellitus (T2DM): Insights from network pharmacology, molecular docking, and dynamics simulations. Food Biosci. 2025, 68, 106406. [Google Scholar] [CrossRef]
- Kario, K.; Hoshide, S.; Mogi, M. Hypertension treatment up-date on World Hypertension Day 2024: Current status and future prospects in Asia. Hypertens. Res. 2024, 47, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.L.; Ernst, M.E.; Cohen, J.D. Hydrochlorothiazide Versus Chlorthalidone: Evidence Supporting Their Interchangeability. Hypertension 2004, 43, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Suchard, M.A.; Krumholz, H.M.; Schuemie, M.J.; Shea, S.; Duke, J.; Pratt, N.; Reich, C.G.; Madigan, D.; You, S.C.; et al. Comparative First-Line Effectiveness and Safety of ACE (Angiotensin-Converting Enzyme) Inhibitors and Angiotensin Receptor Blockers: A Multinational Cohort Study. Hypertension 2021, 78, 591–603. [Google Scholar] [CrossRef]
- Smeets, N.J.L.; Schreuder, M.F.; Dalinghaus, M.; Male, C.; Lagler, F.B.; Walsh, J.; Laer, S.; De Wildt, S.N. Pharmacology of enalapril in children: A review. Drug Discov. Today 2020, 25, 1957–1970. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef]
- Ahmad, I.; Kuznetsov, A.E.; Pirzada, A.S.; Alsharif, K.F.; Daglia, M.; Khan, H. Computational pharmacology and computational chemistry of 4-hydroxyisoleucine: Physicochemical, pharmacokinetic, and DFT-based approaches. Front. Chem. 2023, 11, 1145974. [Google Scholar] [CrossRef]
- Nishinarizki, V.; Hardianto, A.; Gaffar, S.; Muchtaridi, M.; Herlina, T. Virtual screening campaigns and ADMET evaluation to unlock the potency of flavonoids from Erythrina as 3CLpro SARS-COV-2 inhibitors. J. Appl. Pharm. Sci. 2023, 13, 78–88. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Kim, J.-H. Ginseng and ginsenosides on cardiovascular and pulmonary diseases; Pharmacological potentials for the coronavirus (COVID-19). J. Ginseng Res. 2024, 48, 113–121. [Google Scholar] [CrossRef]
- Zheng, W.; Tian, E.; Liu, Z.; Zhou, C.; Yang, P.; Tian, K.; Liao, W.; Li, J.; Ren, C. Small molecule angiotensin converting enzyme inhibitors: A medicinal chemistry perspective. Front. Pharmacol. 2022, 13, 968104. [Google Scholar] [CrossRef]
- Prieto, M.C.; Gonzalez, A.A.; Visniauskas, B.; Navar, L.G. The evolving complexity of the collecting duct renin–angiotensin system in hypertension. Nat. Rev. Nephrol. 2021, 17, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Khan, H.; Haque, S.; Ahmad, S.; Srivastava, N.; Khan, A.; Olatunji, L.A. Angiotensin-Converting Enzyme and Hypertension: A Systemic Analysis of Various ACE Inhibitors, Their Side Effects, and Bioactive Peptides as a Putative Therapy for Hypertension. J. Renin-Angiotensin-Aldosterone Syst. 2023, 2023, 7890188. [Google Scholar] [CrossRef] [PubMed]
