1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Alleviate TNBS-Induced Colitis and Exhibit No Significant Testicular Toxicity
Abstract
1. Introduction
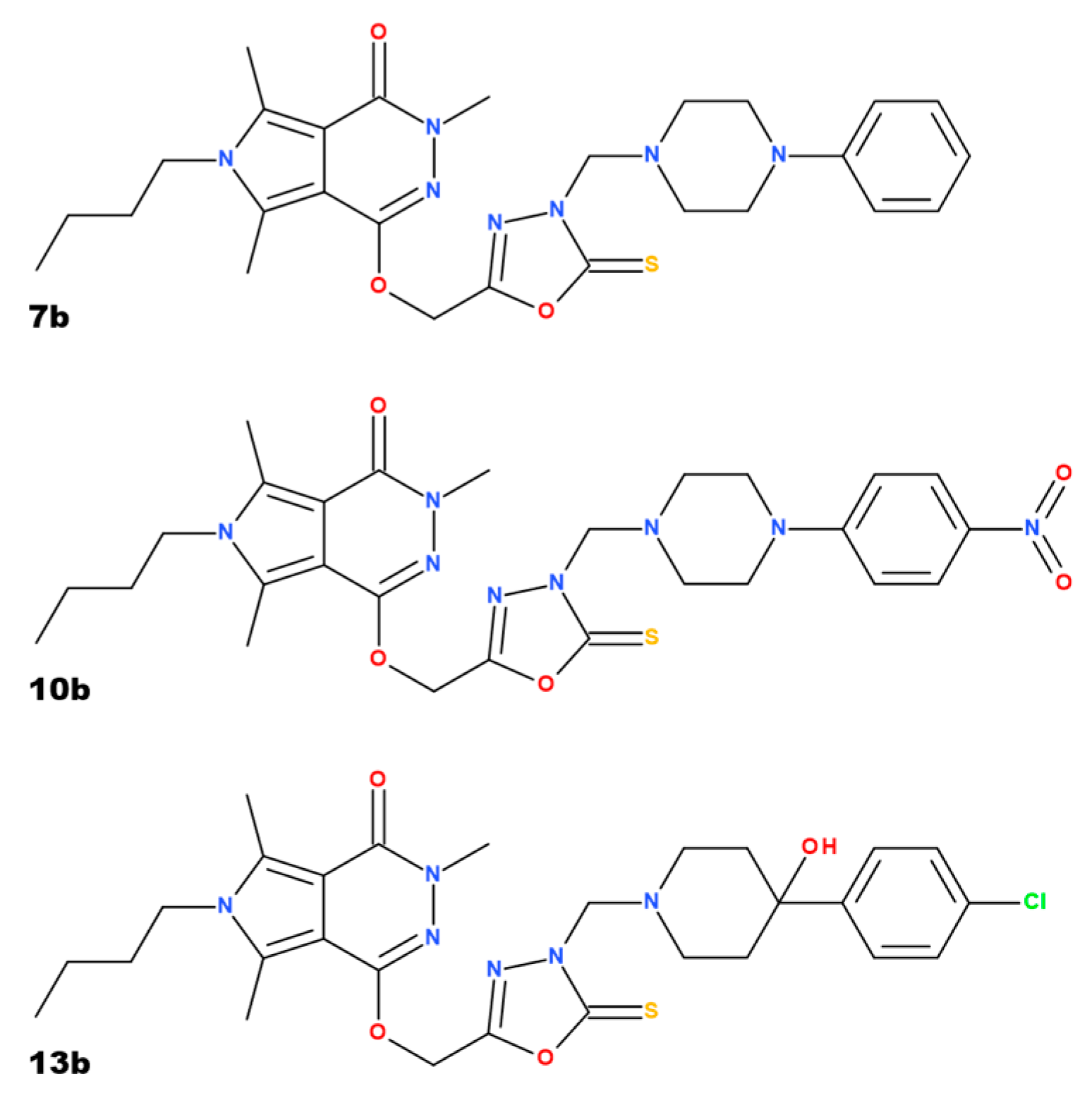
2. Results
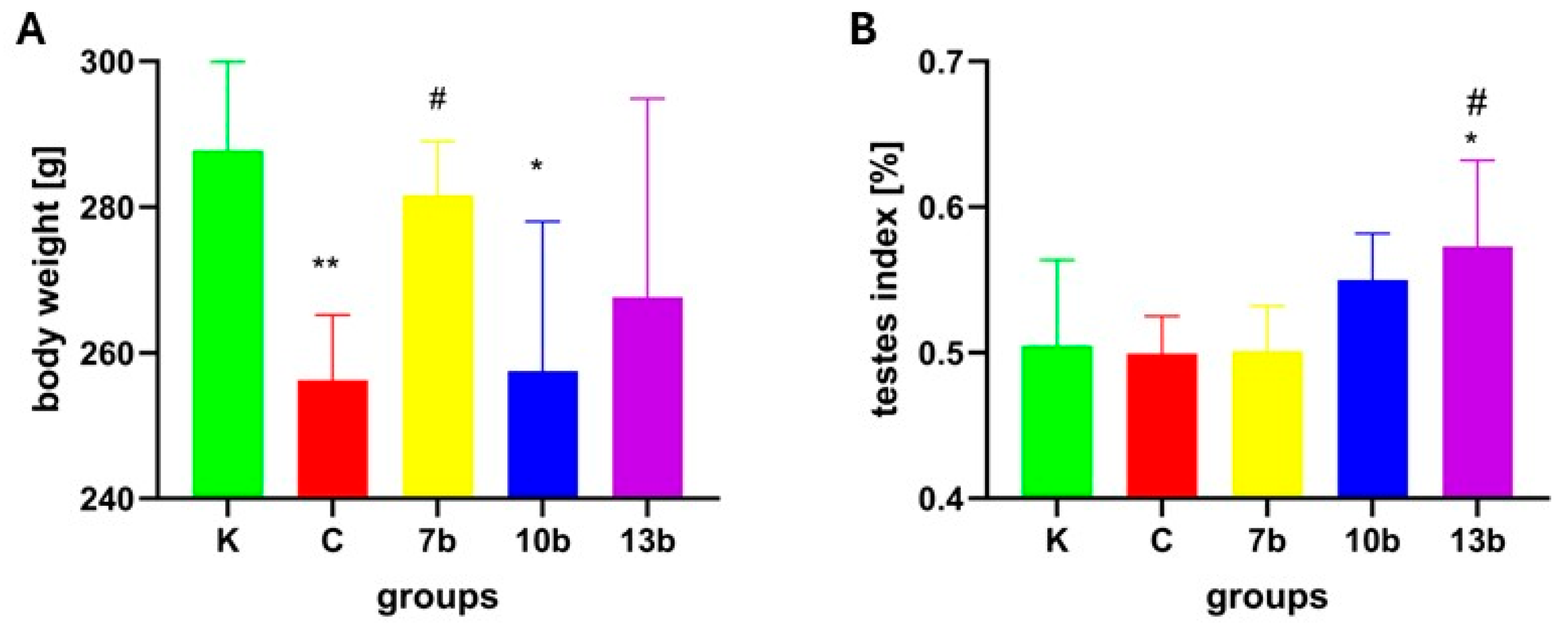
2.1. Body Weight and Testes Index
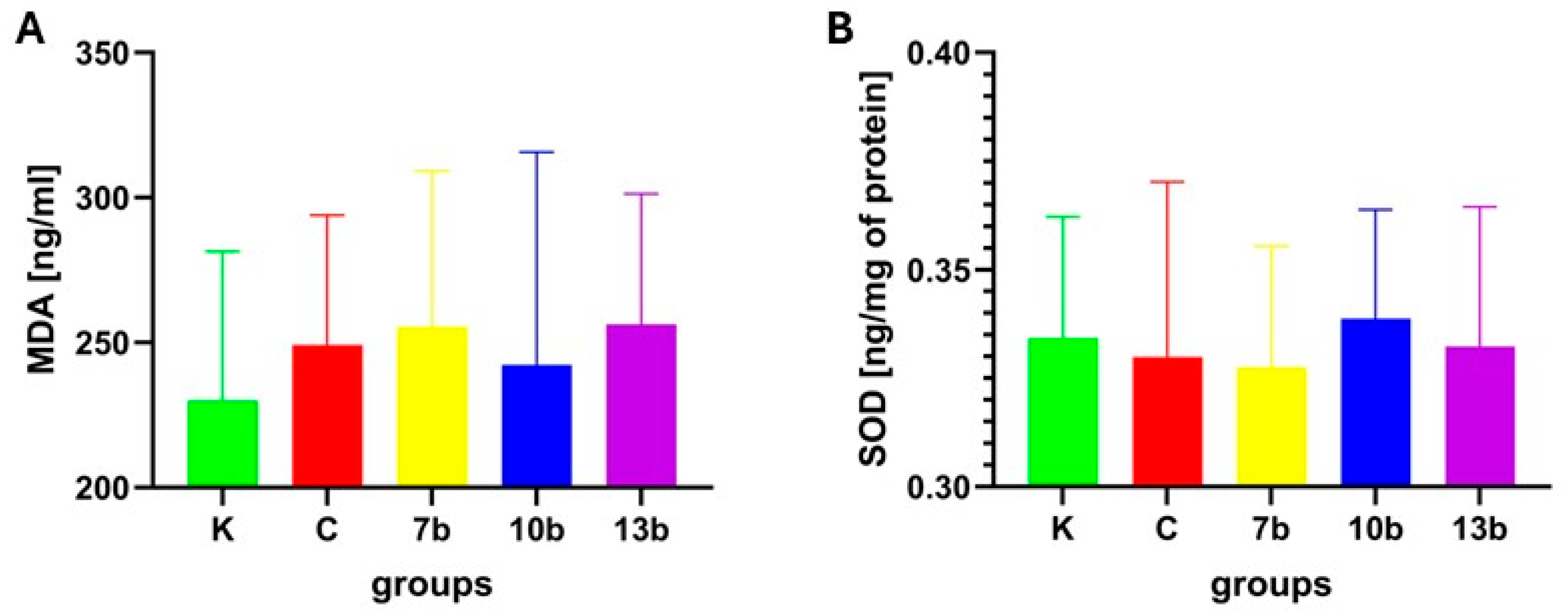
2.2. Oxidative Stress Parameters
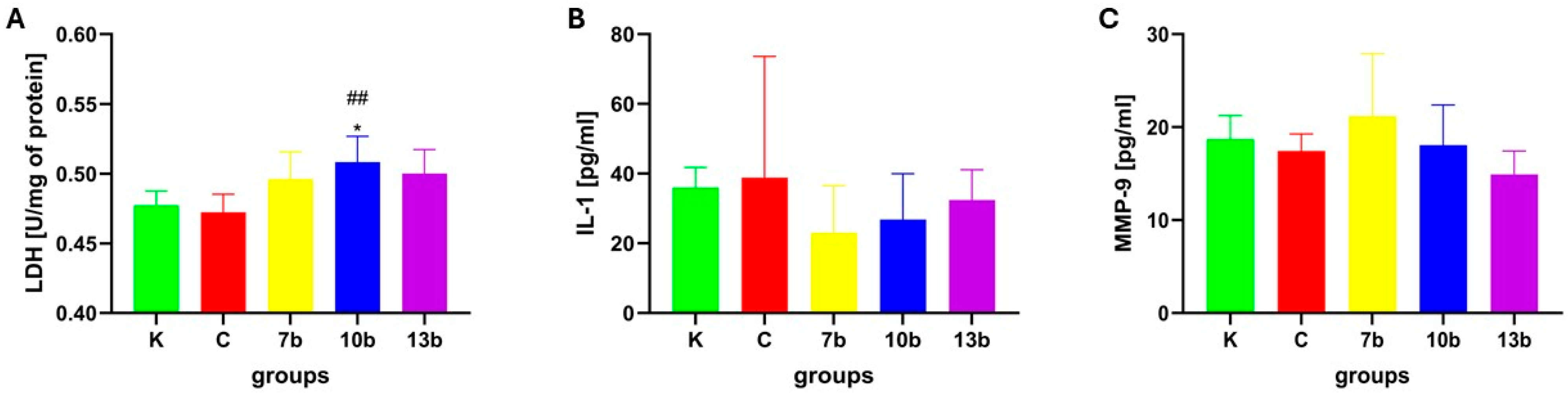
2.3. Tissue Injury and Inflammatory Parameters
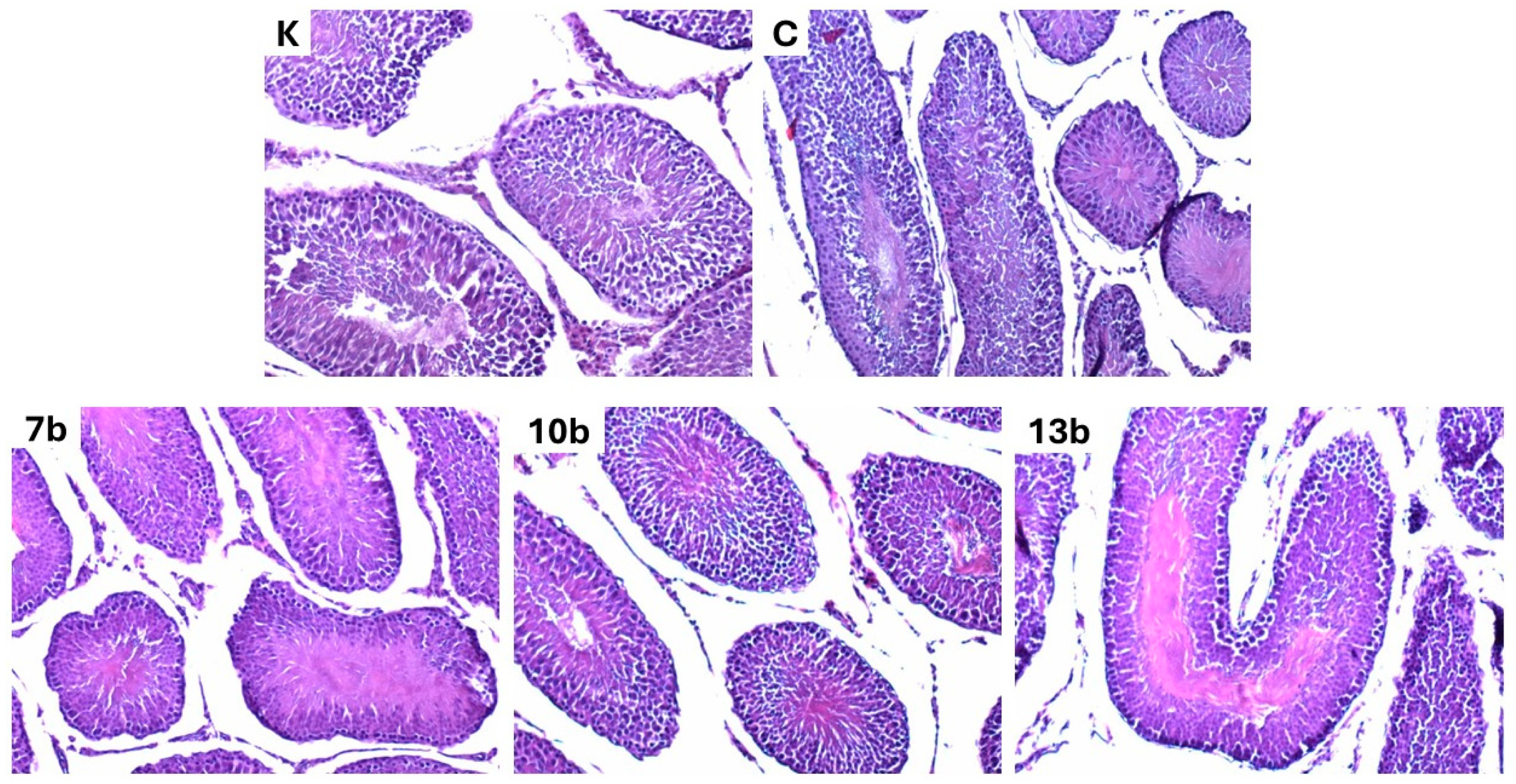
2.4. Histological Evaluation
3. Discussion
4. Materials and Methods
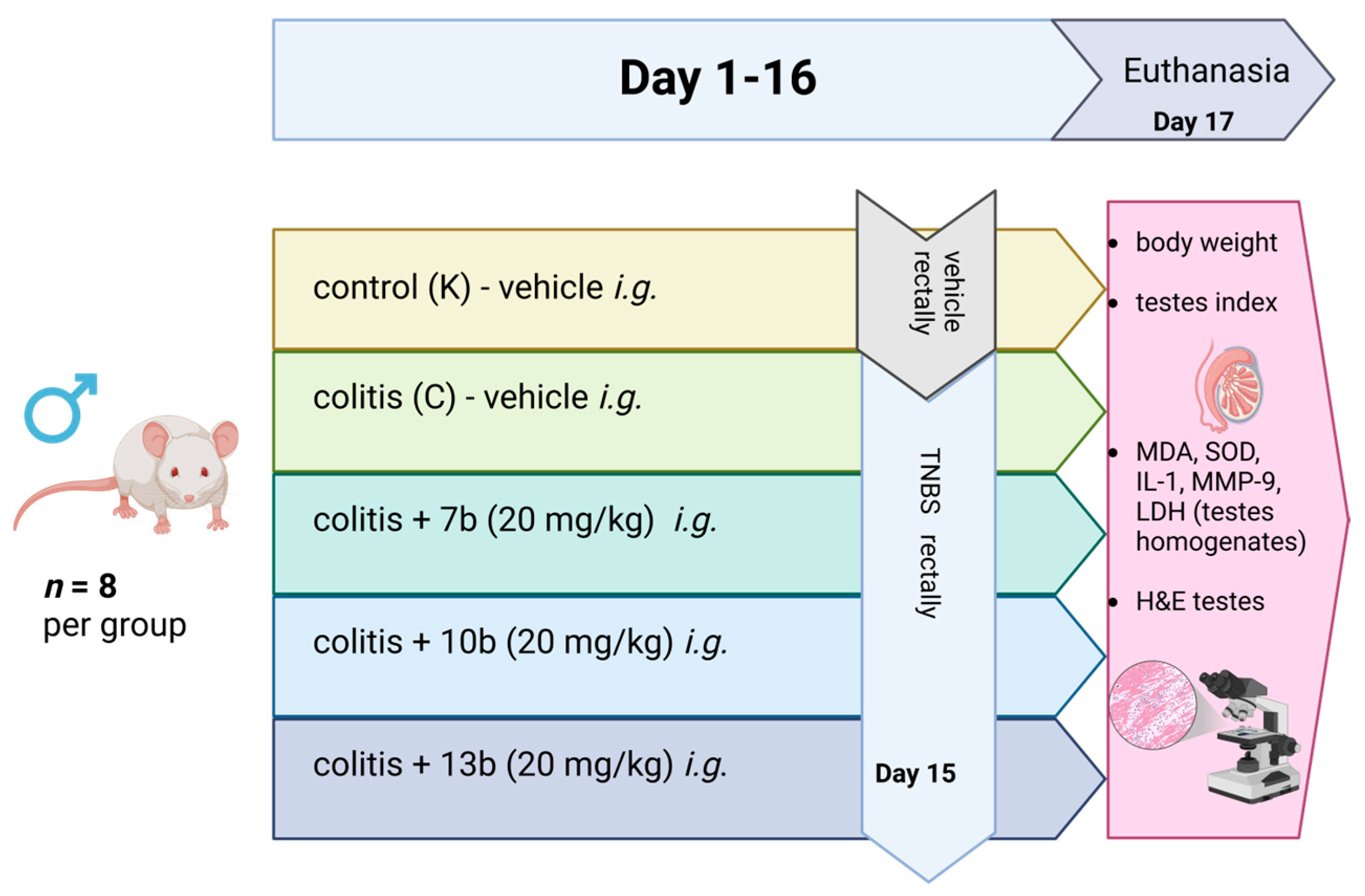
4.1. General Organization of the Study
4.2. Tissue Homogenates Evaluation
4.3. Histological Evaluation
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, A. Growing Share of Childless Adults in U.S. Don’t Expect to Ever Have Children; Pew Research Center: Washington, DC, USA, 2021. [Google Scholar]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Jenaro, C.; Rosset, C. Couple Therapy in Infertility. Papeles Psicol. 2008, 29, 205–212. [Google Scholar]
- Malik, J.; Choudhary, S.; Mandal, S.C.; Sarup, P.; Pahuja, S. Oxidative Stress and Male Infertility: Role of Herbal Drugs. Adv. Exp. Med. Biol. 2022, 1391, 137–159. [Google Scholar] [CrossRef]
- McLaren, J.F. Infertility Evaluation. Obstet. Gynecol. Clin. N. Am. 2012, 39, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Bablok, L.; Dziadecki, W.; Szymusik, I.; Wolczynski, S.; Kurzawa, R.; Pawelczyk, L.; Jedrzejczak, P.; Hanke, W.; Kaminski, P.; Wielgos, M. Patterns of Infertility in Poland—Multicenter Study. Neuro Endocrinol. Lett. 2011, 32, 799–804. [Google Scholar]
