Natural Antifungal Alkaloids for Crop Protection: An Overview of the Latest Synthetic Approaches
Abstract
1. Introduction
2. Quinoline- and Isoquinoline-Containing Alkaloids
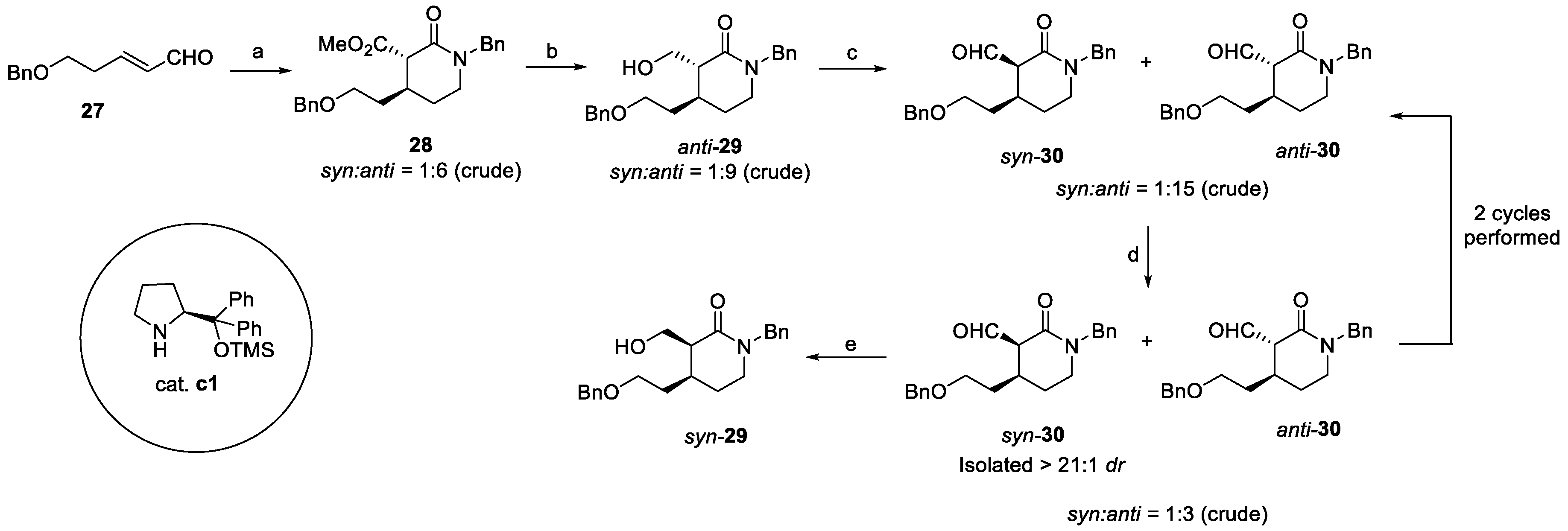
2.1. Quinine and Quinine Analogs (from Plants)
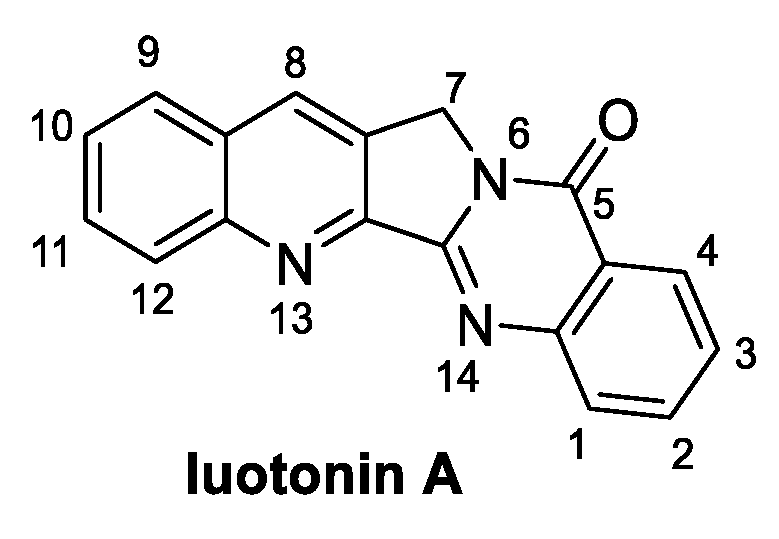
2.2. Luotonin A (from Plants)
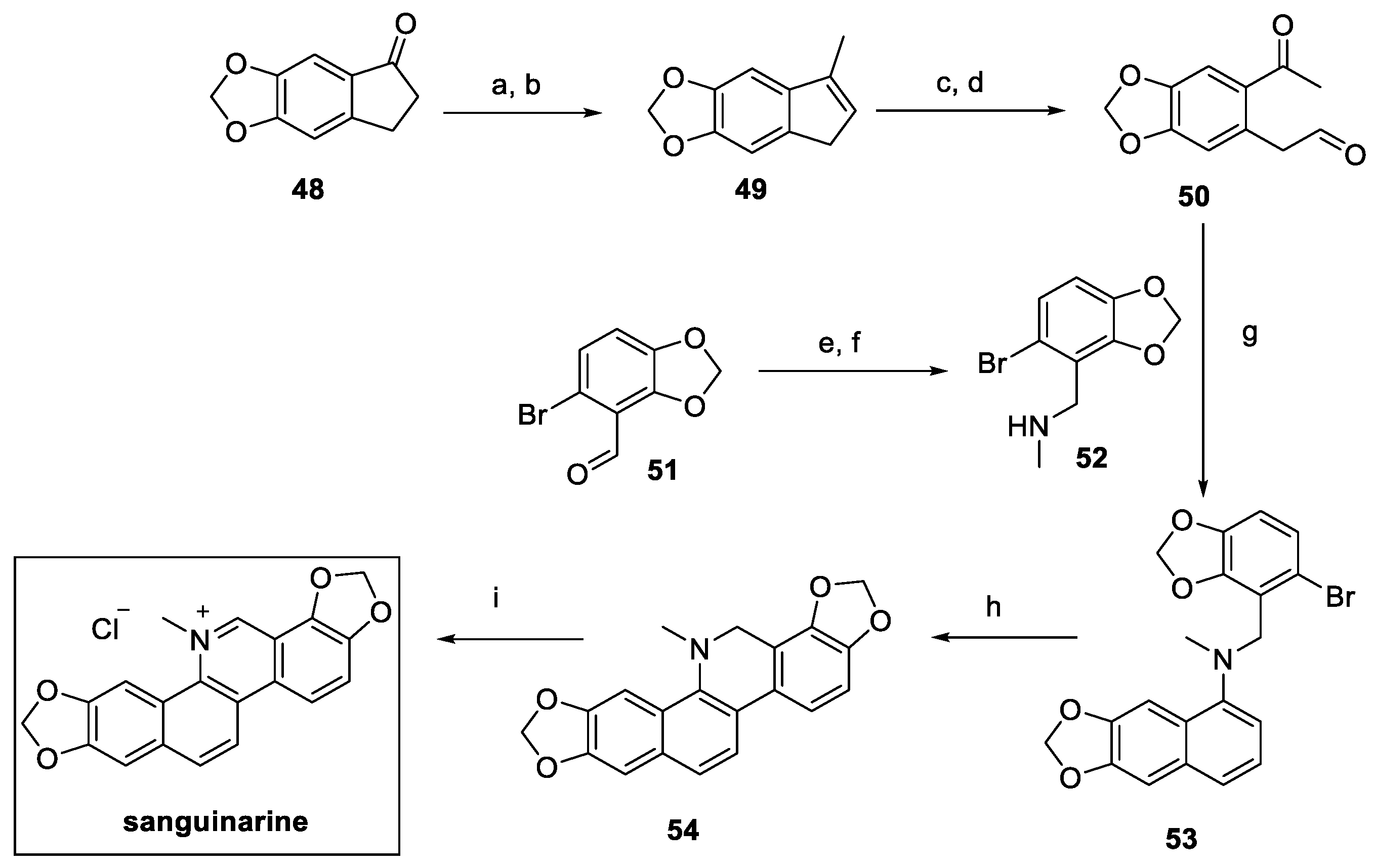
2.3. Sanguinarine (from Plants)
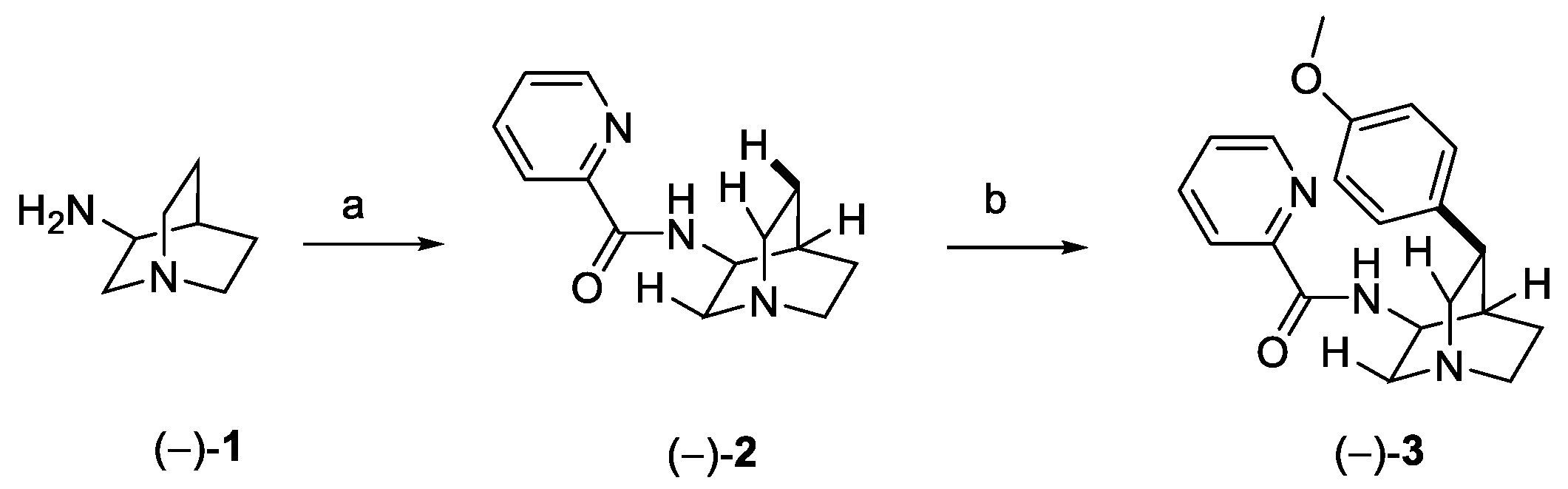
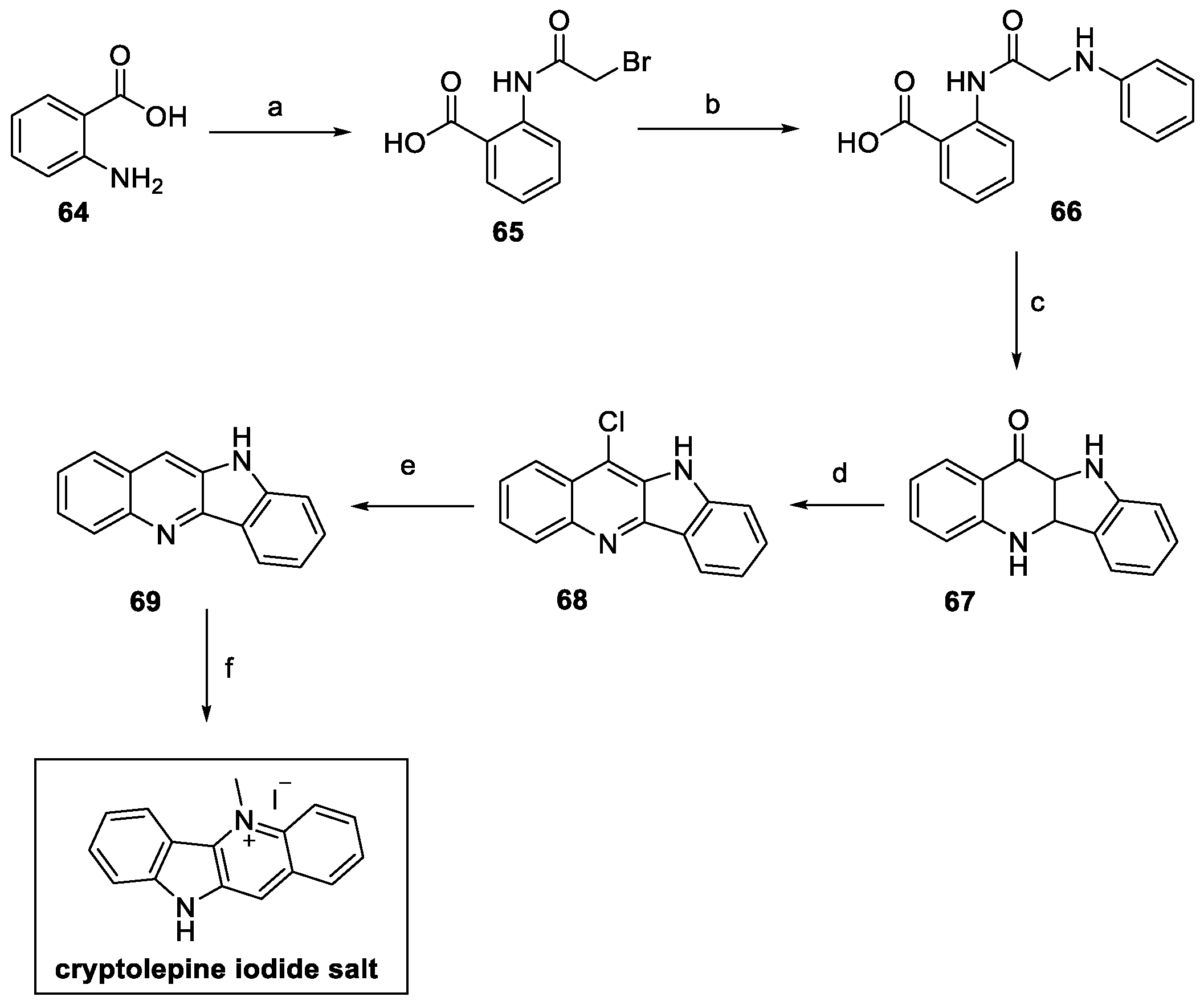
2.4. Cryptolepine, Neocryptolepine, and Isocryptolepine (from Plants)
2.4.1. Cryptolepine
2.4.2. Neocryptolepine
2.4.3. Isocryptolepine
3. Indole-Containing Alkaloids
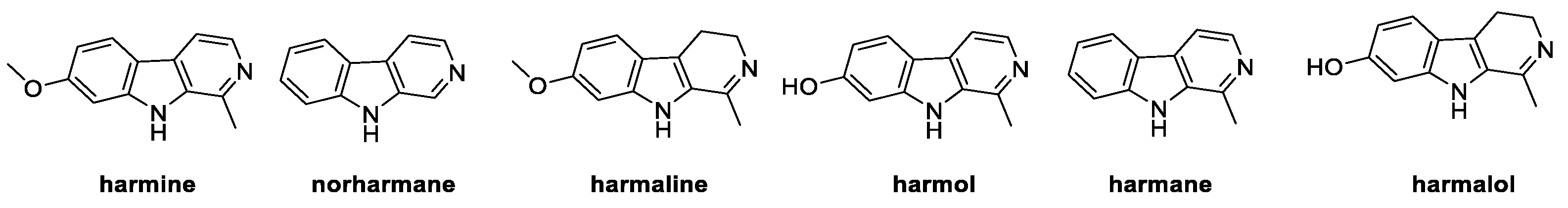
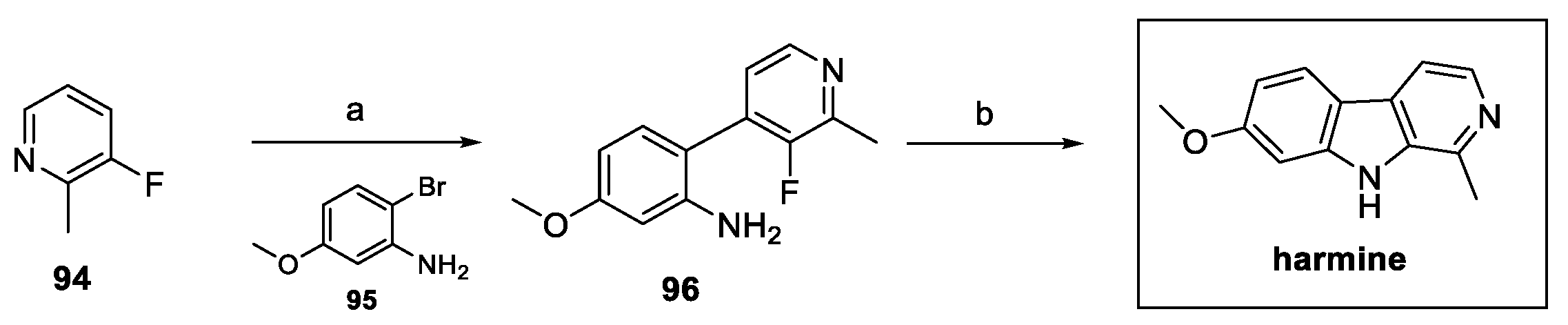
3.1. β-Carbolines: Harmine, Norharmane, Harmaline, Harmol, Harmane, and Harmalol (from Plants)
3.2. Pityriacitrin and Pityriacitrin B (from Bacteria and Marine Environment)
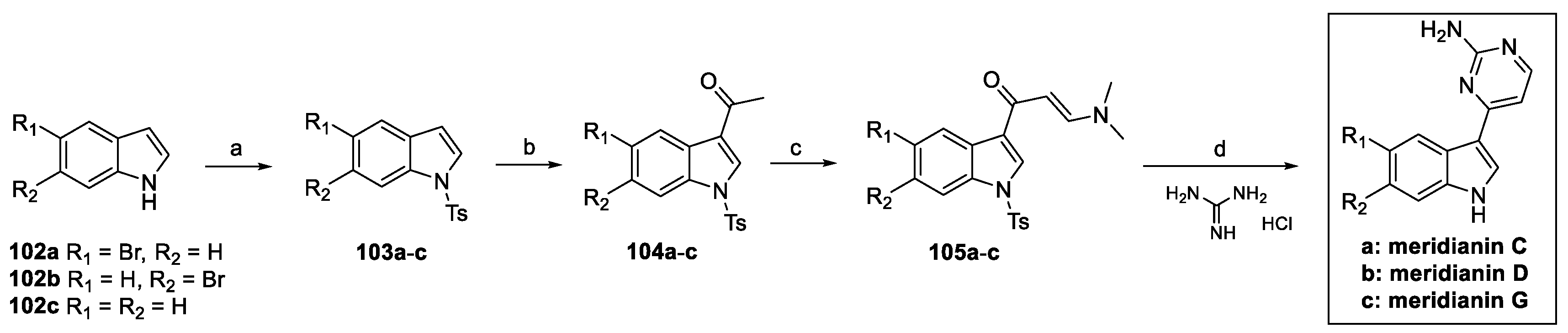
3.3. Meridianin (from Marine Environment)
4. Miscellanea
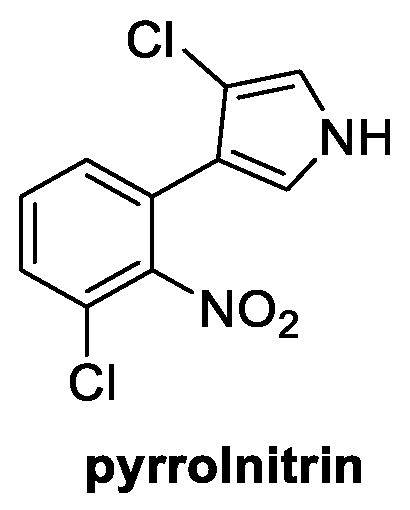
4.1. Phenylpirrole: Pyrrolnitrin (from Bacteria)
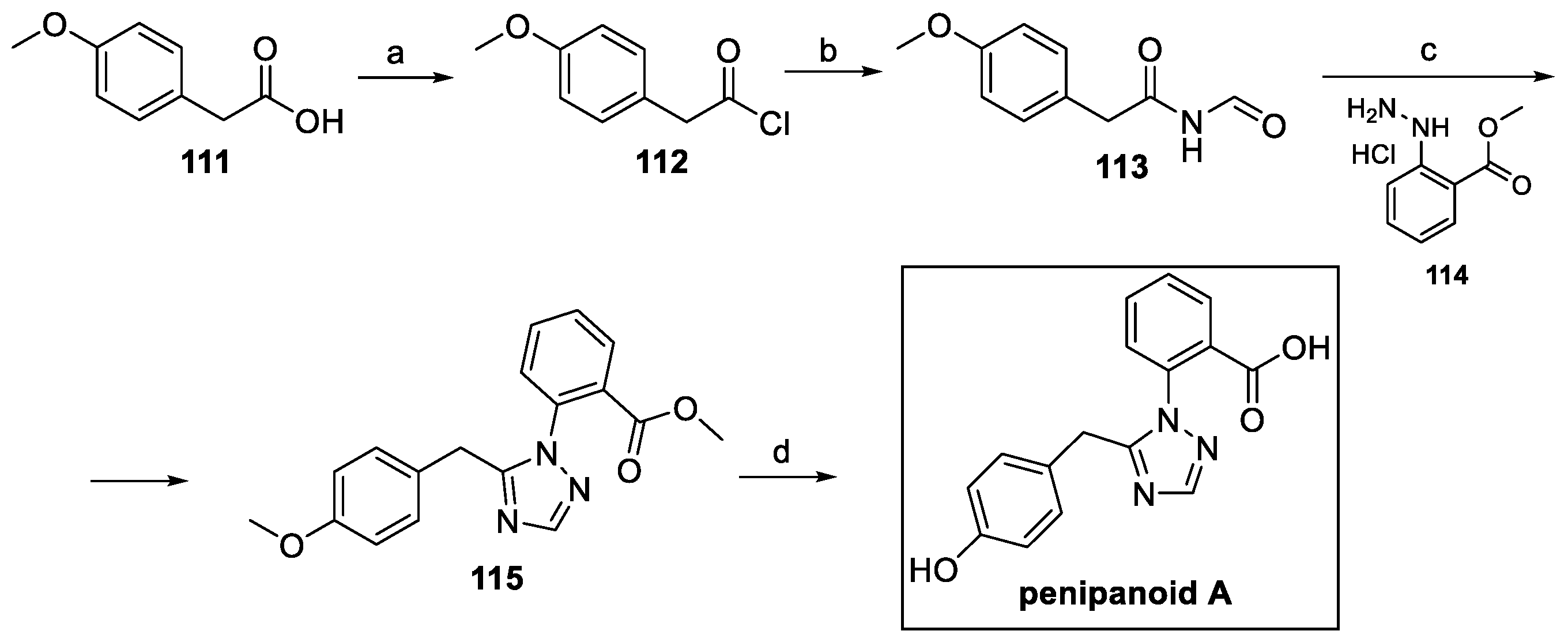
4.2. Triazoles: Penipanoid A (from Bacteria)
4.3. Naphthimidazoles: Kealiinines (from Marine Environment)
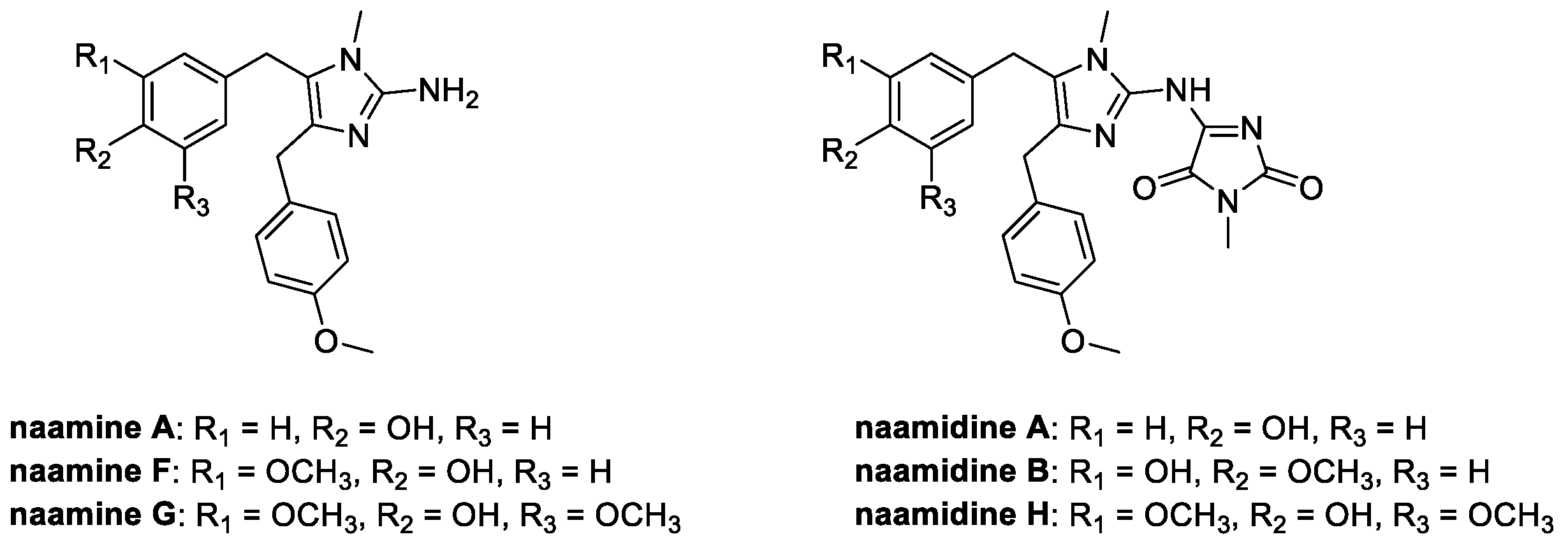
4.4. Aminoimidazoles: Naamines and Naamidines (from Marine Environment)
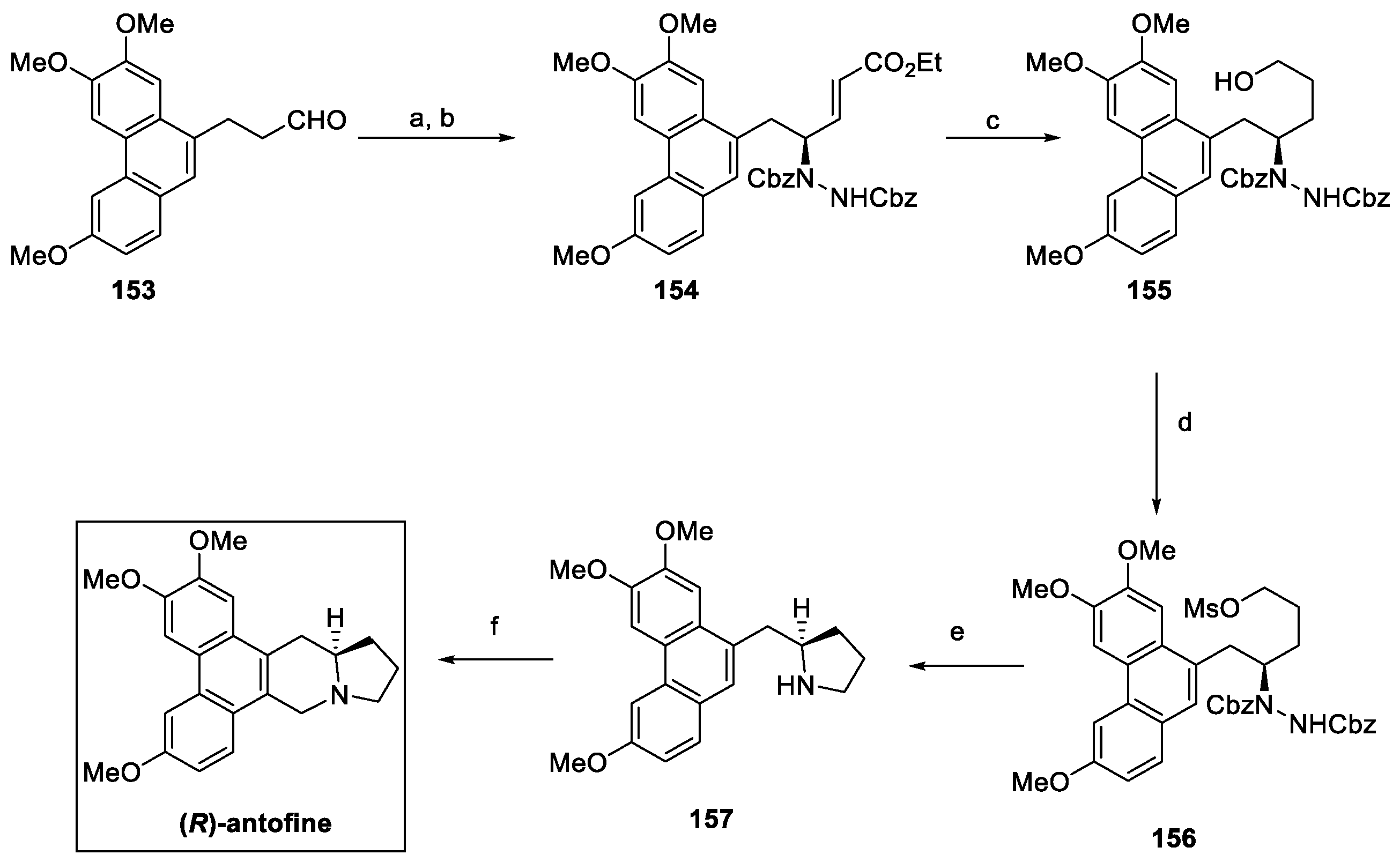
4.5. Indolizidines: Antofine (from Plants)
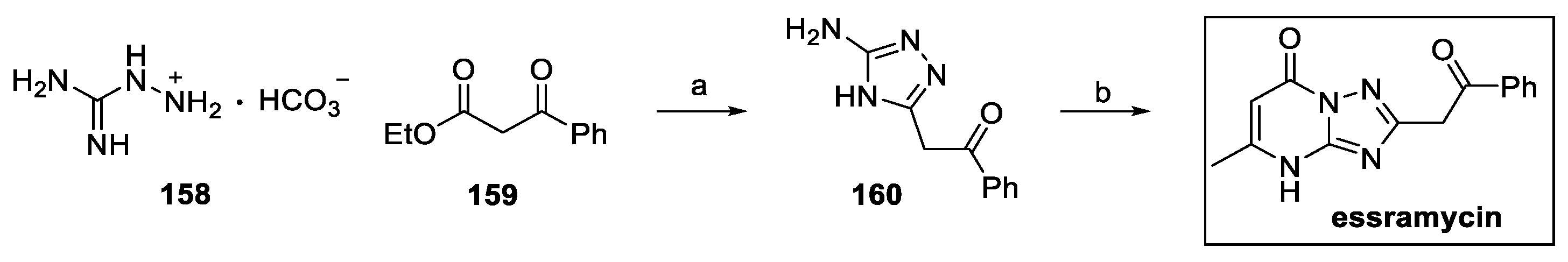
4.6. Pyrimidines: Essramycin (from Marine Environment)
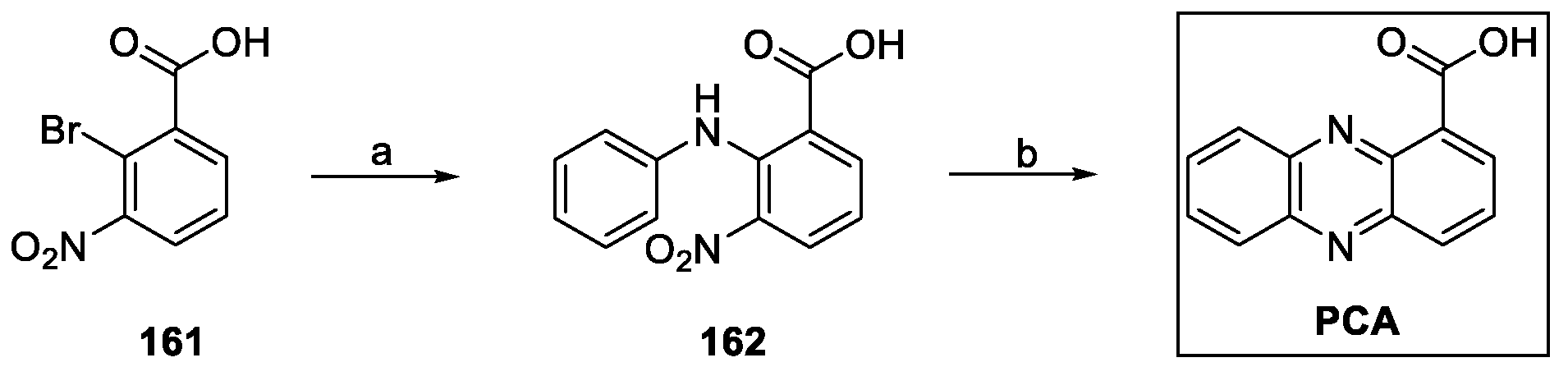
4.7. Phenazines: Phenazine-1-Carboylic Acid, PCA (from Bacteria)
5. Antifungal Activity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Achieving Zero Hunger: The Critical Role of Investments in Social Protection and Agriculture; FAO: Rome, Italy, 2015. [Google Scholar]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Sautua, F.J.; Szparaga, A.; Bohata, A.; Kocira, S.; Carmona, M.A. New Tools for the Management of Fungal Pathogens in Extensive Cropping Systems for Friendly Environments. CRC Crit. Rev. Plant Sci. 2024, 43, 63–93. [Google Scholar] [CrossRef]
- Tsalidis, G.A. Human Health and Ecosystem Quality Benefits with Life Cycle Assessment Due to Fungicides Elimination in Agriculture. Sustainability 2022, 14, 846. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-Derived Bioactives; Springer: Singapore, 2020; pp. 85–117. [Google Scholar]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of Secondary Metabolites in Defense Mechanisms of Plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities. Biomedicines 2021, 9, 485. [Google Scholar] [CrossRef]
- Hashim, S.E.; Basar, N.; Abd Samad, A.; Jamil, S.; Bakar, M.B.; Jamalis, J.; Abd-Aziz, N.; Wagiran, A.; Razak, M.R.M.A.; Samad, A.F.A. Advancing Alkaloid-Based Medicines: Medical Applications, Scalable Production and Synthetic Innovations. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, D.; Gao, C.; Li, J.; Du, B.; Wang, Z.; Qian, S. Recent Advances for Alkaloids as Botanical Pesticides for Use in Organic Agriculture. Int. J. Pest. Manag. 2023, 69, 288–298. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh, M.; Nowak, M.; Kima, H.; Selmar, D. Horizontal Natural Product Transfer: A Potential Source of Alkaloidal Contaminants in Phytopharmaceuticals. Phytomedicine 2017, 34, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Zhu, J.K.; Yin, X.D.; Yan, Y.F.; Wang, Y.L.; Shang, X.F.; Liu, Y.Q.; Zhao, Z.M.; Peng, J.W.; Liu, H. Design, Synthesis, and Antifungal Evaluation of Novel Quinoline Derivatives Inspired from Natural Quinine Alkaloids. J. Agric. Food Chem. 2019, 67, 11340–11353. [Google Scholar] [CrossRef]
