Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation
Abstract
1. Introduction
2. Results
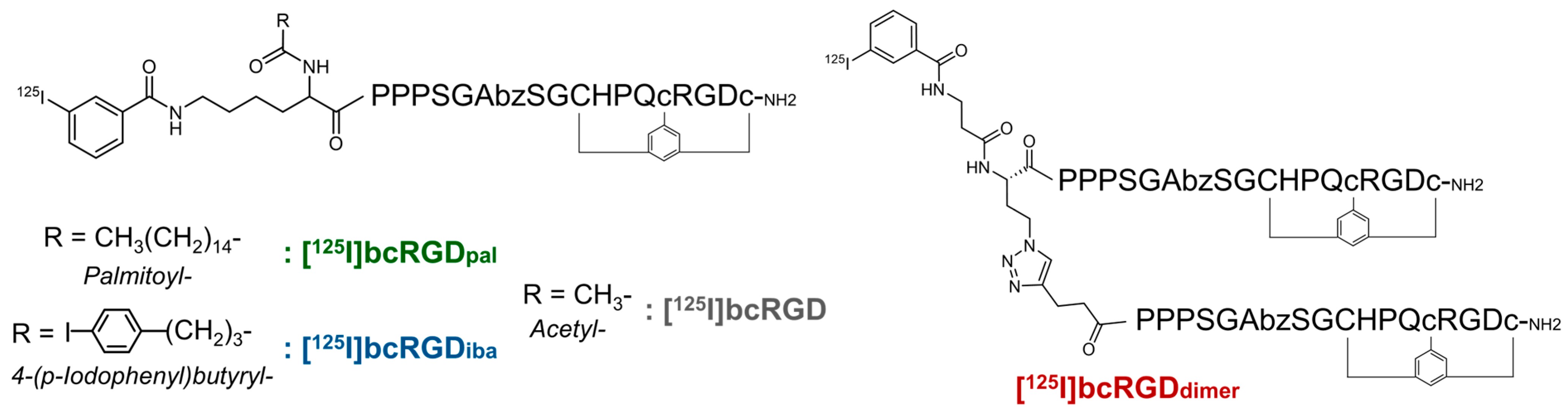
2.1. Properties of Prepared Peptides
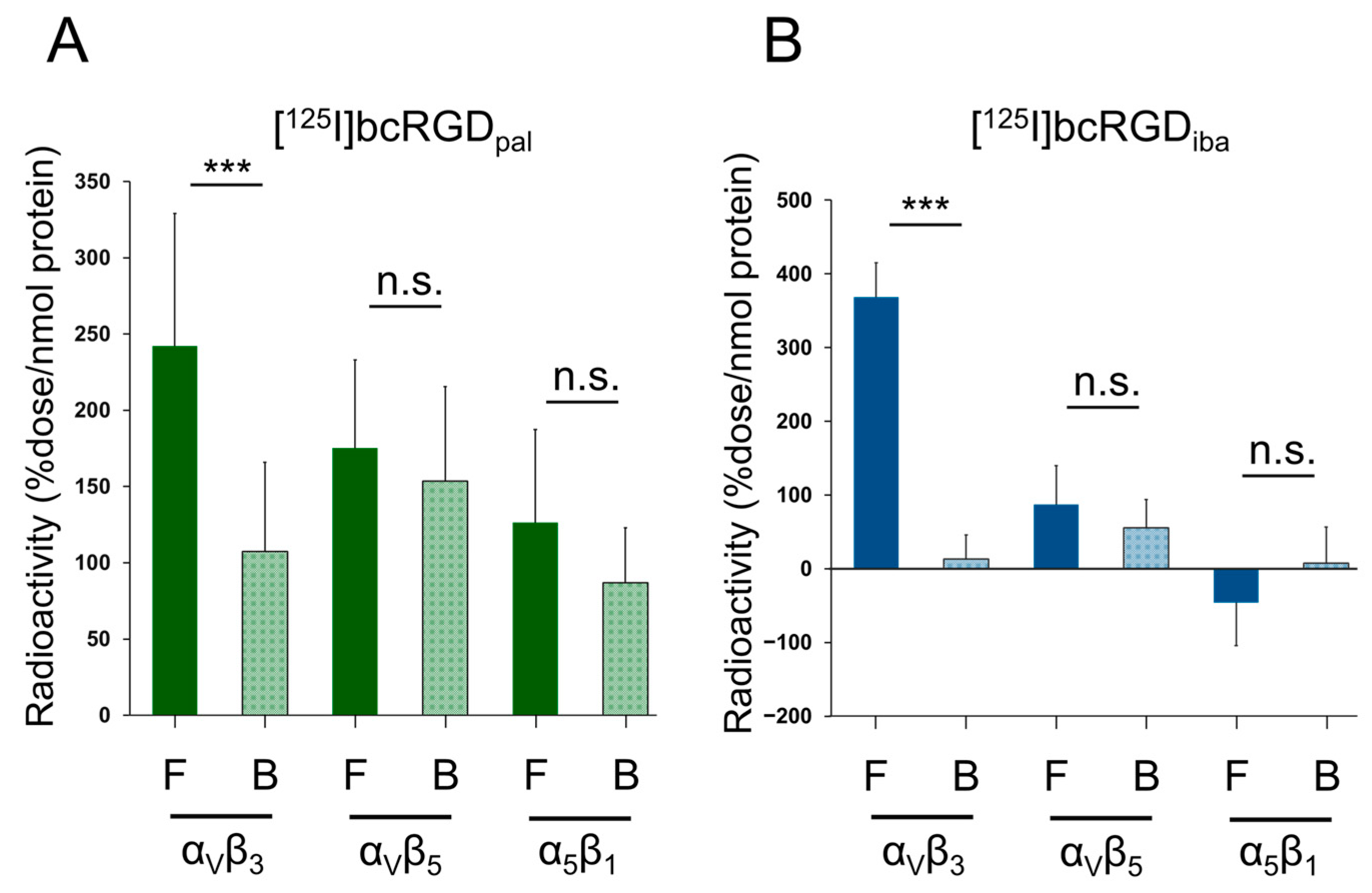
2.2. In Vitro αVβ3 Selectivity
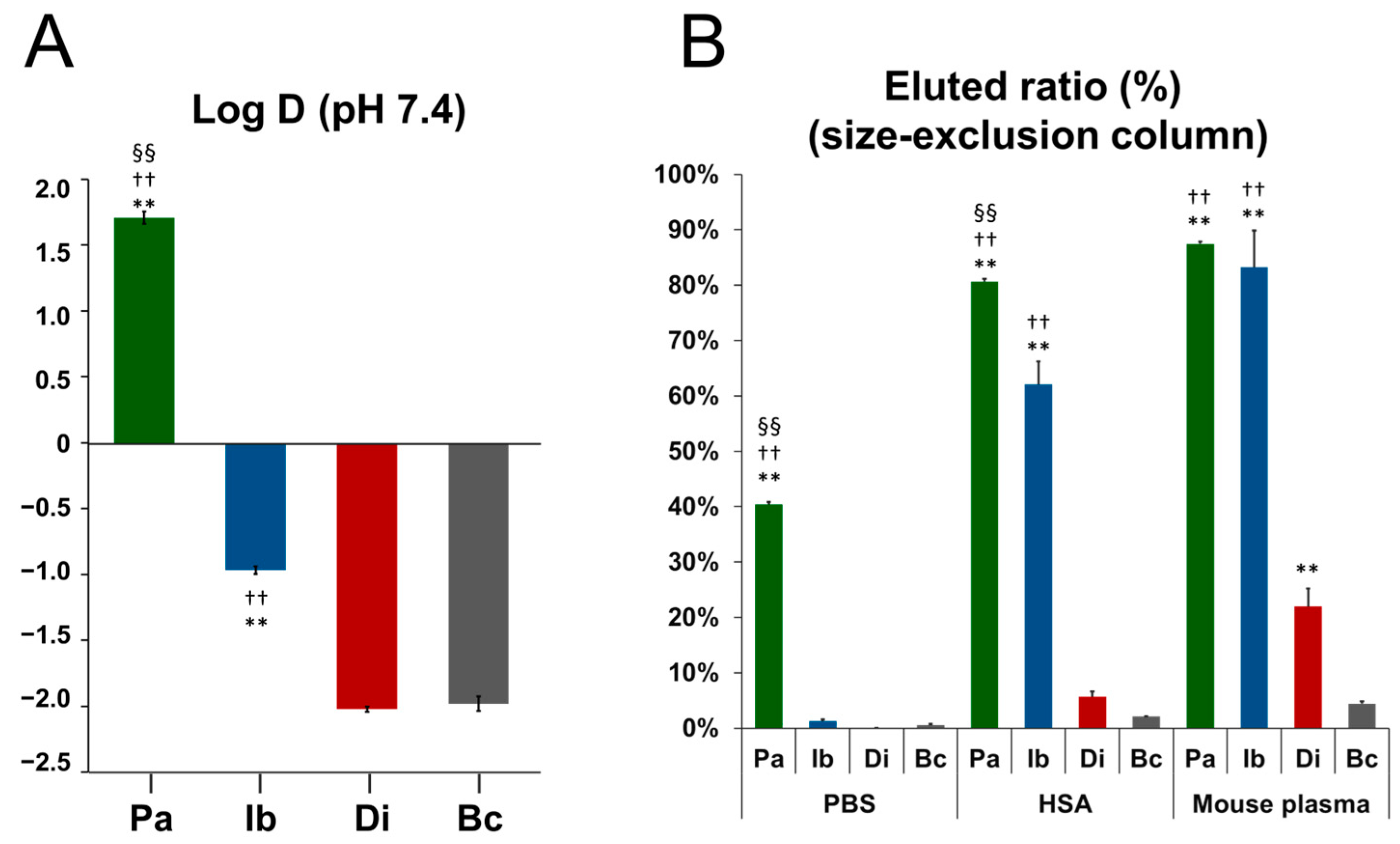
2.3. Octanol–Water Distribution Coefficient (Log D)
2.4. Albumin Binding Property
2.5. In Vivo Study
3. Discussion
4. Materials and Methods
4.1. Preparation of Peptides
4.2. Radiolabeling
4.3. In Vitro Selectivity Assay
4.4. Log D and Albumin Binding Measurement
4.5. In Vivo Study
4.5.1. Cell Lines
4.5.2. Animal Preparation
4.5.3. Biodistribution Study
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAL | Palmitic acid |
| IBA | 4-(p-iodophenyl)butyric acid |
| RCY | Radiochemical yield |
| RCP | Radiochemical purity |
| HPLC | High-performance liquid chromatography |
| ESI-MS | Electrospray ionization mass spectrometry |
| PBS | Phosphate-buffered saline |
| HSA | Human serum albumin |
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Chen, X. Integrin αvβ3-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Schnell, O.; Krebs, B.; Wagner, E.; Romagna, A.; Beer, A.J.; Grau, S.J.; Thon, N.; Goetz, C.; Kretzschmar, H.A.; Tonn, J.C.; et al. Expression of integrin αvβ3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008, 18, 378–386. [Google Scholar] [CrossRef]
- Boger, C.; Warneke, V.S.; Behrens, H.M.; Kalthoff, H.; Goodman, S.L.; Becker, T.; Rocken, C. Integrins αvβ3 and αvβ5 as prognostic, diagnostic, and therapeutic targets in gastric cancer. Gastric Cancer 2015, 18, 784–795. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Preat, V. RGD-based strategies to target αvβ3 integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Krajcovicova, S.; Daniskova, A.; Bendova, K.; Novy, Z.; Soural, M.; Petrik, M. [68Ga]Ga-DFO-c(RGDyK): Synthesis and Evaluation of Its Potential for Tumor Imaging in Mice. Int. J. Mol. Sci. 2021, 22, 7391. [Google Scholar] [CrossRef]
- Ogawa, K.; Echigo, H.; Mishiro, K.; Hirata, S.; Washiyama, K.; Kitamura, Y.; Takahashi, K.; Shiba, K.; Kinuya, S. 68Ga- and 211At-Labeled RGD Peptides for Radiotheranostics with Multiradionuclides. Mol. Pharm. 2021, 18, 3553–3562. [Google Scholar] [CrossRef]
