1. Introduction
Stroke is identified as a spectrum of disorders characterized by the interruption of blood circulation within the brain, leading to compromised neurological functions. These disorders are generally categorized into two principal types: hemorrhagic strokes and ischemic strokes (ISs). The latter, ischemic stroke, arises from the occlusion or constriction of arteries that are responsible for the cerebral blood supply, accounting for over seventy percent of all stroke instances [
1,
2]. Ischemic stroke is associated with a significant incidence of death, disability, and morbidity, posing a severe risk to patients’ lives and markedly affecting their quality of life [
3]. Globally, stroke remains a leading cause of mortality and long-term disability, imposing substantial public health and economic burdens. Therefore, effective therapeutic strategies are urgently needed to mitigate its impact.
In the realm of physicochemical analysis, the development of ischemic stroke is predominantly attributed to mechanisms such as glutamate excitotoxicity, calcium accumulation, oxidative stress, apoptosis, and inflammation, among others [
4,
5,
6,
7]. The Keap1/Nrf2 signaling pathway is a key mechanism for maintaining the redox balance in the body [
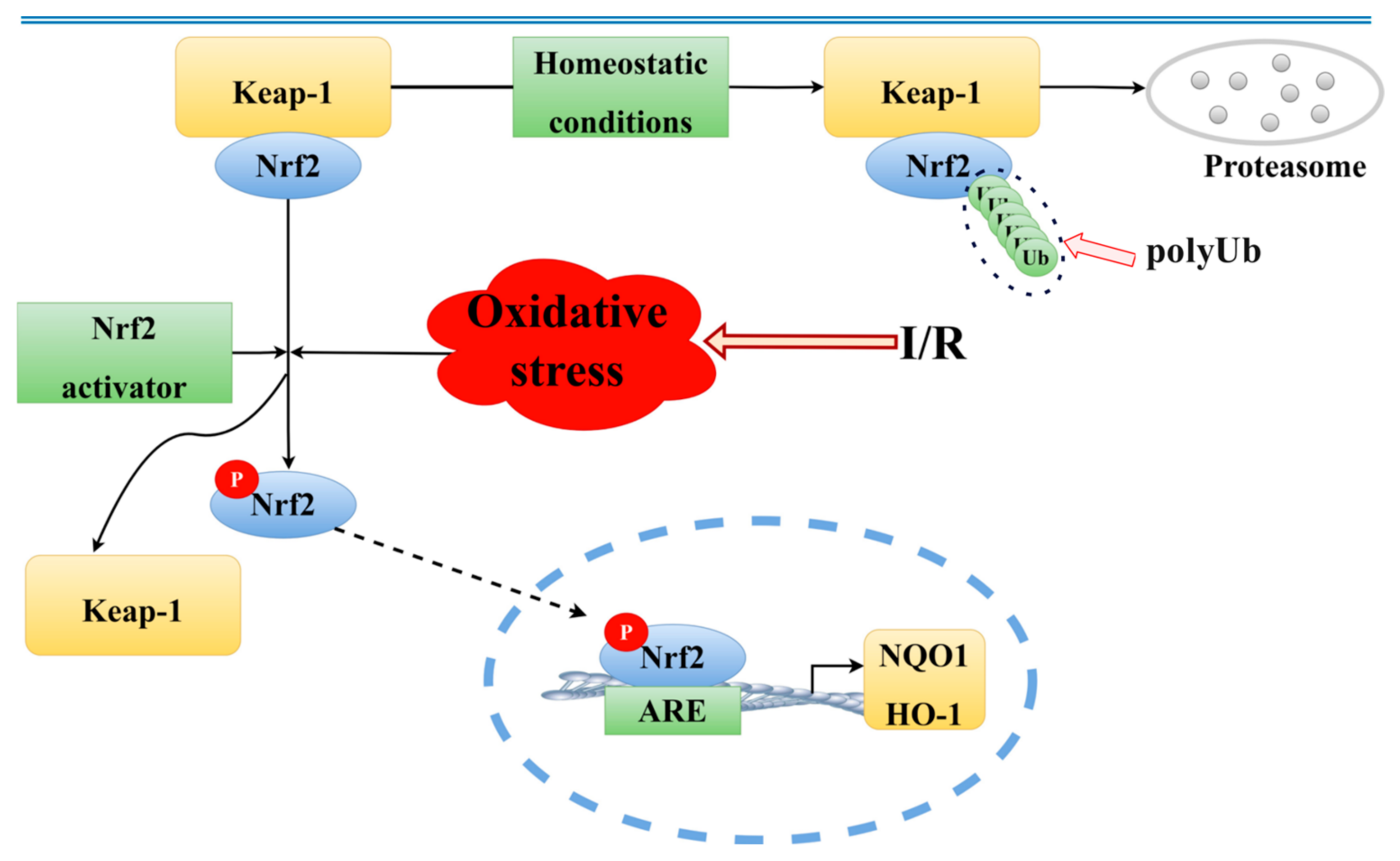
8]. Under normal physiological conditions, when cells are exposed to harmful external stimuli, such as environmental toxins or oxidative stress, reactive oxygen species (ROS) and electrophilic compounds can cause structural changes in Kelch-like, ECH-associated protein 1 (Keap1). This modification leads to the activation and nuclear translocation of a protein called nuclear factor erythroid 2–related factor 2 (Nrf2). Once inside the nucleus, Nrf2 functions as a transcription factor, turning on the expression of several protective genes that help the body defend itself against oxidative damage. These genes produce antioxidant and detoxifying enzymes, which play essential roles in neutralizing harmful molecules. Among these are enzymes like heme oxygenase-1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1), which together help reduce oxidative stress and restore the cellular balance [
9,
10,
11]. This signaling cascade is not only crucial for protection during acute injuries such as ischemic stroke but has also been implicated in the development and progression of a wide range of both cancerous and non-cancerous diseases [
11]. Given its pivotal role in cellular defense, the Keap1/Nrf2 pathway has emerged as a promising therapeutic target for neuroprotection in ischemic stroke. However, effective modulators of this pathway remain limited, particularly among natural compounds.
Currently, the interest in natural medicinal research has surged globally. Evidence from numerous studies indicates a clear beneficial impact of botanicals and their bioactive constituents against ischemic stroke [
12]. Soybeans, belonging to the legume family, have been identified as a significant source of isoflavones. Notably, soy isoflavones (SI), recognized as bioactive compounds, exhibit a range of biological activities. These include mimicking estrogen, combating aging and cancer, offering antioxidant and anti-inflammatory benefits, lowering lipid levels, preventing cardiovascular diseases, and improving memory functions [
13,
14]. Despite these diverse bioactivities, the neuroprotective potential of soy isoflavones in ischemic stroke is still underexplored. Their ability to modulate oxidative stress-related pathways, particularly Keap1/Nrf2, may provide a novel avenue for therapeutic development.
Network pharmacology integrates the principles of systems biology, omics technologies, and computational biology. This integration facilitates a comprehensive and synergistic exploration of the modes of action of pharmaceuticals, offering insights into their pharmacological effects [
15]. Molecular docking techniques enable the prediction of protein–ligand interactions, including binding conformations and associated binding free energies, thereby supporting the functional validation of candidate compounds and clarifying their mechanisms of action [
16]. The combined application of network pharmacology and molecular docking thus provides a powerful platform for investigating the multi-target and multi-pathway actions of complex natural products, such as soy isoflavones (SI), in ischemic stroke models.
Building on this integrated approach, the present study explored the potential role of SI in the prevention and treatment of ischemic stroke (IS), with a specific focus on redox regulation and oxidative stress modulation via the Nrf2 signaling pathway. An in vitro model of ischemic injury was established using Na2S2O4-induced hypoxic damage in PC12 cells, and a rat model of cerebral ischemia–reperfusion injury was induced via the embolization of the common carotid artery.
The primary objective of this study is to elucidate the neuroprotective mechanisms of soy isoflavones (SIs) in ischemic stroke (IS) by integrating in silico, in vitro, and in vivo approaches. First, network pharmacology and molecular docking were employed to systematically predict the putative targets and signaling pathways through which SI may exert therapeutic effects against IS. Next, a hypoxia-induced injury model in PC12 cells was established using sodium dithionite (Na2S2O4) to assess the in vitro protective effects of SI. Additionally, a rat model of cerebral ischemia–reperfusion injury was induced via common carotid artery embolization to evaluate the in vivo efficacy of SI. Particular attention was given to the Keap1/Nrf2/HO-1/NQO1 signaling axis to determine whether SI modulates oxidative stress through this pathway. By combining computational prediction with experimental validation, this study aims to provide mechanistic insights and preclinical evidence supporting the therapeutic potential of SI in ischemic stroke.
3. Discussion
Ischemic stroke has emerged as a critical health issue worldwide. The primary treatment options available are thrombolytic drugs, which, despite their efficacy, pose significant risks to health. These drugs are only effective within a narrow therapeutic window of 3 to 6 h following a stroke; administration beyond this period risks causing ischemia–reperfusion injury [
17]. Studies have elucidated that the cessation of blood flow results in ischemia, and the subsequent restoration of circulation, or reperfusion, leads to an influx of oxygen, generating excessive oxygen free radicals [
18]. Concurrently, this process suppresses the activity of antioxidant enzymes, precipitating oxidative stress that exacerbates brain damage. Although thrombolytic agents offer crucial benefits for acute ischemic stroke, potentially saving lives, their long-term application is hindered by their chemical composition, which can interfere with patient metabolism. On the other hand, natural biological agents present a more favorable profile, characterized by gentler effects and reduced toxicity and side effects, making them more suitable for extended use [
19].