- Antika, L.D.; Triana, D.; Ernawati, T. Antimicrobial Activity of Quinine Derivatives against Human Pathogenic Bacteria. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012006. [Google Scholar] [CrossRef]
- Stork, G.; Niu, D.; Fujimoto, A.; Koft, E.R.; Balkovec, J.M.; Tata, J.R.; Dake, G.R. The First Stereoselective Total Synthesis of Quinine. J. Am. Chem. Soc. 2001, 123, 3239–3242. [Google Scholar] [CrossRef]
- Shiomi, S.; Misaka, R.; Kaneko, M.; Ishikawa, H. Enantioselective Total Synthesis of the Unnatural Enantiomer of Quinine. Chem. Sci. 2019, 10, 9433–9437. [Google Scholar] [CrossRef]
- Nair, V.; Menon, R.S.; Vellalath, S. Asymmetric Synthesis of Quinine: A Landmark in Organic Synthesis. Nat. Prod. Comm. 2006, 1, 1934578X0600101009. [Google Scholar] [CrossRef]
- O’Donovan, D.H.; Aillard, P.; Berger, M.; de la Torre, A.; Petkova, D.; Knittl-Frank, C.; Geerdink, D.; Kaiser, M.; Maulide, N. C−H Activation Enables a Concise Total Synthesis of Quinine and Analogues with Enhanced Antimalarial Activity. Angew. Chem. Int. Ed. 2018, 57, 10737–10741. [Google Scholar] [CrossRef]
- Lee, J.; Chen, D.Y.-K. A Local-Desymmetrization-Based Divergent Synthesis of Quinine and Quinidine. Angew. Chem. Int. Ed. 2019, 58, 488–493. [Google Scholar] [CrossRef]
- Jiang, Y.; Deiana, L.; Zhang, K.; Lin, S.; Córdova, A. Total Asymmetric Synthesis of Quinine, Quinidine, and Analogues via Catalytic Enantioselective Cascade Transformations. Eur. J. Org. Chem. 2019, 2019, 6016–6023. [Google Scholar] [CrossRef]
- Cagir, A.; Jones, S.H.; Gao, R.; Eisenhauer, B.M.; Hecht, S.M. Luotonin A. A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Zhang, J.; Peng, J.W.; Zhang, Z.J.; Zhao, W.B.; Wang, R.X.; Ma, K.Y.; Li, J.C.; Liu, Y.Q.; Zhao, Z.M.; et al. Discovery of Luotonin A Analogues as Potent Fungicides and Insecticides: Design, Synthesis and Biological Evaluation Inspired by Natural Alkaloid. Eur. J. Med. Chem. 2020, 194, 112253. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Seo, H.A.; Cheon, C.H. Total Synthesis of Luotonin A and Rutaecarpine from an Aldimine via the Designed Cyclization. Org. Lett. 2016, 18, 5280–5283. [Google Scholar] [CrossRef] [PubMed]
- Baguia, H.; Deldaele, C.; Romero, E.; Michelet, B.; Evano, G. Copper-Catalyzed Photoinduced Radical Domino Cyclization of Ynamides and Cyanamides: A Unified Entry to Rosettacin, Luotonin A, and Deoxyvasicinone. Synthesis 2018, 50, 3022–3030. [Google Scholar] [CrossRef]
- Maiti, M.; Nandi, R.; Chaudhuri, K. The effect of ph on the absorption and fluorescence spectra of sanguinarine. Photochem. Photobiol. 1983, 38, 245–249. [Google Scholar] [CrossRef]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria Canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. Int. J. Mol. Sci. 2016, 17, 1414. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Shang, X.F.; Lawoe, R.K.; Liu, Y.Q.; Zhou, R.; Sun, Y.; Yan, Y.F.; Li, J.C.; Yang, G.Z.; Yang, C.J. Anti-Phytopathogenic Activity and the Possible Mechanisms of Action of Isoquinoline Alkaloid Sanguinarine. Pestic. Biochem. Physiol. 2019, 159, 51–58. [Google Scholar] [CrossRef]
