Oncoral Follow-Up for Outpatients Treated with Oral Anticancer Drugs Assessed by Relative Dose Intensity
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Medication and Complementary and Alternative Medicines (CAM)
2.3. Pharmacist and Nurse Interventions
2.4. Relative Dose Intensity
2.5. Corrected Relative Dose Intensity
2.6. Factors for Early Treatment Termination
2.7. Progression-Free and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Endpoints
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourmaud, A.; Henin, E.; Tinquaut, F.; Regnier, V.; Hamant, C.; Colomban, O.; You, B.; Ranchon, F.; Guitton, J.; Girard, P.; et al. Adherence to oral anticancer chemotherapy: What influences patients’ over or non-adherence? Analysis of the OCTO study through quantitative-qualitative methods. BMC Res. Notes 2015, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- Valgus, J.; Jarr, S.; Schwartz, R.; Rice, M.; Bernard, S.A. Pharmacist-led, interdisciplinary model for delivery of supportive care in the ambulatory cancer clinic setting. J. Oncol. Pract. 2010, 6, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Zerbit, J.; Kroemer, M.; Fuchs, B.; Detroit, M.; Decroocq, J.; Vignon, M.; Willems, L.; Deau-Fischer, B.; Franchi, P.; Deschamps, P.; et al. Pharmaceutical cancer care for haematology patients on oral anticancer drugs: Findings from an economic, clinical and organisational analysis. Eur. J. Cancer Care 2022, 31, e13753. [Google Scholar] [CrossRef] [PubMed]
- Colombo, L.R.P.; Aguiar, P.M.; Lima, T.M.; Storpirtis, S. The effects of pharmacist interventions on adult outpatients with cancer: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 414–424. [Google Scholar] [CrossRef]
- Zerbit, J.; Chevret, S.; Bernard, S.; Kroemer, M.; Ablard, C.; Harel, S.; Brice, P.; Madelaine, I.; Thieblemont, C. Improved time to treatment failure and survival in ibrutinib-treated malignancies with a pharmaceutical care program: An observational cohort study. Ann. Hematol. 2020, 99, 1615–1625. [Google Scholar] [CrossRef]
- Carvalho da Silva, S.P.; Jesus, M.; Roque, F.; Herdeiro, M.T.; Costa e Sousa, R.; Duarte, A.P.; Morgado, M. Active Pharmacovigilance Study: A Follow-Up Model of Oral Anti-Cancer Drugs under Additional Monitoring. Curr. Oncol. 2023, 30, 4139–4152. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef]
- Doolin, J.W.; Berry, J.L.; Forbath, N.S.; Tocci, N.X.; Dechen, T.; Li, S.; Hartwell, R.A.; Espiritu, J.K.; Roberts, D.A.; Zerillo, J.A.; et al. Implementing Electronic Patient-Reported Outcomes for Patients With New Oral Chemotherapy Prescriptions at an Academic Site and a Community Site. JCO Clin. Cancer Inform. 2021, 5, 631–640. [Google Scholar] [CrossRef]