Soybean isoflavones possess multiple pharmacological effects, significantly contributing to antioxidation, mitigating inflammation following cerebral ischemia, cholesterol reduction, and the prevention of cardiovascular diseases [
20,
21]. This study utilized the reflux method to extract isoflavones from soybean, with the constituents of the soybean extract subsequently identified through HPLC-DAD-MS. The primary components identified were daidzin, glycitin, genistin, acetyldaidzin, acetylcitin, malonylgenistin, and acetylgenistin. The network pharmacology analysis suggested that soy isoflavones may modulate the oxidative stress in brain tissues induced by stroke through their influence on redox signaling pathways, thereby offering potential prevention and treatment for ischemic stroke. In vivo experiments entailed establishing a rat model of transient global cerebral ischemia–reperfusion by occluding the bilateral common carotid arteries. Preliminary findings revealed that soybean isoflavone pretreatment markedly ameliorated neurological damages in cerebral ischemia–reperfusion rats, significantly diminishing the brain water content and the infarct volume. Additionally, there was a significant elevation in the activities of SOD, GSH, and CAT within the brain tissues, alongside a marked reduction in MDA and LDH levels. Morphological observations further confirmed the significant mitigation of cerebral lesions by soybean isoflavones in rats subjected to cerebral ischemia–reperfusion injury.
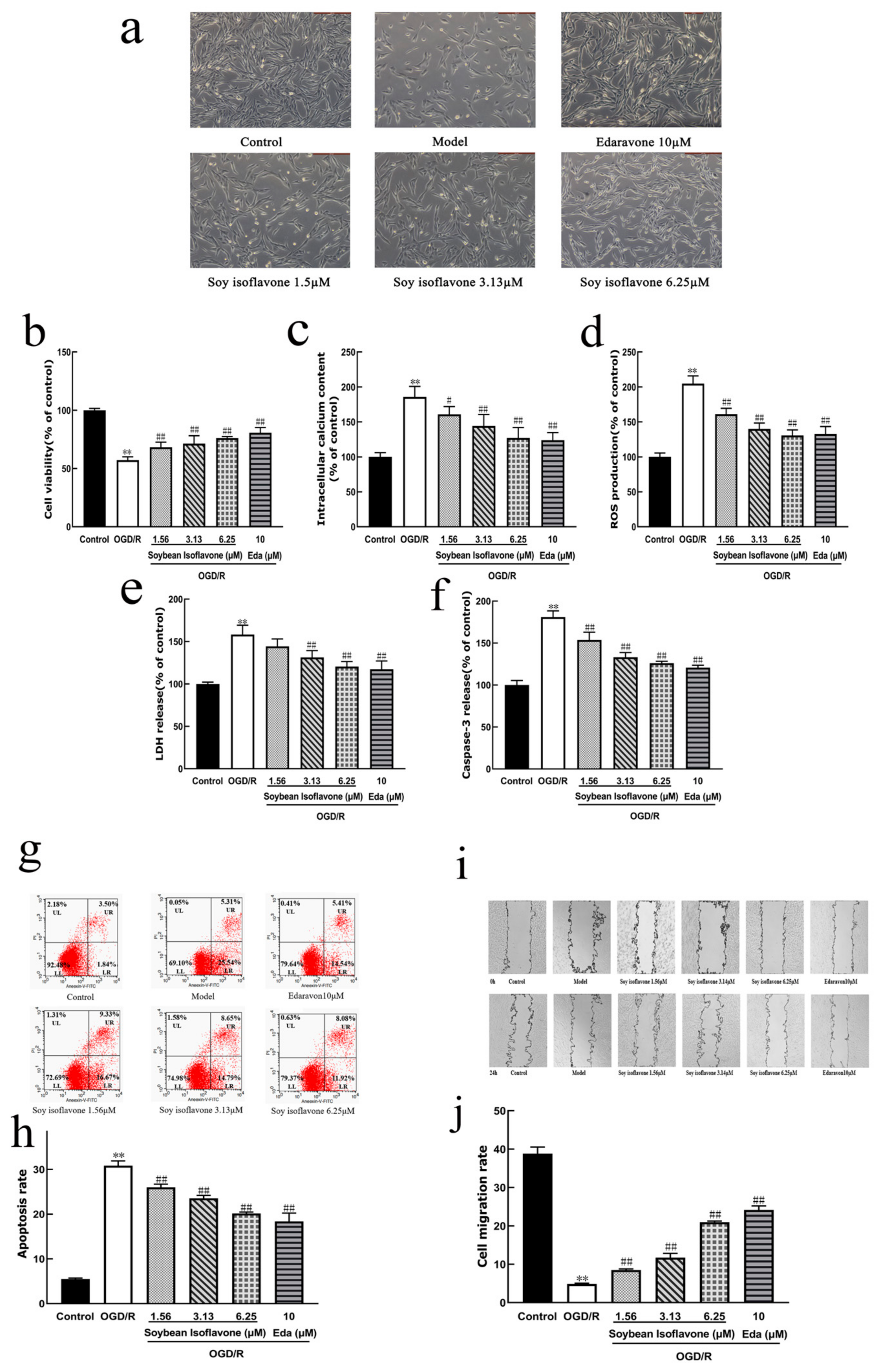
An imbalance between the generation of ROS and the capacity of the organism to counteract their negative effects with antioxidants leads to oxidative stress [
22]. The body possesses an elaborate defense system against free radicals, comprising antioxidant enzymes such as SOD, CAT, and GSH-px [
23]. SOD, a metalloproteinase found across various tissues, cells, and organelles, plays a crucial role in scavenging excess free radicals, thus maintaining the cellular peroxidation balance. Its activity is often used as an indicator of the body’s antioxidant capability [
24]. Furthermore, GSH-px and CAT effectively catalyze the breakdown of hydrogen peroxide, eliminating harmful superoxide metabolites to reduce peroxidative damage [
25]. Therefore, the body’s antioxidant capacity can be gauged by the activities of SOD, GSH-px, and CAT and the levels of ROS. Ischemia/reperfusion injury induces a surge in ROS, leading to an increase in lipid peroxides, which are then converted into MDA (malondialdehyde) [
26]. Simultaneously, the activity of Ca
2+ pumps declines, causing an elevation in cellular Ca
2+ concentration and accumulation, which interferes with Ca
2+ signal transduction [
27]. An increase in Ca
2+ concentration triggers the production of free fatty acids, leading to a surge in free radicals [
28], which disturbs both the energy and the intracellular environmental equilibrium. This process activates Caspase-3, fostering neuronal apoptosis, exacerbating cellular damage, and heightening the risk of myocardial infarction. Concurrently, oxidative stress can escalate serum LDH (lactate dehydrogenase) levels, causing further cellular and tissue damage. Thus, serum concentrations of MDA, Ca
2+, Caspase-3, and LDH serve as indicators of oxidative harm. It was discovered that SI significantly mitigates the damage to PC12 cells induced by oxygen–glucose deprivation/reoxygenation, reduces apoptosis, boosts cell viability, and diminishes levels of Ca
2+, LDH, and ROS. Hence, SI exerts a notable protective effect against Na2S2O4-induced injury in PC12 cells, likely through mitigating oxidative stress. Additionally, this investigation reveals that pretreatment with soybean isoflavones markedly lowers MDA and LDH levels in the brain tissue of rats suffering from cerebral ischemia–reperfusion injury while notably enhancing the activities of SOD, GSH-px, and CAT. Consequently, soybean isoflavones effectively elevate antioxidant enzyme activity in rats subjected to cerebral ischemia–reperfusion injury, thereby alleviating oxidative stress-induced damage.