- Tatton, M.R.; Simpson, I.; Donohoe, T.J. New Methods for the Synthesis of Naphthyl Amines; Application to the Synthesis of Dihydrosanguinarine, Sanguinarine, Oxysanguinarine and (±)-Maclekarpines B and C. Chem. Commun. 2014, 50, 11314–11316. [Google Scholar] [CrossRef]
- Parvatkar, P.T.; Parameswaran, P.S. Indoloquinolines: Possible Biogenesis from Common Indole Precursors and Their Synthesis Using Domino Strategies. Curr. Org. Synth. 2016, 15, 58–72. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, H.; Zhang, S.Y.; Li, H.; Ma, K.Y.; Liu, Y.Q.; Yin, X.D.; Zhou, R.; Yan, Y.F.; Wang, R.X.; et al. Design, Synthesis, and Antifungal Evaluation of Cryptolepine Derivatives against Phytopathogenic Fungi. J. Agric. Food Chem. 2021, 69, 1259–1271. [Google Scholar] [CrossRef]
- Shang, X.F.; Dai, L.X.; Zhang, Z.J.; Yang, C.J.; Du, S.S.; Wu, T.L.; He, Y.H.; Zhu, J.K.; Liu, Y.Q.; Yan, Y.F.; et al. Integrated Proteomics and Transcriptomics Analyses Reveals the Possible Antifungal Mechanism of an Indoloquinoline Alkaloid Neocryptolepine against Rhizoctonia Solani. J. Agric. Food Chem. 2021, 69, 6455–6464. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; Jin, Y.R.; Luo, X.F.; Zhang, S.Y.; Dai, T.L.; Ma, L.; Zhang, Z.J.; Wu, Z.R.; Jin, C.X.; Liu, Y.Q. Design, Synthesis, and Antimicrobial Activity Evaluation of Novel Isocryptolepine Derivatives against Phytopathogenic Fungi and Bacteria. J. Agric. Food Chem. 2024, 72, 20831–20841. [Google Scholar] [CrossRef]
- Abe, T.; Suzuki, T.; Anada, M.; Matsunaga, S.; Yamada, K. 2-Hydroxyindoline-3-Triethylammonium Bromide: A Reagent for Formal C3-Electrophilic Reactions of Indoles. Org. Lett. 2017, 19, 4275–4278. [Google Scholar] [CrossRef] [PubMed]
- Mudududdla, R.; Mohanakrishnan, D.; Bharate, S.S.; Vishwakarma, R.A.; Sahal, D.; Bharate, S.B. Orally Effective Aminoalkyl 10H-Indolo[3,2-b]Quinoline-11-Carboxamide Kills the Malaria Parasite by Inhibiting Host Hemoglobin Uptake. ChemMedChem 2018, 13, 2581–2598. [Google Scholar] [CrossRef]
- Majhi, B.; Ganguly, S.; Palit, S.; Parwez, A.; Saha, R.; Basu, G.; Dutta, S. Sequence-Specific Dual DNA Binding Modes and Cytotoxicities of N-6-Functionalized Norcryptotackieine Alkaloids. J. Nat. Prod. 2023, 86, 1667–1676. [Google Scholar] [CrossRef]
- Zhu, J.K.; Gao, J.M.; Yang, C.J.; Shang, X.F.; Zhao, Z.M.; Lawoe, R.K.; Zhou, R.; Sun, Y.; Yin, X.D.; Liu, Y.Q. Design, Synthesis, and Antifungal Evaluation of Neocryptolepine Derivatives against Phytopathogenic Fungi. J. Agric. Food Chem. 2020, 68, 2306–2315. [Google Scholar] [CrossRef]
- Thobokholt, E.N.; Larghi, E.L.; Bracca, A.B.J.; Kaufman, T.S. Isolation and Synthesis of Cryptosanguinolentine (Isocryptolepine), a Naturally-Occurring Bioactive Indoloquinoline Alkaloid. RSC Adv. 2020, 10, 18978–19002. [Google Scholar] [CrossRef]
- Akitake, M.; Noda, S.; Miyoshi, K.; Sonoda, M.; Tanimori, S. Access to γ-Carbolines: Synthesis of Isocryptolepine. J. Org. Chem. 2021, 86, 17727–17737. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal Activity of β-Carbolines on Penicillium Digitatum and Botrytis Cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef]
- Kuang, Y.; Huang, J.; Chen, F. Practical and Phase Transfer–Catalyzed Synthesis of 6-Methoxytryptamine. Synth. Commun. 2006, 36, 1515–1519. [Google Scholar] [CrossRef]
