Neuroprotective Effects of Peanut Skin Extract Against Oxidative Injury in HT-22 Neuronal Cells
Abstract
1. Introduction
2. Results
2.1. Peanut Skin Extract (PSE) Showed Antioxidant Activity in Chemical-Based Systems
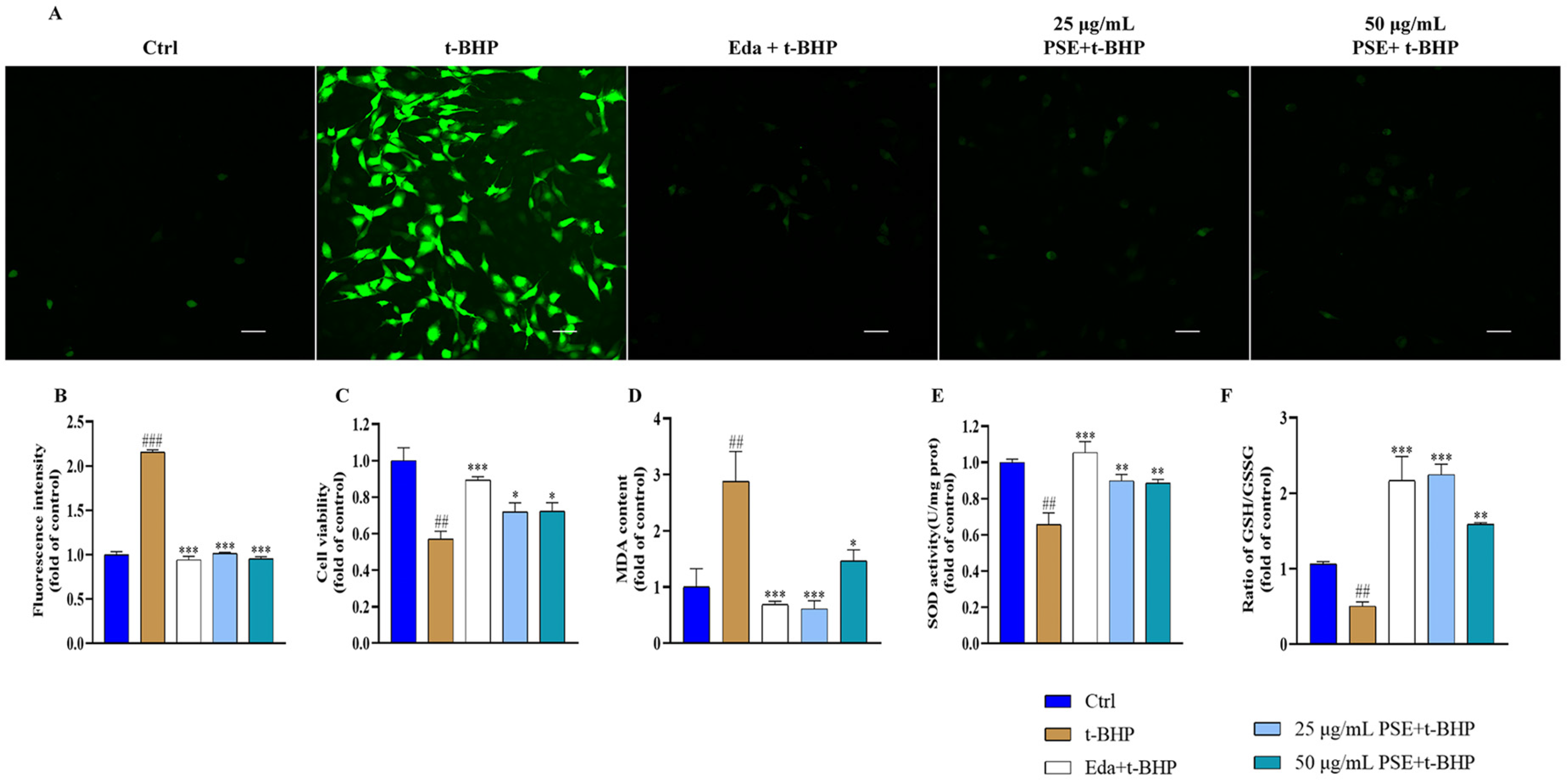
2.2. PSE Prevented Tert-Butyl Hydroperoxide (t-BHP)-Induced Oxidative Stress in HT-22 Cells
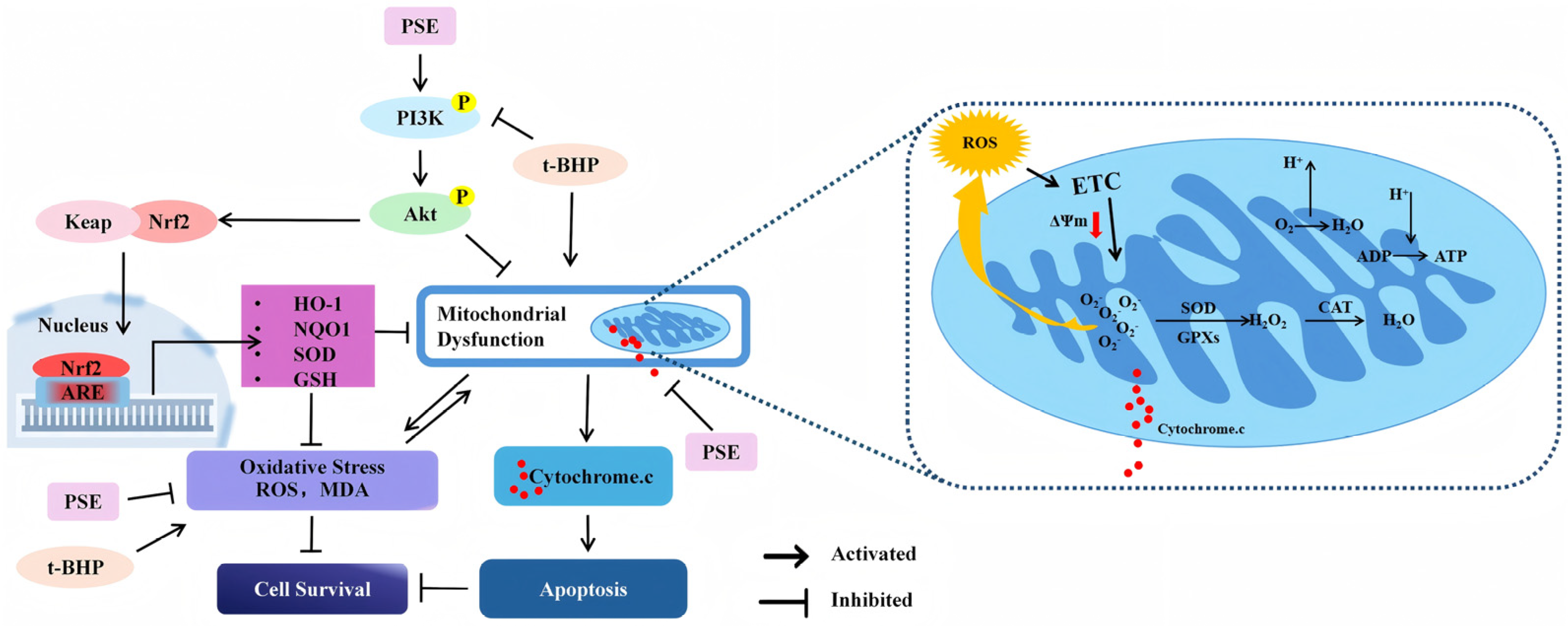
2.3. PSE Attenuated t-BHP-Induced Mitochondrial Dysfunction and Apoptosis in HT-22 Cells
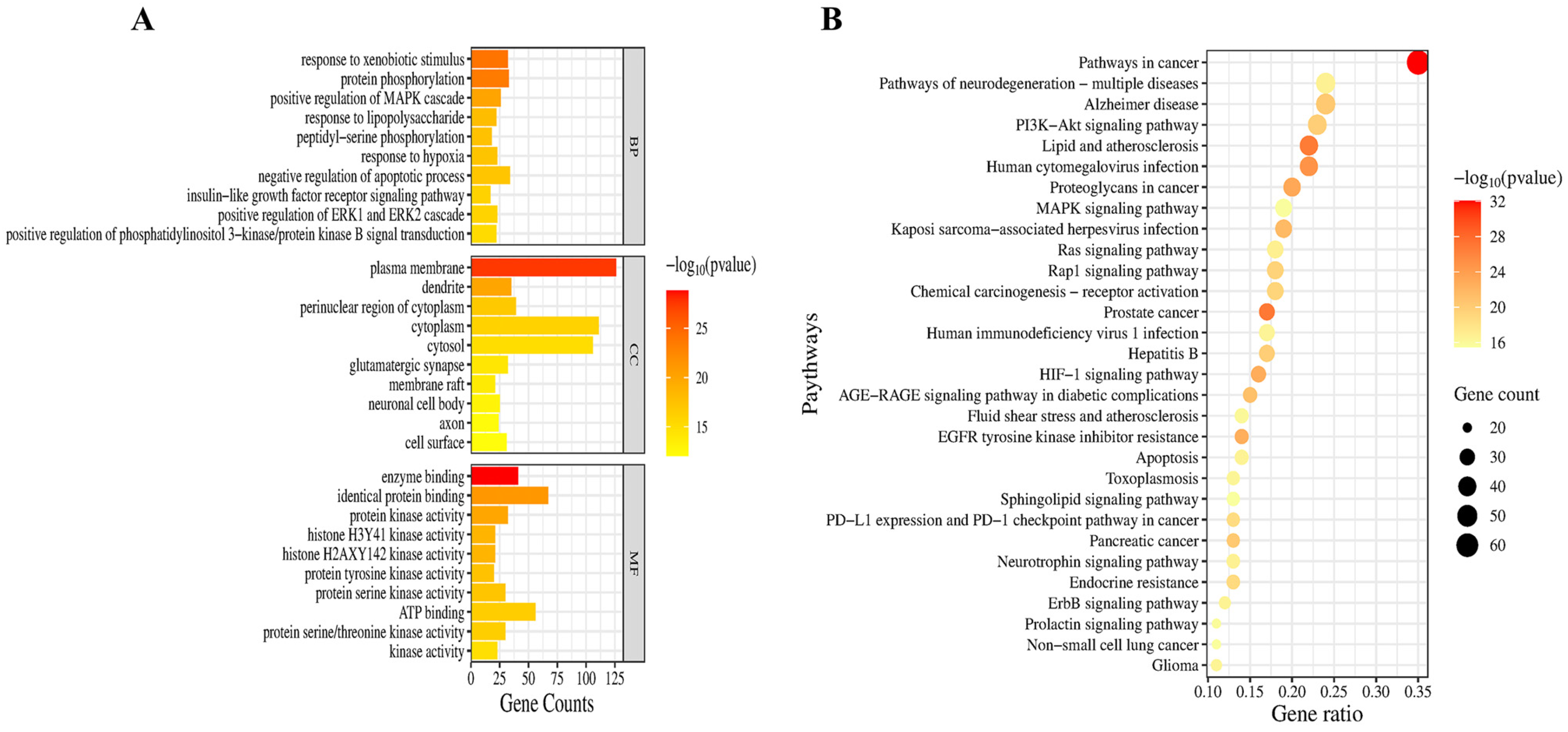
2.4. Network Pharmacological Analysis of the Neuroprotective Mechanisms and Targets of PSE
2.5. The PSE Upregulated PI3K/Akt/Nrf2 Pathway in HT-22 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of PSE
4.3. TPC, TFC, and TPAC Content Analysis
4.4. Chemical-Based Antioxidant Activity Assays
4.5. Cell Culture and Treatment
4.6. Cell Viability Assay
4.7. Detection of Cell Apoptosis
4.8. Detection of Intracellular ROS Levels
4.9. Mitochondrial Membrane Potential (ΔΨm) Assay
4.10. Measurement of SOD Activity and Levels of MDA, GSSG, and GSH
4.11. Identification of the Potential Neuroprotective Targets of Phenolic Compounds in Peanut Skins
4.12. Construction of the PPI Network
4.13. GO and KEGG Enrichment Analysis
4.14. Western Blot Analysis
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liu, T.; Li, X.; Zhou, X.; Chen, W.; Wen, A.; Liu, M.; Ding, Y. PI3K/AKT signaling and neuroprotection in ischemic stroke: Molecular mechanisms and therapeutic perspectives. Neural Regen. Res. 2025, 20, 2758–2775. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [PubMed]
- Cao, D.F.; Zhou, X.Y.; Guo, Q.; Xiang, M.Y.; Bao, M.H.; He, B.S.; Mao, X.Y. Unveiling the role of histone deacetylases in neurological diseases: Focus on epilepsy. Biomark. Res. 2024, 12, 142. [Google Scholar]