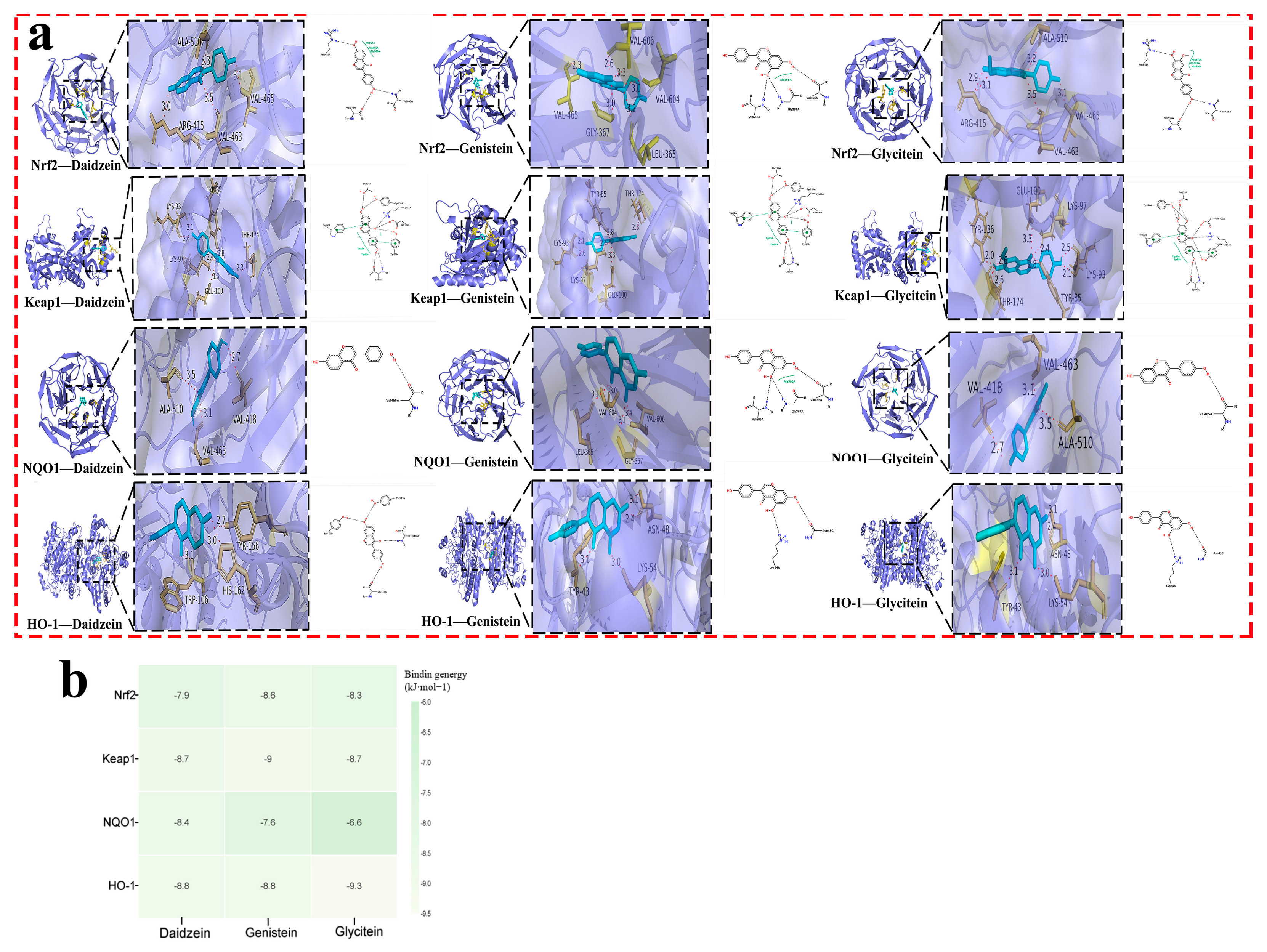
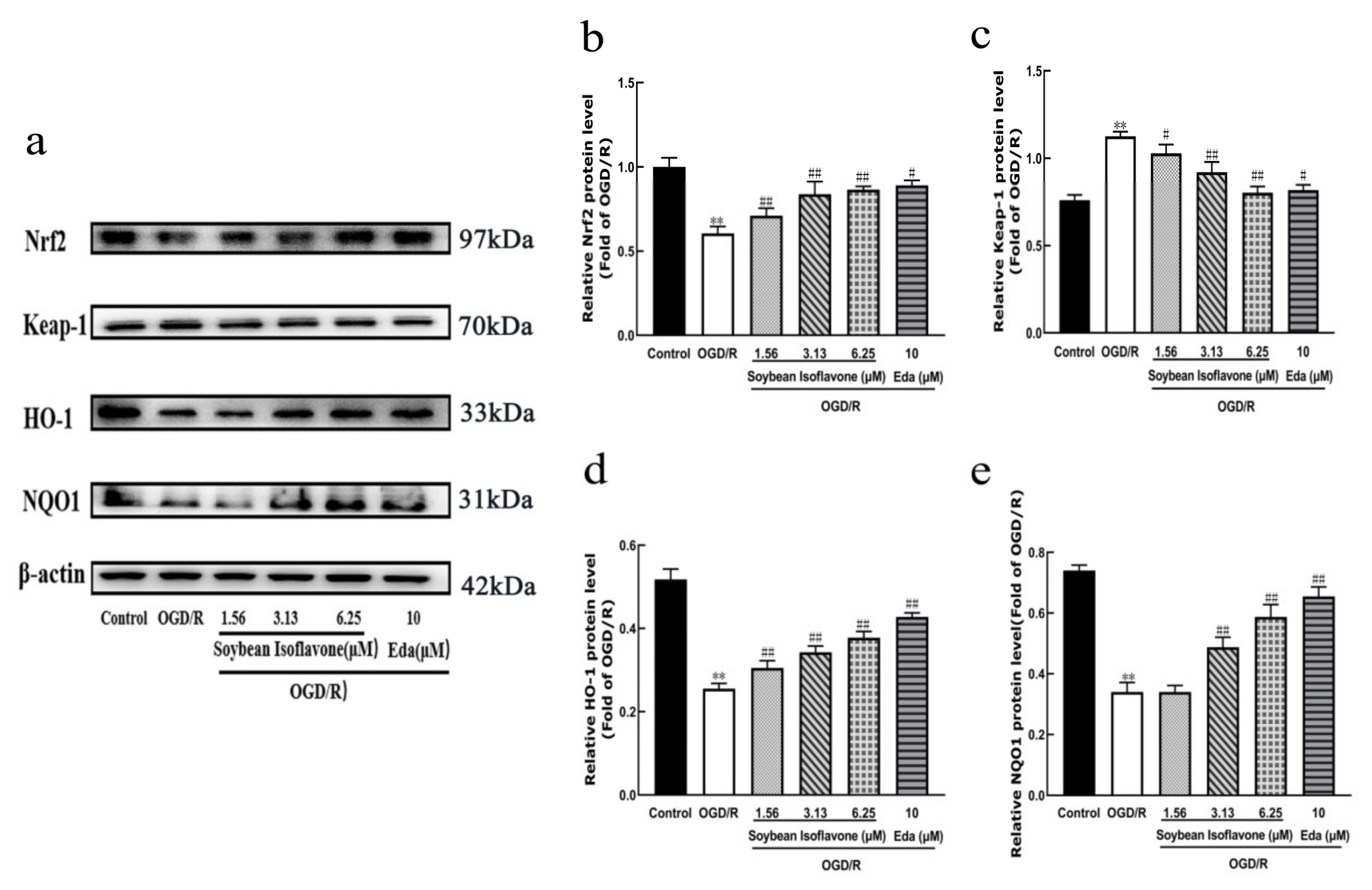
Nrf2 is recognized as a key defender against oxidative stress in ischemic stroke [
29,
30]. Research indicates that Nrf2 not only indirectly modulates the expression of various transcription factors but also contributes to neuroprotection by dampening inflammatory responses, combating oxidative stress, diminishing apoptosis and necrosis, and fostering cell growth and differentiation [
31]. Moreover, the Nrf2 signaling pathway plays a crucial role in neuroprotection, as evidenced by studies on numerous traditional Chinese medicinal compounds [
32]. Normally, Nrf2 forms an inactive complex with Keap1 [
33]. Upon activation, Nrf2 separates from Keap1, migrates from the cytoplasm to the nucleus, and binds to the ARE, thereby activating antioxidant enzymes like HO-1 and NQO1 [
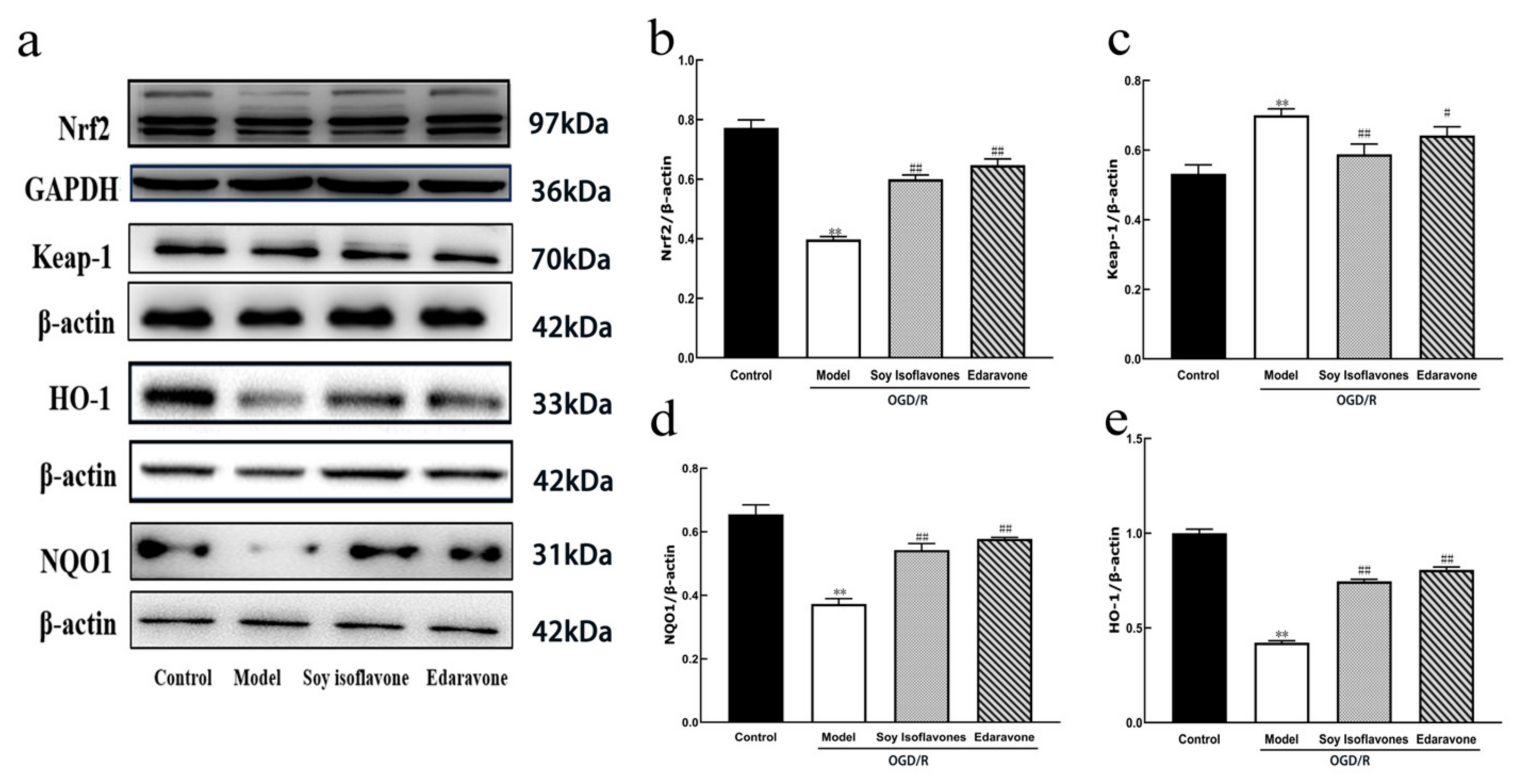
34]. This investigation highlighted that soy isoflavones notably increase the concentrations of Nrf2, HO-1, and NQO1, while significantly reducing Keap1 levels (
Figure 10). It suggests that the neuroprotective mechanisms of soy isoflavones could be linked to stimulating the Nrf2 signaling pathway. Through this pathway, the regulation of antioxidant responses to oxidative stress from cerebral ischemia–reperfusion is achieved, potentially facilitating cellular growth, differentiation, and angiogenesis, thus contributing to neuroprotection.