- Du, H.; Tian, S.; Chen, J.; Gu, H.; Li, N.; Wang, J. Synthesis and Biological Evaluation of N9-Substituted Harmine Derivatives as Potential Anticancer Agents. Bioorg Med. Chem. Lett. 2016, 26, 4015–4019. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, X.; Tian, L.; Gao, Y.; Liu, W.; Chen, H.; Jiang, X.; Xu, Z.; Ding, H.; Zhao, Q. Design, Synthesis and Biological Evaluation of Harmine Derivatives as Potent GSK-3β/DYRK1A Dual Inhibitors for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2021, 222, 113554. [Google Scholar] [CrossRef] [PubMed]
- Sathiyalingam, S.; Roesner, S. Synthesis of A- and Β-Carbolines by a Metalation/Negishi Cross-Coupling/S N Ar Reaction Sequence. Adv. Synth. Catal. 2022, 364, 1769–1774. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, X.; Xu, B.; Bijian, K.; Wan, S.; Li, G.; Alaoui-Jamali, M.; Jiang, T. Total Synthesis and Bioactivity of the Marine Alkaloid Pityriacitrin and Some of Its Derivatives. Eur. J. Med. Chem. 2011, 46, 6089–6097. [Google Scholar] [CrossRef]
- Wang, T.N.; Yang, S.; Shi, S.Y.; Yuan, W.Y.; Chen, J.X.; Duan, Z.Y.; Lu, A.D.; Wang, Z.W.; Wang, Q.M. Pityriacitrin Marine Alkaloids as Novel Antiviral and Anti-Phytopathogenic-Fungus Agents. Pest. Manag. Sci. 2021, 77, 4691–4700. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, M.; Cai, Q.; Jia, F.; Wu, A. A Cascade Coupling Strategy for One-Pot Total Synthesis of Β-Carboline and Isoquinoline-Containing Natural Products and Derivatives. Chem.—Eur. J. 2013, 19, 10132–10137. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Li, Y.; Liu, F.; Huang, W.; Min, Y.; Wang, K.; Yang, J.; Cao, C.; Gong, Y.; et al. Carboline Derivatives Based on Natural Pityriacitrin as Potential Antifungal Agents. Phytochem. Lett. 2022, 48, 100–105. [Google Scholar] [CrossRef]
- Franco, L.H.; Joffé, E.B.D.K.; Puricelli, L.; Tatian, M.; Seldes, A.M.; Palermo, J.A. Indole Alkaloids from the Tunicate Aplidium meridianum. J. Nat. Prod. 1998, 61, 1130–1132. [Google Scholar] [CrossRef]
- Dong, J.; Huang, S.S.; Hao, Y.N.; Wang, Z.W.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Marine-Natural-Products for Biocides Development: First Discovery of Meridianin Alkaloids as Antiviral and Anti-Phytopathogenic-Fungus Agents. Pest. Manag. Sci. 2020, 76, 3369–3376. [Google Scholar] [CrossRef]
- Xiao, L. A Review: Meridianins and Meridianins Derivatives. Molecules 2022, 27, 8714. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, C.G. Synthesis of Indolylpyrimidines via Cross-Coupling of Indolylboronic Acid with Chloropyrimidines: Facile Synthesis of Meridianin D. Heterocycles 2000, 53, 1489–1498. [Google Scholar] [CrossRef]
- Fresneda, P.M.; Molina, P.; Bleda, J.A. Synthesis of the Indole Alkaloids Meridianins from the Tunicate Aplidium Meridianum. Tetrahedron 2001, 57, 2355–2363. [Google Scholar] [CrossRef]
- Karpov, A.S.; Merkul, E.; Rominger, F.; Müller, T.J.J. Concise Syntheses of Meridianins by Carbonylative Alkynylation and a Four-Component Pyrimidine Synthesis. Angew. Chem. Int. Ed. 2005, 44, 6951–6956. [Google Scholar] [CrossRef] [PubMed]
- Linares-Otoya, L.; Liu, Y.; Linares-Otoya, V.; Armas-Mantilla, L.; Crüsemann, M.; Ganoza-Yupanqui, M.L.; Campos-Florian, J.; König, G.M.; Schäberle, T.F. Biosynthetic Basis for Structural Diversity of Aminophenylpyrrole-Derived Alkaloids. ACS Chem. Biol. 2019, 14, 176–181. [Google Scholar] [CrossRef]