- Niepłodność Męska Częstsza niż Cukrzyca [Male Infertility More Common than Diabetes]. Available online: https://www.termedia.pl/ginekologia/Nieplodnosc-meska-czestsza-niz-cukrzyca,55901.html (accessed on 18 November 2024). (In Polish).
- Mann, U.; Shiff, B.; Patel, P. Reasons for Worldwide Decline in Male Fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef]
- Mima, M.; Greenwald, D.; Ohlander, S. Environmental Toxins and Male Fertility. Curr. Urol. Rep. 2018, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Witherspoon, L.; Dunne, C.; Flannigan, R. Global Decline of Male Fertility: Fact or Fiction? A Broad Summary of the Published Evidence on Sperm-Count and Fertility Trends. Br. Columbia Med. J. 2022, 64, 126–130. [Google Scholar]
- Nasser, M.; Haider, A.; Saad, F.; Kurtz, W.; Doros, G.; Fijak, M.; Vignozzi, L.; Gooren, L. Testosterone Therapy in Men with Crohn’s Disease Improves the Clinical Course of the Disease: Data from Long-Term Observational Registry Study. Horm. Mol. Biol. Clin. Investig. 2015, 22, 111–117. [Google Scholar] [CrossRef]
- Caron, B.; Honap, S.; Peyrin-Biroulet, L. Epidemiology of Inflammatory Bowel Disease across the Ages in the Era of Advanced Therapies. J. Crohn’s Colitis 2024, 18, ii3–ii15. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, B.; Bordiga, M.; Li, H.; Travaglia, F.; Bai, S.; Chen, J.; Bai, W. Cyanidin-3-O-Glucoside Supplement Improves Sperm Quality and Spermatogenesis in a Mice Model of Ulcerative Colitis. Nutrients 2022, 14, 984. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Jess, T. Implications of the Changing Epidemiology of Inflammatory Bowel Disease in a Changing World. United Eur. Gastroenterol. J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, T.O. Sexual Dysfunction and Fertility Problems in Men with Inflammatory Bowel Disease. World J. Mens. Health 2020, 38, 285–297. [Google Scholar] [CrossRef]
- Ma, S.; Veysey, M.; Ersser, S.; Mason-Jones, A.; Galdas, P. The Impact of Inflammatory Bowel Disease on Sexual Health in Men: A Scoping Review. J. Clin. Nurs. 2020, 29, 3638–3651. [Google Scholar] [CrossRef] [PubMed]
- Mountifield, R.; Bampton, P.; Prosser, R.; Muller, K.; Andrews, J.M. Fear and Fertility in Inflammatory Bowel Disease: A Mismatch of Perception and Reality Affects Family Planning Decisions. Inflamm. Bowel Dis. 2009, 15, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Kotkowicz-Szczur, M.; Szymańska, E.; Kisielewski, R.; Kierkuś, J. Sexual Functions in Individuals with Inflammatory Bowel Diseases. Gastroenterol. Rev. Przegląd Gastroenterol. 2023, 18, 56–60. [Google Scholar] [CrossRef]
- Wdowiak, A.; Gujski, M.; Bojar, I.; Raczkiewicz, D.; Bartosińska, J.; Wdowiak-Filip, A.; Filip, R. Chronic Inflammation Impairs Male Fertility-A Case-Control Study in Ulcerative Colitis Patients. J. Clin. Med. 2021, 10, 1460. [Google Scholar] [CrossRef]
- Shafiey, S.I.; Ahmed, K.A.; Abo-Saif, A.A.; Abo-Youssef, A.M.; Mohamed, W.R. Galantamine Mitigates Testicular Injury and Disturbed Spermatogenesis in Adjuvant Arthritic Rats via Modulating Apoptosis, Inflammatory Signals, and IL-6/JAK/STAT3/SOCS3 Signaling. Inflammopharmacology 2024, 32, 405–418. [Google Scholar] [CrossRef]