- Beer, A.J. Positron Emission Tomography Using [18F]Galacto-RGD Identifies the Level of Integrin αvβ3 Expression in Man. Clin. Cancer Res. 2006, 12, 3942–3949. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef]
- Haubner, R.; Maschauer, S.; Prante, O. PET radiopharmaceuticals for imaging integrin expression: Tracers in clinical studies and recent developments. BioMed Res. Int. 2014, 2014, 871609. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Beck, J.G.; Doedens, L.; Frank, A.O.; Marinelli, L.; Cosconati, S.; Novellino, E.; Kessler, H. Increasing αvβ3 selectivity of the anti-angiogenic drug cilengitide by N-methylation. Angew. Chem. Int. Ed. 2011, 50, 9496–9500. [Google Scholar] [CrossRef]
- Hariharan, S.; Gustafson, D.; Holden, S.; McConkey, D.; Davis, D.; Morrow, M.; Basche, M.; Gore, L.; Zang, C.; O’Bryant, C.L.; et al. Assessment of the biological and pharmacological effects of the αvβ3 and αvβ5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann. Oncol. 2007, 18, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenovic, A.; Boro, A.; Meier, D.; Bode-Lesniewska, B.; Born, W.; Muff, R.; Fuchs, B. Targeting αvβ3 and αvβ5 integrins inhibits pulmonary metastasis in an intratibial xenograft osteosarcoma mouse model. Oncotarget 2016, 7, 55141–55154. [Google Scholar] [CrossRef]
- Fabricius, E.M.; Wildner, G.P.; Kruse-Boitschenko, U.; Hoffmeister, B.; Goodman, S.L.; Raguse, J.D. Immunohistochemical analysis of integrins αvβ3, αvβ5 and α5β1, and their ligands, fibrinogen, fibronectin, osteopontin and vitronectin, in frozen sections of human oral head and neck squamous cell carcinomas. Exp. Ther. Med. 2011, 2, 9–19. [Google Scholar] [CrossRef]
- Friedlander, M.; Brooks, P.C.; Shaffer, R.W.; Kincaid, C.M.; Varner, J.A.; Cheresh, D.A. Definition of two angiogenic pathways by distinct αv integrins. Science 1995, 270, 1500–1502. [Google Scholar] [CrossRef]
- Ma, Y.; Ai, G.; Zhang, C.; Zhao, M.; Dong, X.; Han, Z.; Wang, Z.; Zhang, M.; Liu, Y.; Gao, W.; et al. Novel Linear Peptides with High Affinity to αvβ3 Integrin for Precise Tumor Identification. Theranostics 2017, 7, 1511–1523. [Google Scholar] [CrossRef]