- Hu, E.; Li, Z.; Li, T.; Yang, X.; Ding, R.; Jiang, H.; Su, H.; Cheng, M.; Yu, Z.; Li, H.; et al. A novel microbial and hepatic biotransformation-integrated network pharmacology strategy explores the therapeutic mechanisms of bioactive herbal products in neurological diseases: The effects of Astragaloside IV on intracerebral hemorrhage as an example. Chin. Med. 2023, 18, 40. [Google Scholar]
- Chamorro, Á.; Lo, E.H.; Renú, A.; van Leyen, K.; Lyden, P.D. The future of neuroprotection in stroke. J. Neurol. Neurosurg. Psychiatry 2021, 92, 129–135. [Google Scholar] [CrossRef]
- Hui, Z.; Lai-Fa, W.; Xue-Qin, W.; Ling, D.; Bin-Sheng, H.; Li, J.M. Mechanisms and therapeutic potential of chinonin in nervous system diseases. J. Asian Nat. Prod. Res. 2024, 26, 1405–1420. [Google Scholar] [CrossRef]
- Kung, H.C.; Lin, K.J.; Kung, C.T.; Lin, T.K. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines 2021, 9, 918. [Google Scholar] [CrossRef]
- Li, J.-L.; Lin, T.-Y.; Chen, P.-L.; Guo, T.-N.; Huang, S.-Y.; Chen, C.-H.; Lin, C.-H.; Chan, C.-C. Mitochondrial Function and Parkinson’s Disease: From the Perspective of the Electron Transport Chain. Front. Mol. Neurosci. 2021, 14, 797833. [Google Scholar] [CrossRef]
- Yang, N.; Guan, Q.W.; Chen, F.H.; Xia, Q.X.; Yin, X.X.; Zhou, H.H.; Mao, X.Y. Antioxidants Targeting Mitochondrial Oxidative Stress: Promising Neuroprotectants for Epilepsy. Oxidative Med. Cell. Longev. 2020, 2020, 6687185. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Z.; Zhou, J.; Ke, C.; Li, D. Significance of Programmed Cell Death Pathways in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 9947. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jiang, T.; Ma, H.; Zhu, Y.; Wang, M. Targeting PI3K/Akt in Cerebral Ischemia Reperfusion Injury Alleviation: From Signaling Networks to Targeted Therapy. Mol. Neurobiol. 2024, 61, 7930–7949. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.J.; Kim, K. Effects of the Edaravone, a Drug Approved for the Treatment of Amyotrophic Lateral Sclerosis, on Mitochondrial Function and Neuroprotection. Antioxidants 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, J.; Xiao, J.; Chen, F.; Wang, M. Neuroprotective effect of cajaninstilbene acid against cerebral ischemia and reperfusion damages by activating AMPK/Nrf2 pathway. J. Adv. Res. 2021, 34, 199–210. [Google Scholar] [CrossRef]
- Xu, H.; Wang, E.; Chen, F.; Xiao, J.; Wang, M.; Vergara, D. Neuroprotective Phytochemicals in Experimental Ischemic Stroke: Mechanisms and Potential Clinical Applications. Oxidative Med. Cell. Longev. 2021, 2021, 6687386. [Google Scholar] [CrossRef]
- Toomer, O.T. A comprehensive review of the value-added uses of peanut (Arachis hypogaea) skins and by-products. Crit. Rev. Food Sci. Nutr. 2020, 60, 341–350. [Google Scholar] [CrossRef]
- Sato, T.; Akiyama, M.; Nakahama, K.-i.; Seo, S.; Watanabe, M.; Tatsuzaki, J.; Morita, I. A novel mode of stimulating platelet formation activity in megakaryocytes with peanut skin extract. J. Nat. Med. 2018, 72, 211–219. [Google Scholar] [CrossRef]
- Bodoira, R.; Cecilia Cittadini, M.; Velez, A.; Rossi, Y.; Montenegro, M.; Martínez, M.; Maestri, D. An overview on extraction, composition, bioactivity and food applications of peanut phenolics. Food Chem. 2022, 381, 132250. [Google Scholar] [CrossRef]
- Sampson Kofi, K.; William Iheanyi, E.; Hajara, A.-K.; Godfred, D.; Onyewuchi, A. Phytochemicals from Peanut (Arachis hypogaea L.) Skin Extract with Potential for Pharmacological Activity. Curr. Bioact. Compd. 2021, 17, 5–23. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, X.; Zhang, L.; Zhao, A.; Zheng, Z.; Gao, H.; You, S.; Zhang, J.; Sun, J. Depolymerized peanut skin-derived proanthocyanidins alleviate cognitive dysfunction by inhibiting Aβ42 aggregation in Alzheimer’s disease. Food Res. Int. 2025, 203, 115747. [Google Scholar] [CrossRef]
