Bioactivity of Synthesized Trifluoromethyl Thioxanthone Analogues
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antioxidant Activity Results
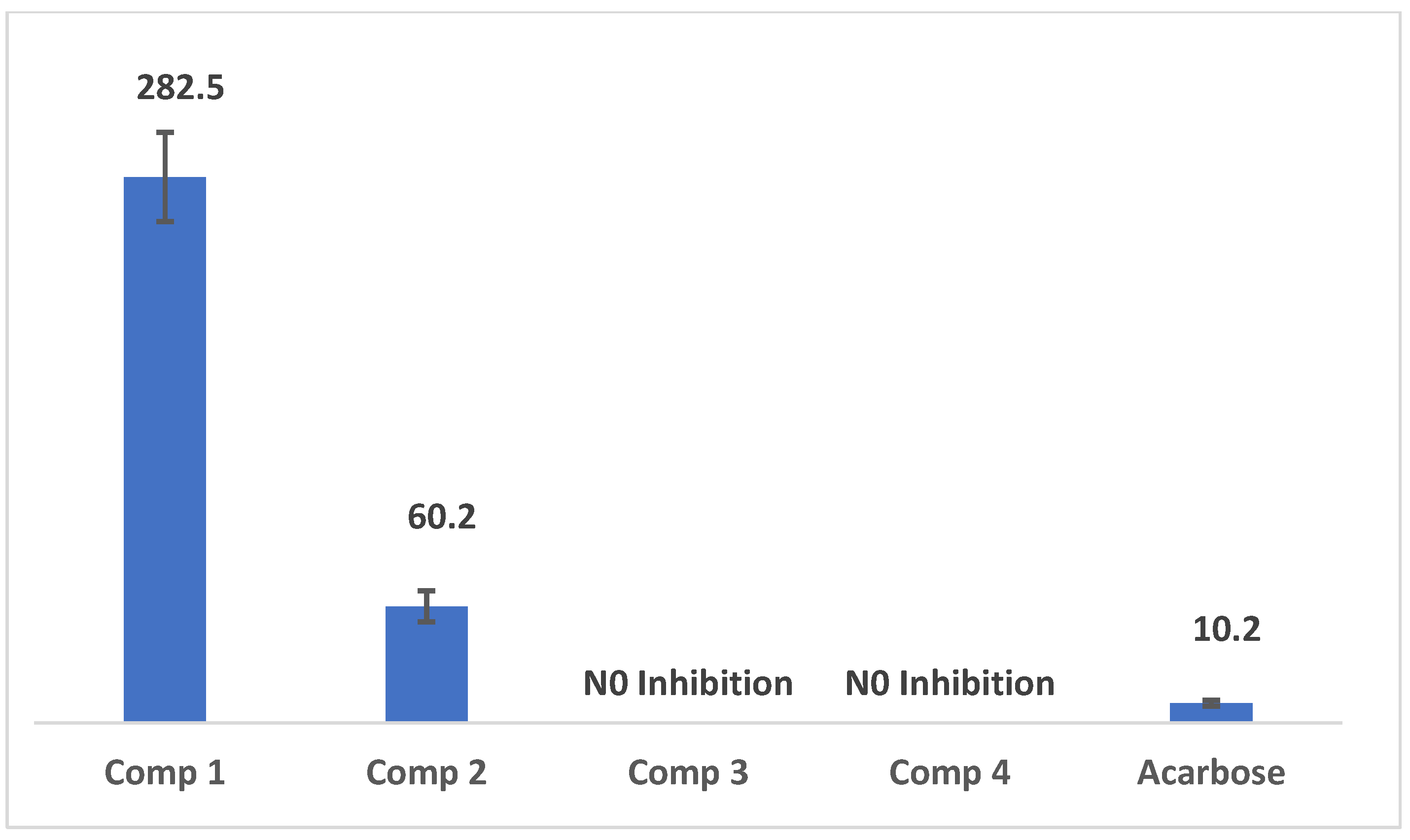
2.3. Results of α-Amylase Inhibition Assay
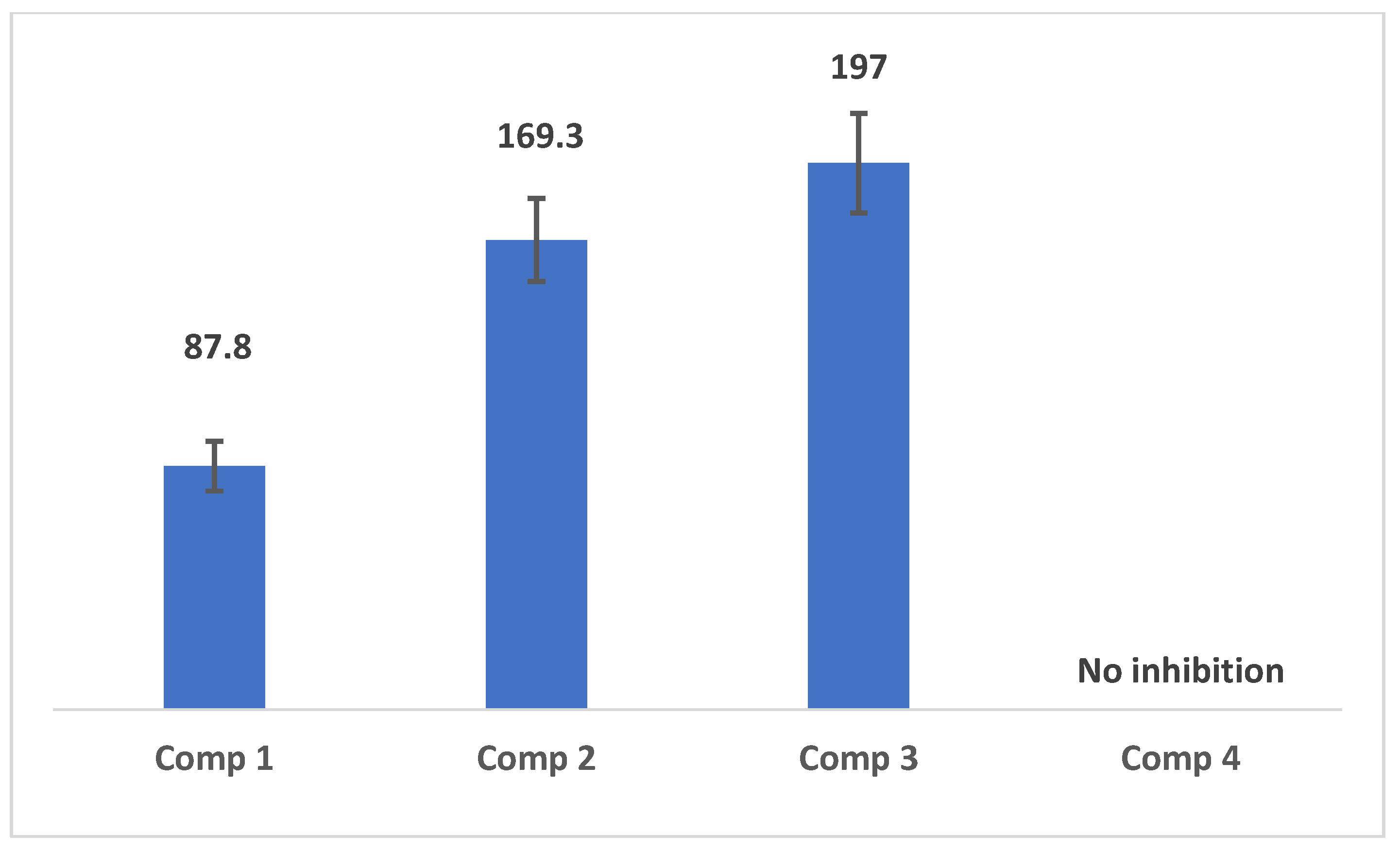
2.4. Results of Inhibition Assay of Pancreatic Lipase Enzyme
2.5. Results of the Cytotoxicity Results
2.6. Cyclooxygenase Inhibition Activity
3. Materials and Methods
3.1. Reagents and Materials
3.2. Statistical Analysis and IC50 Calculations
3.3. Chemical Synthesis and Characterization of the Products
3.3.1. Synthesis of Tertiary Alcohols
3.3.2. General Synthesis of Thioxanthene Analogues
3.4. Antioxidant Activity
3.5. α-Amylase Inhibition Assay
3.6. Inhibition Assay of Pancreatic Lipase Enzyme
3.7. Cytotoxicity Procedure
3.8. COX Inhibition Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bubendorf, L.; Schopfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.P.; Zager, J.S. Future perspectives: Cancer metastases. Clin. Exp. Metastasis 2018, 35, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Ain, D.; Shaikh, T.; Manimala, S.; Ghebrehiwet, B. The role of complement in the tumor microenvironment. Fac. Rev. 2021, 10, 80. [Google Scholar] [CrossRef]
- Chaudhry, G.E.; Jan, R.; Akim, A.; Zafar, M.N.; Sung, Y.Y.; Muhammad, T.S.T. Breast Cancer: A Global Concern, Diagnostic and Therapeutic Perspectives, Mechanistic Targets in Drug Development. Adv. Pharm. Bull. 2021, 11, 580–594. [Google Scholar] [CrossRef]
- Huang, W.; Kong, L.; Cao, Y.; Yan, L. Identification and Quantification, Metabolism and Pharmacokinetics, Pharmacological Activities, and Botanical Preparations of Protopine: A Review. Molecules 2021, 27, 215. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Kobayashi, H.; Higashiura, Y.; Shigetomi, H.; Kajihara, H. Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation (Review). Mol. Med. Rep. 2014, 9, 9–15. [Google Scholar] [CrossRef]
- Alderton, G.; Scanlon, S.T. Inflammation. Science 2021, 374, 1068–1069. [Google Scholar] [CrossRef]
- Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef]