- Silverman, A.P.; Levin, A.M.; Lahti, J.L.; Cochran, J.R. Engineered cystine-knot peptides that bind αvβ3 integrin with antibody-like affinities. J. Mol. Biol. 2009, 385, 1064–1075. [Google Scholar] [CrossRef]
- Bernhagen, D.; Jungbluth, V.; Quilis, N.G.; Dostalek, J.; White, P.B.; Jalink, K.; Timmerman, P. Bicyclic RGD Peptides with Exquisite Selectivity for the Integrin αvβ3 Receptor Using a “Random Design” Approach. ACS Comb. Sci. 2019, 21, 198–206. [Google Scholar] [CrossRef]
- Kondo, N.; Wakamori, K.; Hirata, M.; Temma, T. Radioiodinated bicyclic RGD peptide for imaging integrin αvβ3 in cancers. Biochem. Biophys. Res. Commun. 2020, 528, 168–173. [Google Scholar] [CrossRef]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled peptides: Valuable tools for the detection and treatment of cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Middendorp, S.J.; Wilbs, J.; Deyle, K.; Heinis, C. Acylated heptapeptide binds albumin with high affinity and application as tag furnishes long-acting peptides. Nat. Commun. 2017, 8, 16092. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Hausner, S.H.; Harris, R.; Sutcliffe, J.L. A Comparison of Evans Blue and 4-(p-Iodophenyl)butyryl Albumin Binding Moieties on an Integrin αvβ6 Binding Peptide. Pharmaceutics 2022, 14, 745. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, P.; Ding, J.; Chen, J.; Huo, L.; Liu, Z. Albumin Binder-Conjugated Fibroblast Activation Protein Inhibitor Radiopharmaceuticals for Cancer Therapy. J. Nucl. Med. 2022, 63, 952–958. [Google Scholar] [CrossRef]
- Eder, M.; Pavan, S.; Bauder-Wust, U.; van Rietschoten, K.; Baranski, A.C.; Harrison, H.; Campbell, S.; Stace, C.L.; Walker, E.H.; Chen, L.; et al. Bicyclic Peptides as a New Modality for Imaging and Targeting of Proteins Overexpressed by Tumors. Cancer Res. 2019, 79, 841–852. [Google Scholar] [CrossRef]
- Holtke, C.; Alsibai, W.; Grewer, M.; Stolting, M.; Geyer, C.; Eisenblatter, M.; Wildgruber, M.; Helfen, A. How Different Albumin-Binders Drive Probe Distribution of Fluorescent RGD Mimetics. Front. Chem. 2021, 9, 689850. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, M.; Ding, J.; Chen, J.; Zhang, T.; Huo, L.; Liu, Z. Fatty acid-conjugated radiopharmaceuticals for fibroblast activation protein-targeted radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1985–1996. [Google Scholar] [CrossRef]
