Neuroprotective Potential of Tetraselmis chuii Compounds: Insights into Blood–Brain Barrier Permeability and Intestinal Transport
Abstract
1. Introduction
2. Results
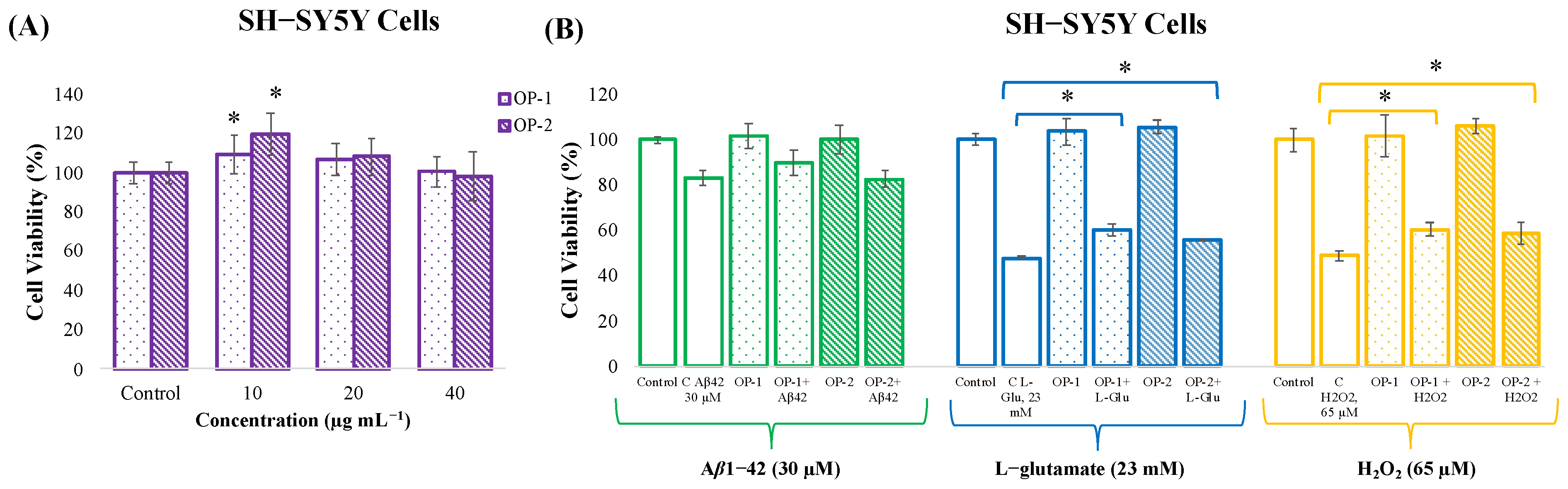
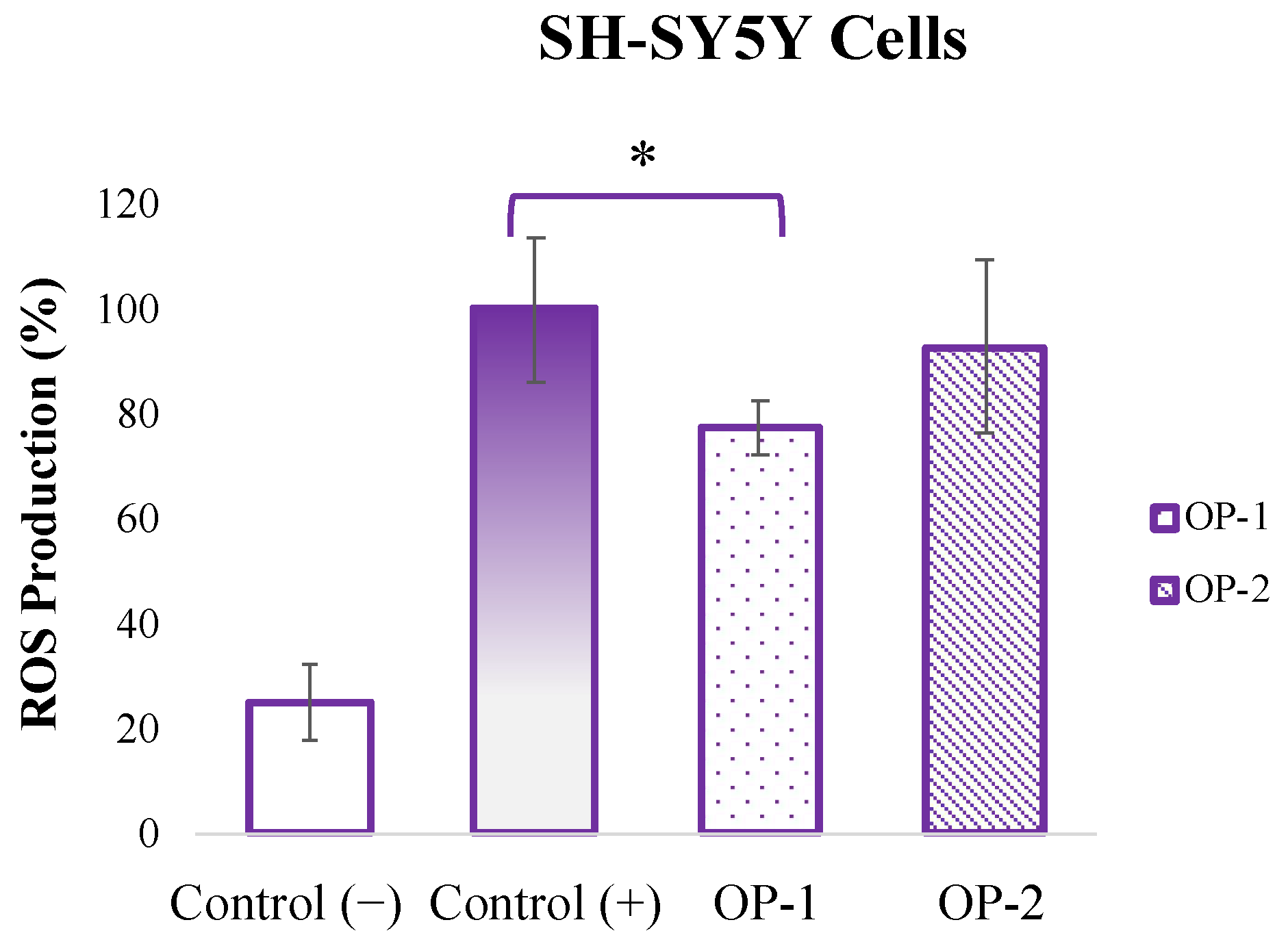
2.1. In Vitro Neuroprotective Potential of T. chuii Extracts in SH-SY5Y Cells
2.2. Parallel Artificial Membrane Permeability Assay for the Blood–Brain Barrier (PAMPA-BBB)
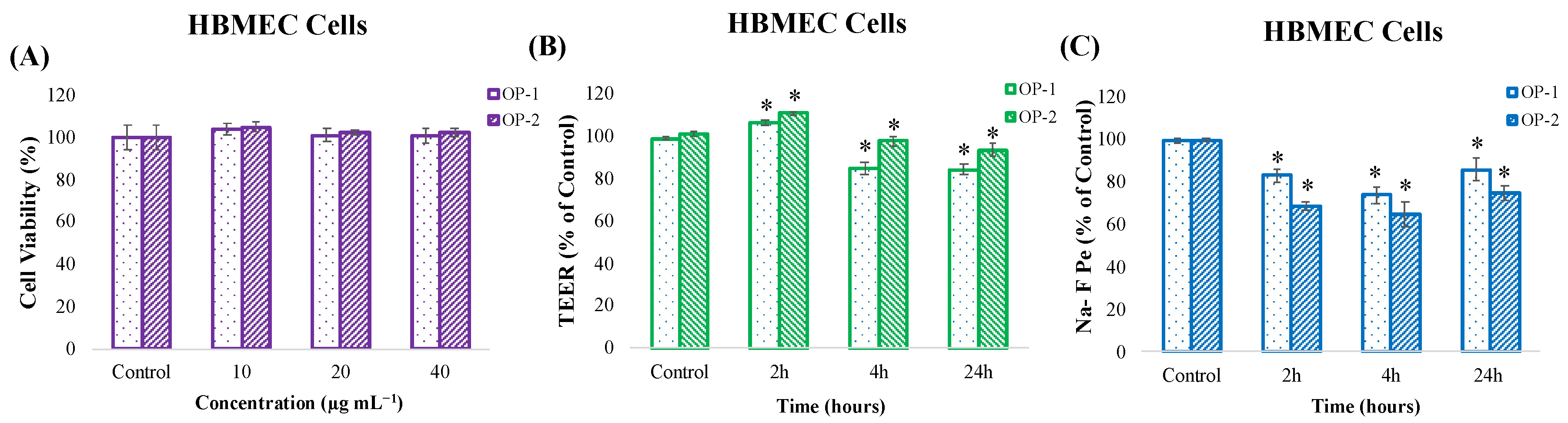
2.3. In Vitro Toxicity and Cell Barrier Integrity Assay of T. chuii Extracts in HBMEC Cells
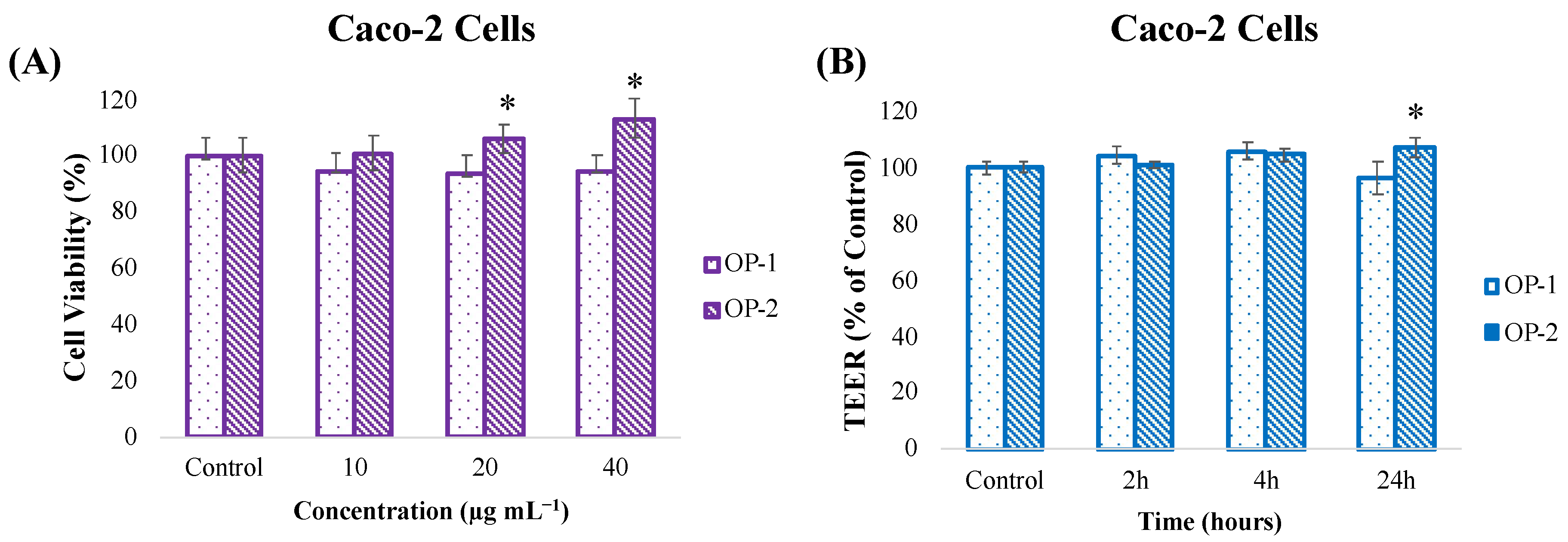
2.4. In Vitro Toxicity and Cell Barrier Integrity Assay of T. chuii Extracts in Caco-2 Cells
2.5. Evaluation of Carotenoids Transport Across the BBB and the Intestinal Barrier Endothelium
3. Discussion
4. Materials and Methods
4.1. Samples, Chemicals, and Reagents
4.2. Carotenoids-Enriched Microalga Extract
4.3. Cell Culture Assays in SH-SY5Y Cells
4.3.1. Toxicity Evaluation of T. chuii Extracts
4.3.2. Neuroprotection Evaluation of T. chuii Extracts
4.3.3. Antioxidant Capacity of T. chuii Extracts
4.4. Parallel Artificial Membrane Permeability for the Blood–Brain Barrier (PAMPA-BBB)
4.5. Cell Culture Assays in HBMEC Cells
4.5.1. Toxicity Evaluation of T. chuii Extracts
4.5.2. Blood–Brain Barrier Transport Study of T. chuii Extracts
4.5.3. Cell Barrier Integrity
Transendothelial Electrical Resistance (TEER)
Sodium Fluorescein (Na-F) Paracellular Permeability