- Gallo, C.G.; Fiorino, S.; Posabella, G.; Antonacci, D.; Tropeano, A.; Pausini, E.; Pausini, C.; Guarniero, T.; Hong, W.; Giampieri, E.; et al. The function of specialized pro-resolving endogenous lipid mediators, vitamins, and other micronutrients in the control of the inflammatory processes: Possible role in patients with SARS-CoV-2 related infection. Prostaglandins Other Lipid Mediat. 2022, 159, 106619. [Google Scholar] [CrossRef]
- Habtemariam, S. The anti-obesity potential of sigmoidin A. Pharm. Biol. 2012, 50, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Azziz, S.S.S.A.; Wong, C.F.; Din, W.N.I.W.M.; Mahamod, W.R.W.; Bakri, Y.M.; Ahmad, M.S.; Yahaya, R.; Ismail, N.H.; Salleh, W.M.N.H.W. evaluation of anti-lipase activity of leaf and bark extracts from Aquilaria subintegra and A. malaccensis. Marmara Pharm. J. 2017, 22, 91–95. [Google Scholar]
- Chang, Y.; Zhang, D.; Yang, G.; Zheng, Y.; Guo, L. Screening of Anti-Lipase Components of Artemisia argyi Leaves Based on Spectrum-Effect Relationships and HPLC-MS/MS. Front. Pharmacol. 2021, 12, 675396. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Somtimuang, C.; Olatunji, O.J.; Ovatlarnporn, C. Evaluation of In Vitro alpha-Amylase and alpha-Glucosidase Inhibitory Potentials of 14 Medicinal Plants Constituted in Thai Folk Antidiabetic Formularies. Chem. Biodivers. 2018, 15, e1800025. [Google Scholar] [CrossRef]
- West, I.C. Radicals and oxidative stress in diabetes. Diabet. Med. A J. Br. Diabet. Assoc. 2000, 17, 171–180. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Rajagopalan, R. Diabetes and insulin resistance associated disorders: Disease and the therapy. Curr. Sci. 2002, 83, 1533–1538. [Google Scholar]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef]
- Gulshan Singh, P.K. Bioassay Guided Fractionation and α-Amylase Inhibitory Activity of Flavanoid Isolated from Pinus roxburghii Sarg. Nat. Prod. Chem. Res. 2015, 3, 2. [Google Scholar] [CrossRef]
- Agarwal, P.; Gupta, R. Alpha-amylase inhibition can treat diabetes mellitus. J. Med. Phys. 2016, 5, 1–8. [Google Scholar]
