3D-Printed Organ-Realistic Phantoms to Verify Quantitative SPECT/CT Accuracy for 177Lu-PSMA-617 Treatment Planning
Abstract
1. Introduction
2. Results
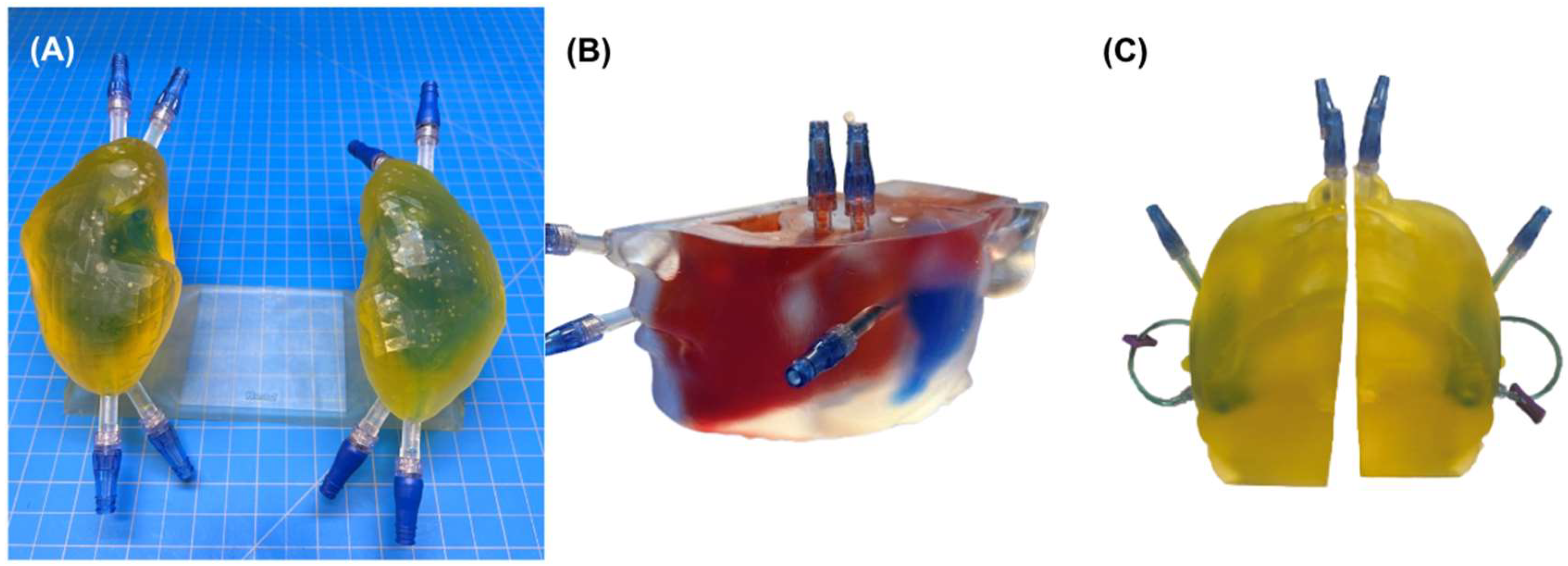
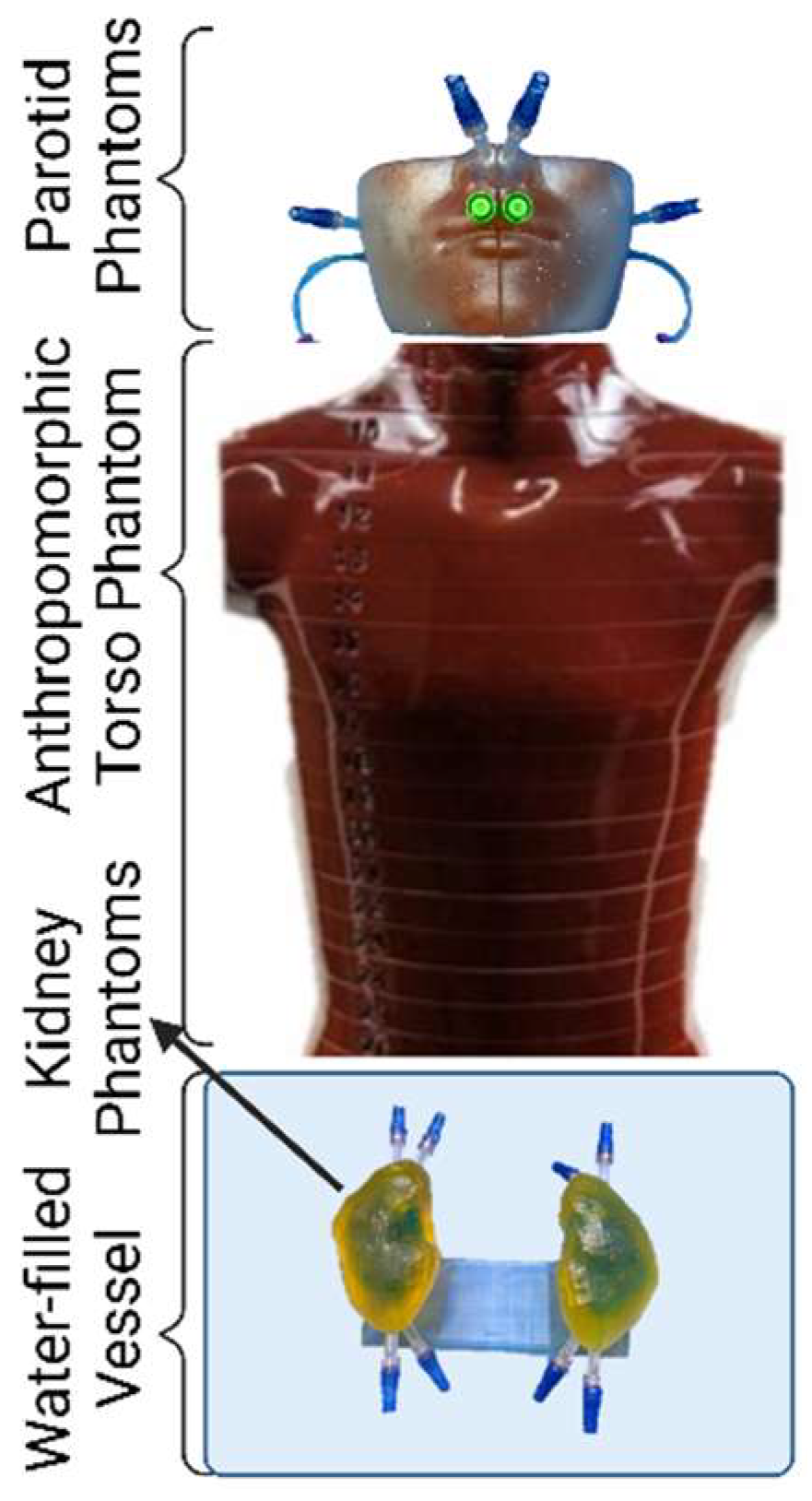
2.1. Phantom Design and Fabrication
2.2. Geometric Verification
2.3. Leak Testing
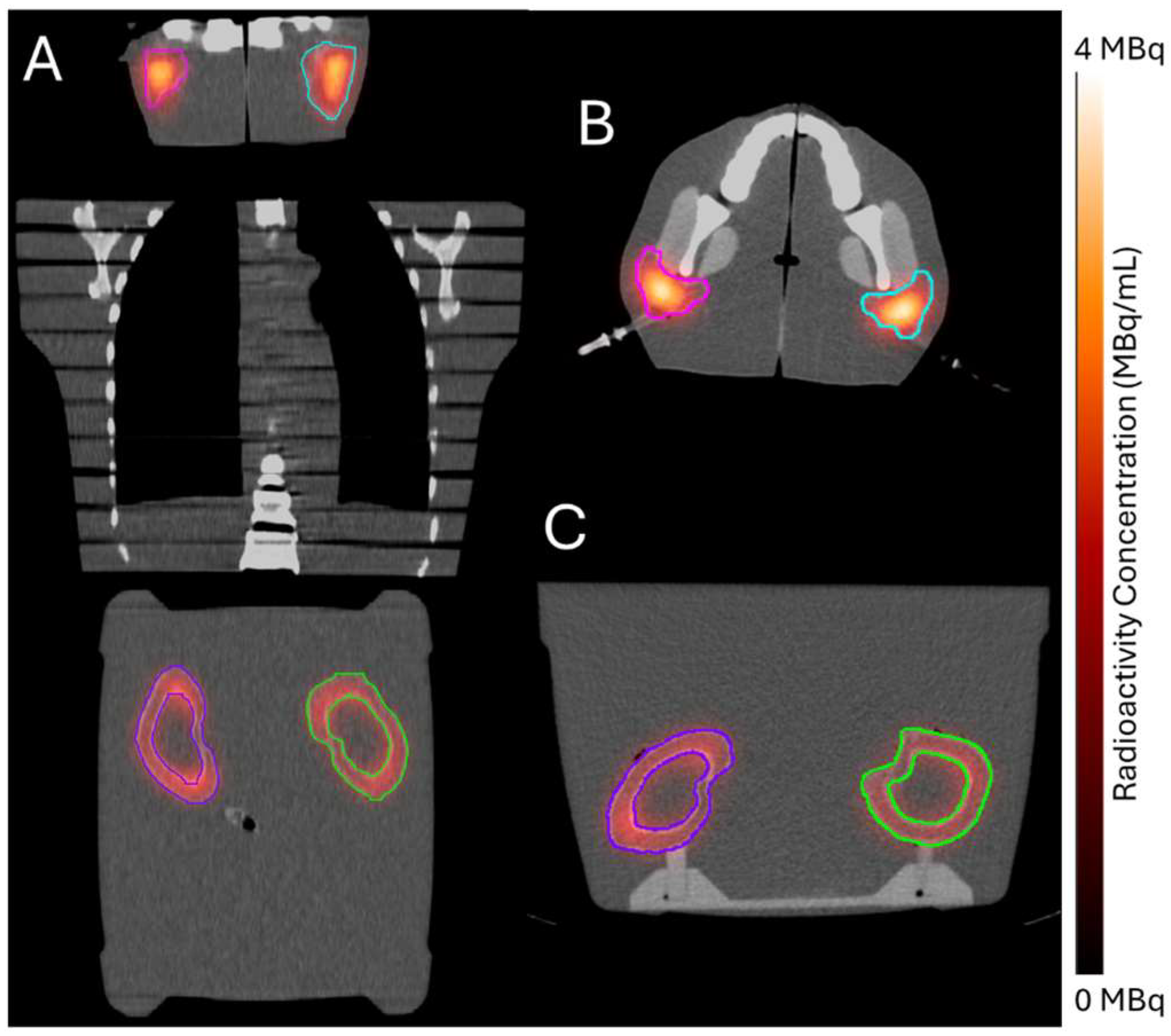
2.4. Clinical Feasibility Testing
Radioactivity Verification
3. Discussion
4. Materials and Methods
4.1. Phantom Design and Fabrication
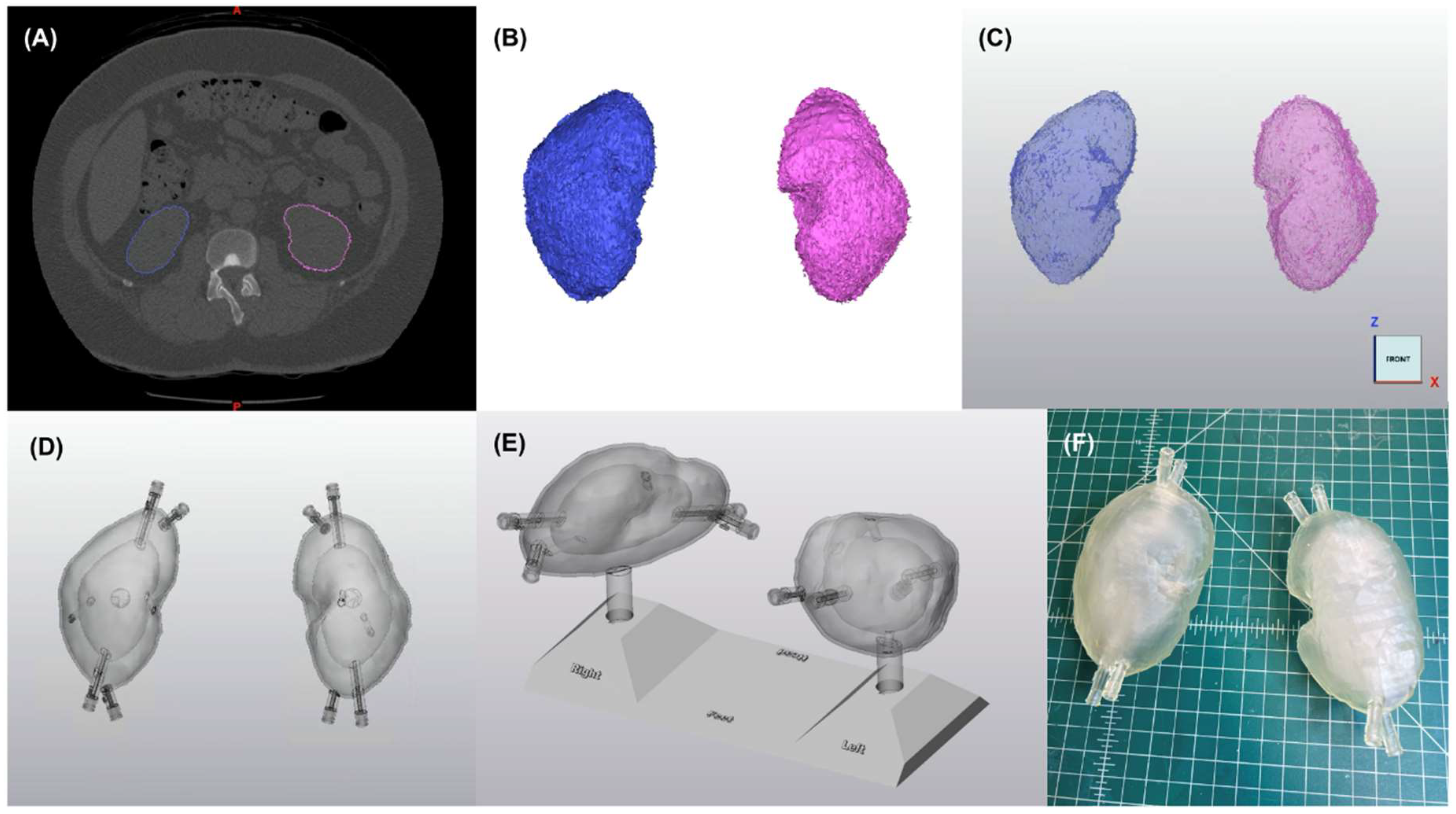
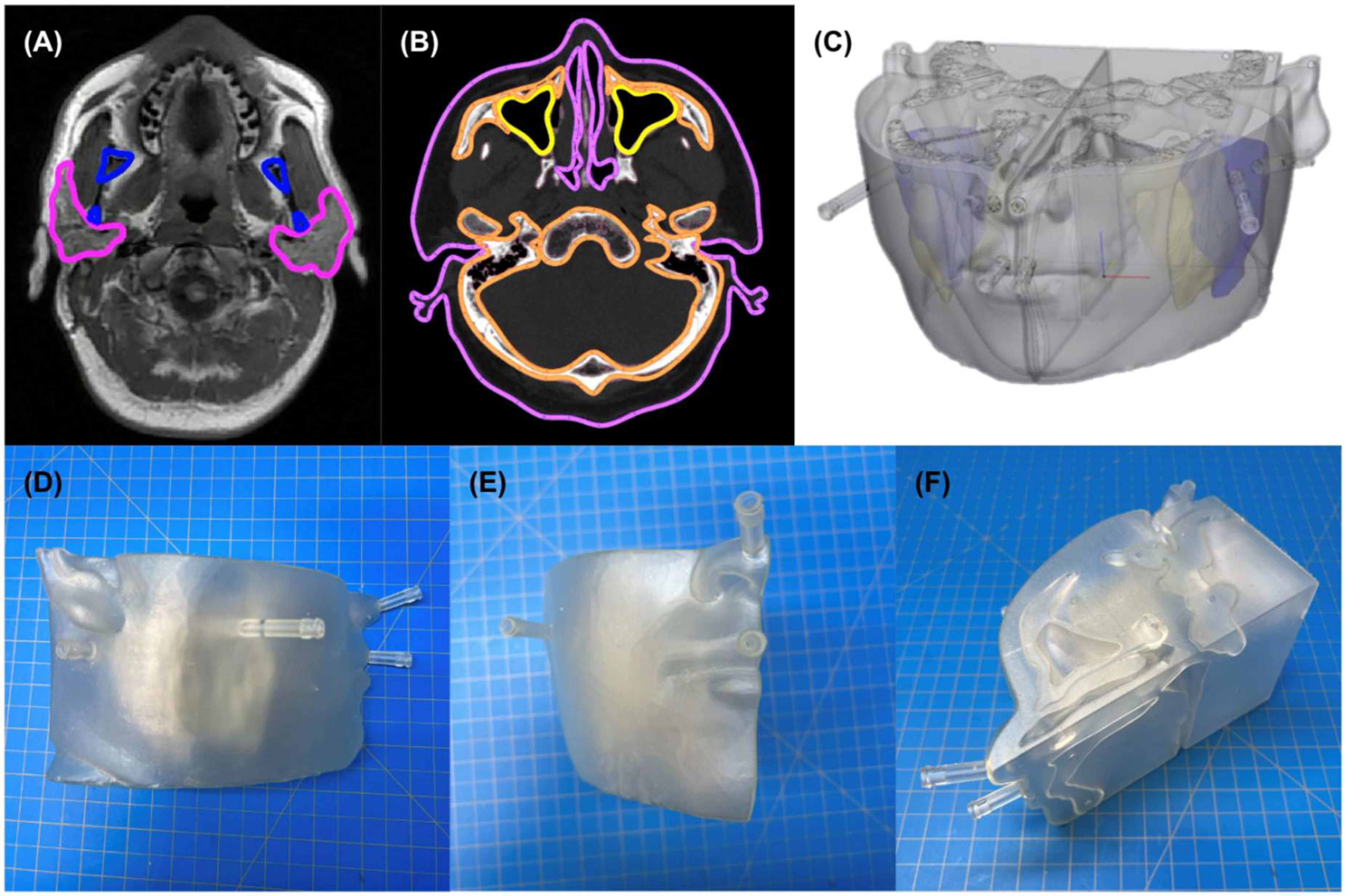
4.1.1. Representative Anatomy
4.1.2. Segmentation and Computer Aided Design