4.6. Cell Culture Assays in Caco-2 Cells
4.6.1. Toxicity Evaluation of T. chuii Extracts
4.6.2. Intestinal Transepithelial Transport Study of T. chuii Extracts
4.7. Quantification of Carotenoids in the Transport Assays
4.7.1. Carotenoid Extraction
4.7.2. Liquid Chromatography–Tandem Mass Spectrometry (UHPLC-Q-TOF-MS)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamisa, A.B.; Ahammad, I.; Bhattacharjee, A.; Hossain, M.U.; Ishtiaque, A.; Chowdhury, Z.M.; Das, K.C.; Salimullah, M.; Keya, C.A. A Meta-Analysis of Bulk RNA-Seq Datasets Identifies Potential Biomarkers and Repurposable Therapeutics against Alzheimer’s Disease. Sci. Rep. 2024, 14, 24717. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Albratty, M. An Update on the Novel and Approved Drugs for Alzheimer Disease. Saudi Pharm. J. 2022, 30, 1755–1764. [Google Scholar] [CrossRef]
- Dingezweni, S. The Blood–Brain Barrier. South. Afr. J. Anaesth. Analg. 2020, 26, S32–S34. [Google Scholar] [CrossRef]
- Kam, A.; Li, M.; Razmovski-Naumovski, V.; Nammi, S.; Chan, K.; Li, Y.; Li, Q. The Protective Effects of Natural Products on Blood-Brain Barrier Breakdown. Curr. Med. Chem. 2012, 19, 1830–1845. [Google Scholar] [CrossRef]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation With Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef]