Research indicates that soy isoflavones exhibit a protective capacity in the context of cerebral ischemia–reperfusion injuries. This protective effect is attributed to the enhancement of antioxidant enzyme activities and the suppression of oxidative stress. Furthermore, soy isoflavones’ defense against ischemic stroke is linked to the stimulation of Nrf2 and the reduction of Keap1 activity. These findings are consistent with previous in vivo and in vitro studies, further supporting the multi-target neuroprotective potential of SI.
In vitro, Zhang et al. (2022) reported that SI protected PC12 cells from CoCl
2-induced hypoxic injury by activating Nrf2 and suppressing the p38 MAPK and AKT–mTOR pathways, thereby reducing ROS levels, enhancing cell viability, and inhibiting apoptosis [
35]. These mechanisms align with our observations in the OGD/R model, where SI demonstrated antioxidant and anti-apoptotic effects. In vivo, Schreihofer et al. (2005) found that a high-soy diet significantly reduced the infarct size in female rats subjected to permanent middle cerebral artery occlusion (MCAO), indicating notable neuroprotective effects under physiological conditions [
36]. Similarly, Huang et al. (2009) showed that SI alleviated brain tissue damage and improved neurological function in ischemic rats by modulating the Notch signaling pathway [
37]. Moreover, Li et al. (2025) demonstrated that genistein, a major component of SI, mitigated cerebral ischemia–reperfusion injury in rats by inhibiting the Wnt/Ca
2+ signaling pathway, emphasizing its role in calcium homeostasis [
38]. These studies reinforce our findings that SI exerts neuroprotective effects through multiple signaling pathways, particularly those related to oxidative stress and apoptosis regulation.
In conclusion, our findings are in strong agreement with existing in vivo and in vitro research, underscoring the multifaceted mechanisms by which soy isoflavones alleviate ischemic brain injury. These results support the therapeutic potential of SI and provide a solid foundation for its future clinical application. Although this study provides evidence supporting the neuroprotective role of soy isoflavones through the activation of the Nrf2 signaling pathway, several limitations should be noted. First, the study was conducted using a single animal model (BCCAO) and PC12 cells, which may not fully reflect the complexity of human ischemic stroke pathology. Second, the exact active monomer(s) within the soy isoflavone extract responsible for the observed effects remain to be identified. Third, the long-term safety and efficacy of soy isoflavones were not assessed. Future studies should explore the effects of individual isoflavone components, investigate additional models of stroke (e.g., MCAO), and assess chronic outcomes such as cognitive function and neuroregeneration.
4. Materials and Methods
4.1. Chemical, Reagents, and Software
Soy isoflavones standards, including Daidzin, Glycitin, Genistein, Acetyldaidzin, Acetylglycitin, Malonylgenistein, and Acetylgenistein, were purchased from Aladdin Industrial Corporation (Cat# various; Shanghai, China). Acetonitrile (HPLC grade) was obtained from Thermo Fisher Scientific (Cat# A955-4; Waltham, MA, USA). Ultrapure water was prepared using a Milli-Q system (Millipore, Billerica, MA, USA). Analytical-grade reagents were provided by Beijing Chemical Works (Beijing, China). Kits for superoxide dismutase (SOD), glutathione peroxidase (GSH-px), malondialdehyde (MDA), and total protein were from the Nanjing Jiancheng Bioengineering Institute (Cat# A001-3, A005, A003-1, A045-2-2; Nanjing, Jiangsu, China). Kits for catalase (CAT), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), lactate dehydrogenase (LDH), reactive oxygen species (ROS), Fluo-3 acetoxymethyl ester (Fluo-3AM), and caspase-3 were obtained from Beyotime Biotechnology (Shanghai, China). Other chemicals, including internal standard, Earle’s Balanced Salt Solution (EBSS), phosphate-buffered saline (PBS), sodium dithionite (Na2S2O4), and edaravone, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Primary antibodies: Anti-Nrf2 (Cat# 12721, Cell Signaling Technology, Danvers, MA, USA); Anti-Keap1 (Cat# ab227828), Anti-HO-1 (Cat# ab68477), Anti-NQO1 (Cat# ab34173), and Anti-β-actin (Cat# ab8227) (all from Abcam, Cambridge, UK). The GraphPad Prism version 9.5.1 (GraphPad Software, San Diego, CA, USA) was used for the statistical analysis. ImageJ version 1.53 (National Institutes of Health, Bethesda, MD, USA) was used for the densitometric analysis. FlowJo version 10.8.1 (BD Biosciences, Ashland, OR, USA) was used for the flow cytometry data analysis. Cytoscape version 3.9.1 (The Cytoscape Consortium, San Diego, CA, USA), AutoDockTools version 1.5.7, AutoDock Vina version 1.2.3, and PyMOL version 2.5.4 (Schrödinger, LLC, New York, NY, USA) were used for molecular docking and visualization.
4.2. Extraction and Identification of Soybean Isoflavones
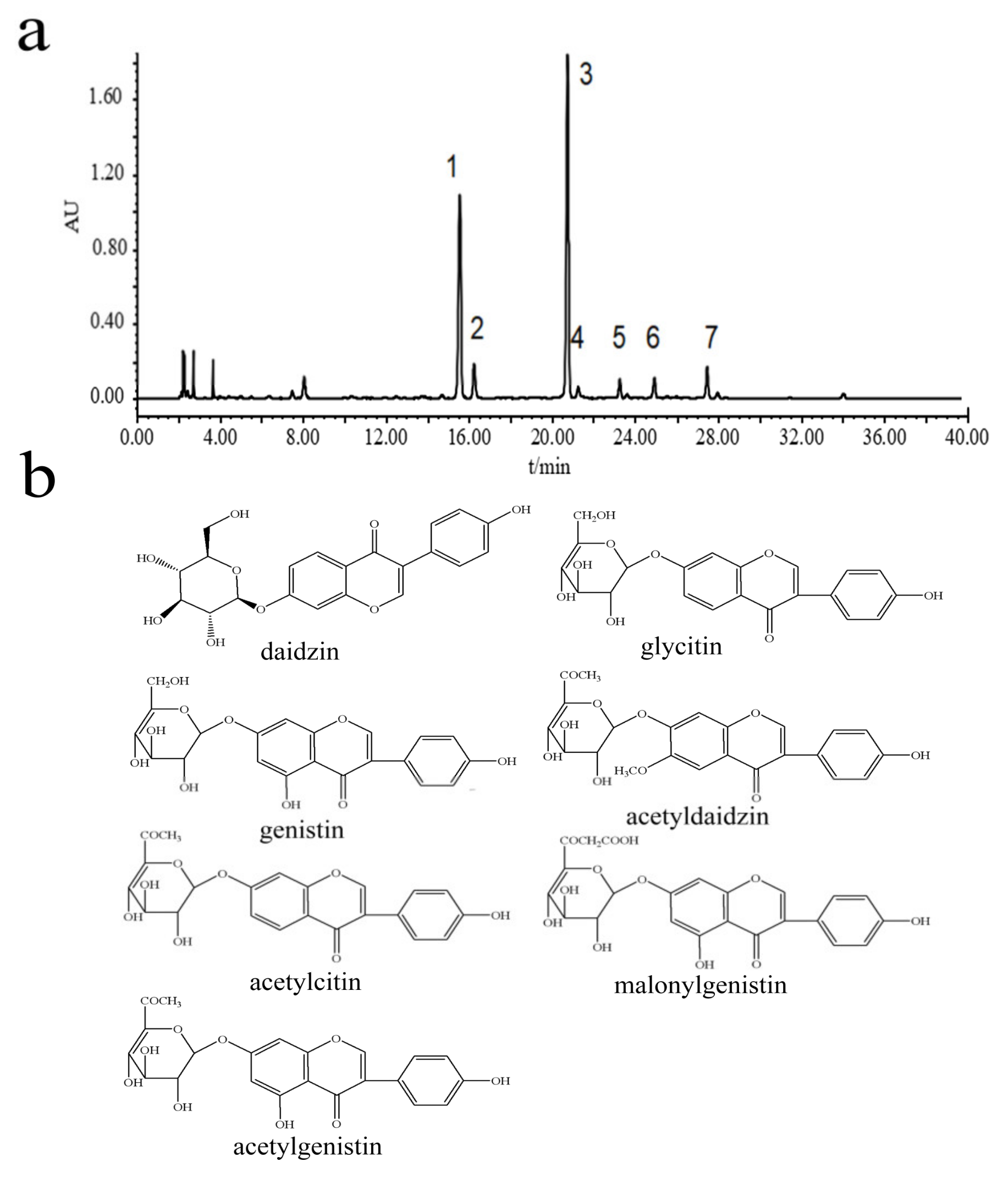
A precise 10 g sample of soybean was measured and subjected to a reflux extraction process using 100 mL of 65% ethanol, conducted twice for a duration of 2 h each. The resultant filtrate was then concentrated and pooled together, followed by dilution to a fixed volume of 10 mL with methanol. Subsequent to comprehensive homogenization, the filtration of the solution through a membrane with a pore size of 0.45 μm was performed in preparation for the analytical procedures. The identification of chemical components within the soybean extracts was carried out utilizing HPLC-DAD-MS [
39].