- Deng, Y.; Efremov, A.K.; Yan, J. Modulating binding affinity, specificity, and configurations by multivalent interactions. Biophys. J. 2022, 121, 1868–1880. [Google Scholar] [CrossRef]
- Kaeopookum, P.; Petrik, M.; Summer, D.; Klinger, M.; Zhai, C.; Rangger, C.; Haubner, R.; Haas, H.; Hajduch, M.; Decristoforo, C. Comparison of 68Ga-labeled RGD mono- and multimers based on a clickable siderophore-based scaffold. Nucl. Med. Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled Cyclic RGD Peptide Bioconjugates as Radiotracers Targeting Multiple Integrins. Bioconjug. Chem. 2015, 26, 1413–1438. [Google Scholar] [CrossRef]
- Nakamura, I.; Duong, L.T.; Rodan, S.B.; Rodan, G.A. Involvement of αvβ3 integrins in osteoclast function. J. Bone Miner. Metab. 2007, 25, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Fu, C.; Bhattacharya, J. Vascular expression of the αvβ3-integrin in lung and other organs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L217–L226. [Google Scholar] [CrossRef] [PubMed]
- Menke-van der Houven van Oordt, C.W.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-PET Imaging to Assess Target Engagement: Experience from 89Zr-Anti-HER3 mAb (GSK2849330) in Patients with Solid Tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef]
- Wang, T.; Deng, Y.; Wang, S.; He, J.; Wang, S. Kinetic 18F-FDG PET/CT imaging of hepatocellular carcinoma: A dual input four-compartment model. EJNMMI Phys. 2024, 11, 20. [Google Scholar] [CrossRef]
- Simard, J.R.; Zunszain, P.A.; Ha, C.E.; Yang, J.S.; Bhagavan, N.V.; Petitpas, I.; Curry, S.; Hamilton, J.A. Locating high-affinity fatty acid-binding sites on albumin by X-ray crystallography and NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2005, 102, 17958–17963. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, E.; Xu, P.; Lin, J.; Hu, L.; Zhang, J.; Dai, S.; Ding, Z.; Xiao, Y.; Chen, Z. Novel albumin-binding photothermal agent ICG-IBA-RGD for targeted fluorescent imaging and photothermal therapy of cancer. RSC Adv. 2021, 11, 7226–7230. [Google Scholar] [CrossRef]