- García-Llorca, A.; Carta, F.; Supuran, C.T.; Eysteinsson, T. Carbonic anhydrase, its inhibitors and vascular function. Front. Mol. Biosci. 2024, 11, 1338528. [Google Scholar] [CrossRef] [PubMed]
- Carre, G.; Ouedraogo, M.; Magaud, C.; Carreyre, H.; Becq, F.; Bois, P.; Supuran, C.T.; Thibaudeau, S.; Vandebrouck, C.; Bescond, J. Vasorelaxation induced by dodoneine is mediated by calcium channels blockade and carbonic anhydrase inhibition on vascular smooth muscle cells. J. Ethnopharmacol. 2015, 169, 8–17. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Mei, Y.; Liu, L.; Li, L.; Yang, L.; Wang, Z. Rare ginsenosides: A unique perspective of ginseng research. J. Adv. Res. 2024, 66, 303–328. [Google Scholar] [CrossRef]
- Wang, K.; Miao, X.; Kong, F.; Huang, S.; Mo, J.; Jin, C.; Zheng, Y. Integrating Network Pharmacology and Experimental Verification to Explore the Mechanism of Effect of Zuojin Pills in Pancreatic Cancer Treatment. DDDT 2021, 15, 3749–3764. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Chen, G.; Mao, J.; Tian, X.; Zhan, S.; Peng, Z.; Zhu, Y.; Wang, W. Integration of network pharmacology, bioinformatics and experimental verification strategy to discover the pharmacological mechanisms of mogroside acts against pharyngitis. J. Ethnopharmacol. 2025, 344, 119499. [Google Scholar] [CrossRef]
- Sourav, C.; Chong, K.T.; Tayara, H. Exploring Nigella sativa anticancerous properties using network pharmacology, molecular docking and molecular dynamics simulation approach for non-small cell lung cancer. Food Biosci. 2025, 63, 105525. [Google Scholar] [CrossRef]
- Mouli, H.M.C.; Harini, D.; Shaikh, N.; Khemchandani, R.; Shreya, S.; Jana, A.; Samanthula, G. In silico characterization of indole-substituted densely functionalized pyrrole against breast cancer: Integrating DFT, molecular docking, MD simulations, and ADME analysis. J. Mol. Struct. 2025, 1328, 141375. [Google Scholar] [CrossRef]
- Enejoh, O.A.; Okonkwo, C.H.; Nortey, H.; Kemiki, O.A.; Moses, A.; Mbaoji, F.N.; Yusuf, A.S.; Awe, O.I. Machine learning and molecular dynamics simulations predict potential TGR5 agonists for type 2 diabetes treatment. Front. Chem. 2025, 12, 1503593. [Google Scholar] [CrossRef]