- Escudero-Vilaplana, V.; Ribed, A.; Romero-Jimenez, R.M.; Herranz-Alonso, A.; Sanjurjo-Saez, M. Pharmacotherapy follow-up of key points in the safety of oral antineoplastic agents. Eur. J. Cancer Care 2017, 26, e12463. [Google Scholar] [CrossRef]
- Yeoh, T.T.; Tay, X.Y.; Si, P.; Chew, L. Drug-related problems in elderly patients with cancer receiving outpatient chemotherapy. J. Geriatr. Oncol. 2015, 6, 280–287. [Google Scholar] [CrossRef]
- Umar, R.M.; Can, Z.Y.; Güven, E.; Koçberber, E.K.; Olmez, O.F. The Prevalence of Drug-Drug Interactions and Reported Therapy Related Side Effects in Oncology Out-Patients. Clin. Exp. Health Sci. 2023, 13, 212–217. [Google Scholar] [CrossRef]
- Bulsink, A.; Imholz, A.L.T.; Brouwers, J.R.B.J.; Jansman, F.G.A. Characteristics of potential drug-related problems among oncology patients. Int. J. Clin. Pharm. 2013, 35, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.S.; Cheung, N. Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. J. Oncol. Pharm. Pract. 2016, 22, 741–748. [Google Scholar] [CrossRef]
- Sun, W.; Reeve, R.; Ouellette, T.; Stutsky, M.; De Jesus, R.; Huffer, M.J.; Mougalian, S.S. Novel Tool to Monitor Adherence to Oral Oncolytics: A Pilot Study. JCO Clin. Cancer Inform. 2021, 5, 701–708. [Google Scholar] [CrossRef]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. New Oral Anti-Cancer Drugs and Medication Safety. Dtsch. Arztebl. Int. 2019, 116, 775–782. [Google Scholar] [CrossRef]
- Mir, O.; Ferrua, M.; Fourcade, A.; Mathivon, D.; Duflot-Boukobza, A.; Dumont, S.; Baudin, E.; Delaloge, S.; Malka, D.; Albiges, L.; et al. Digital remote monitoring plus usual care versus usual care in patients treated with oral anticancer agents: The randomized phase 3 CAPRI trial. Nat. Med. 2022, 28, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.E.; Basumallik, N.; Tian, X.; Soto, S.; Wiestner, A. Clinically indicated ibrutinib dose interruptions and reductions do not compromise long-term outcomes in CLL. Blood 2019, 133, 2452–2455. [Google Scholar] [CrossRef]
- Denduluri, N.; Patt, D.A.; Wang, Y.; Bhor, M.; Li, X.; Favret, A.M.; Morrow, P.K.; Barron, R.L.; Asmar, L.; Saravanan, S.; et al. Dose Delays, Dose Reductions, and Relative Dose Intensity in Patients with Cancer Who Received Adjuvant or Neoadjuvant Chemotherapy in Community Oncology Practices. J. Natl. Compr. Cancer Netw. JNCCN 2015, 13, 1383–1393. [Google Scholar] [CrossRef]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1609–e1618. [Google Scholar] [CrossRef]