- Soares, P.M.F.; Borba, E.F.; Bonfa, E.; Hallak, J.; Corrêa, A.L.; Silva, C.A.A. Gonad Evaluation in Male Systemic Lupus Erythematosus. Arthritis Rheum. 2007, 56, 2352–2361. [Google Scholar] [CrossRef]
- Salah, M.; Ismail, K.A.; Khadrawy, S.M. Nobiletin Protects against Diabetes-Induced Testicular Injury via Hypophysis-Gonadal Axis Upregulation and Amelioration of Oxidative Stress. Mol. Biol. Rep. 2022, 49, 189–203. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Yue, X.; Yin, R.; Wu, L.; Li, J.; Wang, J.; Yan, B.; Dai, L.; Shen, C.; Zhi, Y.; Wan, L.; et al. Diminazene Aceturate, an ACE2 Activator, Ameliorates Testicular Injury in Diet-Induced Obese Mice. Reproduction 2025, 169, e240242. [Google Scholar] [CrossRef]
- Akin, A.T.; Kaymak, E.; Ceylan, T.; Ozturk, E.; Basaran, K.E.; Karabulut, D.; Ozdamar, S.; Yakan, B. Chloroquine Attenuates Chronic Hypoxia-Induced Testicular Damage via Suppressing Endoplasmic Reticulum Stress and Apoptosis in Experimental Rat Model. Clin. Exp. Pharmacol. Physiol. 2022, 49, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Chartres, N.; Cooper, C.B.; Bland, G.; Pelch, K.E.; Gandhi, S.A.; BakenRa, A.; Woodruff, T.J. Effects of Microplastic Exposure on Human Digestive, Reproductive, and Respiratory Health: A Rapid Systematic Review. Environ. Sci. Technol. 2024, 58, 22843–22864. [Google Scholar] [CrossRef]