- Peele, K.A.; Durthi, C.P.; Srihansa, T.; Krupanidhi, S.; Ayyagari, V.S.; Babu, D.J.; Indira, M.; Reddy, A.R.; Venkateswarulu, T.C. Molecular docking and dynamic simulations for antiviral compounds against SARS-CoV-2: A computational study. Inform. Med. Unlocked 2020, 19, 100345. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, D.; Gu, C.; Huang, W. Biological Effects of Calceolarioside A as a Natural Compound: Anti-Ovarian Cancer, Anti-Tyrosinase, and Anti-HMG-CoA Reductase Potentials with Molecular Docking and Dynamics Simulation Studies. Mol. Biotechnol. 2025, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Padi, N.; Mathura, S.; Achilonu, I. Unravelling selectivity discrepancies of protoporphyrin binding to glutathione transferase: A comparative analysis of molecular dynamic simulated versus implicit solvent-minimized protein models. J. Mol. Graph. Model. 2025, 136, 108971. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.; Aljahdali, M.; Sumon, M.; Asseri, A.; Altayb, H.; Islam, M.; Alsaiari, A.; Opo, F.; Jahan, N.; Ahammad, F.; et al. Integrative Ligand-Based Pharmacophore Modeling, Virtual Screening, and Molecular Docking Simulation Approaches Identified Potential Lead Compounds against Pancreatic Cancer by Targeting FAK1. Pharmaceuticals 2023, 16, 120. [Google Scholar] [CrossRef]
- Kumar, A.; Rajput, D.; Gupta, N.; Singh, H.; Chopra, S.; Chopra, H. In Silico Identification of Promising PDE5 Inhibitors Against Hepatocellular Carcinoma Among Natural Derivatives: A Study Involving Docking and ADMET Analysis. Drug Res. 2025, 75, 21–33. [Google Scholar] [CrossRef]
- Jurowski, K.; Niżnik, Ł.; Frydrych, A.; Kobylarz, D.; Noga, M.; Krośniak, A.; Fijałkowska, O.; Świdniak, A.; Ahuja, V. Toxicological profile of Acovenoside A as an active pharmaceutical ingredient–prediction of missing key toxicological endpoints using in silico toxicology methodology. Chem.-Biol. Interact. 2025, 408, 111404. [Google Scholar] [CrossRef]
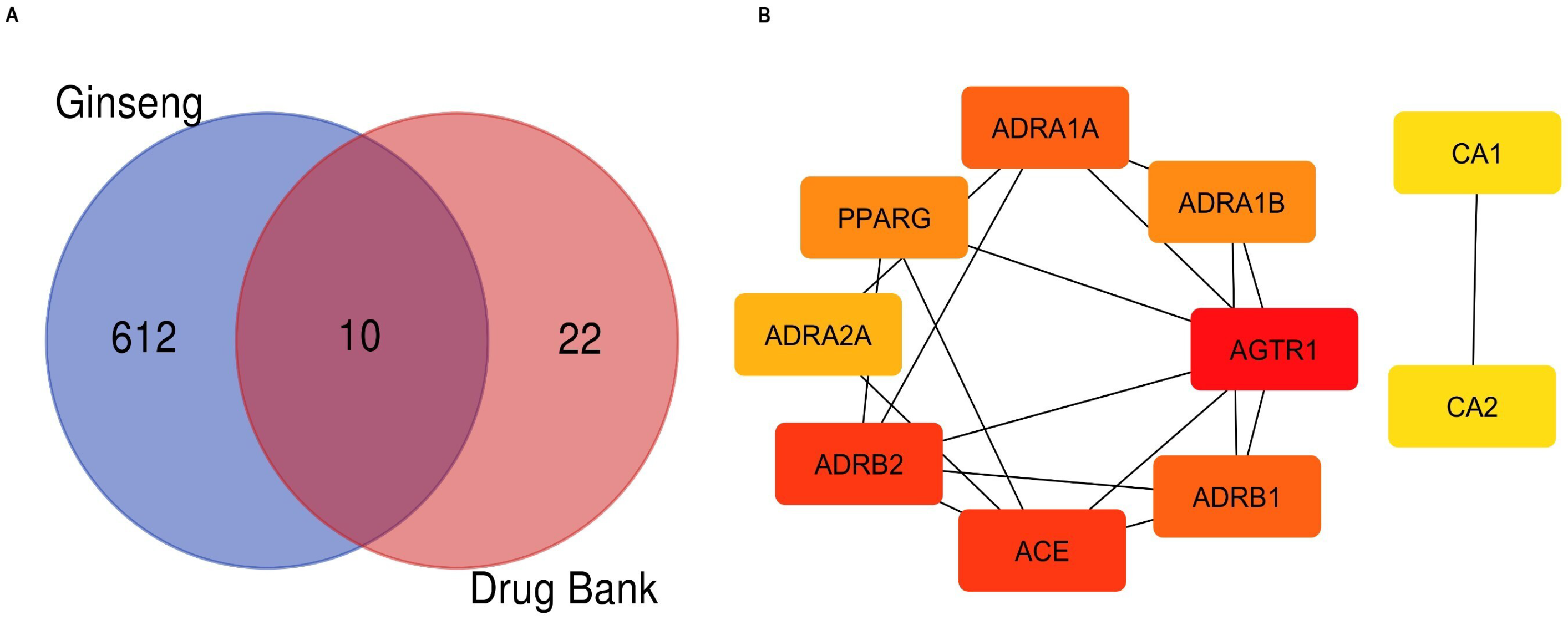



| S. No | Genes | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|
| 1 | AGTR1 | 6 | 0.158 | 0.875 |
| 2 | ADRB2 | 5 | 0.071 | 0.778 |
| 3 | ACE | 5 | 0.040 | 0.778 |
| 4 | ADRA1A | 4 | 0.111 | 0.700 |
| 5 | ADRB1 | 4 | 0.040 | 0.700 |
| 6 | ADRA1B | 3 | 0.016 | 0.636 |
| 7 | PPARG | 3 | 0.000 | 0.636 |
| 8 | ADRA2A | 2 | 0.016 | 0.583 |
| 9 | CA1 | 1 | 0.000 | 1.000 |
| 10 | CA2 | 1 | 0.000 | 1.000 |
| S. No | Compound Name | CID Number | Docking Score (kcal/mol) | Molecular Weight (g/mol) |
|---|---|---|---|---|
| 1 | Floralquinquenoside C | 23652173 | −7.7578 | 817.0 |
| 2 | Ginsenoside Rg6 | 91895489 | −7.5202 | 767.0 |
| 3 | Ginsenoside Km | 102294900 | −6.7204 | 668.9 |
| 4 | Notoginsenoside T1 | 131752527 | −6.7279 | 652.9 |
| 5 | Ginsenoside Ki | 102294899 | −6.0429 | 668.9 |
| 6 | Floralginsenoside M | 101423540 | −6.6718 | 963.2 |
| 7 | Floralquinquenoside B | 23652021 | −6.5276 | 817.0 |