- Armstrong, D.L. The hippocampal tissue slice in animal models of CNS disorders. Neurosci. Biobehav. Rev. 1991, 15, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Chukwumah, Y.; Walker, L.T.; Verghese, M. Peanut Skin Color: A Biomarker for Total Polyphenolic Content and Antioxidative Capacities of Peanut Cultivars. Int. J. Mol. Sci. 2009, 10, 4941–4952. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Ouyang, L.; Yao, R.; He, D.; Han, Z.; Li, W.; Ding, Y.; Wang, Z.; Kang, Y.; et al. Metabolomics combined with transcriptomics analyses of mechanism regulating testa pigmentation in peanut. Front. Plant Sci. 2022, 13, 1065049. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Krewenka, C.; Radad, K.; Kranner, B.; Huber, A.; Duvigneau, J.C.; Miller, I.; Moldzio, R. THC (Δ9-Tetrahydrocannabinol) Exerts Neuroprotective Effect in Glutamate-affected Murine Primary Mesencephalic Cultures Through Restoring Mitochondrial Membrane Potential and Anti-apoptosis Involving CB(1) Receptor-dependent Mechanism. Phytother. Res. 2016, 30, 2044–2052. [Google Scholar] [CrossRef]
- Cordeiro-Massironi, K.A.-O.; Soares Freitas, R.A.M.; Vieira da Silva Martins, I.A.-O.; de Camargo, A.C.; Torres, E. Bioactive compounds of peanut skin in prevention and adjunctive treatment of chronic non-communicable diseases. Food Funct. 2024, 15, 6304–6323. [Google Scholar] [CrossRef]
- Putra, N.A.-O.X.; Rizkiyah, D.N.; Che Yunus, M.A.-O.; Abdul Aziz, A.A.-O.; Md Yasir, A.A.-O.; Irianto, I.A.-O.X.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L. Valorization of Peanut Skin as Agricultural Waste Using Various Extraction Methods: A Review. Molecules 2023, 28, 4325. [Google Scholar] [CrossRef]
- Sorita, G.D.; Leimann, F.V.; Ferreira, S.R.S. Phenolic Fraction from Peanut (Arachis hypogaea L.) By-product: Innovative Extraction Techniques and New Encapsulation Trends for Its Valorization. Food Bioprocess. Technol. 2023, 16, 726–748. [Google Scholar]