- Kilani, J.; Fillinger, S. Phenylpyrroles: 30 Years, Two Molecules and (Nearly) No Resistance. Front. Microbiol. 2016, 7, 2014. [Google Scholar] [CrossRef]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial Pyrrolnitrin: Natural Metabolite with Immense Practical Utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef]
- Nakano, H.; Umio, S.; Kariyone, K.; Tanaka, K.; Kishimoto, T.; Noguchi, H.; Ueda, I.; Nakamura, H.; Morimoto, T. Total Synthesis of Pyrrolnitrin, a New Antibiotic. Tetrahedron Lett. 1966, 7, 737–740. [Google Scholar] [CrossRef]
- Morrison, M.D.; Hanthorn, J.J.; Pratt, D.A. Synthesis of Pyrrolnitrin and Related Halogenated Phenylpyrroles. Org. Lett. 2009, 11, 1051–1054. [Google Scholar] [CrossRef]
- Bray, B.L.; Mathies, P.H.; Naef, R.; Solas, D.R.; Tidwell, T.T.; Artis, D.R.; Muchowski, J.M. N-(Triisopropylsilyl)Pyrrole. A Progenitor “Par Excellence” of 3-Substituted Pyrroles. J. Org. Chem. 1990, 55, 6317–6328. [Google Scholar] [CrossRef]
- Li, C.-S.; An, C.-Y.; Li, X.-M.; Gao, S.-S.; Cui, C.-M.; Sun, H.-F.; Wang, B.-G. Triazole and Dihydroimidazole Alkaloids from the Marine Sediment-Derived Fungus Penicillium Paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef]
- Reddy, A.S.; Reddy, V.K.; Rao, G.N.; Basavaiah, K. An Efficient Total Synthesis of a Natural Penipanoid A. Tetrahedron Lett. 2022, 90, 153612. [Google Scholar] [CrossRef]
- Reddy, A.S.; Reddy, V.K.; Rao, G.N.; Basavaiah, K.; Nagulapalli Venkata, K.C. A Novel and Efficient One-Pot Strategy for the Synthesis of 1,2,4-Triazoles: Access to Synthesis of Penipanoid A and Its Analogues. RSC Adv. 2023, 13, 2680–2682. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Bhan, A.; Mandal, S.S.; Lovely, C.J. Total Syntheses and Cytotoxicity of Kealiiquinone, 2-Deoxy-2-Aminokealiiquinone and Analogs. Bioorg Med. Chem. Lett. 2013, 23, 6183–6187. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Guo, J.; Wang, Z.; Liu, Y.; Song, H.; Wang, Q. Marine Natural Products for Drug Discovery: First Discovery of Kealiinines A-C and Their Derivatives as Novel Antiviral and Antiphytopathogenic Fungus Agents. J. Agric. Food Chem. 2018, 66, 7310–7318. [Google Scholar] [CrossRef]
- Das, J.; Koswatta, P.B.; Jones, J.D.; Yousufuddin, M.; Lovely, C.J. Total Syntheses of Kealiinines A–C. Org. Lett. 2012, 14, 6210–6213. [Google Scholar] [CrossRef]
- Guo, P.; Li, G.; Liu, Y.; Lu, A.; Wang, Z.; Wang, Q. Naamines and Naamidines as Novel Agents against a Plant Virus and Phytopathogenic Fungi. Mar. Drugs 2018, 16, 311. [Google Scholar] [CrossRef]
- Ohta, S.; Tsuno, N.; Nakamura, S.; Taguchi, N.; Yamashita, M.; Kawasaki, I.; Fujieda, M. Total Syntheses of Naamine A and Naamidine A, Marine Imidazole Alkaloids. Heterocycles-Sendai Inst. Heterocycl. Chem. 2000, 53, 1939–1956. [Google Scholar] [CrossRef]
- Kawasaki, I.; Nakamura, S.; Yanagitani, S.; Kakuno, A.; Yamashita, M.; Ohta, S. New Access to 1,3-Dialkyl-2,3-Dihydro-2-Imino-1H-Imidazoles and Their Application to the First Total Synthesis of Naamine B, a Marine 2,3-Dihydro-2-Imino-1,3-Dimethyl-1H-Imidazole Alkaloid. J. Chem. Soc. Perkin Trans. 2001, 1, 3095–3099. [Google Scholar] [CrossRef]
- Ermolat’ev, D.S.; Bariwal, J.B.; Steenackers, H.P.L.; De Keersmaecker, S.C.J.; Van der Eycken, E.V. Concise and Diversity-Oriented Route toward Polysubstituted 2-Aminoimidazole Alkaloids and Their Analogues. Angew. Chem. 2010, 122, 9655–9658. [Google Scholar] [CrossRef]