The procedure for high-performance liquid chromatography (HPLC) involved using a binary linear gradient elution technique [
40]. Here, acetonitrile served as mobile phase A, while mobile phase B consisted of water containing 0.05% phosphoric acid. The gradient schedule for the mobile phases was meticulously programmed: starting with mobile phase B at 90%, it was gradually reduced to 82% over the first 10 min, then further decreased to 80% between 10 and 13 min, followed by a drop to 60% from 13 to 30 min, and was ultimately adjusted to 50% for the final 10 min, from 30 to 40 min. The flow rate was consistently held at 1.0 mL/min, with a sample injection volume of 10 μL, and the detection was conducted at a wavelength of 254 nm, all while maintaining the column at a stable temperature of 30 °C.
For the mass spectrometry, the diode array detector (DAD) was linked to the mass spectrometer via a three-way valve, employing an electrospray ionization (ESI) source in positive ion mode. The scan range was set between M/Z 50 and 1000. The ion source spray voltage was established at 4.5 kV, with a sheath gas flow rate of nitrogen set at 20 L/min and an ion trap pressure at 3.1 × 107 Pa. The temperature for the metal capillary was 250 °C with a voltage of 20 V. The resolution for ESI-MS^n was maintained at 1.0 Da, and the collision-induced dissociation (CID) collision energy ranged from 20 to 30%.
4.3. Network Pharmacology Research
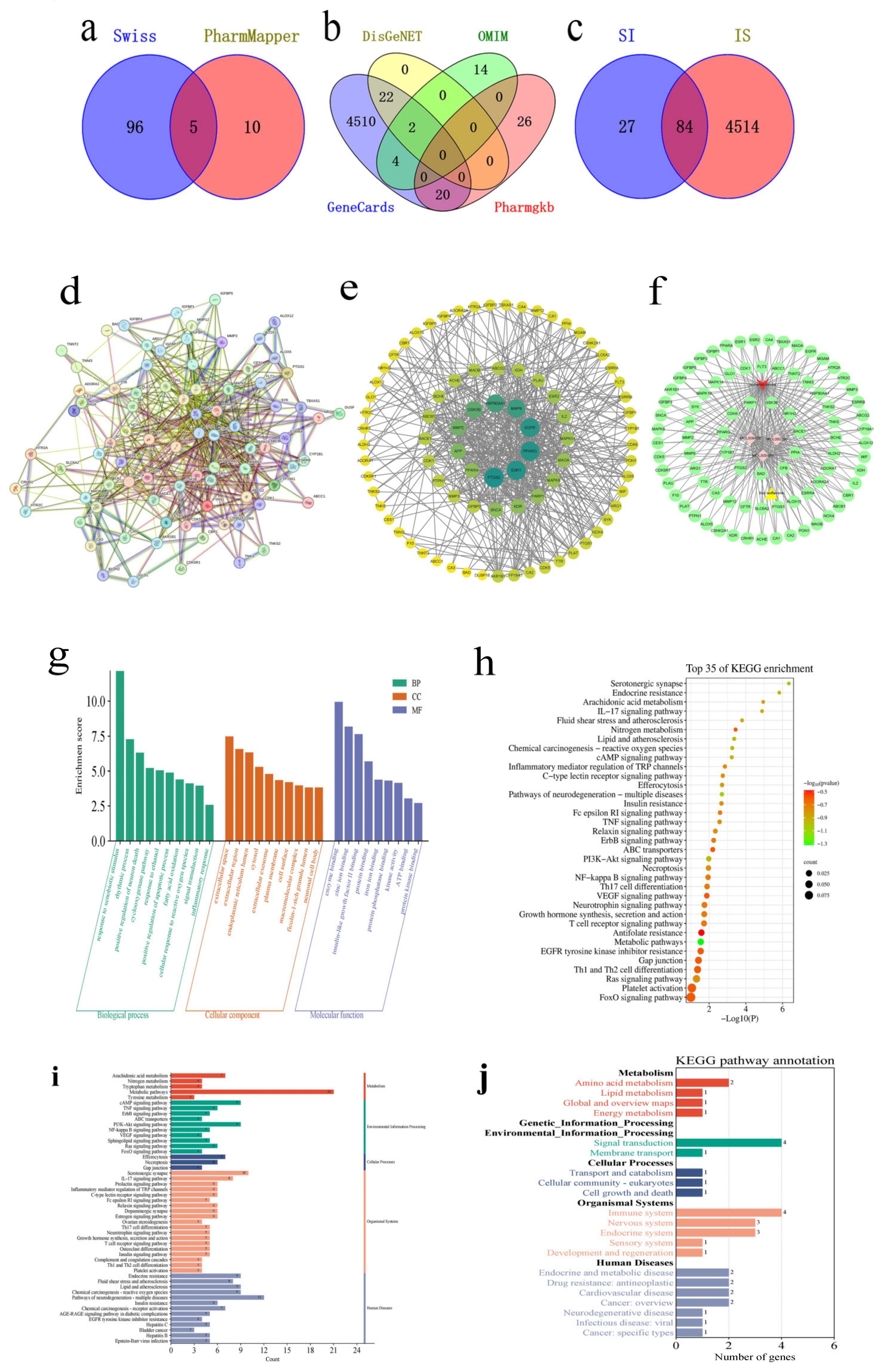
The research on soy isoflavone was initiated by exploring the CNKI database using “soy isoflavone” as the search term to compile a list of soy isoflavone components. The SwissADME online server was utilized to screen soy isoflavone’s active ingredients, evaluating each component’s pharmacokinetic parameters and adherence to the Lipinski Rule of Five [
15]. The potential targets of these active ingredients were then predicted through the Swiss Target Prediction and PharmMapper databases. To identify relevant targets for ischemic stroke, searches were conducted in the GeneCards, OMIM, PharmGkb, and DisGeNET databases. The identified soy isoflavone targets were aligned with those related to drug actions to pinpoint common targets, and a Venn diagram was created to visualize these intersections. Subsequently, these intersecting targets were analyzed for Protein–Protein Interactions (PPIs) using the String database (version 12.0), with the findings visualized in Cytoscape 3.8.0. The intersection targets underwent further analysis of the GO and KEGG pathways using the DAVID website. The top 50 KEGG pathways were classified and summarized according to the first 6 classifications in the KEGG pathway database. Molecular docking between the core components and the core targets was performed based on the above analysis. The 3D crystal structures of the target proteins were obtained from the Protein Data Bank (PDB). Their pdb format files were downloaded, de-watered, and hydrogenated in AutoDockTools (version: 1.5.7) and then selected as receptors and saved as pdbqt files. The mol2 files of the active components were obtained through the TCMSP database, hydrogenated in AutoDockTools, selected as ligands, and exported as pdbqt files. Molecular docking was carried out through AutoDock Vina (version: 1.2.3) and the affinity of each component for the target was obtained and visualized by PyMOL (version: 2.7.0) and the ZBH-Center for Bioinformatics. The results were illustrated through histograms and bubble maps representing the pathway information, which were crafted by the bioinformatics.com.cn platform, accessed last on 28 December 2023 [
41].