- Busslinger, S.D.; Becker, A.E.; Vaccarin, C.; Deberle, L.M.; Renz, M.L.; Groehn, V.; Schibli, R.; Muller, C. Investigations Using Albumin Binders to Modify the Tissue Distribution Profile of Radiopharmaceuticals Exemplified with Folate Radioconjugates. Cancers 2023, 15, 4259. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Solomon, L.A.; Kamath, G.; Fry, H.C.; Sankaranarayanan, S.K. Water ordering controls the dynamic equilibrium of micelle-fibre formation in self-assembly of peptide amphiphiles. Nat. Commun. 2016, 7, 12367. [Google Scholar] [CrossRef]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. 177Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.R.; Kurosawa, Y.; Saga, T. Evaluation of 89Zr-Labeled Human Anti-CD147 Monoclonal Antibody as a Positron Emission Tomography Probe in a Mouse Model of Pancreatic Cancer. PLoS ONE 2013, 8, e61230. [Google Scholar] [CrossRef]
- Tolmachev, V.; Tran, T.A.; Rosik, D.; Sjoberg, A.; Abrahmsen, L.; Orlova, A. Tumor targeting using affibody molecules: Interplay of affinity, target expression level, and binding site composition. J. Nucl. Med. 2012, 53, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Pallarola, D.; Platzman, I.; Bochen, A.; Cavalcanti-Adam, E.A.; Axmann, M.; Kessler, H.; Geiger, B.; Spatz, J.P. Focal adhesion stabilization by enhanced integrin-cRGD binding affinity. BioNanoMaterials 2017, 18, 20160014. [Google Scholar] [CrossRef]
- Hedhli, J.; Czerwinski, A.; Schuelke, M.; Ploska, A.; Sowinski, P.; Hood, L.; Mamer, S.B.; Cole, J.A.; Czaplewska, P.; Banach, M.; et al. Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of αvβ3 Integrin Activity. Sci. Rep. 2017, 7, 3185. [Google Scholar] [CrossRef] [PubMed]
- Echigo, H.; Mishiro, K.; Fuchigami, T.; Shiba, K.; Kinuya, S.; Ogawa, K. Synthesis and Evaluation of a Dimeric RGD Peptide as a Preliminary Study for Radiotheranostics with Radiohalogens. Molecules 2021, 26, 6107. [Google Scholar] [CrossRef]
- Nakamura, I.; Pilkington, M.F.; Lakkakorpi, P.T.; Lipfert, L.; Sims, S.M.; Dixon, S.J.; Rodan, G.A.; Duong, L.T. Role of αvβ3 integrin in osteoclast migration and formation of the sealing zone. J. Cell Sci. 1999, 112 Pt 22, 3985–3993. [Google Scholar] [CrossRef]