| Properties | Parameters | Floralquinquenoside C | Ginsenoside Rg6 | Notoginsenoside T1 | Floralquinquenoside B | Decision | Unit |
|---|---|---|---|---|---|---|---|
| Absorption | Water Solubility | −2.953 | −3.43 | −4.378 | −2.988 | Numeric | log mol/L |
| CaCO-2 Permeability | −0.642 | 0.569 | 0.393 | −0.63 | Numeric | log Papp (10−6 cm/s) | |
| Intestinal Absorption (Human) | 19.012 | 42.19 | 41.082 | 18.969 | Numeric | % Absorbed | |
| Skin Permeability | −2.735 | −2.735 | −2.744 | −2.735 | Numeric | log Kp | |
| P-glycoprotein Substrate | Yes | Yes | Yes | Yes | Categorical | Yes/No | |
| Distribution | Volume Distribution (VDss) | −0.556 | −0.719 | −0.749 | −0.578 | Numeric | log L/kg |
| Fraction Unbound (Human) | 0.422 | 0.312 | 0.308 | 0.42 | Numeric | Fu | |
| BBB Permeability | −1.613 | −1.111 | −1.157 | −1.683 | Numeric | log BB | |
| Metabolism | CYP1A2 Inhibitor | No | No | No | No | Categorical | Yes/No |
| CYP2C19 | No | No | No | No | Categorical | Yes/No | |
| CYP2C9 | No | No | No | No | Categorical | Yes/No | |
| CYP2D6 | No | No | No | No | Categorical | Yes/No | |
| CYP3A4 | No | No | No | No | Categorical | Yes/No | |
| Excretion | Total Clearance | 0.458 | 0.485 | 0.334 | 0.593 | Numeric | log mL/min/kg |
| Renal OCT2 Substrate | No | No | No | No | Categorical | Yes/No |
| Ligands | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Cytotoxicity |
|---|---|---|---|---|---|
| Floralquinquenoside C | Inactive | Inactive | Inactive | Inactive | Inactive |
| Ginsenoside Rg6 | Inactive | Inactive | Inactive | Inactive | Inactive |
| Notoginsenoside T1 | Inactive | Inactive | Inactive | Inactive | Inactive |
| Floralquinquenoside B | Inactive | Inactive | Inactive | Inactive | Inactive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurmi, S.; Majhi, R.; Tayara, H.; Chong, K.T. Exploring Ginseng Bioactive Compound’s Role in Hypertension Remedy: An In Silico Approach. Pharmaceuticals 2025, 18, 648. https://doi.org/10.3390/ph18050648
Kurmi S, Majhi R, Tayara H, Chong KT. Exploring Ginseng Bioactive Compound’s Role in Hypertension Remedy: An In Silico Approach. Pharmaceuticals. 2025; 18(5):648. https://doi.org/10.3390/ph18050648
Chicago/Turabian StyleKurmi, Sagar, Rita Majhi, Hilal Tayara, and Kil To Chong. 2025. "Exploring Ginseng Bioactive Compound’s Role in Hypertension Remedy: An In Silico Approach" Pharmaceuticals 18, no. 5: 648. https://doi.org/10.3390/ph18050648
APA StyleKurmi, S., Majhi, R., Tayara, H., & Chong, K. T. (2025). Exploring Ginseng Bioactive Compound’s Role in Hypertension Remedy: An In Silico Approach. Pharmaceuticals, 18(5), 648. https://doi.org/10.3390/ph18050648