- Koswatta, P.B.; Lovely, C.J. Total Syntheses of Naamidine G and 14-Methoxynaamidine G. Tetrahedron Lett. 2010, 51, 164–166. [Google Scholar] [CrossRef]
- Koswatta, P.B.; Lovely, C.J. Concise Total Synthesis of Naamine G and Naamidine H. Chem. Commun. 2010, 46, 2148. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; OuYang, Q.; Wan, C.; Che, J.; Li, L.; Chen, J.; Tao, N. Isolation of Antofine from Cynanchum Atratum BUNGE (Asclepiadaceae) and Its Antifungal Activity against Penicillium Digitatum. Postharvest. Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Oh, J.; Kim, G.D.; Kim, S.; Lee, S.K. Antofine, a Natural Phenanthroindolizidine Alkaloid, Suppresses Angiogenesis via Regulation of AKT/MTOR and AMPK Pathway in Endothelial Cells and Endothelial Progenitor Cells Derived from Mouse Embryonic Stem Cells. Food Chem. Toxicol. 2017, 107, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, Y.; Liu, J.; Kang, Y.; Li, R.; Wang, J. The Anti-Tumor Activity and Mechanism of Alkaloids from Aconitum Szechenyianum Gay. Bioorg Med. Chem. Lett. 2016, 26, 380–387. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Lu, L.; Gong, Y.; Han, W.; Piao, G. Natural Indole-containing Alkaloids and Their Antibacterial Activities. Arch. Pharm. 2020, 353, 2000120. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Wang, J.; Lu, J.; Huo, Y. Extraction Separation and Antifungal Activities of Cynanchum Komarovii Al. Iljinski. World Sci. Res. J. 2023, 9, 52–59. [Google Scholar]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium Italicum and Its Possible Mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef]
- Yi, M.; Gu, P.; Kang, X.Y.; Sun, J.; Li, R.; Li, X.Q. Total Synthesis of (±)-Antofine. Tetrahedron Lett. 2014, 55, 105–107. [Google Scholar] [CrossRef]
- Ansari, A.; Ramapanicker, R. Enantioselective Synthesis of (R)-Antofine and (R)-Cryptopleurine. ChemistrySelect 2018, 3, 12591–12594. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; Shaaban, M.; Shaaban, K.A.; El-Bondkly, A.M.; Laatsch, H. Essramycin: A First Triazolopyrimidine Antibiotic Isolated from Nature†. J. Antibiot. 2008, 61, 149–157. [Google Scholar] [CrossRef]
- Battaglia, U.; Moody, C.J. A Short Synthesis of the Triazolopyrimidine Antibiotic Essramycin. J. Nat. Prod. 2010, 73, 1938–1939. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Gouda, M.A. Synthesis and Antimicrobial Activity of Some Novel Quinoline, Chromene, Pyrazole Derivatives Bearing Triazolopyrimidine Moiety. J. Heterocycl. Chem. 2017, 54, 850–858. [Google Scholar] [CrossRef]
- Tee, E.H.L.; Karoli, T.; Ramu, S.; Huang, J.X.; Butler, M.S.; Cooper, M.A. Synthesis of Essramycin and Comparison of Its Antibacterial Activity. J. Nat. Prod. 2010, 73, 1940–1942. [Google Scholar] [CrossRef]
- Wang, T.; Yang, S.; Li, H.; Lu, A.; Wang, Z.; Yao, Y.; Wang, Q. Discovery, Structural Optimization, and Mode of Action of Essramycin Alkaloid and Its Derivatives as Anti-Tobacco Mosaic Virus and Anti-Phytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 471–484. [Google Scholar] [CrossRef]
- Shtark, O.Y.; Shaposhnikov, A.I.; Kravchenko, L.V. The Production of Antifungal Metabolites by Pseudomonas Chlororaphis Grown on Different Nutrient Sources. Microbiology 2003, 72, 574–578. [Google Scholar] [CrossRef]