- Wrapping, smoothing, and reduction in triangles on the surface of the kidney parts to produce a more natural appearance;
- Hollowing to create a 1.5 mm thick wall, minimizing potential radiation attenuation from the 3D-printed material;
- Creation of two chambers in each kidney by duplicating the hollow parts and reducing the organ size by 0.31 to represent (1) the cortical (outer) chamber, containing 67% of the model’s volume and (2) the medulla (inner) chamber, containing 31% of the model’s volume [30];
- Adding angled support structures to secure the inner medulla chamber to the outer chamber wall;
- Creation of two luer ports per chamber (i.e., four in total per kidney) to be used with standard one-way valves to inject water and/or 177Lu-PSMA-617 into each chamber;
- Design of a peg and base system (180-degree lock-and-key design) to position kidneys in their anatomic orientations (i.e., distance between kidneys and distance above scanner bed) while imaged and immersed in water; and
- Labeling the base with indicators of left, right, head, and feet, to ensure proper and consistent orientation.
- Wrapping, smoothing, and reduction in triangles on the surface of the bone, soft tissue, and parotid parts to produce a more natural appearance;
- Boolean subtraction of the sinuses and esophagus from the soft tissue (to simulate naturally occurring air pockets);
- Mirroring of the left parotid across the midline to produce a matched right parotid structure;