- Lim, E.Y.; Park, J.; Kim, Y.T.; Kim, M.J. Imipramine Inhibits Migration and Invasion in Metastatic Castration-Resistant Prostate Cancer PC-3 Cells via AKT-Mediated NF-kappaB Signaling Pathway. Molecules 2020, 25, 4619. [Google Scholar] [CrossRef] [PubMed]
- Onyango, E.O.; Fu, L.; Gribble, G.W. Synthesis of a dicyano abietane, a key intermediate for the anti-inflammatory agent TBE-31. Org. Lett. 2014, 16, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.M.; Deng, Y.; Dai, X.Y.; Liu, J.; Ouyang, L.; Wei, Y.Q.; Zhao, Y.L. Synthesis and biological evaluation of novel acenaphthene derivatives as potential antitumor agents. Molecules 2011, 16, 2519–2526. [Google Scholar] [CrossRef]
- Hassan, A.A.; Aly, A.A.; Mohamed, N.K.; El Shaieb, K.M.; Makhlouf, M.M.; Abdelhafez, E.M.N.; Brase, S.; Nieger, M.; Dalby, K.N.; Kaoud, T.S. Design, synthesis, and DNA interaction studies of furo-imidazo[3.3.3]propellane derivatives: Potential anticancer agents. Bioorg. Chem. 2019, 85, 585–599. [Google Scholar] [CrossRef]
- Araujo, P.C.O.; Sari, M.H.M.; Jardim, N.S.; Jung, J.T.K.; Bruning, C.A. Effect of m-trifluoromethyl-diphenyl diselenide on acute and subchronic animal models of inflammatory pain: Behavioral, biochemical and molecular insights. Chem.-Biol. Interact. 2020, 317, 108941. [Google Scholar] [CrossRef]
- Luzina, E.L.; Popov, A.V. Anticancer activity of N-bis(trifluoromethyl)alkyl-N′-(polychlorophenyl) and N′-(1,2,4-triazolyl) ureas. Eur. J. Med. Chem. 2010, 45, 5507–5512. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y. l-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar] [CrossRef]
- Daher, B.; Vucetic, M.; Pouyssegur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system xc−: Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An Update on the Anticancer Activity of Xanthone Derivatives: A Review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef]
- Saraswathy, S.U.P.; Lalitha, L.C.P.; Rahim, S.; Gopinath, C.; Haleema, S.; SarojiniAmma, S.; Aboul-Enein, H.Y. A Review on Synthetic and Pharmacological Potential of Compounds Isolated from Garcinia mangostana Linn. Phytomedicine Plus 2022, 2, 100253. [Google Scholar] [CrossRef]