- Bonadonna, G.; Valagussa, P.; Moliterni, A.; Zambetti, M.; Brambilla, C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. N. Engl. J. Med. 1995, 332, 901–906. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Reiner, M.; Morrow, P.K.; Watson, H.; Crawford, J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit. Rev. Oncol. Hematol. 2015, 93, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Dale, D.C.; Tomita, D.; Whittaker, S.; Crawford, J. A retrospective evaluation of chemotherapy dose intensity and supportive care for early-stage breast cancer in a curative setting. Breast Cancer Res. Treat. 2013, 139, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Veitch, Z.N.; Farooq Khan, O.; Tilley, D.; Xanthoula, K.; Tang, P.A.; King, K.M.; Lupichuk, S.M. Adjustments in relative dose intensity (RDI) for FECD chemotherapy in breast cancer: A population analysis. J. Clin. Oncol. 2017, 35, 15. [Google Scholar] [CrossRef]
- Mir, O. The Capri Program Proves Clinical and Economic Efficiency for Personalized Remote Monitoring of Patients Treated with Oral Anticancer Drugs. ASCO. 2020. Available online: https://www.gustaveroussy.fr/sites/default/files/cp-asco-2020-capri-mir-en-29052020.pdf (accessed on 12 October 2020).
- Bosly, A.; Bron, D.; Van Hoof, A.; De Bock, R.; Berneman, Z.; Ferrant, A.; Kaufman, L.; Dauwe, M.; Verhoef, G. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann. Hematol. 2008, 87, 277–283. [Google Scholar] [CrossRef]
- Hryniuk, W.; Levine, M.N. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J. Clin. Oncol. 1986, 4, 1162–1170. [Google Scholar] [CrossRef]
- Hryniuk, W.; Bush, H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J. Clin. Oncol. 1984, 2, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Davis, R.B.; Schiffer, C.A.; Berg, D.T.; Powell, B.L.; Schulman, P.; Omura, G.A.; Moore, J.O.; McIntyre, O.R.; Frei, E. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N. Engl. J. Med. 1994, 331, 896–903. [Google Scholar] [CrossRef]
- Lepage, E.; Gisselbrecht, C.; Haioun, C.; Sebban, C.; Tilly, H.; Bosly, A.; Morel, P.; Herbrecht, R.; Reyes, F.; Coiffier, B. Prognostic significance of received relative dose intensity in non-Hodgkin’s lymphoma patients: Application to LNH-87 protocol. The GELA. (Groupe d’Etude des Lymphomes de l’Adulte). Ann. Oncol. 1993, 4, 651–656. [Google Scholar] [CrossRef]
- Wildiers, H.; Reiser, M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit. Rev. Oncol. Hematol. 2011, 77, 221–240. [Google Scholar] [CrossRef]
- Luciani, A.; Bertuzzi, C.; Ascione, G.; Di Gennaro, E.; Bozzoni, S.; Zonato, S.; Ferrari, D.; Foa, P. Dose intensity correlate with survival in elderly patients treated with chemotherapy for advanced non-small cell lung cancer. Lung Cancer Amst. Neth. 2009, 66, 94–96. [Google Scholar] [CrossRef]
- O’Bryan, R.M.; Baker, L.H.; Gottlieb, J.E.; Rivkin, S.E.; Balcerzak, S.P.; Grumet, G.N.; Salmon, S.E.; Moon, T.E.; Hoogstraten, B. Dose response evaluation of adriamycin in human neoplasia. Cancer 1977, 39, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Kirino, S.; Tsuchiya, K.; Kurosaki, M.; Kaneko, S.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Okada, M.; et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS ONE 2020, 15, e0231828. [Google Scholar] [CrossRef] [PubMed]