- Valdés, A.; Sánchez-Martínez, J.D.; Gallego, R.; Ibáñez, E.; Herrero, M.; Cifuentes, A. In Vivo Neuroprotective Capacity of a Dunaliella salina Extract—Comprehensive Transcriptomics and Metabolomics Study. NPJ Sci. Food 2024, 8, 4. [Google Scholar] [CrossRef]
- Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Suárez-Montenegro, Z.J.; Ibáñez, E.; Cifuentes, A.; Herrero, M. Study of the Potential Neuroprotective Effect of Dunaliella salina Extract in SH-SY5Y Cell Model. Anal. Bioanal. Chem. 2022, 414, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Bhat, I.; Bangera Sheshappa, M. Lutein and the Underlying Neuroprotective Promise against Neurodegenerative Diseases. Mol. Nutr. Food Res. 2024, 68, 2300409. [Google Scholar] [CrossRef]
- Medoro, A.; Davinelli, S.; Milella, L.; Willcox, B.J.; Allsopp, R.C.; Scapagnini, G.; Willcox, D.C. Dietary Astaxanthin: A Promising Antioxidant and Anti-Inflammatory Agent for Brain Aging and Adult Neurogenesis. Mar. Drugs 2023, 21, 643. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Z.; Chen, F.; Li, B.; Jin, H. Lutein Inhibits Glutamate-Induced Apoptosis in HT22 Cells via the Nrf2/HO-1 Signaling Pathway. Front. Neurosci. 2024, 18, 1432969. [Google Scholar] [CrossRef] [PubMed]
- Pruccoli, L.; Balducci, M.; Pagliarani, B.; Tarozzi, A. Antioxidant and Neuroprotective Effects of Fucoxanthin and Its Metabolite Fucoxanthinol: A Comparative In Vitro Study. Curr. Issues Mol. Biol. 2024, 46, 5984–5998. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, H.; Ding, Y.; Liu, S.; Ding, Y.; Lu, B.; Xiao, J.; Zhou, X. Dietary Protective Potential of Fucoxanthin as an Active Food Component on Neurological Disorders. J. Agric. Food Chem. 2023, 71, 3599–3619. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Kim, M.R. Neuroprotective Effect of Carotenoid-Rich Enteromorpha Prolifera Extract via TrkB/Akt Pathway against Oxidative Stress in Hippocampal Neuronal Cells. Mar. Drugs 2020, 18, 372. [Google Scholar] [CrossRef]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2023, 15, 92. [Google Scholar] [CrossRef]
- Ikeda, C.; Manabe, Y.; Tomonaga, N.; Wada, T.; Maoka, T.; Sugawara, T. Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Mar. Drugs 2020, 18, 588. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sugahara, K.; Takemoto, Y.; Tsuda, J.; Hirose, Y.; Hashimoto, M.; Yamashita, H. Protective Effect of Astaxanthin Nanoemulsion on Mammalian Inner Ear Hair Cells. PeerJ 2023, 9, 364–371. [Google Scholar] [CrossRef]
- Zanoni, F.; Vakarelova, M.; Zoccatelli, G. Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles. Mar. Drugs 2019, 17, 627. [Google Scholar] [CrossRef]
- Freitas, M.A.; Ferreira, J.; Nunes, M.C.; Raymundo, A. The Chemistry and Bioactive Properties behind Microalgae-Enriched Gluten-Free Breads. Int. J. Food Sci. Technol. 2024, 59, 872–885. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- FDA. GRAS Notice Inventory; US Food and Drug Administration: Silver Spring, MD, USA, 2016; Volume 26.