- Lima, R.T.; Sousa, D.; Gomes, A.S.; Mendes, N.; Matthiesen, R.; Pedro, M.; Marques, F.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. The Antitumor Activity of a Lead Thioxanthone is Associated with Alterations in Cholesterol Localization. Molecules 2018, 23, 3301. [Google Scholar] [CrossRef]
- Abualhasan, M.N.; Hawash, M.; Aqel, S.; Al-Masri, M.; Mousa, A.; Issa, L. Biological Evaluation of Xanthene and Thioxanthene Derivatives as Antioxidant, Anticancer, and COX Inhibitors. ACS Omega 2023, 8, 38597–38606. [Google Scholar]
- Chen, F.; Romero-Canelon, I.; Habtemariam, A.; Song, J.I.; Banerjee, S.; Clarkson, G.J.; Song, L.; Prokes, I.; Sadler, P.J. Effect of cysteine thiols on the catalytic and anticancer activity of Ru(II) sulfonyl-ethylenediamine complexes. Dalton Trans. 2022, 51, 4447–4457. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Perevozova, T.V.; Ivanova, A.I.; Averkin, D.V.; Khizhnyak, S.D.; Pakhomov, P.M. l-Cysteine as a reducing/capping/gel-forming agent for the preparation of silver nanoparticle composites with anticancer properties. Soft Matter 2022, 18, 3031–3040. [Google Scholar]
- Jaradat, N.; Al-Lahham, S.; Abualhasan, M.N.; Bakri, A.; Zaide, H.; Hammad, J.; Hussein, F.; Issa, L.; Mousa, A.; Speih, R. Chemical Constituents, Antioxidant, Cyclooxygenase Inhibitor, and Cytotoxic Activities of Teucrium pruinosum Boiss. Essential Oil. Biomed. Res. Int. 2018, 2018, 4034689. [Google Scholar] [CrossRef]
- Francis, P.; Chakraborty, K. Clathriketal, a new tricyclic spiroketal compound from marine sponge Clathria prolifera attenuates serine exopeptidase dipeptidyl peptidase-IV. Nat. Prod. Res. 2022, 36, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Lee, S.S.; Chung, B.Y.; Cho, J.Y.; Lee, I.C.; Ahn, S.R.; Jang, S.J.; Kim, T.H. Pancreatic lipase inhibition by C-glycosidic flavones Isolated from Eremochloa ophiuroides. Molecules 2010, 15, 8251–8259. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.-A.S.; Abd El-Karim, S.S. Design, synthesis and anticervical cancer activity of new benzofuran–pyrazol-hydrazono-thiazolidin-4-one hybrids as potential EGFR inhibitors and apoptosis inducing agents. Bioorg. Chem. 2019, 89, 103035. [Google Scholar]