- Shirotake, S.; Yasumizu, Y.; Ito, K.; Masunaga, A.; Ito, Y.; Miyazaki, Y.; Hagiwara, M.; Kanao, K.; Mikami, S.; Nakagawa, K.; et al. Impact of Second-Line Targeted Therapy Dose Intensity on Patients with Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2016, 14, e575–e583. [Google Scholar] [CrossRef]
- Barr, P.M.; Brown, J.R.; Hillmen, P.; O’Brien, S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.; Mulligan, S.P.; Jaeger, U.; Furman, R.R.; et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood 2017, 129, 2612–2615. [Google Scholar] [CrossRef]
- Ribed, A.; Romero-Jiménez, R.M.; Escudero-Vilaplana, V.; Iglesias-Peinado, I.; Herranz-Alonso, A.; Codina, C.; Sanjurjo-Sáez, M. Pharmaceutical care program for onco-hematologic outpatients: Safety, efficiency and patient satisfaction. Int. J. Clin. Pharm. 2016, 38, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Gervès-Pinquié, C.; Daumas-Yatim, F.; Lalloué, B.; Girault, A.; Ferrua, M.; Fourcade, A.; Lemare, F.; Dipalma, M.; Minvielle, E. Impacts of a navigation program based on health information technology for patients receiving oral anticancer therapy: The CAPRI randomized controlled trial. BMC Health Serv. Res. 2017, 17, 133. [Google Scholar] [CrossRef]
- Kim, S.H.; Suh, Y.; Ah, Y.-M.; Jun, K.; Lee, J.-Y. Real-world prevalence of potential drug-drug interactions involving oral antineoplastic agents: A population-based study. Support. Care Cancer 2019, 28, 3617–3626. [Google Scholar] [CrossRef]
- Mir, O.; Touati, N.; Lia, M.; Litière, S.; Le Cesne, A.; Sleijfer, S.; Blay, J.-Y.; Leahy, M.; Young, R.; Mathijssen, R.H.J.; et al. Impact of Concomitant Administration of Gastric Acid-Suppressive Agents and Pazopanib on Outcomes in Soft-Tissue Sarcoma Patients Treated within the EORTC 62043/62072 Trials. Clin. Cancer Res. 2019, 25, 1479–1485. [Google Scholar] [CrossRef]
- Vacher, L.; Thivat, E.; Poirier, C.; Mouret-Reynier, M.-A.; Chollet, P.; Devaud, H.; Dubray-Longeras, P.; Kwiatkowski, F.; Durando, X.; van Praagh-Doreau, I.; et al. Improvement in adherence to Capecitabine and Lapatinib by way of a therapeutic education program. Support. Care Cancer 2019, 28, 3313–3322. [Google Scholar] [CrossRef]
- Gebbia, V.; Bellavia, M.; Banna, G.L.; Russo, P.; Ferraù, F.; Tralongo, P.; Borsellino, N. Treatment monitoring program for implementation of adherence to second-line erlotinib for advanced non-small-cell lung cancer. Clin. Lung Cancer 2013, 14, 390–398. [Google Scholar] [CrossRef]