- Hollowing and creation of three chambers in each side of the head representing parotid, skull, and soft tissue, respectively, each with 1.5 mm walls;
- Creation of two luer ports in each of the soft tissue and parotid chambers to be used with standard one-way valves to inject water and/or 177Lu-PSMA-617; and
- Creation of simple access holes for injecting bone-mimicking material into the skull chamber.
4.1.3. 3D Printing
4.1.4. Tissue Heterogeneity Representation
4.1.5. Phantom Watertight Sealing
4.2. Geometric Verification
4.3. Leak Testing
4.4. Clinical Feasibility Testing
4.4.1. Radioactivity Injection
- Removing 50 mL of water from each of the kidney cortex and parotid chambers to create space for 177Lu-PSMA-617;
- Drawing 177Lu-PSMA-617 into a 20 mL syringe;
- Measuring the radioactivity using a dose calibrator (Capintec CRC-15R) that had previously been cross-calibrated using a reference radioactivity of 177Lu-PSMA-617 following manufacturer guidelines;
- Injecting the radioactivity into the partially emptied (from step 1) chamber;
- Flushing the syringe with water at least twice to transfer residual radioactivity from the syringe and luer valve system into the chamber;
- Measuring the residual radioactivity left in the syringe using the dose calibrator, and
- Filling the chamber to capacity with water and removing any air bubbles.