- Hau, R.K.; Wright, S.H.; Cherrington, N.J. Drug Transporters at the Human Blood-Testis Barrier. Drug Metab. Dispos. 2023, 51, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Cheng, C.Y. The Blood-Testis Barrier: Its Biology, Regulation, and Physiological Role in Spermatogenesis. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2005; Volume 71, pp. 263–296. [Google Scholar]
- Huang, M.; Wang, C.; Yao, Y.; Li, H.; Yao, Y.; Zhu, Y.; Cui, Y.; Yuan, Y.; Sha, J. Mebendazole-Induced Blood-Testis Barrier Injury in Mice Testes by Disrupting Microtubules in Addition to Triggering Programmed Cell Death. Int. J. Mol. Sci. 2022, 23, 4220. [Google Scholar] [CrossRef]
- Zhou, J.; Xi, Y.; Zhang, J.; Tang, J.; Zhou, X.; Chen, J.; Nie, C.; Zhu, Z.; Ma, B. Protective Effect of Dioscorea Zingiberensis Ethanol Extract on the Disruption of Blood-Testes Barrier in High-Fat Diet/Streptozotocin-Induced Diabetic Mice by Upregulating ZO-1 and Nrf2. Andrologia 2020, 52, e13508. [Google Scholar] [CrossRef]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The Impact of Drugs on Male Fertility: A Review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. The Physiology of Male Reproduction: Impact of Drugs and Their Abuse on Male Fertility. Andrologia 2020, 52, e13672. [Google Scholar] [CrossRef]
- Banerjee, A.; Scarpa, M.; Pathak, S.; Burra, P.; Sturniolo, G.C.; Russo, F.P.; Murugesan, R.; D’Incá, R. Inflammatory Bowel Disease Therapies Adversely Affect Fertility in Men- A Systematic Review and Meta-Analysis. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Methotrexate 2.5 Mg Tablets—Summary of Product Characteristics (SmPC)—(Emc). Available online: https://www.medicines.org.uk/emc/product/511/smpc#gref (accessed on 20 November 2024).