- Akhtar, O.S.; Attwood, K.; Lund, I.; Hare, R.; Hernandez-Ilizaliturri, F.J.; Torka, P. Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL). Leuk. Lymphoma 2019, 60, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Timlin, C.; Ujjani, C.; Skarbnik, A.; Howlett, C.; Banerjee, R.; Nabhan, C.; Schuster, S.J. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: Results from a multi-centre study. Br. J. Haematol. 2018, 181, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Forum, U.C. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: A UK and Ireland analysis of outcomes in 315 patients. Haematologica 2016, 101, 1563. [Google Scholar] [CrossRef]
- Coutre, S.E.; Byrd, J.C.; Hillmen, P.; Barrientos, J.C.; Barr, P.M.; Devereux, S.; Robak, T.; Kipps, T.J.; Schuh, A.; Moreno, C.; et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019, 3, 1799–1807. [Google Scholar] [CrossRef]
- Sandy, J.; Della-Fiorentina, S. Relative dose intensity in early stage breast cancer chemotherapy: A retrospective analysis of incidence, risk factors and outcomes at a south-west Sydney cancer clinic. Asia Pac. J. Clin. Oncol. 2013, 9, 365–372. [Google Scholar] [CrossRef]
- van Egdom, L.S.E.; Oemrawsingh, A.; Verweij, L.M.; Lingsma, H.F.; Koppert, L.B.; Verhoef, C.; Klazinga, N.S.; Hazelzet, J.A. Implementing Patient-Reported Outcome Measures in Clinical Breast Cancer Care: A Systematic Review. Value Health 2019, 22, 1197–1226. [Google Scholar] [CrossRef] [PubMed]
- Hammersen, F.; Pursche, T.; Fischer, D.; Katalinic, A.; Waldmann, A. Use of Complementary and Alternative Medicine among Young Patients with Breast Cancer. Breast Care 2020, 15, 163–170. [Google Scholar] [CrossRef]
- Clairet, A.-L.; Boiteux-Jurain, M.; Curtit, E.; Jeannin, M.; Gérard, B.; Nerich, V.; Limat, S. Interaction between phytotherapy and oral anticancer agents: Prospective study and literature review. Med. Oncol. 2019, 36, 45. [Google Scholar] [CrossRef]
- Kaye, A.D.; Clarke, R.C.; Sabar, R.; Vig, S.; Dhawan, K.P.; Hofbauer, R.; Kaye, A.M. Herbal medicines: Current trends in anesthesiology practice--a hospital survey. J. Clin. Anesth. 2000, 12, 468–471. [Google Scholar] [CrossRef]
- Adusumilli, P.S.; Ben-Porat, L.; Pereira, M.; Roesler, D.; Leitman, I.M. The prevalence and predictors of herbal medicine use in surgical patients. J. Am. Coll. Surg. 2004, 198, 583–590. [Google Scholar] [CrossRef]
- Kawasumi, K.; Kujirai, A.; Matsui, R.; Kawano, Y.; Yamaguchi, M.; Aoyama, T. Survey of serious adverse events and safety evaluation of oral anticancer drug treatment in Japan: A retrospective study. Mol. Clin. Oncol. 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Schlichtig, K.; Dürr, P.; Dörje, F.; Fromm, M.F. Medication Errors During Treatment with New Oral Anticancer Agents: Consequences for Clinical Practice Based on the AMBORA Study. Clin. Pharmacol. Ther. 2021, 110, 1075–1086. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Schrag, D.; Henson, S.; Jansen, J.; Ginos, B.; Stover, A.M.; Carr, P.; Spears, P.A.; Jonsson, M.; Deal, A.M.; et al. Effect of Electronic Symptom Monitoring on Patient-Reported Outcomes Among Patients With Metastatic Cancer: A Randomized Clinical Trial. JAMA 2022, 327, 2413–2422. [Google Scholar] [CrossRef]