- Niu, J.; Chen, J.; Xu, Z.; Zhu, X.; Wu, Q.; Li, J. Synthesis and Bioactivities of Amino Acid Ester Conjugates of Phenazine-1-Carboxylic Acid. Bioorg Med. Chem. Lett. 2016, 26, 5384–5386. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, X.; Hu, J.; Wang, Y.; Du, X.; Li, J.; Wu, Q. Effect of Introducing Amino Acids into Phenazine-1-Carboxylic Acid on Phloem Mobility. Nat. Prod. Res. 2021, 35, 4373–4379. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J.; Cheng, X.; Zhang, H.; Zhang, C.; Xia, Z.; Wu, Z.; Zhang, L.; Zheng, Y.; Gao, Z.; et al. Design, synthesis and biological evaluations of diverse Michael acceptor-based phenazine hybrid molecules as TrxR1 inhibitors. Bioorg Chem. 2021, 109, 104736. [Google Scholar] [CrossRef]
- Lv, P.; Chen, Y.; Shi, T.; Wu, X.; Li, Q.X.; Hua, R. Synthesis and fungicidal activities of sanguinarine derivatives. Pestic. Biochem. Physiol. 2018, 147, 3–10. [Google Scholar] [CrossRef]























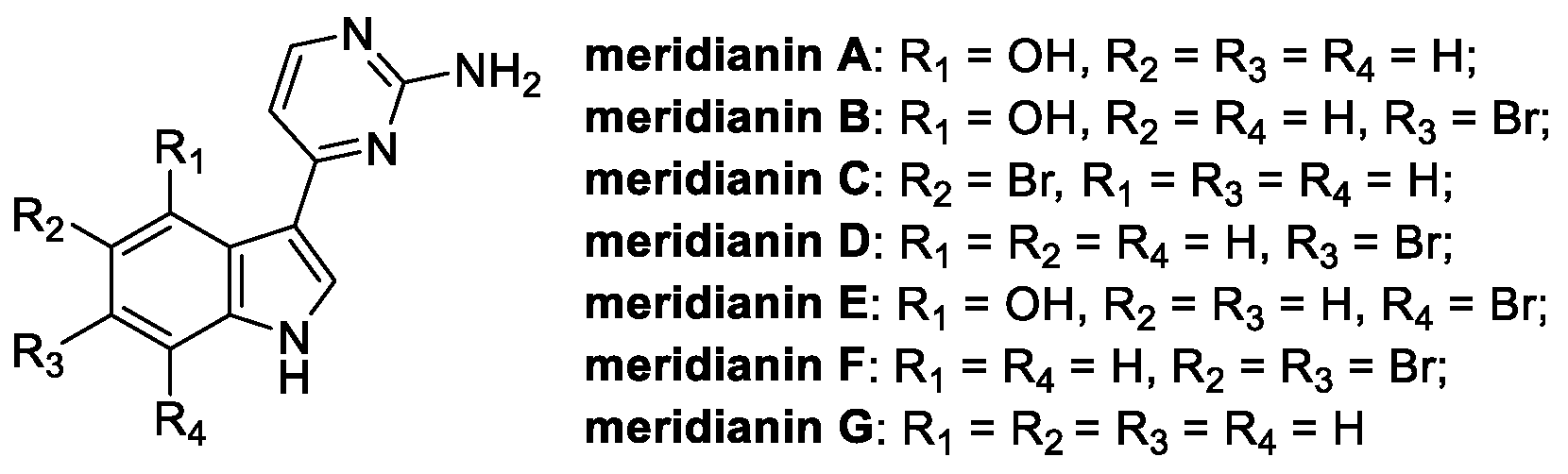












| Phytopathogens | Natural Alkaloids | Mycelia Growth Inhibition | MIC | EC50 | Ref. |
|---|---|---|---|---|---|
| F. graminearum | Quinine | 46% at 100 μg/mL | [15] | ||
| Neocryptolepine | 16.31 μg/mL | [38] | |||
| Meridianin C, D, G | 58% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 36% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 24% at 50 mg/kg | [66] | |||
| P. zeae | Quinine | 30% at 100 μg/mL | [15] | ||
| Isocryptolepine | 35% at 100 μg/mL | [34] | |||
| R. solani | Quinine | 30% at 100 μg/mL | [15] | ||
| Sanguinarine | 77% at 50 μg/mL | 11.6 μg/mL | [91] | ||
| Pyrrolnitrin | 1 μg/mL | [58] | |||
| Neocryptolepine | 9 μg/mL | [38] | |||
| Isocryptolepine | 6% at 100 μg/mL | [34] | |||
| Meridianin C, D, G | 72% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 47% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 61% at 50 mg/kg | [66] | |||
| PCA | 25.66 ± 1.75 μg/mL | [88] | |||
| M. melonis | Quinine | 30% at 100 μg/mL | [15] | ||
| Neocryptolepine | 1.16 μg/mL | [38] | |||
| B. cinerea | Luotonin A | 0.50 mM | [24] | ||
| Cryptolepine | 0.050 μg/mL | [32] | |||
| Neocryptolepine | 4.44 μg/mL | [38] | |||
| Harmane | 1 mM | [41] | |||
| Harmine | 0.5 mM | [41] | |||
| Harmaline and harmalol | 30% at 1 mM | [41] | |||
| Pityriacitrin B | 82% at 1 mg/mL | [47] | |||
| Essramycin | 46% at 50 mg/kg | [86] | |||
| Pyrrolnitrin | 5 μg/mL | [58] | |||
| Meridianin C, D, G | 68% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 38% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 11% at 50 mg/kg | [66] | |||
| M. oryzae | Quinine | 30% at 100 μg/mL | [15] | ||
| Luotonin A | 0.50 mM | [24] | |||
| Neocryptolepine | 17.63 μg/mL | [38] | |||
| Isocryptolepine | 12% at 100 μg/mL | [34] | |||
| Sanguinarine | 6.96 μg/mL | [29] | |||
| S. sclerotiorum | Cryptolepine | 5.507 μg/mL | [32] | ||
| Neocryptolepine | 17.65 μg/mL | [38] | |||
| Pityriacitrin | 52% at 50 μg/mL | [47] | |||
| Meridianin C, D, G | 70% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 38% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 35% at 50 mg/kg | [66] | |||
| P. digitatum | Antofine | [78] | |||
| Harmane | 1.56 μg/mL | [41] | |||
| Harmine | 0.5 mM | [41] | |||
| R. cerealis | Pityriacitrin | 42% at 50 μg/mL | 0.5 mM | [47] | |
| Essramycin | 58% at 50 mg/kg | [86] | |||
| Meridianin C, D, G | 66% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 70% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 41% at 50 mg/kg | [66] | |||