- Mantecón, L.; Moyano, R.; Cameán, A.M.; Jos, A. Safety Assessment of a Lyophilized Biomass of Tetraselmis chuii (TetraSOD®) in a 90 Day Feeding Study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef] [PubMed]
- Conlon, T.; Aranyos, A.; Luck, T.; Touzet, N. The Effects of Trophic Mode and Medium Composition on the Biochemical Profile and Antioxidant Capacity of Tetraselmis chuii (CCAP 66/21B). Biocatal. Agric. Biotechnol. 2024, 61, 103362. [Google Scholar] [CrossRef]
- Paterson, S.; Villanueva-Bermejo, D.; Hernández-Ledesma, B.; Gómez-Cortés, P.; de la Fuente, M.A. Supercritical CO2 Extraction Increases the Recovery Levels of Omega-3 Fatty Acids in Tetraselmis chuii Extracts. Food Chem. 2024, 453, 139692. [Google Scholar] [CrossRef]
- Cocksedge, S.P.; Mantecón, L.; Castaño, E.; Infante, C.; Bailey, S.J. The Potential of Superoxide Dismutase-Rich Tetraselmis chuii as a Promoter of Cellular Health. Int. J. Mol. Sci. 2025, 26, 1693. [Google Scholar] [CrossRef]
- Cokdinleyen, M.; Alvarez-Rivera, G.; Tejera, J.L.G.; Mendiola, J.A.; Valdés, A.; Kara, H.; Ibáñez, E.; Cifuentes, A. Tetraselmis chuii Edible Microalga as a New Source of Neuroprotective Compounds Obtained Using Fast Biosolvent Extraction. Int. J. Mol. Sci. 2024, 25, 3897. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In Vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Isabel, U.V.; de la Riera, M.; Belén, A.; Dolores, R.S.; Elena, G.B. A New Frontier in Neuropharmacology: Recent Progress in Natural Products Research for Blood–Brain Barrier Crossing. Curr. Res. Biotechnol. 2024, 8, 100235. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Garcia, A.R.; Alvarez-Rivera, G.; Valdés, A.; Brito, M.A.; Cifuentes, A. In Vitro Study of the Blood–Brain Barrier Transport of Natural Compounds Recovered from Agrifood By-Products and Microalgae. Int. J. Mol. Sci. 2023, 24, 533. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Ed.; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar] [CrossRef]
- Cokdinleyen, M.; dos Santos, L.C.; de Andrade, C.J.; Kara, H.; Colás-Ruiz, N.R.; Ibañez, E.; Cifuentes, A. A Narrative Review on the Neuroprotective Potential of Brown Macroalgae in Alzheimer’s Disease. Nutrients 2024, 16, 4394. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Georgiopoulou, I.; Antoniou, N.; Tzima, S.; Kontou, M.; Louli, V.; Fatouros, C.; Magoulas, K.; Kolisis, F.N. Extracts from Chlorella vulgaris Protect Mesenchymal Stromal Cells from Oxidative Stress Induced by Hydrogen Peroxide. Plants 2023, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.X.; Qiu, Y.H.; Liu, Y.; Yu, L.L.; Zhang, X.; Tsim, K.W.K.; Qin, Q.W.; Hu, W.H. Fucoxanthin Attenuates Angiogenesis by Blocking the VEGFR2-Mediated Signaling Pathway through Binding the Vascular Endothelial Growth Factor. J. Agric. Food Chem. 2024, 72, 21610–21623. [Google Scholar] [CrossRef]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef]
- Pang, Q.Q.; Kim, J.H.; Kim, H.Y.; Kim, J.H.; Cho, E.J. Protective effects and mechanisms of pectolinarin against H2O2-induced oxidative stress in SH-SY5Y neuronal cells. Molecules 2023, 28, 5826. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Manochkumar, J.; Doss, C.G.P.; El-Seedi, H.R.; Efferth, T.; Ramamoorthy, S. The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine 2021, 91, 153676. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.; Rout, S.; Mali, S.N.; Oliveira, M. Neuroprotective Potential of Carotenoids. In Carotenoids; Campos Chisté, R., Helena de Aguiar Andrade, E., Santana de Oliveira, M., Eds.; Springer: Cham, Switzerland, 2024; pp. 259–278. [Google Scholar] [CrossRef]