- Fiore, V.F.; Wong, S.S.; Tran, C.; Tan, C.; Xu, W.; Sulchek, T.; White, E.S.; Hagood, J.S.; Barker, T.H. αvβ3 Integrin drives fibroblast contraction and strain stiffening of soft provisional matrix during progressive fibrosis. JCI Insight 2018, 3, e97597. [Google Scholar] [CrossRef]
- Liolios, C.; Sachpekidis, C.; Kolocouris, A.; Dimitrakopoulou-Strauss, A.; Bouziotis, P. PET Diagnostic Molecules Utilizing Multimeric Cyclic RGD Peptide Analogs for Imaging Integrin αvβ3 Receptors. Molecules 2021, 26, 1792. [Google Scholar] [CrossRef]
- Ye, X.; Gaucher, J.F.; Hu, H.; Wang, L.; Broussy, S. Dimer Peptide Ligands of Vascular Endothelial Growth Factor: Optimizing Linker Length for High Affinity and Antiangiogenic Activity. J. Med. Chem. 2023, 66, 9753–9765. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef]
- Yang, G.; Gao, H.; Luo, C.; Zhao, X.; Luo, Q.; Shi, J.; Wang, F. Palmitic Acid-Conjugated Radiopharmaceutical for Integrin αvβ3-Targeted Radionuclide Therapy. Pharmaceutics 2022, 14, 1327. [Google Scholar] [CrossRef]
- Kondo, Y.; Kimura, H.; Fukumoto, C.; Yagi, Y.; Hattori, Y.; Kawashima, H.; Yasui, H. Copper-mediated radioiodination reaction through aryl boronic acid or ester precursor and its application to direct radiolabeling of a cyclic peptide. J. Label. Compd. Radiopharm. 2021, 64, 336–345. [Google Scholar] [CrossRef]
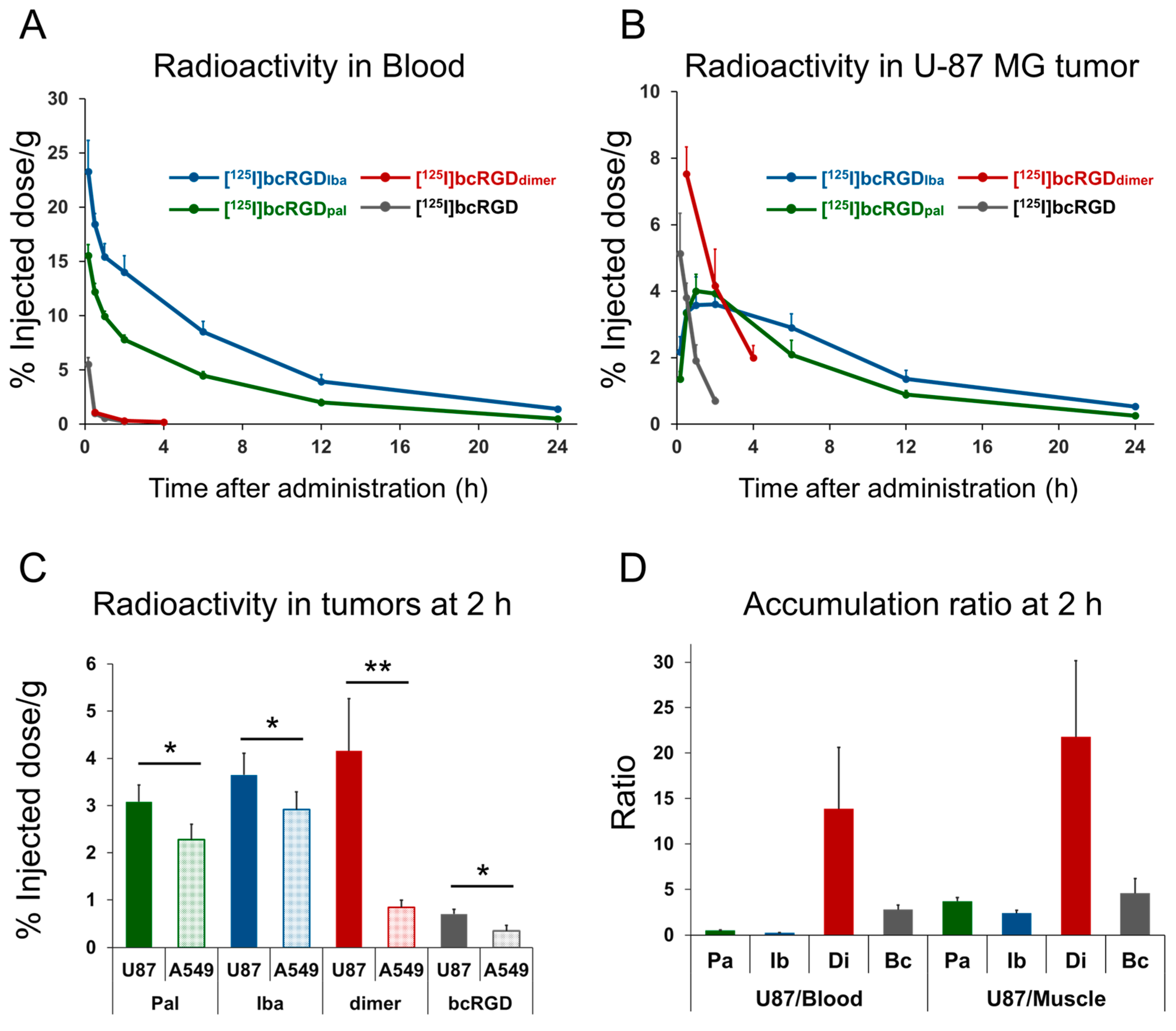
| Time After Administration | |||||||
|---|---|---|---|---|---|---|---|
| 5 min | 30 min | 1 h | 2 h | 6 h | 12 h | 24 h | |
| Blood | 15.6 ± 1.0 | 12.2 ± 0.8 | 9.9 ± 0.5 | 7.8 ± 0.4 | 4.5 ± 0.4 | 2.0 ± 0.3 | 0.5 ± 0.0 |
| Heart | 6.6 ± 0.1 | 4.7 ± 0.4 | 4.3 ± 0.3 | 3.1 ± 0.2 | 1.7 ± 0.0 | 0.8 ± 0.1 | 0.2 ± 0.0 |
| Lung | 11.8 ± 1.3 | 11.2 ± 1.0 | 9.6 ± 1.3 | 7.8 ± 1.0 | 4.4 ± 0.5 | 2.2 ± 0.3 | 0.6 ± 0.1 |
| Liver | 17.6 ± 0.9 | 19.0 ± 2.1 | 15.8 ± 0.9 | 12.3 ± 0.3 | 6.0 ± 0.2 | 1.9 ± 0.2 | 0.5 ± 0.0 |
| Kidneys | 9.0 ± 0.2 | 8.3 ± 0.9 | 7.7 ± 0.2 | 6.7 ± 0.6 | 4.2 ± 0.6 | 1.7 ± 0.2 | 0.4 ± 0.0 |
| Stomach ¶ | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.3 | 0.1 ± 0.1 |
| Small intestine | 2.3 ± 0.2 | 4.5 ± 0.6 | 7.0 ± 0.3 | 9.3 ± 0.9 | 4.0 ± 0.2 | 1.9 ± 0.5 | 0.3 ± 0.0 |
| Large intestine | 0.9 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.1 | 6.9 ± 1.1 | 11.8 ± 2.0 | 7.5 ± 0.8 | 2.3 ± 1.1 |