- Goyal, A.; Agrawal, A.; Verma, A.; Dubey, N. The PI3K-AKT pathway: A plausible therapeutic target in Parkinson’s disease. Exp. Mol. Pathol. 2023, 129, 104846. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A. NRF2 in neurodegenerative diseases. Curr. Opin. Toxicol. 2016, 1, 46–53. [Google Scholar] [CrossRef]
- Poh Loh, K.; Hong Huang, S.; De Silva, R.; HTan, B.K.; Zhun Zhu, Y. Oxidative Stress: Apoptosis in Neuronal Injury. Curr. Alzheimer Res. 2006, 3, 327–337. [Google Scholar]
- Shohag, S.; Akhter, S.; Islam, S.; Sarker, T.; Sifat, M.K.; Rahman, M.M.; Islam, M.R.; Sharma, R.; Kumar, G. Perspectives on the Molecular Mediators of Oxidative Stress and Antioxidant Strategies in the Context of Neuroprotection and Neurolongevity: An Extensive Review. Oxidative Med. Cell. Longev. 2022, 2022, 7743705. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Li, T.; Li, Z.; Su, H.; Yan, Q.; Wang, L.; Li, H.; Zhang, W.; Tang, T.; Wang, Y. Metabolomics reveals the effects of hydroxysafflor yellow A on neurogenesis and axon regeneration after experimental traumatic brain injury. Pharm. Biol. 2023, 61, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Takahashi, C.; Ohya, R.; Ano, Y. β-Lactolin improves mitochondrial function in Aβ-treated mouse hippocampal neuronal cell line and a human iPSC-derived neuronal cell model of Alzheimer’s disease. FASEB J. 2022, 36, e22277. [Google Scholar] [CrossRef] [PubMed]
- Batu Öztürk, A.; Can Öztürk, N.; Ayaz, F. Conditioned media of mouse macrophages modulates neuronal dynamics in mouse hippocampal cells. Int. Immunopharmacol. 2023, 114, 109548. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, D.; Reece, E.A.; Wang, A.R.; Yang, P. Oxidative stress-induced miR-27a targets the redox gene nuclear factor erythroid 2-related factor 2 in diabetic embryopathy. Am. J. Obstet. Gynecol. 2018, 218, 136.e1–136.e10. [Google Scholar] [CrossRef]