4.4.2. SPECT/CT Image Acquisition
4.4.3. Quantitative Image Reconstruction
4.4.4. Radioactivity Verification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrmann, K.; Schwaiger, M.; Lewis, J.S.; Solomon, S.B.; McNeil, B.J.; Baumann, M.; Gambhir, S.S.; Hricak, H.; Weissleder, R. Radiotheranostics: A roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Bednarz, B. Theranostics and Patient-Specific Dosimetry. Semin. Radiat. Oncol. 2023, 33, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Hebert, K.; Santoro, L.; Monnier, M.; Castan, F.; Berkane, I.; Assénat, E.; Fersing, C.; Gélibert, P.; Pouget, J.-P.; Bardiès, M.; et al. Absorbed Dose–Response Relationship in Patients with Gastroenteropancreatic Neuroendocrine Tumors Treated with 177Lu]Lu-DOTATATE: One Step Closer to Personalized Medicine. J. Nucl. Med. 2024, 65, 923–930. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Roth, D.; Gustafsson, J.; Warfvinge, C.F.; Sundlöv, A.; Åkesson, A.; Tennvall, J.; Gleisner, K.S. Dosimetric Quantities in Neuroendocrine Tumors over Treatment Cycles with 177Lu-DOTATATE. J. Nucl. Med. 2022, 63, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Fletcher, S.L. Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50), ICRU-62; ICRU: Bethesda, MD, USA, 1999. [Google Scholar]
- van der Merwe, D.; Van Dyk, J.; Healy, B.; Zubizarreta, E.; Izewska, J.; Mijnheer, B.; Meghzifene, A. Accuracy requirements and uncertainties in radiotherapy: A report of the International Atomic Energy Agency. Acta Oncol. 2017, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, D.; Gear, J.I.; Fioroni, F.; Flux, G.D.; Murray, I.; Castellani, G.; Versari, A.; Iori, M.; Grassi, E. Uncertainty analysis of tumour absorbed dose calculations in molecular radiotherapy. EJNMMI Phys. 2020, 7, 63. [Google Scholar] [CrossRef]
- Ritt, P. Recent Developments in SPECT/CT. Semin. Nucl. Med. 2022, 52, 276–285. [Google Scholar] [CrossRef]
- Ritt, P.; Vija, H.; Hornegger, J.; Kuwert, T. Absolute quantification in SPECT. Eur. J. Nucl. Med. Mol. Imaging 2011, 38 (Suppl. S1), S69–S77. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Z.; Chen, G.; Shi, K.; Mok, G.S.P. Partial volume correction for Lu-177-PSMA SPECT. EJNMMI Phys. 2024, 11, 93. [Google Scholar] [CrossRef]
- Willowson, K.; Bailey, D.L.; Baldock, C. Quantitative SPECT reconstruction using CT-derived corrections. Phys. Med. Biol. 2008, 53, 3099. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.M.; Dhurairaj, T.; Basu, S.; Bural, G.; Surti, S.; Alavi, A. A recovery coefficient method for partial volume correction of PET images. Ann. Nucl. Med. 2009, 23, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Tran-Gia, J.; Schlögl, S.; Lassmann, M. Design and Fabrication of Kidney Phantoms for Internal Radiation Dosimetry Using 3D Printing Technology. J. Nucl. Med. 2016, 57, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Tran-Gia, J.; Lassmann, M. Optimizing Image Quantification for 177Lu SPECT/CT Based on a 3D Printed 2-Compartment Kidney Phantom. J. Nucl. Med. 2018, 59, 616–624. [Google Scholar] [CrossRef]
- Jessen, L.; Gustafsson, J.; Ljungberg, M.; Curkic-Kapidzic, S.; Imsirovic, M.; Sjögreen-Gleisner, K. 3D printed non-uniform anthropomorphic phantoms for quantitative SPECT. EJNMMI Phys. 2024, 11, 8. [Google Scholar] [CrossRef]
- Salvadori, J.; Allegrini, O.; Opsommer, T.; Carullo, J.; Sarrut, D.; Porot, C.; Ritzenthaler, F.; Meyer, P.; Namer, I.-J. Anatomy-based correction of kidney PVE on 177Lu SPECT images. EJNMMI Phys. 2024, 11, 15. [Google Scholar] [CrossRef]
- Grings, A.; Jobic, C.; Kuwert, T.; Ritt, P. The magnitude of the partial volume effect in SPECT imaging of the kidneys: A phantom study. EJNMMI Phys. 2022, 9, 18. [Google Scholar] [CrossRef]
- QIBA SPECT Biomarker Committee. Quantifying Dopamine Transporters with 123-Iodine Labeled Ioflupane in Neurodegenerative Disease Profile; Quantitative Imaging Biomarkers Alliance: Oak Brook, IL, USA, 2017; Available online: https://qibawiki.rsna.org/index.php/SPECT_Biomarker_Ctte (accessed on 4 March 2025).