- Riley, S.A.; Lecarpentier, J.; Mani, V.; Goodman, M.J.; Mandal, B.K.; Turnberg, L.A. Sulphasalazine Induced Seminal Abnormalities in Ulcerative Colitis: Results of Mesalazine Substitution. Gut 1987, 28, 1008–1012. [Google Scholar] [CrossRef]
- Ommati, M.M.; Heidari, R.; Jamshidzadeh, A.; Zamiri, M.J.; Sun, Z.; Sabouri, S.; Wang, J.; Ahmadi, F.; Javanmard, N.; Seifi, K.; et al. Dual Effects of Sulfasalazine on Rat Sperm Characteristics, Spermatogenesis, and Steroidogenesis in Two Experimental Models. Toxicol. Lett. 2018, 284, 46–55. [Google Scholar] [CrossRef]
- Osawe, S.O.; Farombi, E.O. Quercetin and Rutin Ameliorates Sulphasalazine-Induced Spermiotoxicity, Alterations in Reproductive Hormones and Steroidogenic Enzyme Imbalance in Rats. Andrologia 2018, 50, e12981. [Google Scholar] [CrossRef]
- Finelli, R.; Leisegang, K.; Finocchi, F.; De Masi, S.; Agarwal, A.; Damiani, G. The Impact of Autoimmune Systemic Inflammation and Associated Medications on Male Reproductive Health in Patients with Chronic Rheumatological, Dermatological, and Gastroenterological Diseases: A Systematic Review. Am. J. Reprod. Immunol. 2021, 85, e13389. [Google Scholar] [CrossRef] [PubMed]
- Chermesh, I.; Eliakim, R. Mesalazine-Induced Reversible Infertility in a Young Male. Dig. Liver Dis. 2004, 36, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, M.C.; Paoluzi, O.A.; Pica, R.; Iacopini, F.; Crispino, P.; Rivera, M.; Spera, G.; Paoluzi, P. Sulphasalazine and 5-Aminosalicylic Acid in Long-Term Treatment of Ulcerative Colitis: Report on Tolerance and Side-Effects. Dig. Liver Dis. 2001, 33, 563–569. [Google Scholar] [CrossRef]
- Habib, R.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Infliximab Abrogates Cadmium-Induced Testicular Damage and Spermiotoxicity via Enhancement of Steroidogenesis and Suppression of Inflammation and Apoptosis Mediators. Ecotoxicol. Environ. Saf. 2019, 182, 109398. [Google Scholar] [CrossRef]
- Suominen, J.; Wang, Y.; Kaipia, A.; Toppari, J. Tumor Necrosis Factor-Alpha (TNF-Alpha) Promotes Cell Survival during Spermatogenesis, and This Effect Can Be Blocked by Infliximab, a TNF-Alpha Antagonist. Eur. J. Endocrinol. 2004, 151, 629–640. [Google Scholar] [CrossRef]
- Honap, S.; Agorogianni, A.; Colwill, M.J.; Mehta, S.K.; Donovan, F.; Pollok, R.; Poullis, A.; Patel, K. JAK Inhibitors for Inflammatory Bowel Disease: Recent Advances. Frontline Gastroenterol. 2024, 15, 59–69. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Merwid-Ląd, A.; Wiatrak, B.; Danielewski, M.; Dzimira, S.; Szkudlarek, D.; Szczukowski, Ł.; Świątek, P.; Szeląg, A. Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo [3,4-d]Pyridazinone Exert Anti-Inflammatory Activity without Acute Gastrotoxicity in the Carrageenan-Induced Rat Paw Edema Test. J. Inflamm. Res. 2021, 14, 5739–5756. [Google Scholar] [CrossRef] [PubMed]
- Szandruk-Bender, M.; Wiatrak, B.; Szczukowski, Ł.; Świątek, P.; Rutkowska, M.; Dzimira, S.; Merwid-Ląd, A.; Danielewski, M.; Szeląg, A. Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo [3,4-d]Pyridazinone Exert Antinociceptive Activity in the Tail-Flick and Formalin Test in Rodents and Reveal Reduced Gastrotoxicity. Int. J. Mol. Sci. 2020, 21, E9685. [Google Scholar] [CrossRef] [PubMed]
- Szandruk-Bender, M.; Wiatrak, B.; Dzimira, S.; Merwid-Ląd, A.; Szczukowski, Ł.; Świątek, P.; Szeląg, A. Targeting Lineage-Specific Transcription Factors and Cytokines of the Th17/Treg Axis by Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo [3,4-d]Pyridazinone Attenuates TNBS-Induced Experimental Colitis. Int. J. Mol. Sci. 2022, 23, 9897. [Google Scholar] [CrossRef]
- Szczukowski, Ł.; Redzicka, A.; Wiatrak, B.; Krzyżak, E.; Marciniak, A.; Gębczak, K.; Gębarowski, T.; Świątek, P. Design, Synthesis, Biological Evaluation and in Silico Studies of Novel Pyrrolo [3,4-d]Pyridazinone Derivatives with Promising Anti-Inflammatory and Antioxidant Activity. Bioorg Chem. 2020, 102, 104035. [Google Scholar] [CrossRef]
- Langenbach, R.; Loftin, C.D.; Lee, C.; Tiano, H. Cyclooxygenase-Deficient Mice. A Summary of Their Characteristics and Susceptibilities to Inflammation and Carcinogenesis. Ann. N. Y. Acad. Sci. 1999, 889, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Mayerhofer, A.; Matzkin, M.E. Cyclooxygenase and Prostaglandins in Somatic Cell Populations of the Testis. Reproduction 2015, 149, R169–R180. [Google Scholar] [CrossRef]
- Tran-Guzman, A.; Khan, A.; Culty, M. Differential Roles of Cyclooxygenase Enzymes in the Regulation of Murine Juvenile Undifferentiated Spermatogonia. Andrology 2024, 12, 899–917. [Google Scholar] [CrossRef]
- Neeraja, S.; Sreenath, A.; Reddy, P.; Reddanna, P. Expression of Cyclooxygenase-2 in Rat Testis. Reprod. BioMedicine Online 2003, 6, 302–309. [Google Scholar] [CrossRef]
- Parillo, F.; Catone, G.; Boiti, C.; Zerani, M. Immunopresence and Enzymatic Activity of Nitric Oxide Synthases, Cyclooxygenases and PGE2-9-Ketoreductase and in Vitro Production of PGF2α, PGE2 and Testosterone in the Testis of Adult and Prepubertal Alpaca (Lama pacos). Gen. Comp. Endocrinol. 2011, 171, 381–388. [Google Scholar] [CrossRef]
- Mousa, A.A.; Elweza, A.E.; Elbaz, H.T.; Tahoun, E.A.E.-A.; Shoghy, K.M.; Elsayed, I.; Hassan, E.B. Eucalyptus Globulus Protects against Diclofenac Sodium Induced Hepatorenal and Testicular Toxicity in Male Rats. J. Tradit. Complement. Med. 2020, 10, 521–528. [Google Scholar] [CrossRef]