| P. infestans | Essramycin | 58% at 50 mg/kg | [86] | ||
| Meridianin C, D, G | 46% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 25% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 44% at 50 mg/kg | [66] | |||
| Alternaria sp. | Pyrrolnitrin | 5 μg/mL | [58] | ||
| A. solani | Sanguinarine | 68% at 50 μg/mL | [91] | ||
| Meridianin C, D, G | 62% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 50% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 13% at 50 mg/kg | [66] | |||
| P. aphanidermatum | Pyrrolnitrin | 1 μg/mL | [58] | ||
| P. ultimun | Pyrrolnitrin | 5 μg/mL | [58] | ||
| Rhizopus sp. | Pyrrolnitrin | 1 μg/mL | [58] | ||
| F. oxysporum | Sanguinarine | 15% at 50 μg/mL | [91] | ||
| Pyrrolnitrin | 85% at 20 μg/mL | [58] | |||
| F. oxysporumf. sp. cucumeris | Meridianin C, D, G | 41% at 50 μg/mL | [51] | ||
| Naamines and naamidines | 20% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 42% at 50 mg/kg | [66] | |||
| P. expansum | Pyrrolnitrin | 20 μg/mL | [58] | ||
| S. rolsfii | Pyrrolnitrin | 10 μg/mL | [58] | ||
| P. piricola | Meridianin C, D, G | 88% at 50 μg/mL | [51] | ||
| Naamines and naamidines | 67% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 63% at 50 mg/kg | [66] | |||
| P. capsici | Pityriacitrin B | 78% at 1 mg/mL | [47] | ||
| Kealiinine A, B, C | 68% at 50 mg/kg | [66] | |||
| Meridianin C, D, G | 82% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 17% at 50 μg/mL | [68] | |||
| C. arachidicola Hori | Sanguinarine | 67% at 50 μg/mL | [91] | ||
| Meridianin C, D, G | 59% at 50 μg/mL | [51] | |||
| Naamines and naamidines | 47% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 29% at 50 mg/kg | [66] | |||
| B. maydis | Meridianin C, D, G | 36% at 50 μg/mL | [51] | ||
| Naamines and naamidines | 28% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 22% at 50 mg/kg | [66] | |||
| W. anthracnose | Meridianin C, D, G | 54% at 50 μg/mL | [51] | ||
| Naamines and naamidines | 33% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 34% at 50 mg/kg | [66] | |||
| F. moniliforme | Meridianin C, D, G | 24% at 50 μg/mL | [51] | ||
| Naamines and naamidines | 26% at 50 μg/mL | [68] | |||
| Kealiinine A, B, C | 19% at 50 mg/kg | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dozio, D.; Sacchi, F.; Pinto, A.; Dallavalle, S.; Annunziata, F.; Princiotto, S. Natural Antifungal Alkaloids for Crop Protection: An Overview of the Latest Synthetic Approaches. Pharmaceuticals 2025, 18, 589. https://doi.org/10.3390/ph18040589
Dozio D, Sacchi F, Pinto A, Dallavalle S, Annunziata F, Princiotto S. Natural Antifungal Alkaloids for Crop Protection: An Overview of the Latest Synthetic Approaches. Pharmaceuticals. 2025; 18(4):589. https://doi.org/10.3390/ph18040589
Chicago/Turabian StyleDozio, Denise, Francesca Sacchi, Andrea Pinto, Sabrina Dallavalle, Francesca Annunziata, and Salvatore Princiotto. 2025. "Natural Antifungal Alkaloids for Crop Protection: An Overview of the Latest Synthetic Approaches" Pharmaceuticals 18, no. 4: 589. https://doi.org/10.3390/ph18040589
APA StyleDozio, D., Sacchi, F., Pinto, A., Dallavalle, S., Annunziata, F., & Princiotto, S. (2025). Natural Antifungal Alkaloids for Crop Protection: An Overview of the Latest Synthetic Approaches. Pharmaceuticals, 18(4), 589. https://doi.org/10.3390/ph18040589