- Robinson, A.P.; Tipping, J.; Cullen, D.M.; Hamilton, D.; Brown, R.; Flynn, A.; Oldfield, C.; Page, E.; Price, E.; Smith, A.; et al. Organ-specific SPECT activity calibration using 3D printed phantoms for molecular radiotherapy dosimetry. EJNMMI Phys. 2016, 3, 12. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; Valstar, M.H.; de Keizer, B.; Willems, S.M.; Smit, L.A.; Al-Mamgani, A.; Smeele, L.E.; van Es, R.J.J.; de Bree, R.; Vogel, W.V. Physiologic distribution of PSMA-ligand in salivary glands and seromucous glands of the head and neck on PET/CT. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 478–486. [Google Scholar] [CrossRef]
- De Jong, M.; Valkema, R.; Van Gameren, A.; Van Boven, H.; Bex, A.; Van De Weyer, E.P.; Burggraaf, J.D.; Körner, M.; Reubi, J.C.; Krenning, E.P. Inhomogeneous localization of radioactivity in the human kidney after injection of [111In-DTPA]octreotide. J. Nucl. Med. 2004, 45, 1168–1171. [Google Scholar]
- Ravi, P.; Chepelev, L.; Lawera, N.; Haque, K.M.A.; Chen, V.C.P.; Ali, A.; Rybicki, F.J. A systematic evaluation of medical 3D printing accuracy of multi-pathological anatomical models for surgical planning manufactured in elastic and rigid material using desktop inverted vat photopolymerization. Med. Phys. 2021, 48, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Ells, Z.; Grogan, T.R.; Czernin, J.; Dahlbom, M.; Calais, J. Dosimetry of [177Lu]Lu-PSMA-Targeted Radiopharmaceutical Therapies in Patients with Prostate Cancer: A Comparative Systematic Review and Metaanalysis. J. Nucl. Med. 2024, 65, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Kwon, T.E.; Han, H.; Choi, C.; Kitahara, C.M.; Lee, C. Enhanced Internal Dosimetry for Alimentary Tract Organs in Nuclear Medicine based on the ICRP Mesh-Type Reference Phantoms. Radiat. Phys. Chem. 2024, 224, 112009. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, T.; Ittermann, T.; Ihler, F.; Koch, C.; Blaurock, M.; Bülow, R.; Völzke, H.; Busch, C.J.; Beule, A.G. Population Based Average Parotid Gland Volume and Prevalence of Incidental Tumors in T1-MRI. Healthcare 2022, 10, 2310. [Google Scholar] [CrossRef]
- Okur, A.; Serin, H.I.; Zengin, K.; Erkoc, M.F.; Tanık, S.; Yıldırım, U.; Karaçavus, S.; Akyol, L. Relationship between kidney volume and body indexes in the Turkish population determined using ultrasonography. Int. Braz. J. Urol. 2014, 40, 816–822. [Google Scholar] [CrossRef]
- Cheong, B.; Muthupillai, R.; Rubin, M.F.; Flamm, S.D. Normal values for renal length and volume as measured by magnetic resonance imaging. Clin. J. Am. Soc. Nephrol. 2007, 2, 38–45. [Google Scholar] [CrossRef]
- Wang, X.; Vrtiska, T.J.; Avula, R.T.; Walters, L.R.; Chakkera, H.A.; Kremers, W.K.; Lerman, L.O.; Rule, A.D. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014, 85, 677–685. [Google Scholar] [CrossRef]
- Agrawal, A.P.; Kumar, V.; Kumar, J.; Paramasivam, P.; Dhanasekaran, S.; Prasad, L. An investigation of combined effect of infill pattern, density, and layer thickness on mechanical properties of 3D printed ABS by fused filament fabrication. Heliyon 2023, 9, e16531. [Google Scholar] [CrossRef]
- Yao, T.; Deng, Z.; Zhang, K.; Li, S. A method to predict the ultimate tensile strength of 3D printing polylactic acid (PLA) materials with different printing orientations. Compos. Part B Eng. 2019, 163, 393–402. [Google Scholar] [CrossRef]
- Ma, X.; Figl, M.; Unger, E.; Buschmann, M.; Homolka, P. X-ray attenuation of bone, soft and adipose tissue in CT from 70 to 140 kV and comparison with 3D printable additive manufacturing materials. Sci. Rep. 2022, 12, 14580. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, J.; Esfandiari, N.; Baracos, V.E.; Buteau, F.A.; Frenette, J.; Putman, C.T.; Mazurak, V.C. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014, 210, 489–497. [Google Scholar] [CrossRef] [PubMed]
- ASTM F1929-15; Standard Test Method for Detecting Seal Leaks in Porous Medical Packaging by Dye Penetration. ASTM International: West Conshohocken, PA, USA, 2012.