- Owumi, S.E.; Aliyu-Banjo, N.O.; Odunola, O.A. Selenium Attenuates Diclofenac-Induced Testicular and Epididymal Toxicity in Rats. Andrologia 2020, 52, e13669. [Google Scholar] [CrossRef]
- Tella, T.; Adegbegi, A.; Emeninwa, C.; Odola, A.; Ayangbenro, A.; Adaramoye, O. Evaluation of the Antioxidative Potential of Diisopropyldithiocarbamates Sodium Salt on Diclofenac-Induced Toxicity in Male Albino Rats. Toxicol. Rep. 2022, 9, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Purohit, A.; Ram, H. Assessment of Dose-Dependent Reproductive Toxicity of Diclofenac Sodium in Male Rats. Drug Chem. Toxicol. 2019, 42, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Waly, H.; Abd-Elkareem, M.; Raheem, S.A.; Khalil, N.S.A. Berberine Protects against Diclofenac Sodium-Induced Testicular Impairment in Mice by Its Anti-Oxidant and Anti-Apoptotic Activities. Iran. J. Basic Med. Sci. 2022, 25, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Uzun, B.; Atli, O.; Perk, B.O.; Burukoglu, D.; Ilgin, S. Evaluation of the Reproductive Toxicity of Naproxen Sodium and Meloxicam in Male Rats. Hum. Exp. Toxicol. 2015, 34, 415–429. [Google Scholar] [CrossRef]
- Ugochukwu, A.P.; Ebere, O.O.; Okwuoma, A. Effects of Nimesulide on Testicular Functions in Prepubertal Albino Rats. J. Basic. Clin. Physiol. Pharmacol. 2011, 22, 137–140. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Missiry, M.A.; Amer, M.A. The Protective Action of Melatonin on Indomethacin-Induced Gastric and Testicular Oxidative Stress in Rats. Redox Report. 2001, 6, 173–177. [Google Scholar] [CrossRef]
- Ghosh, R.; Prosad Banik, S. Protective Effect of Indomethacin on Vanadium-Induced Adrenocortical and Testicular Damages in Rat. Toxicol. Mech. Methods 2022, 32, 114–122. [Google Scholar] [CrossRef]
- Winnall, W.R.; Muir, J.A.; Liew, S.; Hirst, J.J.; Meachem, S.J.; Hedger, M.P. Effects of Chronic Celecoxib on Testicular Function in Normal and Lipopolysaccharide-Treated Rats. Int. J. Androl. 2009, 32, 542–555. [Google Scholar] [CrossRef]
- Kale, O.E.; Oyesola, T.O.; Raji, F.S. Celecoxib, a Cyclooxygenase-2 Inhibitor, Offers Chemoprevention against Reproductive and Neurobehavioural Abnormalities Induced by Atrazine in Male Wistar Rats. Environ. Toxicol. Pharmacol. 2018, 58, 84–97. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Hass, U.; Lesné, L.; Lottrup, G.; Jacobsen, P.R.; Desdoits-Lethimonier, C.; Boberg, J.; Petersen, J.H.; Toppari, J.; Jensen, T.K.; et al. Intrauterine Exposure to Mild Analgesics Is a Risk Factor for Development of Male Reproductive Disorders in Human and Rat. Hum. Reprod. 2011, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Altindağ, F.; Rağbetli, M.Ç. The Effect of Maternal Treatment with Diclofenac Sodium and Thymoquinone on Testicular Parameters in Rat Offspring. Rev. Int. Androl. 2021, 19, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Azmy, A.M.; Abd Elbaki, B.T.; Ali, M.A.; Mahmoud, A.A. Effect of Ozone versus Naringin on Testicular Injury in Experimentally Induced Ulcerative Colitis in Adult Male Albino Rats. Ultrastruct. Pathol. 2022, 46, 439–461. [Google Scholar] [CrossRef]
- Farombi, E.; Adedara, I.; Ajayi, B.; Idowu, T.; Eriomala, O.; Akinbote, F. 6-Gingerol Improves Testicular Function in Mice Model of Chronic Ulcerative Colitis. Hum. Exp. Toxicol. 2018, 37, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative Stress and Male Infertility: Current Knowledge of Pathophysiology and Role of Antioxidant Therapy in Disease Management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Elsherbini, D.M.A.; Ebrahim, H.A. Effect of Meloxicam (Cyclooygenase-2 Inhibitor) versus Vitamin D3 (Cholecalciferol) as Ameliorating Agents of Progressive Doxorubicin-Induced Nephrotoxicity in Rats. Anat. Cell Biol. 2020, 53, 169–182. [Google Scholar] [CrossRef]
- Sun, L. Low-Dose Cyclooxygenase-2 (COX-2) Inhibitor Celecoxib Plays a Protective Role in the Rat Model of Neonatal Necrotizing Enterocolitis. Bioengineered 2021, 12, 7234–7245. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- El-Demerdash, F.M.; Ahmed, M.M.; Baghdadi, H.H. Punica Granatum Peel Extract Modulates Levofloxacin-Induced Oxidative Stress and Testicular Damage in Rats. Tissue Cell 2023, 85, 102227. [Google Scholar] [CrossRef]
- Goldberg, E.; Eddy, E.M.; Duan, C.; Odet, F. LDHC: The Ultimate Testis-Specific Gene. J. Androl. 2010, 31, 86–94. [Google Scholar] [CrossRef]
- Shetty, P.; Dsouza, R.; Kumar, B.V. Matrix Metalloproteinase-9 as a Predictor of Healing in Diabetic Foot Ulcers. Cureus 2024, 16, e75521. [Google Scholar] [CrossRef]
- Wątroba, S.; Wiśniowski, T.; Bryda, J.; Kurzepa, J. The Role of Matrix Metalloproteinases in Pathogenesis of Human Bladder Cancer. Acta Biochim. Pol. 2021, 68, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.Y.; Lee, W.M.; Cheng, C.Y. The Interplay of Collagen IV, Tumor Necrosis Factor-Alpha, Gelatinase B (Matrix Metalloprotease-9), and Tissue Inhibitor of Metalloproteases-1 in the Basal Lamina Regulates Sertoli Cell-Tight Junction Dynamics in the Rat Testis. Endocrinology 2003, 144, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Qian, Y.; Liu, Z.; Wang, C.; Qu, J.; Wang, X.; Wang, S. Perfluorooctane Sulfonate (PFOS) Disrupts Blood-Testis Barrier by down-Regulating Junction Proteins via P38 MAPK/ATF2/MMP9 Signaling Pathway. Toxicology 2016, 373, 1–12. [Google Scholar] [CrossRef]