- Bozorov, K.A.; Mamadalieva, N.Z.; Elmuradov, B.Z.; Triggiani, D.; Egamberdieva, D.; Tiezzi, A.; Aisa, H.A.; Shakhidoyatov, K.M.; Arslan, H. Synthesis of Substituted Thieno[2,3-d]pyrimidin-4-ones and Their Testing for Evaluation of Cytotoxic Activity on Mammalian Cell Models. J. Chem. 2012, 2013, 1–6. [Google Scholar] [CrossRef]
- Cerri, F.; Saliu, F.; Maggioni, D.; Montano, S.; Seveso, D.; Lavorano, S.; Zoia, L.; Gosetti, F.; Lasagni, M.; Orlandi, M.; et al. Cytotoxic Compounds from Alcyoniidae: An Overview of the Last 30 Years. Mar. Drugs 2022, 20, 134. [Google Scholar] [CrossRef]
- Ghodsi, R.; Zarghi, A.; Daraei, B.; Hedayati, M. Design, synthesis and biological evaluation of new 2, 3-diarylquinoline derivatives as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 1029–1033. [Google Scholar]
- Abualhasan, M.N.; Good, J.A.; Wittayanarakul, K.; Anthony, N.G.; Berretta, G.; Rath, O.; Kozielski, F.; Sutcliffe, O.B.; Mackay, S.P. Doing the methylene shuffle--further insights into the inhibition of mitotic kinesin Eg5 with S-trityl L-cysteine. Eur. J. Med. Chem. 2012, 54, 483–498. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Diaz-Sanchez, A.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of alpha-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta. Part. A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, C.S.; Park, K.M.; Chang, P.S. Inhibitory characteristics of flavonol-3-O-glycosides from Polygonum aviculare L. (common knotgrass) against porcine pancreatic lipase. Sci. Rep. 2019, 9, 18080. [Google Scholar] [CrossRef]
- Meerwein, M.; Tarnutzer, A.; Boni, M.; Van Bambeke, F.; Hombach, M.; Zinkernagel, A.S. Increased Azithromycin Susceptibility of Multidrug-Resistant Gram-Negative Bacteria on RPMI-1640 Agar Assessed by Disk Diffusion Testing. Antibiotics 2020, 9, 218. [Google Scholar] [CrossRef]