4.4. In Vitro Studies
4.4.1. PC12 Cell Culture
PC12 cells, acquired from the Chinese Academy of Sciences in Shanghai, were cultivated in a nutrient-rich Dulbecco’s Modified Eagle Medium (DMEM), a high-glucose medium, which was further supplemented with 10% fetal bovine serum and a 1% mixture of penicillin and streptomycin antibiotics. These cells were then incubated at a constant temperature of 37 °C in an environment enriched with 5% CO
2 for a duration spanning between three and four d, until they reached a confluence of 70–80%. Following this phase of growth, a solution containing 0.25% trypsin was employed to gently detach the cells, facilitating their transfer to new culture containers for continued propagation. In preparation for a range of experimental activities, the cells were subsequently allocated into 96-well plates, with 100 μL of the cell culture introduced into each well. Post-seeding, an additional period of incubation was undertaken in a 5% CO
2 atmosphere at 37 °C, extending from one to two d, to allow the cells to achieve a confluence between 80 and 90%, thereby rendering them suitable for further experimental investigations [
42].
4.4.2. Establishment of Soy Isoflavone Concentrations
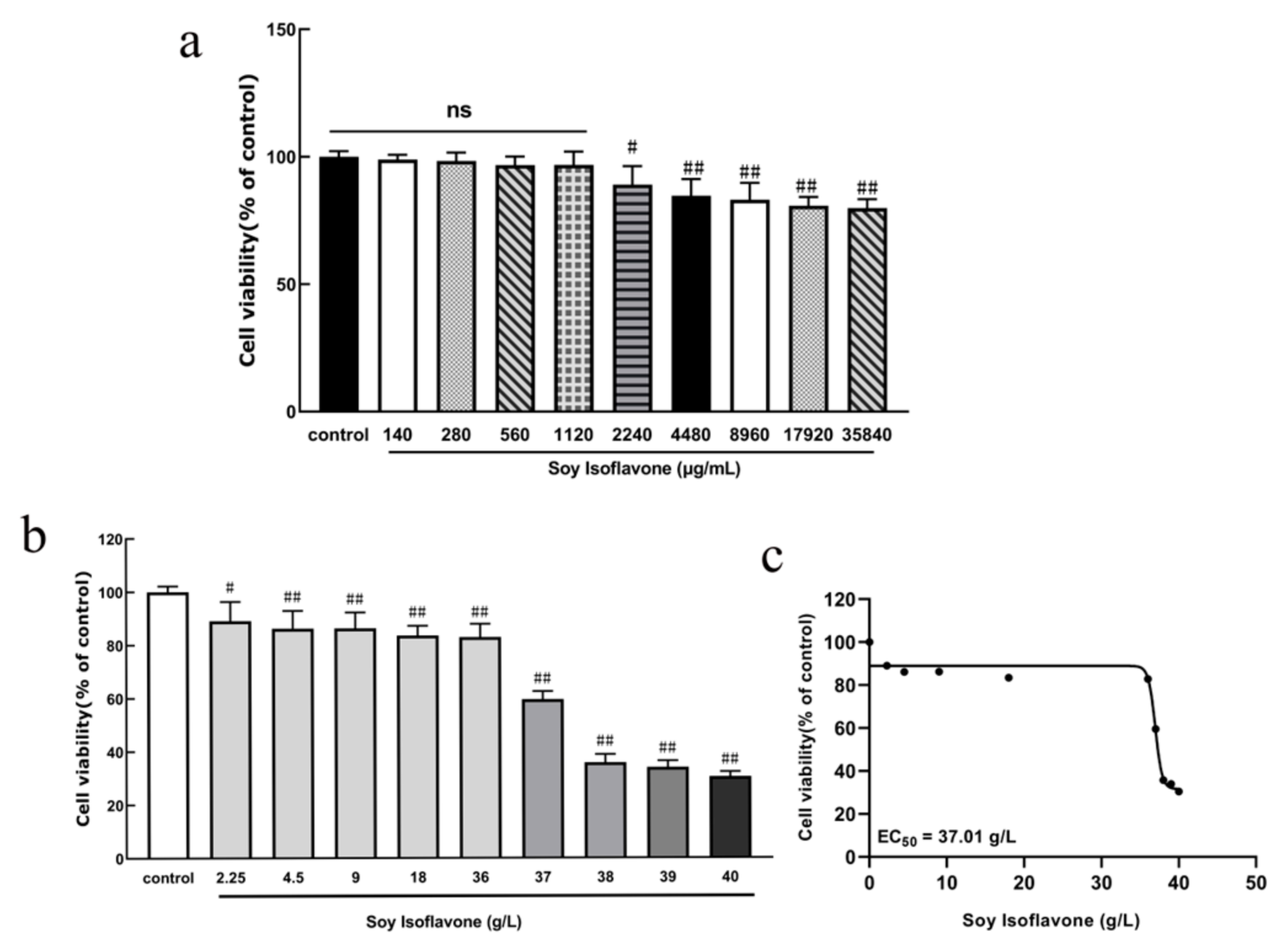
PC12 cells were inoculated into 96-well culture plates at a density of 9 × 104/mL, 100 μL per well, and incubated at 37 °C with 5% CO2 for 24 h. Afterwards, the original culture medium was discarded, and different concentrations of soy isoflavones (140, 280, 560, 1120, 2240, 4480, 8960, 17,920, and 35,840 μg/mL) were added to incubate the cells for 24 h. At the end of the incubation period, the cytotoxicity of the PC12 cells was detected by MTT.
4.4.3. The Establishment and Experimental Grouping of a Glucose–Oxygen Deprivation/Reoxygenation (OGD/R) Ex Vivo Stroke Model
To evaluate the protective impact of a natural isoflavone, SI, on PC12 cells subjected to OGD/R-induced injury, the cells, prepared as previously mentioned, were allocated to various groups: the control group received no OGD/R injury treatment. In the model group, the cells were treated with 100 µL of glucose-free EBSS solution enriched with 10 mmol/L Na
2S
2O
4 for 2 h. Subsequently, the medium was replaced with a standard culture medium to allow reoxygenation, and the cells were further incubated for 24 h, establishing an OGD/R injury model to mimic ischemic brain damage. For the treatment groups, OGD/R conditions were amended with a standard culture medium containing different concentrations of SI (1.56 µM, 3.13 µM, and 6.25 µM) for 24 h. Additionally, a positive control group was treated with 10 µM edaravone under OGD/R conditions (
n = 6), to compare the efficacy of SI against a known neuroprotective agent [
43].
4.4.4. Morphological Observation
Following the incorporation of various treatment agents, the PC12 cells were maintained at 37 °C for a 24 h period. Post-treatment, the cells underwent examination under an inverted microscope to assess any morphological alterations across the specified groups (n = 6).
4.4.5. MTT Method to Measure Cellular Activity
Following treatment, each well in a 96-well cell culture plate was treated with 10 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 g/L concentration) in a light-protected environment. After incubating for 4 h at 37 °C in a 5% CO2 atmosphere, the supernatant was discarded. Subsequently, to dissolve the resultant formazan crystals, 100 µL of dimethyl sulfoxide (DMSO) was added to each well. The optical density of the solutions in each well was measured at 490 nm wavelength using an enzyme-linked immunosorbent assay, with measurements taken within 10 to 15 min. All the data are presented as the mean ± SD (n = 6 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.6. Detection of Ca2+ Content in PC12 Cells
After applying the treatment, the cells were incubated for one hour at 37 °C in a 5% CO
2 atmosphere with the culture medium that included the Fluo-3AM fluorescent probe at a concentration of 2.5 µmol/L. Following this incubation period, any residual probe was meticulously washed away, and the fluorescence indicative of intracellular Ca
2+ levels was detected using a fluorescence microscope [
44]. The intensity of this fluorescence, with the excitation and emission wavelengths set at 488 nm and 530 nm, respectively, was quantitatively measured through enzyme-linked assay techniques. To account for differences in cell density, an MTT assay was utilized to standardize the cell density before calculating the concentrations of intracellular Ca
2+ in PC12 cells.