- Lee, J.; Hyun, D.H. NAD(P)H-quinone oxidoreductase 1 induces complicated effects on mitochondrial dysfunction and ferroptosis in an expression level-dependent manner. Biosci. Trends 2024, 18, 153–164. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Moon, S.; Choi, B.Y.; Kim, S.; Jeon, H.S.; Suh, S.W.; Kim, Y.M.; Choi, Y.K. Korean Red Ginseng Improves Astrocytic Mitochondrial Function by Upregulating HO-1-Mediated AMPKα-PGC-1α-ERRα Circuit after Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 13081. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Stiles, B.L. PI-3-K and AKT: Onto the mitochondria. Adv. Drug Deliv. Rev. 2009, 61, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Deng, X. Nicotine Inactivation of the Proapoptotic Function of Bax through Phosphorylation. J. Biol. Chem. 2005, 280, 10781–10789. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wang, H.G. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Nature 2001, 20, 7779–7786. [Google Scholar]
- Gao, B.; Jing, Y.; Li, X.; Cong, S. Ubiquitin specific peptidase 11 knockdown slows Huntington’s disease progression via regulating mitochondrial dysfunction and neuronal damage depending on PTEN-mediated AKT pathway. Mol. Med. 2025, 31, 7. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.-P.; Liu, S.-Y.; Sun, Q.-Y.; Ren, J.; Liu, H.-X. Proanthocyanidin B2 attenuates high-glucose-induced neurotoxicity of dorsal root ganglion neurons through the PI3K/Akt signaling pathway. Neural Regen. Res. 2018, 13, 1628–1636. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Idham, Z.; Veza, I.; Qomariyah, L.; Yunus, M.A.C. Optimization and modelling in flavonoid and phenolic compounds recovery from peanut skin by subcritical water. Biomass Convers. Biorefinery 2024, 14, 12299–12309. [Google Scholar] [CrossRef]
- Nayak, S.P.; Prasad, P.; Singh, V.; Tripathi, A.M.; Bag, S.K.; Mohanty, C.S. Role of miRNAs in the regulation of proanthocyanidin biosynthesis in the legume Psophocarpus tetragonolobus (L.) DC. Plant Growth Regul. 2024, 102, 23–38. [Google Scholar] [CrossRef]