- Banerjee, S.; Baghel, D.; Pacheco de Oliveira, A.; Ghosh, A. β-Carotene, a Potent Amyloid Aggregation Inhibitor, Promotes Disordered Aβ Fibrillar Structure. Int. J. Mol. Sci. 2023, 24, 5175. [Google Scholar] [CrossRef]
- de Oliveira Caland, R.B.; Cadavid, C.O.M.; Carmona, L.; Peña, L.; de Paula Oliveira, R. Pasteurized Orange Juice Rich in Carotenoids Protects Caenorhabditis elegans against Oxidative Stress and β-Amyloid Toxicity through Direct and Indirect Mechanisms. Oxid. Med. Cell. Longev. 2019, 2019, 5046280. [Google Scholar] [CrossRef]
- Brasil, F.B.; de Almeida, F.J.S.; Luckachaki, M.D.; Dall’Oglio, E.L.; de Oliveira, M.R. Astaxanthin prevents mitochondrial impairment in the dopaminergic SH-SY5Y cell line exposed to glutamate-mediated excitotoxicity: Role for the Nrf2/HO-1/CO-BR axis. Eur. J. Pharmacol. 2021, 908, 174336. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Inan, B.; Mutlu, B.; Cakır, R.; Balkanlı, D. From ice to neurons: Investigating the neuroprotective effects of Antarctic microalgae Chlorella variabilis and Chlorella pyrenoidosa extracts. 3 Biotech. 2024, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Carotenoids from Mamey (Pouteria sapota) and Carrot (Daucus carota) Increase the Oxidative Stress Resistance of Caenorhabditis elegans. Biochem. Biophys. Rep. 2021, 26, 100989. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, C.Q.; Yan, Y.F.; Liu, B.R.; Zu, C.L. Effect of Increased UV-B Radiation on Carotenoid Accumulation and Total Antioxidant Capacity in Tobacco (Nicotiana tabacum L.) Leaves. Genet. Mol. Res. 2017, 16, gmr16018438. [Google Scholar] [CrossRef]
- Wei, J.; Ye, Z.; Li, Y.; Li, Y.; Zhou, Z. Citrus Carotenoid Extracts Promote ROS Accumulation and Induce Oxidative Stress to Exert Anti-Proliferative and Pro-Apoptotic Effects in MDA-MB-231 Cells. Antioxidants 2024, 13, 264. [Google Scholar] [CrossRef]
- Islam, F.; Khan, J.; Zehravi, M.; Das, R.; Haque, M.A.; Banu, A.; Parwaiz, S.; Nainu, F.; Nafady, M.H.; Shahriar, S.M.S.; et al. Synergistic effects of carotenoids: Therapeutic benefits on human health. Process Biochem. 2024, 136, 254–272. [Google Scholar] [CrossRef]
- Shi, J.; Kakuda, Y.; Yeung, D. Antioxidative Properties of Lycopene and Other Carotenoids from Tomatoes: Synergistic Effects. Biofactors 2004, 21, 203–210. [Google Scholar] [CrossRef]
- Sowmya, P.; Arathi, B.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Astaxanthin from Shrimp Efficiently Modulates Oxidative Stress and Allied Cell Death Progression in MCF-7 Cells Treated Synergistically with β-Carotene and Lutein from Greens. Food Chem. Toxicol. 2017, 106, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, Z.; Zheng, L.; Zhang, B.; Luo, T.; Li, H. Interaction between Flavonoids and Carotenoids on Ameliorating Oxidative Stress and Cellular Uptake in Different Cells. Foods 2021, 10, 3096. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High Throughput Artificial Membrane Permeability Assay for Blood–Brain Barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered From Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, T.C.; Pinheiro, P.N.; Fernandes, A.S.; Murador, D.C.; Neves, B.V.; de Menezes, C.R.; de Rosso, V.V.; Jacob-Lopes, E.; Zepka, L.Q. Bioaccessibility and Intestinal Uptake of Carotenoids from Microalgae Scenedesmus obliquus. LWT 2021, 140, 110780. [Google Scholar] [CrossRef]
- Cornelissen, F.M.G.; Markert, G.; Deutsch, G.; Antonara, M.; Faaij, N.; Bartelink, I.; Noske, D.; Vandertop, W.P.; Bender, A.; Westerman, B.A. Explaining Blood-Brain Barrier Permeability of Small Molecules by Integrated Analysis of Different Transport Mechanisms. J. Med. Chem. 2023, 66, 7253–7267. [Google Scholar] [CrossRef]