| Pancreas | 2.8 ± 0.2 | 2.5 ± 0.3 | 1.9 ± 0.1 | 1.6 ± 0.1 | 1.2 ± 0.3 | 0.4 ± 0.0 | 0.1 ± 0.0 |
| Spleen | 5.1 ± 0.4 | 5.9 ± 0.8 | 4.6 ± 0.6 | 3.7 ± 0.1 | 1.9 ± 0.2 | 0.8 ± 0.1 | 0.2 ± 0.0 |
| Muscle | 1.4 ± 0.3 | 1.6 ± 0.2 | 1.4 ± 0.2 | 1.1 ± 0.1 | 0.6 ± 0.1 | 0.3 ± 0.0 | 0.1 ± 0.0 |
| Bone | 1.4 ± 0.1 | 1.7 ± 0.6 | 1.1 ± 0.3 | 1.3 ± 0.4 | 0.7 ± 0.1 | 0.4 ± 0.0 | 0.1 ± 0.1 |
| Brain | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| Thyroid ¶ | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| U-87 MG | 1.4 ± 0.2 | 3.4 ± 0.5 | 4.0 ± 0.5 | 3.9 ± 0.2 | 2.1 ± 0.4 | 0.9 ± 0.1 | 0.3 ± 0.1 |
| U87/Blood | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.1 |
| U87/Muscle | 3.0 ± 0.1 | 2.1 ± 0.3 | 2.9 ± 0.2 | 3.7 ± 0.4 | 3.3 ± 0.8 | 3.4 ± 0.5 | 2.4 ± 0.4 |
| Time After Administration | |||||||
|---|---|---|---|---|---|---|---|
| 5 min | 30 min | 1 h | 2 h | 6 h | 12 h | 24 h | |
| Blood | 23.3 ± 2.9 | 18.5 ± 1.0 | 15.4 ± 1.2 | 14.0 ± 1.5 | 8.5 ± 1.0 | 3.9 ± 0.7 | 1.4 ± 0.1 |
| Heart | 7.4 ± 1.4 | 5.3 ± 0.5 | 4.5 ± 0.8 | 4.2 ± 0.1 | 2.7 ± 0.2 | 1.3 ± 0.1 | 0.5 ± 0.0 |
| Lung | 10.7 ± 1.9 | 9.4 ± 0.3 | 8.2 ± 0.6 | 7.5 ± 0.4 | 5.3 ± 0.5 | 2.6 ± 0.3 | 1.2 ± 0.1 |
| Liver | 7.6 ± 0.5 | 6.9 ± 0.2 | 5.7 ± 0.7 | 5.1 ± 0.4 | 3.4 ± 0.4 | 1.4 ± 0.2 | 0.6 ± 0.0 |
| Kidneys | 7.8 ± 0.5 | 7.1 ± 1.4 | 7.3 ± 0.4 | 5.5 ± 0.3 | 3.9 ± 0.4 | 1.8 ± 0.1 | 0.8 ± 0.1 |
| Stomach ¶ | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.3 | 0.8 ± 0.2 | 0.2 ± 0.1 | 0.2 ± 0.0 |
| Small intestine | 2.3 ± 0.4 | 3.0 ± 0.1 | 4.8 ± 0.6 | 4.9 ± 0.3 | 5.6 ± 1.1 | 2.2 ± 0.5 | 0.9 ± 0.1 |
| Large intestine | 1.5 ± 0.7 | 1.0 ± 0.1 | 1.0 ± 0.1 | 7.1 ± 2.0 | 11.3 ± 2.0 | 3.4 ± 1.0 | 2.2 ± 0.2 |
| Pancreas | 2.3 ± 0.4 | 2.0 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.0 ± 0.2 | 0.5 ± 0.1 | 0.2 ± 0.0 |
| Spleen | 2.9 ± 0.5 | 3.0 ± 0.2 | 2.5 ± 0.2 | 2.3 ± 0.3 | 1.7 ± 0.5 | 0.9 ± 0.1 | 0.4 ± 0.0 |
| Muscle | 1.4 ± 0.4 | 1.5 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.2 | 1.2 ± 0.3 | 0.5 ± 0.1 | 0.3 ± 0.0 |
| Bone | 1.7 ± 0.3 | 1.9 ± 0.6 | 1.6 ± 0.4 | 1.5 ± 0.3 | 1.3 ± 0.1 | 0.6 ± 0.2 | 0.3 ± 0.1 |
| Brain | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| Thyroid ¶ | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| U-87 MG | 2.2 ± 0.5 | 3.4 ± 0.5 | 3.6 ± 0.8 | 3.6 ± 0.7 | 2.9 ± 0.4 | 1.4 ± 0.3 | 0.5 ± 0.1 |