- Marino, P.; Bannier, M.; Moulin, J.-F.; Gravis, G. The role and use of Patient Reported Outcomes in the management of cancer patients. Bull. Cancer 2018, 105, 603–609. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Li, L.; Sanoff, H.K.; Carpenter, W.; Schrag, D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J. Clin. Oncol. 2012, 30, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Chavez-MacGregor, M.; Giordano, S.H. Randomized Clinical Trials and Observational Studies: Is There a Battle? J. Clin. Oncol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Morton, S.C.; Costlow, M.R.; Graff, J.S.; Dubois, R.W. Standards and guidelines for observational studies: Quality is in the eye of the beholder. J. Clin. Epidemiol. 2016, 71, 3–10. [Google Scholar] [CrossRef]
- Goh, I.; Lai, O.; Chew, L. Prevalence and Risk of Polypharmacy Among Elderly Cancer Patients Receiving Chemotherapy in Ambulatory Oncology Setting. Curr. Oncol. Rep. 2018, 20, 38. [Google Scholar] [CrossRef]
- Huot, L.; Guerre, P.; Descotes, G.; Caffin, A.-G.; Herledan, C.; Ranchon, F.; Rioufol, C. Cost-effectiveness of the ONCORAL multidisciplinary programme for the management of outpatients taking oral anticancer agents at risk of drug-related event: Protocol for a pragmatic randomised controlled study. BMJ Open 2024, 14, e074956. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). Protocol Development. CTEP. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 3 May 2020).
- Bias Reduction of Maximum Likelihood Estimates. Biometrika. Oxford Academic. Available online: https://academic.oup.com/biomet/article/80/1/27/228364 (accessed on 2 March 2022).
- Lattard, C.; Herledan, C.; Reverdy, T.; Antherieu, G.; Caffin, A.-G.; Cerfon, M.-A.; Maire, M.; Rivat, M.; France, S.; Ghesquières, H.; et al. Early follow-up of outpatients with oral anticancer therapy in the ONCORAL multidisciplinary community-hospital program. Oncologist 2025, 30, oyae241. [Google Scholar] [CrossRef] [PubMed]
- Malifarge, L.; Deppenweiler, M.; Italiano, A.; Lortal, B. Impact of Medication Reconciliation in Oncology Early Phase Studies: A Drug-Drug Interaction Retrospective Study. JCO Oncol. Pract. 2024, 20, 386–392. [Google Scholar] [CrossRef] [PubMed]

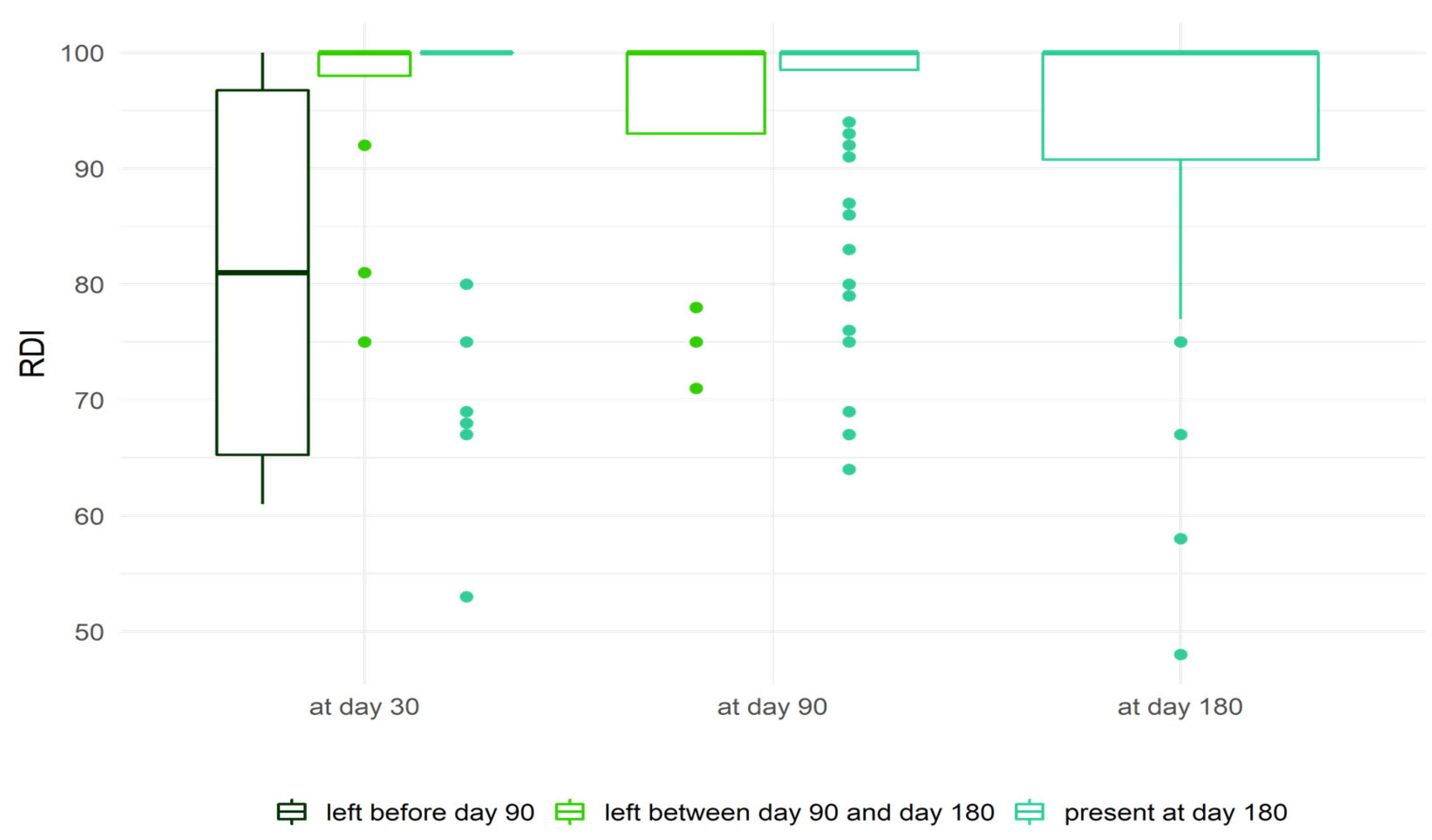
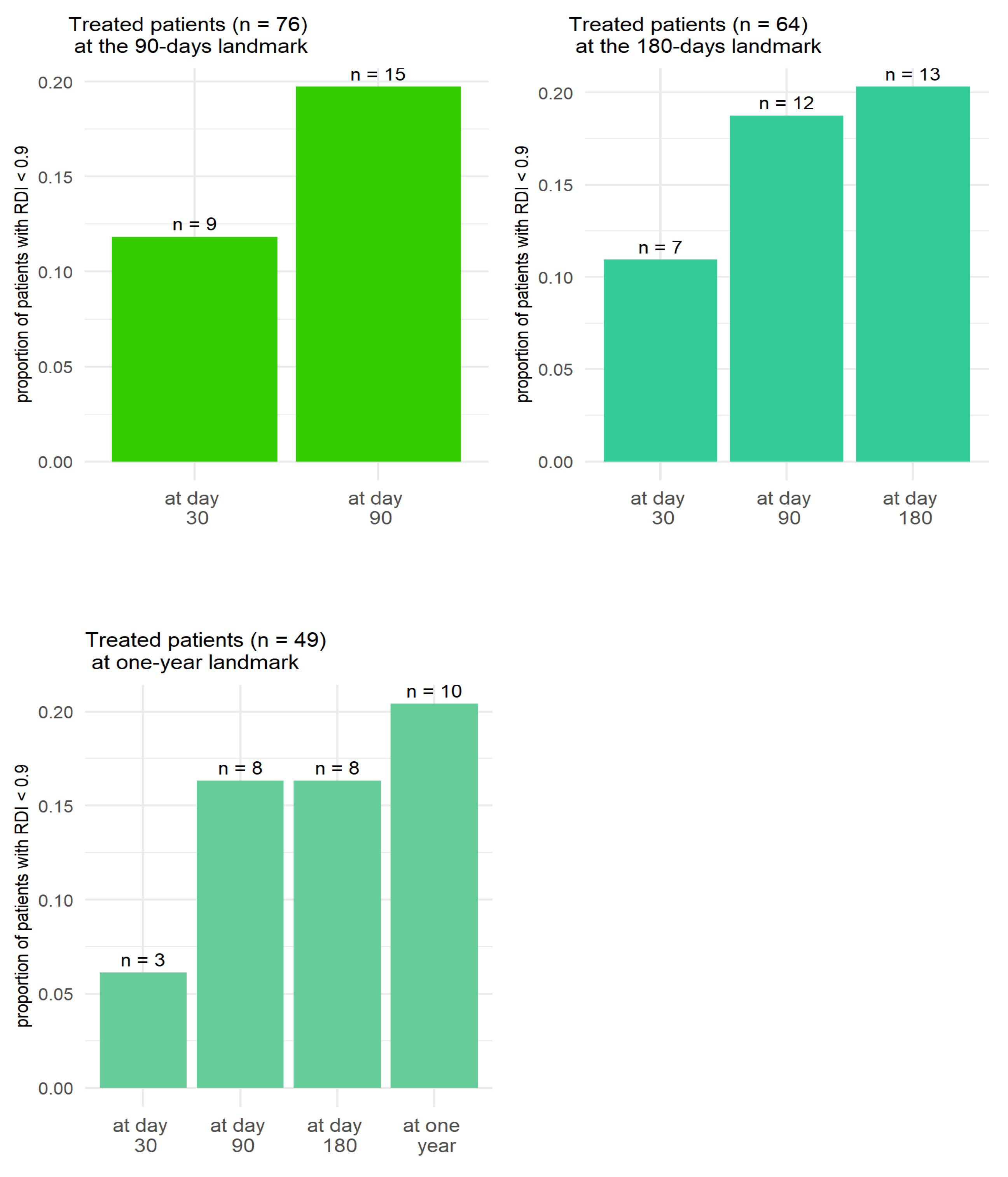
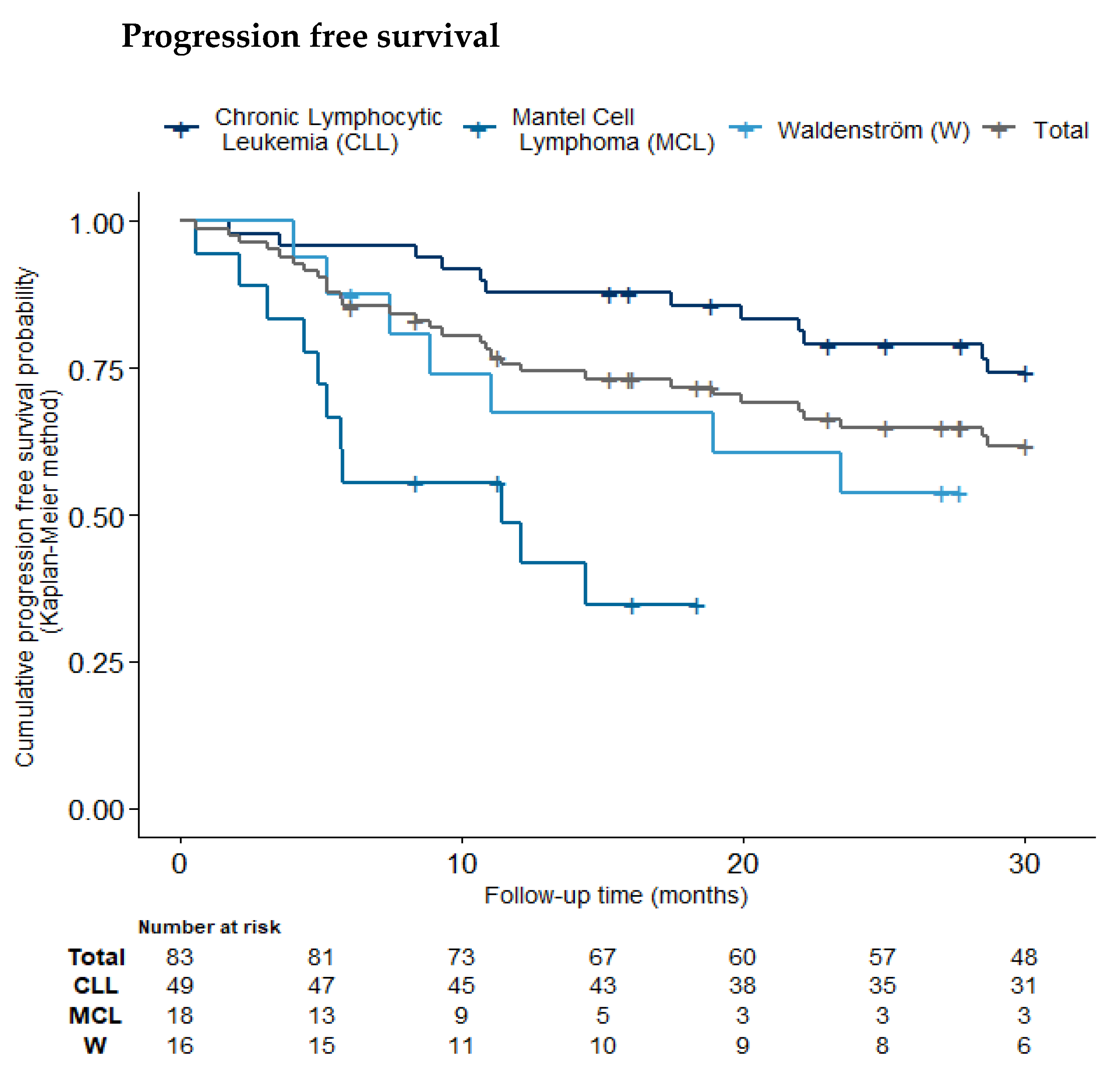
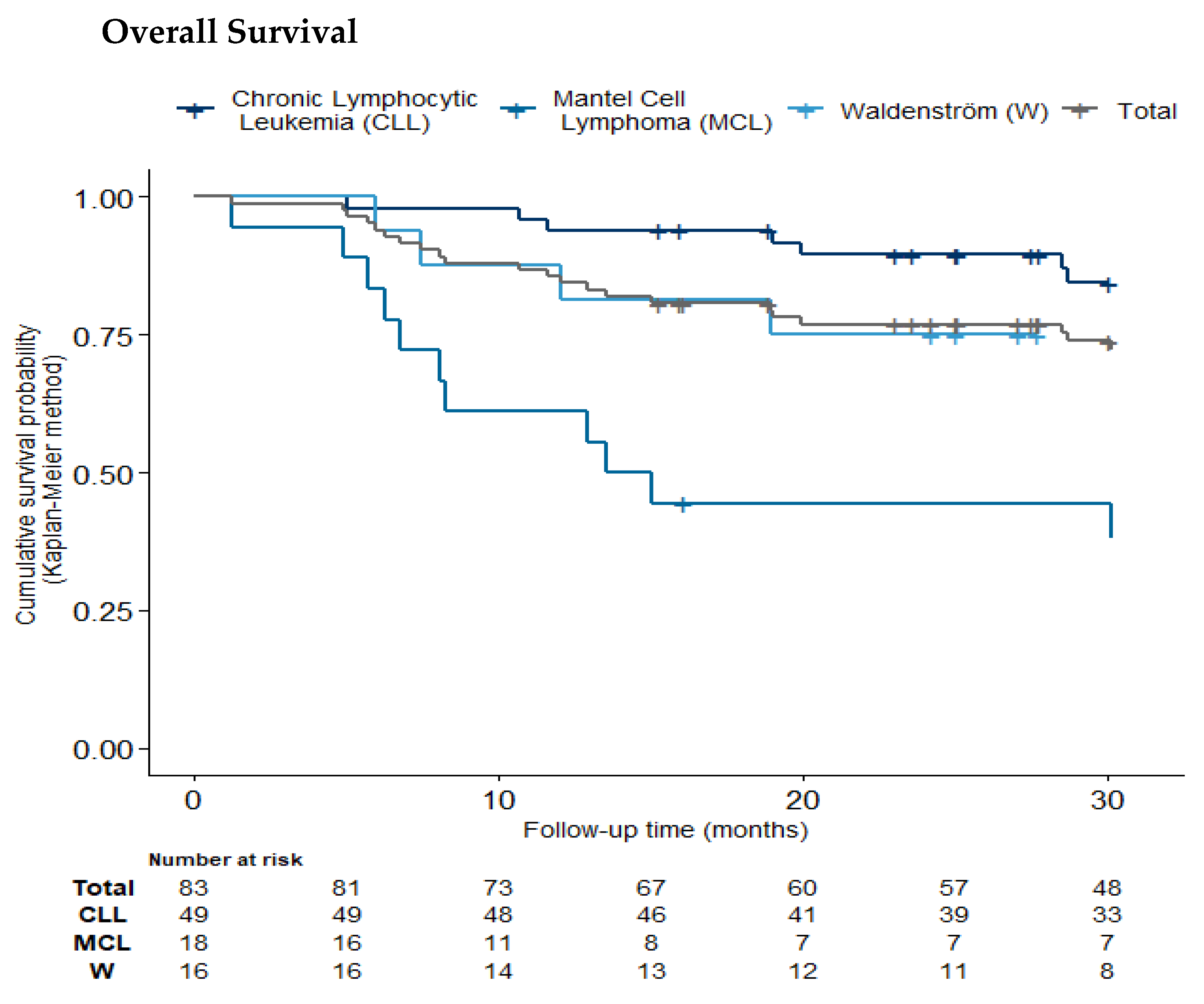
| Pathology | Chronic Lymphocytic Leukemia (n = 49, 59%) | Mantel Cell Lymphoma (n = 18, 22%) | Waldenström (n = 16, 19%) | Total (n = 83) |
|---|---|---|---|---|
| Patient number evolution | ||||
| Month 1 | 49 | 17 | 16 | 82 |