In this methodology, H represents the content of Ca2+ within the sample, A denotes the value obtained from the calcium ion assay for each individual well, and O signifies the absorbance measured for each specific well. Additionally, MTT B refers to the average value derived from the MTT assay across all the wells within a given treatment group, with each group comprising six replicates. All the data are presented as the mean ± SD (n = 6 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.7. LDH Release Assay in PC12 Cells
Following the completion of the drug treatment, the release of lactate dehydrogenase (LDH) from the PC12 cells was quantitatively assessed as per the protocol provided by the LDH assay kit, with the experiment being replicated six times [
45]. All the data are presented as the mean ± SD (
n = 6 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.8. Detection of ROS Levels in PC12 Cells
Following the treatment, the PC12 cells were exposed to a serum-free culture medium containing a 10 μmol/L DCFH-DA probe for 20 min in an environment of 5% CO
2 at 37 °C. Subsequently, the cells were washed to remove any residual serum-free medium. The levels of ROS within the PC12 cells were then quantified utilizing an enzyme marker, employing the same calculation method as previously described for Ca
2+ levels, with the process repeated in six samples [
46]. All the data are presented as the mean ± SD (
n = 6 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.9. Caspase-3 Release Assay in PC12 Cells
Following the completion of the drug treatment, the release of Caspase-3 from the PC12 cells was quantified in accordance with the guidelines provided by the Caspase-3 assay kit, with the analysis conducted on six replicates [
47]. All the data are presented as the mean ± SD (
n = 6 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.10. Apoptosis Assay
Following the administration of the drug, PC12 cell apoptosis was evaluated using flow cytometry with the assistance of the Annexin V-FITC Apoptosis Assay Kit (Beyotime Biotechnology, Shanghai, China). This kit employs fluorescein isothiocyanate-labeled Annexin V, a phospholipid-binding protein, in conjunction with propidium iodide (PI) for staining. The apoptotic rates were determined using the FACSCanto II flow cytometry system(BD Biosciences, San Jose, CA, USA), and the proportion of apoptotic cells was analyzed and calculated using FlowJo software version 10.6.2, across three experimental replicates. All the data are presented as the mean ± SD (n = 3 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.11. Wound Healing Assay
For the cell scratch assay, a 6-well plate was prepared. Parallel lines spaced 0.5 cm apart were marked on the underside of the plate using a marker, with three lines per well to delineate the observation area. The cells were plated at a concentration of 5 × 105 cells per milliliter in each well. Following this, a pipette tip was utilized to generate a straight scratch through the cell monolayer. Any detached cells resulting from the scratch were removed by washing with PBS. Images of the scratch were captured at 0 and 24 h post-scratch using a microscope to evaluate cell migration. The assessment was based on measuring the changes in the width of the scratch, indicating the cells’ migratory capacity. All the data are presented as the mean ± SD (n = 3 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.4.12. Western Blot Analysis
For protein sample preparation, the cells underwent lysis on ice for approximately 20 min. This was followed by centrifugation at 4 °C at 13,000× g for 5 min. The protein concentrations in the resulting supernatants were accurately measured using the bicinchoninic acid (BCA) protein assay method. Post-separation, the proteins were subjected to a 5–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis process, subsequently being transferred to polyvinylidene fluoride (PVDF) membranes. To inhibit non-specific binding, these membranes underwent incubation with 5% skimmed milk at room temperature for an hour. Overnight incubation at 4 °C was carried out with primary antibodies specifically targeting Nrf2, Keap1, HO-1, β-actin, and NQO1, with dilutions prepared at 1:1000 for all but NQO1, which was diluted to 1:4000. Following thorough washes, the membranes were treated with secondary antibodies diluted to 1:1000 for an hour at room temperature. After additional washing steps, the protein bands were detected with an ECL detection system and captured for analysis. A semi-quantitative assessment of these bands was performed using ImageJ (version 1.53) software, providing an analytical basis for evaluating protein expression levels. All the data are presented as the mean ± SD (n = 3 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.5. In Vivo Studies
4.5.1. Animals and Treatment
Forty-eight adult male ICR rats (average body weight 27.3 ± 1.8 g, age 6–8 weeks) were obtained from the Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). The animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Changchun Normal University (Approval No. 2024003, approved on 13 March 2024). All animal handling followed the guidelines set by the National Institutes of Health (NIHs) and complied with the ARRIVE 2.0 reporting standards.
4.5.2. Housing Conditions and Welfare Measures
Rats were housed in ventilated cages under a controlled environment (temperature: 18–22 °C; relative humidity: 50–60%; 12/12 h light/dark cycle), with ad libitum access to standard chow and water. Daily monitoring was performed for food intake, weight, and clinical symptoms. All efforts were made to minimize animal suffering, and the minimum number of animals required for statistical validity was used.
4.5.3. Experimental Design and Surgical Procedure
A total of 48 rats (body weight 25–30 g) were randomly assigned into four groups (
n = 12 per group) using a random number generator: The sham group underwent identical surgical procedures without carotid occlusion. The model group received bilateral common carotid artery occlusion (BCCAO) to induce cerebral ischemia–reperfusion injury. The SI treatment group was treated with soy isoflavones (100 mg/kg, dissolved in saline) via intragastric administration for 5 consecutive d prior to ischemia [
48,
49]. The edaravone group received edaravone (10 mg/kg, i.p.), a standard neuroprotective agent, once daily for 5 d as a positive control.
Transient cerebral ischemia–reperfusion injury was induced using the bilateral common carotid artery occlusion (BCCAO) model [
50]. The rats were anesthetized with pentobarbital sodium (45 mg/kg, i.p.). A midline neck incision (~3 cm) was made to isolate and occlude the bilateral common carotid arteries with vascular clips for 60 min, followed by reperfusion for 24 h. For the sham group, the arteries were exposed but not occluded. Intraoperatively, the rectal temperature of the rats was maintained at 37.0 ± 0.5 °C using an automated thermostatic heating pad. Postoperatively, the animals were housed in a climate-controlled environment (ambient temperature: 23 ± 1 °C; relative humidity: 55 ± 5%) with sterile bedding and ad libitum access to food/water. Subsequently, behavioral tests were performed on the animals. After the completion of the behavioral tests, the animals were euthanized under deep anesthesia by cervical dislocation, in accordance with the AVMA guidelines. Brain tissues were immediately harvested and processed as follows: 5 rats per group for histopathological examination, 3 rats per group for Western blot analysis, and 4 rats per group for HE staining. All the relevant pairwise comparisons among the four experimental groups (control, model, SI, and edaravone) were pre-defined and included in the statistical analysis to comprehensively assess the treatment effects and control efficacy.