| U87/Blood | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| U87/Muscle | 1.6 ± 0.3 | 2.3 ± 0.6 | 2.3 ± 0.4 | 2.4 ± 0.3 | 2.5 ± 0.3 | 2.6 ± 0.3 | 2.1 ± 0.4 |
| Time After Administration | |||
|---|---|---|---|
| 30 min | 2 h | 4 h | |
| Blood | 1.1 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Heart | 0.6 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Lung | 2.9 ± 0.5 | 1.2 ± 0.3 | 0.4 ± 0.1 |
| Liver | 0.9 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| Kidneys | 10.9 ± 2.6 | 2.0 ± 0.2 | 0.9 ± 0.2 |
| Stomach ¶ | 0.6 ± 0.5 | 0.2 ± 0.0 | 0.3 ± 0.3 |
| Small intestine | 3.6 ± 0.6 | 2.3 ± 1.0 | 1.6 ± 0.4 |
| Large intestine | 0.4 ± 0.2 | 5.5 ± 1.0 | 5.5 ± 1.4 |
| Pancreas | 0.5 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 |
| Spleen | 0.7 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.0 |
| Muscle | 0.7 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Bone | 1.5 ± 0.3 | 0.9 ± 0.3 | 0.4 ± 0.0 |
| Brain | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Thyroid ¶ | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| U-87 MG | 7.5 ± 0.8 | 4.2 ± 1.1 | 2.9 ± 0.4 |
| A549 | 1.7 ± 0.6 | 0.8 ± 0.2 | 0.4 ± 0.1 |
| U87/Blood | 12.3 ± 4.0 | 21.8 ± 8.4 | 18.2 ± 8.0 |
| U87/Muscle | 7.1 ± 1.8 | 13.9 ± 6.8 | 10.3 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, N.; Kato, M.; Oshima, A.; Hirano, F.; Miyazaki, A.; Temma, T. Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation. Pharmaceuticals 2025, 18, 549. https://doi.org/10.3390/ph18040549
Kondo N, Kato M, Oshima A, Hirano F, Miyazaki A, Temma T. Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation. Pharmaceuticals. 2025; 18(4):549. https://doi.org/10.3390/ph18040549
Chicago/Turabian StyleKondo, Naoya, Marika Kato, Aoi Oshima, Fuko Hirano, Anna Miyazaki, and Takashi Temma. 2025. "Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation" Pharmaceuticals 18, no. 4: 549. https://doi.org/10.3390/ph18040549
APA StyleKondo, N., Kato, M., Oshima, A., Hirano, F., Miyazaki, A., & Temma, T. (2025). Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation. Pharmaceuticals, 18(4), 549. https://doi.org/10.3390/ph18040549