- Abualhasan, M.N.; Al-Masri, M.Y.; Manasara, R.; Yadak, L.; Abu-Hasan, N.S. Anti-Inflammatory and Anticoagulant Activities of Synthesized NSAID Prodrug Esters. Scientifica 2020, 2020, 9817502. [Google Scholar] [CrossRef]


| Conc. (µg/mL) | Trolox | Comp 1 | Comp 2 | Comp 3 | Comp 4 |
|---|---|---|---|---|---|
| 1 | 30.5 ± 2.2 | 7.1 ± 1.2 | 36.3 ± 2.4 | 31.3 ± 2.3 | 30.5 ± 1.2 |
| 2 | 23.6 ± 1.3 | 7.6 ± 2.3 | 37.3 ± 3.9 | 36.4 ± 3.5 | 36.4 ± 2.5 |
| 3 | 61.4 ± 5.3 | 8.2 ± 1.5 | 39.3 ± 4.2 | 39.9 ± 3.6 | 39.4 ± 1.5 |
| 5 | 90.7± 11.3 | 8.6 ± 3.1 | 39.5 ± 3.4 | 39.9 ± 4.1 | 41.2 ± 3.1 |
| 7 | 95.3 ± 10.7 | 8.9 ± 2.3 | 39.7 ± 3.6 | 40.1 ± 3.8 | 41.6 ± 3.6 |
| 10 | 95.9± 9.5 | 9.3 ± 1.6 | 40.1 ± 4.9 | 40.2 ± 5.1 | 41.9 ± 2.8 |
| 20 | 96.7 ± 12.5 | 9.9 ± 2.5 | 40.7 ± 2.5 | 40.4 ± 5.2 | 42.2 ± 2.4 |
| 30 | 97.2 ± 8.5 | 12.6 ± 3.2 | 40.7 ± 3.8 | 41.9 ± 4.9 | 43.3 ± 6.1 |
| 50 | 98.1± 9.2 | 12.9 ± 2.6 | 41.6 ± 5.1 | 42.9 ± 3.2 | 45.5 ± 5.1 |
| 80 | 98.9± 11.8 | 16.3 ± 4.3 | 42.2 ± 3.9 | 46.6 ± 2.4 | 45.6 ± 2.6 |
| Compounds | COX-1 IC50 (nM) | COX-2 IC50 (nM) | COX-2 Selectivity |
|---|---|---|---|
| Comp (1) | 40.2 ± 0.41 | 27.1 ± 0.6 | 1.5 |
| Comp (2) | 14.0 ± 0.64 | 74.1 ± 0.48 | 0.2 |
| Comp (3) | 32.5 ± 0.53 | 25.9 ± 0.45 | 1.3 |
| Comp (4) | 10.1± 1.30 | 6.5 ±0.77 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abualhasan, M.; Haider, H.; Odeh, A.; Daraghmeh, A. Bioactivity of Synthesized Trifluoromethyl Thioxanthone Analogues. Pharmaceuticals 2025, 18, 561. https://doi.org/10.3390/ph18040561
Abualhasan M, Haider H, Odeh A, Daraghmeh A. Bioactivity of Synthesized Trifluoromethyl Thioxanthone Analogues. Pharmaceuticals. 2025; 18(4):561. https://doi.org/10.3390/ph18040561
Chicago/Turabian StyleAbualhasan, Murad, Hussein Haider, Ahmad Odeh, and Amer Daraghmeh. 2025. "Bioactivity of Synthesized Trifluoromethyl Thioxanthone Analogues" Pharmaceuticals 18, no. 4: 561. https://doi.org/10.3390/ph18040561
APA StyleAbualhasan, M., Haider, H., Odeh, A., & Daraghmeh, A. (2025). Bioactivity of Synthesized Trifluoromethyl Thioxanthone Analogues. Pharmaceuticals, 18(4), 561. https://doi.org/10.3390/ph18040561