4.5.4. Neurological Function Score
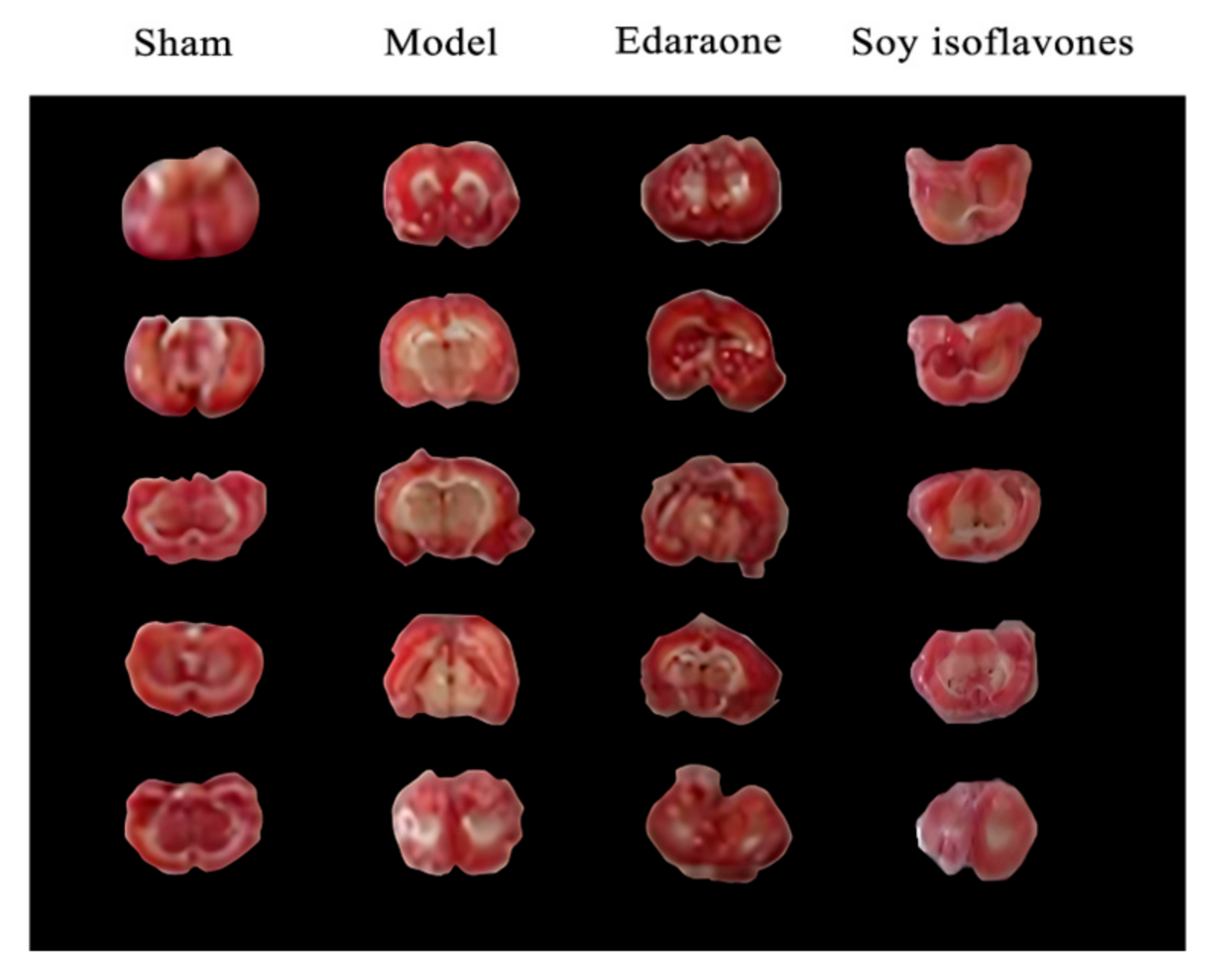
Neurological function was assessed according to the method described by Bederson et al. The assessment was conducted in a blinded manner to exclude an observer bias. Briefly, each rat was initially placed on a flat surface to observe its spontaneous movements. Circling behavior toward the side opposite the ischemic region was scored as 1 point. When the rats were gently lifted by the tail, a flexion of the elbow was scored as 2 points, an internal rotation of the shoulder as 3 points, and a wrist flexion of the forelimb as 1 point. Additionally, pushing resistance tests were performed, with scores from 1 to 3 assigned based on the degree of motor deficit (greater distances corresponded to higher scores). The rats were placed on a metal mesh and were further assessed for contralateral muscle tension reduction, with the severity scored from 1 to 3 points according to the degree of impairment [
16]. Subsequently, the rats were euthanized under deep anesthesia, and their brains were harvested and maintained in cold saline (4 °C). The olfactory bulb, cerebellum, and brainstem were removed, and the remaining brain tissue was briefly frozen at −20 °C for 10 min. Coronal brain slices were made (four cuts, yielding five slices in total), quickly immersed in 2% 2,3,5-triphenyltetrazolium chloride (TTC) solution, and incubated at 37 °C for 20 min in the dark. Viable brain tissues were stained a rose-red color, while ischemic tissues remained unstained. Each brain slice was digitally photographed, and the cerebral infarct area was quantitatively analyzed using the BI-2000 image analysis software,version 3.0 (Taimeng Medical Imaging, Chengdu, China). All the data are presented as the mean ± SD (
n = 10 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.5.5. The Water Content of Brain Tissue Was Measured
Following the ischemia–reperfusion process, the cerebellum, olfactory bulb, and lower brainstem were meticulously removed from the rats. The mass of the brain tissue left, referred to as the wet weight (W), was then accurately determined. This tissue was subsequently placed in an oven set at a constant temperature of 110 °C and dried for a duration of 48 h. Upon the completion of the drying period, the dry weight (D) of the tissue was recorded. The percentage of the water content in the brain tissue was calculated using the formula water content = [(W − D)/W] × 100%. All the data are presented as the mean ± SD (n = 10 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
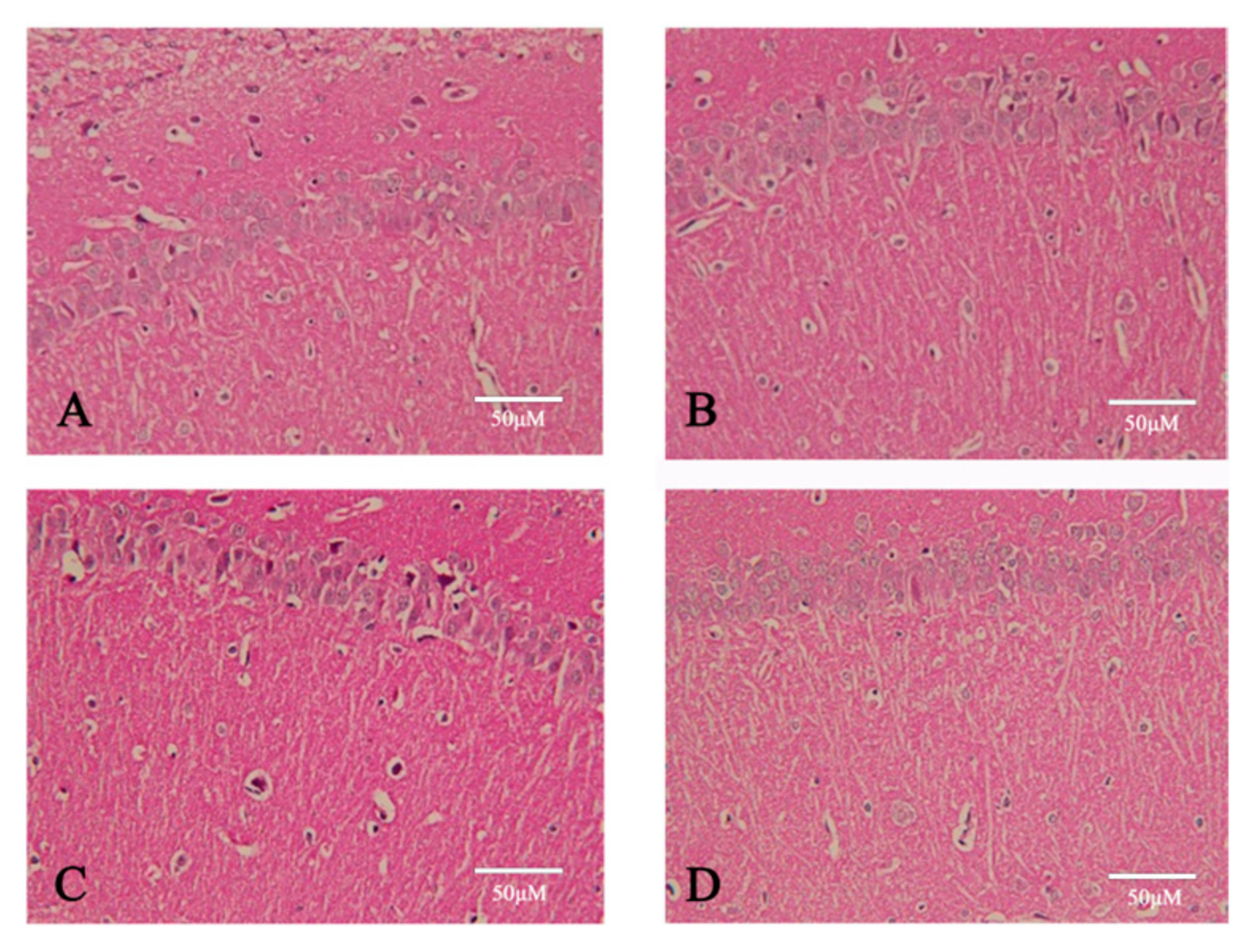
4.5.6. The Pathological Morphology of the Brain Tissue Was Observed
Following euthanasia, the brains of the rats were rapidly dissected and rinsed in cold saline at 4 °C. The olfactory bulb, cerebellum, and brainstem were removed. The brains were then fixed in 4% paraformaldehyde (Solarbio, Beijing, China) for 24–48 h, followed by dehydration in graded ethanol, clearing in xylene, embedding in paraffin, and coronal sectioning into 5 μm thick slices using a microtome (Leica RM2235, Leica Biosystems, Wetzlar, Germany). The sections were stained using standard hematoxylin and eosin (H&E) protocols. Hematoxylin stained cell nuclei dark blue, while eosin stained the cytoplasm and extracellular matrix pink. A microscopic examination was performed under a light microscope (Leica DM3000, Germany) to evaluate the degree of cellular damage, tissue disorganization, and neuronal loss in the ischemic regions. Representative images were captured, and pathological scoring or area quantification was performed using BI-2000 image analysis software,version 3.0 (Taimeng Medical Imaging, Chengdu, China). From each rat (n = 5 per group), three coronal sections were analyzed. For each section, three non-overlapping fields from the cortex and hippocampus were randomly selected and imaged at 200× magnification, yielding a total of nine images per animal for the statistical analysis. (n = 5; histological scores compared using a one-way ANOVA).
Histopathological evaluation focused on key morphological features indicative of ischemic injury, including neuronal cell loss, nuclear condensation and pyknosis, cytoplasmic eosinophilia, edema in the neuropil, vascular congestion or hemorrhage, and inflammatory cell infiltration. A semi-quantitative scoring system was used to assess tissue damage based on the extent and severity of histological alterations in the cortex and hippocampus. Each parameter was scored on a scale from 0 to 3 (0 = normal, 1 = mild, 2 = moderate, 3 = severe). All the sections were assessed blindly by two independent observers.
4.5.7. The Activities of SOD, GSH-px, CAT, MDA, and LDH in the Brain Tissue Were Detected
Following the ischemia–reperfusion procedure in the rats, the olfactory bulb, cerebellum, and brainstem were removed. The remaining brain tissues were then finely chopped on ice. These tissues were mixed with physiological saline in a centrifuge tube at a specified ratio and homogenized using a tissue homogenizer until no visible solid particles remained. The mixture underwent centrifugation at 3000 rpm for 10 min, facilitating the separation of the supernatant, which was then carefully transferred into a fresh centrifuge tube. The concentrations of SOD, GSH-px, CAT, MDA, and LDH within the rat brain tissue were accurately measured using a multifunctional microplate reader [
51]. All the data are presented as the mean ± SD (
n = 10 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.5.8. Detection of Nrf2, Keap1, HO-1, and NQO1 Protein Expression by Western Blot
The brain tissues were processed in a cell lysis buffer that included complete inhibitors for proteases and phosphatases. The nucleoproteins and total proteins were extracted, and their concentrations were determined using the BCA technique. Identical quantities of proteins from each group underwent separation via 10% SDS-PAGE and were subsequently transferred onto a PVDF membrane. The next complete steps follow the method in
Section 4.4.12. All the data are presented as the mean ± SD (
n = 3 per group), and statistical comparisons were performed using a one-way ANOVA followed by Tukey’s post hoc test.
4.5.9. Statistical Analysis
The data from the experiments were processed and analyzed using the GraphPad Prism 9 software, which meant that all the results were presented as the mean ± SD. A one-way ANOVA was used for multiple group comparisons, followed by Tukey’s post hoc test to determine the statistical significance between all the group pairs (sham vs. model, SI vs. model, edaravone vs. model, and SI vs. edaravone). This approach ensured that every possible comparison between the study groups was systematically considered and appropriately tested. A p-value below 0.01 was interpreted as denoting a more substantial level of significance.