- Warinrak, C.; Wu, J.-T.; Hsu, W.-L.; Liao, J.-W.; Chang, S.-C.; Cheng, F.-P. Expression of Matrix Metalloproteinases (MMP-2, MMP-9) and Their Inhibitors (TIMP-1, TIMP-2) in Canine Testis, Epididymis and Semen. Reprod. Domest. Anim. 2015, 50, 48–57. [Google Scholar] [CrossRef]
- Belardin, L.B.; Antoniassi, M.P.; Camargo, M.; Intasqui, P.; Fraietta, R.; Bertolla, R.P. Semen Levels of Matrix Metalloproteinase (MMP) and Tissue Inhibitor of Metalloproteinases (TIMP) Protein Families Members in Men with High and Low Sperm DNA Fragmentation. Sci. Rep. 2019, 9, 903. [Google Scholar] [CrossRef]
- Robinson, L.L.L.; Sznajder, N.A.; Riley, S.C.; Anderson, R.A. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Human Fetal Testis and Ovary. Mol. Hum. Reprod. 2001, 7, 641–648. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; van der Meer, J.W.M. Treating Inflammation by Blocking Interleukin-1 in a Broad Spectrum of Diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Mai, W.; Shang, Y.; Wang, Y.; Chen, Y.; Mu, B.; Zheng, Q.; Liu, H. 1-DNJ Alleviates Obesity-Induced Testicular Inflammation in Mice Model by Inhibiting IKKβ/ NF-kB Pathway. Reprod. Sci. 2024, 31, 2103–2113. [Google Scholar] [CrossRef]
- Vakili-Sadeghi, E.; Najafpour, A.; Mohammadi, R. Protective Effects of Propolis on Ischemia-Reperfusion Injury in a Rat Testicular Torsion and Detorsion Model. Vet. Res. Forum 2023, 14, 389–395. [Google Scholar] [CrossRef]
- Mersal, E.A.; Morsi, A.A.; Alkahtani, J.; Alhalal, R.; Alessa, S.; Shehab, A.; Sakr, E.M.; Sabir, D.K.; Dawood, A.F.; Abdelmoneim, A.M. Pirfenidone Targeted Mechanisms for Alleviating Methotrexate-Induced Testiculopathy in Wistar Rats. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 2003–2014. [Google Scholar] [CrossRef]
- Ishikawa, T.; Morris, P.L. Interleukin-1beta Signals through a c-Jun N-Terminal Kinase-Dependent Inducible Nitric Oxide Synthase and Nitric Oxide Production Pathway in Sertoli Epithelial Cells. Endocrinology 2006, 147, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Walch, L.; Morris, P.L. Cyclooxygenase 2 Pathway Mediates IL-1beta Regulation of IL-1alpha, -1beta, and IL-6 mRNA Levels in Leydig Cell Progenitors. Endocrinology 2002, 143, 3276–3283. [Google Scholar] [CrossRef]
- Selmanoğlu, G.; Koçkaya, E.A.; Akay, M.T.; Kismet, K. Subacute Toxicity of Celecoxib on Thyroid and Testis of Rats: Hormonal and Histopathological Changes. Environ. Toxicol. Pharmacol. 2006, 22, 85–89. [Google Scholar] [CrossRef]
- Rizzoto, G.; Hall, C.; Tyberg, J.V.; Thundathil, J.C.; Caulkett, N.A.; Kastelic, J.P. Increased Testicular Blood Flow Maintains Oxygen Delivery and Avoids Testicular Hypoxia in Response to Reduced Oxygen Content in Inspired Air. Sci. Rep. 2018, 8, 10905. [Google Scholar] [CrossRef]
- Palkar, M.B.; Singhai, A.S.; Ronad, P.M.; Vishwanathswamy, A.H.M.; Boreddy, T.S.; Veerapur, V.P.; Shaikh, M.S.; Rane, R.A.; Karpoormath, R. Synthesis, Pharmacological Screening and in Silico Studies of New Class of Diclofenac Analogues as a Promising Anti-Inflammatory Agents. Bioorg Med. Chem. 2014, 22, 2855–2866. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, K.; Poojary, B.; Lobo, P.L.; Fernandes, J.; Kumari, N.S. Synthesis and Biological Evaluation of Some 1,3,4-Oxadiazole Derivatives. Eur. J. Med. Chem. 2010, 45, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.A.; Nour, M.S.; Salem, M.A.; Arafa, R.K. New Oxadiazoles with Selective-COX-2 and EGFR Dual Inhibitory Activity: Design, Synthesis, Cytotoxicity Evaluation and in Silico Studies. Eur. J. Med. Chem. 2019, 183, 111693. [Google Scholar] [CrossRef]
- Peregrym, K.; Szczukowski, Ł.; Wiatrak, B.; Potyrak, K.; Czyżnikowska, Ż.; Świątek, P. In Vitro and In Silico Evaluation of New 1,3,4-Oxadiazole Derivatives of Pyrrolo [3,4-d]Pyridazinone as Promising Cyclooxygenase Inhibitors. Int. J. Mol. Sci. 2021, 22, 9130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merwid-Ląd, A.; Ziółkowski, P.; Nowak, B.; Świątek, P.; Szczukowski, Ł.; Kwiatkowska, J.; Piasecka, K.; Szeląg, A.; Szandruk-Bender, M. 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Alleviate TNBS-Induced Colitis and Exhibit No Significant Testicular Toxicity. Pharmaceuticals 2025, 18, 546. https://doi.org/10.3390/ph18040546
Merwid-Ląd A, Ziółkowski P, Nowak B, Świątek P, Szczukowski Ł, Kwiatkowska J, Piasecka K, Szeląg A, Szandruk-Bender M. 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Alleviate TNBS-Induced Colitis and Exhibit No Significant Testicular Toxicity. Pharmaceuticals. 2025; 18(4):546. https://doi.org/10.3390/ph18040546
Chicago/Turabian StyleMerwid-Ląd, Anna, Piotr Ziółkowski, Beata Nowak, Piotr Świątek, Łukasz Szczukowski, Joanna Kwiatkowska, Katarzyna Piasecka, Adam Szeląg, and Marta Szandruk-Bender. 2025. "1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Alleviate TNBS-Induced Colitis and Exhibit No Significant Testicular Toxicity" Pharmaceuticals 18, no. 4: 546. https://doi.org/10.3390/ph18040546
APA StyleMerwid-Ląd, A., Ziółkowski, P., Nowak, B., Świątek, P., Szczukowski, Ł., Kwiatkowska, J., Piasecka, K., Szeląg, A., & Szandruk-Bender, M. (2025). 1,3,4-Oxadiazole Derivatives of Pyrrolo[3,4-d]pyridazinone Alleviate TNBS-Induced Colitis and Exhibit No Significant Testicular Toxicity. Pharmaceuticals, 18(4), 546. https://doi.org/10.3390/ph18040546