- Shah, B.; Dong, X. Current Status of In Vitro Models of the Blood-Brain Barrier. Curr. Drug Deliv. 2022, 19, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Liebner, S. Structure and Function of the Blood–Brain Barrier (BBB). Handb. Exp. Pharmacol. 2020, 273, 3–31. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Marques, D.; Figueira, I.; Cankar, K.; Bosch, D.; Brito, M.A.; dos Santos, C.N. Costunolide and Parthenolide: Novel Blood-Brain Barrier Permeable Sesquiterpene Lactones to Improve Barrier Tightness. Biomed. Pharmacother. 2023, 167, 115413. [Google Scholar] [CrossRef]
- Scalise, A.A.; Kakogiannos, N.; Zanardi, F.; Iannelli, F.; Giannotta, M. The Blood–Brain and Gut–Vascular Barriers: From the Perspective of Claudins. Tissue Barriers 2021, 9, 1926190. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Ward, L.J.; Arefin, S.; Ebert, T.; Laucyte-Cibulskiene, A.; Pilote, L.; Norris, C.M.; Raparelli, V.; Kautzky-Willer, A.; Herrero, M.T.; et al. Blood–Brain Barrier and Gut Barrier Dysfunction in Chronic Kidney Disease with a Focus on Circulating Biomarkers and Tight Junction Proteins. Sci. Rep. 2022, 12, 4414. [Google Scholar] [CrossRef]
- Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; Santana-Filho, A.P.; Watanabe, P.D.S.; Sant’Ana, D.D.M.G.; Luciano, F.B.; Bocate, K.C.P.; van den Wijngaard, R.M.; Werner, M.F.D.P.; et al. Rhamnogalacturonan, a Chemically-Defined Polysaccharide, Improves Intestinal Barrier Function in DSS-Induced Colitis in Mice and Human Caco-2 Cells. Sci. Rep. 2018, 8, 12261. [Google Scholar] [CrossRef]
- Amasheh, M.; Schlichter, S.; Amasheh, S.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Quercetin Enhances Epithelial Barrier Function and Increases Claudin-4 Expression in Caco-2 Cells. J. Nutr. 2008, 138, 1067–1073. [Google Scholar] [CrossRef]
- Iftikhar, M.; Iftikhar, A.; Zhang, H.; Gong, L.; Wang, J. Transport, Metabolism and Remedial Potential of Functional Food Extracts (FFEs) in Caco-2 Cells Monolayer: A Review. Food Res. Int. 2020, 136, 109240. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.L.; Rodrigues, D.B.; Chitchumroonchokchai, C. Bioavailability and Metabolism of Carotenoid Esters. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; Mercadante, A., Ed.; Royal Society of Chemistry: London, UK, 2019; Chapter 13; pp. 390–420. [Google Scholar] [CrossRef]
- Flieger, J.F.A.F.W. Carotenoid Supplementation for Alleviating the Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 8982. [Google Scholar] [CrossRef]
- Si, P.; Zhu, C. Biological and Neurological Activities of Astaxanthin (Review). Mol. Med. Rep. 2022, 26, 300. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef]
- Paul, R.; Mazumder, M.K.; Nath, J.; Deb, S.; Paul, S.; Bhattacharya, P.; Borah, A. Lycopene—A Pleiotropic Neuroprotective Nutraceutical: Deciphering Its Therapeutic Potentials in Broad Spectrum Neurological Disorders. Neurochem. Int. 2020, 140, 104823. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin Has Potential for Therapeutic Efficacy in Neurodegenerative Disorders by Acting on Multiple Targets. Nutr. Neurosci. 2022, 25, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Suzuki, S.; Ushida, Y.; Sato, I.; Suganuma, H. Neoxanthin Is Undetectable in Human Blood after Ingestion of Fresh Young Spinach Leaf. PLoS ONE 2023, 18, 0288143. [Google Scholar] [CrossRef]
- Tang, G.F.; Zhang, M.R.; Liu, Q.Q.; Mai, R.R. Applications of nanodiamonds in the diagnosis and treatment of neurological diseases. J. Nanopart. Res. 2022, 24, 55. [Google Scholar] [CrossRef]