- Severo, J.; Tiecher, A.; Chaves, F.C.; Silva, J.A.; Rombaldi, C.V. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chem. 2011, 126, 995–1000. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Chen, X.-X.; Feng, H.-L.; Ding, Y.-M.; Chai, W.-M.; Xiang, Z.-H.; Shi, Y.; Chen, Q.-X. Structure characterization of proanthocyanidins from Caryota ochlandra Hance and their bioactivities. Food Chem. 2014, 155, 1–8. [Google Scholar] [CrossRef]



| The Content of Polyphenols | TPC (mg GAE/g) | TFC (mg RE/g) | TPAC (mg PE/g) | |
| 123.90 ± 0.46 | 75.97 ± 0.23 | 53.34 ± 1.58 | ||
| Antioxidant capacity | ABTS (mg AAE/g) | DPPH (mg TE/g) | •OH-RSA (mg AAE/g) | FRAP (mg AAE/g) |
| 230.36 ± 15.96 | 10.21 ± 0.12 | 136.49 ± 3.26 | 14.59 ± 1.12 |
| Phenolic Acids | Flavonoids | Stilbenes |
|---|---|---|
| Caffeic acid | Catechin | Resveratrol |
| p-Coumaric acid | Epigallocatechin gallate | Piceatannol |
| Ellagic acid | Rutin | Piceid |
| Chicoric acid | Epigallocatechin | |
| Ferulic acid | Naringenin | |
| Gallic acid | Catechin gallate | |
| Eriodictyol | ||
| proanthocyanidin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhou, Y.; Xu, H.; Wang, M. Neuroprotective Effects of Peanut Skin Extract Against Oxidative Injury in HT-22 Neuronal Cells. Pharmaceuticals 2025, 18, 544. https://doi.org/10.3390/ph18040544
Huang J, Zhou Y, Xu H, Wang M. Neuroprotective Effects of Peanut Skin Extract Against Oxidative Injury in HT-22 Neuronal Cells. Pharmaceuticals. 2025; 18(4):544. https://doi.org/10.3390/ph18040544
Chicago/Turabian StyleHuang, Jinlan, Yue Zhou, Hui Xu, and Mingfu Wang. 2025. "Neuroprotective Effects of Peanut Skin Extract Against Oxidative Injury in HT-22 Neuronal Cells" Pharmaceuticals 18, no. 4: 544. https://doi.org/10.3390/ph18040544
APA StyleHuang, J., Zhou, Y., Xu, H., & Wang, M. (2025). Neuroprotective Effects of Peanut Skin Extract Against Oxidative Injury in HT-22 Neuronal Cells. Pharmaceuticals, 18(4), 544. https://doi.org/10.3390/ph18040544