| Month 3 | 45 | 15 | 16 | 76 |
| Month 6 | 42 | 10 | 12 | 64 |
| Age, years | ||||
| Median (IQR) | 71.07 (63.8–80.0) | 78.00 (68.8–81.1) | 77.53 (70.6–79.8) | 74.38 |
| Sex | ||||
| Female | 17 (34.7%) | 2 (11.1%) | 7 (43.8%) | 26 (31.3%) |
| Male | 32 (65.3%) | 16 (88.9%) | 9 (56.2%) | 57 (68.7%) |
| Charlson | ||||
| Median (IQR) | 3.00 (2.00–4.00) | 4.50 (4.00–5.00) | 3.50 (3.00–4.25) | 4.00 |
| Hypertension | 21 (42.9%) | 5 (27.8%) | 4 (25.0%) | 30 (36.1%) |
| Cardio-vascular disease (CVD) | 9 (18.4%) | 4 (22.2%) | 7 (43.8%) | 20 (24.1%) |
| Treatment line | ||||
| 1 | 6 (12.2%) | 0 (0.0%) | 1 (6.2%) | 7 (8.4%) |
| 2 | 26 (53.1%) | 11 (61.1%) | 7 (43.8%) | 44 (53.0%) |
| 3 | 11 (22.4%) | 5 (27.8%) | 1 (6.2%) | 17 (20.5%) |
| 4 and more | 6 (12.2%) | 2 (11.1%) | 7 (43.8%) | 15 (18.1%) |
| Effect of Concomitant Drug and CAM | Drug and CAM | Interaction’s Classification According to Drugs® |
|---|---|---|
| Apixaban | Major | |
| Rivaroxaban | Major | |
| Increased risk of bleeding | Clomipramine | Moderate |
| Diclofenac (topic et ophtalmique) | Moderate | |
| Duloxetine | Moderate | |
| Escitalopram | Moderate | |
| Fluoxetine | Moderate | |
| Paroxetine | Moderate | |
| Sertraline | Moderate | |
| Venlafaxine | Moderate | |
| Vitamine E | / | |
| Enzyme inducer | Carbamazepine Phenobarbital | Major |
| Major | ||
| Diltiazem | Major | |
| Verapamil | Major | |
| Amiodarone | Moderate | |
| Enzyme inhibitor | Turmeric | / |
| Grapefruit | / | |
| Linseed oil | / | |
| Aloe vera | / | |
| Ginger | / | |
| Modification of metabolism by ibrutinib | Loperamide | Moderate |
| Silodosine | Moderate | |
| Sitagliptine | Moderate | |
| Antioxidant, reduction in absorption or other mechanism | Omega 3/6 | / |
| Propolis | / | |
| Psyllium | / | |
| Spirulina | / |
| 180 Day RDI | RDI < 90% | RDI ≥ 90% | Total |
|---|---|---|---|
| N = 13 | N = 51 | N = 64 | |
| age | |||
| Mean (SD) | 73.43 (8.95) | 70.59 (10.13) | 71.17 (9.90) |
| Median | 75.50 | 70.44 | 71.03 |
| Q1–Q3 | 68.35–80.01 | 64.06–78.10 | 65.20–79.68 |
| Min-Max | 50.18–84.08 | 50.10–88.77 | 50.10–88.77 |
| N | 13 | 51 | 64 |
| Sex | |||
| F | 7 (53.8%) | 17 (33.3%) | 24 (37.5%) |
| M | 6 (46.2%) | 34 (66.7%) | 40 (62.5%) |
| Charlson | |||
| Mean (SD) | 3.92 (1.66) | 3.45 (1.91) | 3.55 (1.86) |
| Median | 4.00 | 3.00 | 3.50 |
| Q1–Q3 | 3.00–4.00 | 2.00– 4.50 | 2.00– 4.25 |
| Min-Max | 1.00–7.00 | 1.00–12.00 | 1.00–12.00 |
| N | 13 | 51 | 64 |
| polypharmacy | |||
| 0–5 | 9 (69.2%) | 38 (74.5%) | 47 (73.4%) |
| 5+ | 4 (30.8%) | 13 (25.5%) | 17 (26.6%) |
| CAM | |||
| no | 4 (30.8%) | 12 (23.5%) | 16 (25.0%) |
| yes | 9 (69.2%) | 39 (76.5%) | 48 (75.0%) |
| Adverse events | |||
| Grade 0, 1, 2 | 10 (76.9%) | 36 (70.6%) | 46 (71.9%) |
| Grade 3–4 | 3 (23.1%) | 15 (29.4%) | 18 (28.1%) |
| Live_alone | |||