- D’Arienzo, M.; Cazzato, M.; Cozzella, M.L.; Cox, M.; D’Andrea, M.; Fazio, A.; Fenwick, A.; Iaccarino, G.; Johansson, L.; Strigari, L.; et al. Gamma camera calibration and validation for quantitative SPECT imaging with 177Lu. Appl. Radiat. Isot. 2016, 112, 156–164. [Google Scholar] [CrossRef] [PubMed]

| Chamber | Original Contour (cc) | CAD Model (cc) | CAD Error (%) | CT of Printed Phantom (cc) | Printing Error (%) |
|---|---|---|---|---|---|
| Left Kidney Full | 195.9 | 206.1 | 5.2 | 211.3 | 2.6 |
| Right Kidney Full | 192.4 | 197.7 | 2.8 | 200.9 | 1.6 |
| Left Kidney Cortex | N/A | 155.6 | - | 160.4 | 3.1 |
| Right Kidney Cortex | N/A | 149.0 | - | 151.3 | 1.5 |
| Left Parotid | 28.6 | 28.6 | 0 | 32.0 | 11.8 |
| Right Parotid | 28.6 | 28.6 | 0 | 30.4 | 6.1 |
| Average Error | 2.0 | 4.5 |
| Contour | (MBq) | (MBq) | Recovery Coefficient |
|---|---|---|---|
| Left Kidney (whole) | 167.92 | 155.72 | 0.93 |
| Right Kidney (whole) | 189.59 | 175.92 | 0.93 |
| Left Kidney Cortex | 167.92 | 142.81 | 0.85 |
| Right Kidney Cortex | 189.59 | 156.20 | 0.82 |
| Left Parotid | 57.13 | 48.76 | 0.85 |
| Right Parotid | 53.00 | 43.76 | 0.83 |
| Parameter | Value |
|---|---|
| Density of supports (unitless) | 0.7 |
| Touchpoint size (mm) | 0.4 |
| Internal Supports | Off |
| Layer thickness—Kidneys (µm) | 100 |
| Layer thickness—Parotids (µm) | 50 |
| Chamber | Radioactivity (MBq) |
|---|---|
| Left Kidney Cortex | 253.82 |
| Right Kidney Cortex | 286.57 |
| Left Parotid | 86.36 |
| Right Parotid | 80.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, L.J.; Belko, S.; Gingold, E.; Wan, S.; Monane, R.; Pugliese, R.; Mourtada, F. 3D-Printed Organ-Realistic Phantoms to Verify Quantitative SPECT/CT Accuracy for 177Lu-PSMA-617 Treatment Planning. Pharmaceuticals 2025, 18, 550. https://doi.org/10.3390/ph18040550
Wilson LJ, Belko S, Gingold E, Wan S, Monane R, Pugliese R, Mourtada F. 3D-Printed Organ-Realistic Phantoms to Verify Quantitative SPECT/CT Accuracy for 177Lu-PSMA-617 Treatment Planning. Pharmaceuticals. 2025; 18(4):550. https://doi.org/10.3390/ph18040550
Chicago/Turabian StyleWilson, Lydia J., Sara Belko, Eric Gingold, Shuying Wan, Rachel Monane, Robert Pugliese, and Firas Mourtada. 2025. "3D-Printed Organ-Realistic Phantoms to Verify Quantitative SPECT/CT Accuracy for 177Lu-PSMA-617 Treatment Planning" Pharmaceuticals 18, no. 4: 550. https://doi.org/10.3390/ph18040550
APA StyleWilson, L. J., Belko, S., Gingold, E., Wan, S., Monane, R., Pugliese, R., & Mourtada, F. (2025). 3D-Printed Organ-Realistic Phantoms to Verify Quantitative SPECT/CT Accuracy for 177Lu-PSMA-617 Treatment Planning. Pharmaceuticals, 18(4), 550. https://doi.org/10.3390/ph18040550