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Lopedota, A.; Franco, M.; Laquintana, V. Transferrin Functionalized Liposomes Loading Dopamine HCl: Development and Permeability Studies across an In Vitro Model of Human Blood-Brain Barrier. Nanomaterials 2018, 8, 178. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid. Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Huang, S.; Ding, X. Precise Design Strategies of Nanotechnologies for Controlled Drug Delivery. J. Funct. Biomater. 2022, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Shitara, Y.; Maeda, K.; Ikejiri, K.; Yoshida, K.; Horie, T.; Sugiyama, Y. Clinical Significance of Organic Anion Transporting Polypeptides (OATPs) in Drug Disposition: Their Roles in Hepatic Clearance and Intestinal Absorption. Biopharm. Drug Dispos. 2013, 34, 45–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Chen, P.C.; Ho, C.T.; Lin-Shiau, S.Y. Inhibition of Xanthine Oxidase and Suppression of Intracellular Reactive Oxygen Species in HL-60 Cells by Theaflavin-3,3′-Digallate, (-)- Epigallocatechin-3-Gallate, and Propyl Gallate. J. Agric. Food Chem. 2000, 48, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Pogačnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kwang, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F.M. Potential for Brain Accessibility and Analysis of Stability of Selected Flavonoids in Relation to Neuroprotection in Vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- Deli, M.A.; Ábrahám, C.S.; Kataoka, Y.; Niwa, M. Permeability Studies on in Vitro Blood-Brain Barrier Models: Physiology, Pathology, and Pharmacology. Cell. Mol. Neurobiol. 2005, 25, 59–127. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Khattabi, C.E.; Youl, E.N.H.; Bertolino, M.; Delporte, C.; Pochet, S.; Stévigny, C. Polyphenolic and Methylxanthine Bioaccessibility of Cocoa Bean Shell Functional Biscuits: Metabolomics Approach and Intestinal Permeability through CaCo-2 Cell Models. Antioxidants 2020, 9, 1164. [Google Scholar] [CrossRef]
| Compound | Molecular Formula | Molecular Weight (m/z) | Te% ± SEM | |||||
|---|---|---|---|---|---|---|---|---|
| HBMEC Cells | Caco-2 Cells | |||||||
| 2 h | 4 h | 24 h | 2 h | 4 h | 24 h | |||
| Fucoxanthinol | C40H56O5 | 617.4191 | 4.4 ± 1.1 c | 5.1 ± 0.1 b | 18.9 ± 2.3 a | 7.0 ± 0.2 c | 8.6 ± 2.2 b | 18.4 ± 7.2 a |
| Diatoxanthin | C40H54O2 | 567.4208 | n.d | 1.1 ± 0.1 b | 8.1 ± 3.4 a | 1.4 ± 0.1 c | 2.3 ± 0.3 b | 4.4 ± 0.9 a |
| Neoxanthin | C40H56O4 | 601.4266 | n.d | 1.4 ± 0.2 b | 13.2 ± 4.3 a | 0.5 ± 0.3 b | 0.4 ± 0.1 b | 0.6 ± 0.4 a |
| Violaxanthin | C40H56O4 | 601.4258 | n.d | n.d | 13.9 ± 6.0 | n.d | n.d | n.d |
| Prasinoxanthin | C40H56O4 | 601.4235 | n.d | n.d | 8.9 ± 2.3 | n.d | n.d | n.d |
| Crocoxanthin | C40H54O | 551.4229 | 3.4 ± 3.2 a | 0.9 ± 0.3 b | 2.7 ± 0.6 a | 12.0 ± 9.5 a | 11.8 ± 5.6 a | 12.1 ± 1.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cokdinleyen, M.; Valdés, A.; Kara, H.; Ibáñez, E.; Cifuentes, A. Neuroprotective Potential of Tetraselmis chuii Compounds: Insights into Blood–Brain Barrier Permeability and Intestinal Transport. Pharmaceuticals 2025, 18, 629. https://doi.org/10.3390/ph18050629
Cokdinleyen M, Valdés A, Kara H, Ibáñez E, Cifuentes A. Neuroprotective Potential of Tetraselmis chuii Compounds: Insights into Blood–Brain Barrier Permeability and Intestinal Transport. Pharmaceuticals. 2025; 18(5):629. https://doi.org/10.3390/ph18050629
Chicago/Turabian StyleCokdinleyen, Melis, Alberto Valdés, Huseyin Kara, Elena Ibáñez, and Alejandro Cifuentes. 2025. "Neuroprotective Potential of Tetraselmis chuii Compounds: Insights into Blood–Brain Barrier Permeability and Intestinal Transport" Pharmaceuticals 18, no. 5: 629. https://doi.org/10.3390/ph18050629
APA StyleCokdinleyen, M., Valdés, A., Kara, H., Ibáñez, E., & Cifuentes, A. (2025). Neuroprotective Potential of Tetraselmis chuii Compounds: Insights into Blood–Brain Barrier Permeability and Intestinal Transport. Pharmaceuticals, 18(5), 629. https://doi.org/10.3390/ph18050629