| 0 | 8 (61.5%) | 36 (70.6%) | 44 (68.8%) |
| 1 | 5 (38.5%) | 14 (27.5%) | 19 (29.7%) |
| NA | 0 (0.0%) | 1 (2.0%) | 1 (1.6%) |
| Pathology | |||
| CLL | 7 (53.8%) | 35 (68.6%) | 42 (65.6%) |
| Mantel cell lymphoma | 1 (7.7%) | 9 (17.6%) | 10 (15.6%) |
| Waldenström | 5 (38.5%) | 7 (13.7%) | 12 (18.8%) |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | OR [95% CI] | p Value | OR [95% CI] | p Value | |||
| Age | (continuous) | 19 | 64 | 1.07 [1.01; 1.15] | 0.02 | 1.10 [1.03; 1.19] | >0.01 | |
| Sex | Female | 2 | 24 | 1 (reference) | 1 (reference) | |||
| Male | 17 | 40 | 4.23 [1.19; 22.59] | 0.02 | 6.44 [1.65; 37.21] | 0.01 | ||
| Charlson | (discrete) | 19 | 64 | 1.27 [1.01; 1.65] | 0.04 | |||
| Polypharmacy | 0–5 | 12 | 47 | 1 (reference) | ||||
| 5+ | 7 | 17 | 1.63 [0.55; 4.65] | 0.37 | ||||
| CAM use | No | 5 | 16 | 1 (reference) | ||||
| Yes | 14 | 48 | 0.9 [0.30; 2.97] | 0.85 | ||||
| Live alone | No | 14 | 44 | 1 (reference) | ||||
| Yes | 5 | 19 | 0.87 [0.26; 2.55] | 0.80 | ||||
| Pathology | CLL | 7 | 42 | 1 (reference) | ||||
| MCL | 8 | 10 | 4.59 [1.4; 15.63] | 0.01 | ||||
| WM | 4 | 12 | 2.04 [0.51; 7.6] | 0.30 | ||||
| Hypertension | No | 14 | 39 | 1 (reference) | ||||
| Yes | 5 | 25 | 0.59 [0.18; 1.7] | 0.33 | ||||
| Cardio-vascular disease | No | 11 | 52 | 1 (reference) | ||||
| Yes | 8 | 12 | 3.1 [1.04; 9.23] | 0.04 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larbre, V.; Ranchon, F.; Maucort-Boulch, D.; Coz, E.; Herledan, C.; Caffin, A.-G.; Baudouin, A.; Maire, M.; Romain-Scelle, N.; Vacheron, C.-H.; et al. Oncoral Follow-Up for Outpatients Treated with Oral Anticancer Drugs Assessed by Relative Dose Intensity. Pharmaceuticals 2025, 18, 565. https://doi.org/10.3390/ph18040565
Larbre V, Ranchon F, Maucort-Boulch D, Coz E, Herledan C, Caffin A-G, Baudouin A, Maire M, Romain-Scelle N, Vacheron C-H, et al. Oncoral Follow-Up for Outpatients Treated with Oral Anticancer Drugs Assessed by Relative Dose Intensity. Pharmaceuticals. 2025; 18(4):565. https://doi.org/10.3390/ph18040565
Chicago/Turabian StyleLarbre, Virginie, Florence Ranchon, Delphine Maucort-Boulch, Elsa Coz, Chloé Herledan, Anne-Gaëlle Caffin, Amandine Baudouin, Magali Maire, Nicolas Romain-Scelle, Charles-Hervé Vacheron, and et al. 2025. "Oncoral Follow-Up for Outpatients Treated with Oral Anticancer Drugs Assessed by Relative Dose Intensity" Pharmaceuticals 18, no. 4: 565. https://doi.org/10.3390/ph18040565
APA StyleLarbre, V., Ranchon, F., Maucort-Boulch, D., Coz, E., Herledan, C., Caffin, A.-G., Baudouin, A., Maire, M., Romain-Scelle, N., Vacheron, C.-H., Karlin, L., Salles, G., Ghesquières, H., & Rioufol, C. (2025). Oncoral Follow-Up for Outpatients Treated with Oral Anticancer Drugs Assessed by Relative Dose Intensity. Pharmaceuticals, 18(4), 565. https://doi.org/10.3390/ph18040565







