Biologic Agents in Idiopathic Hypereosinophilic Syndrome †
Abstract
:1. Introduction
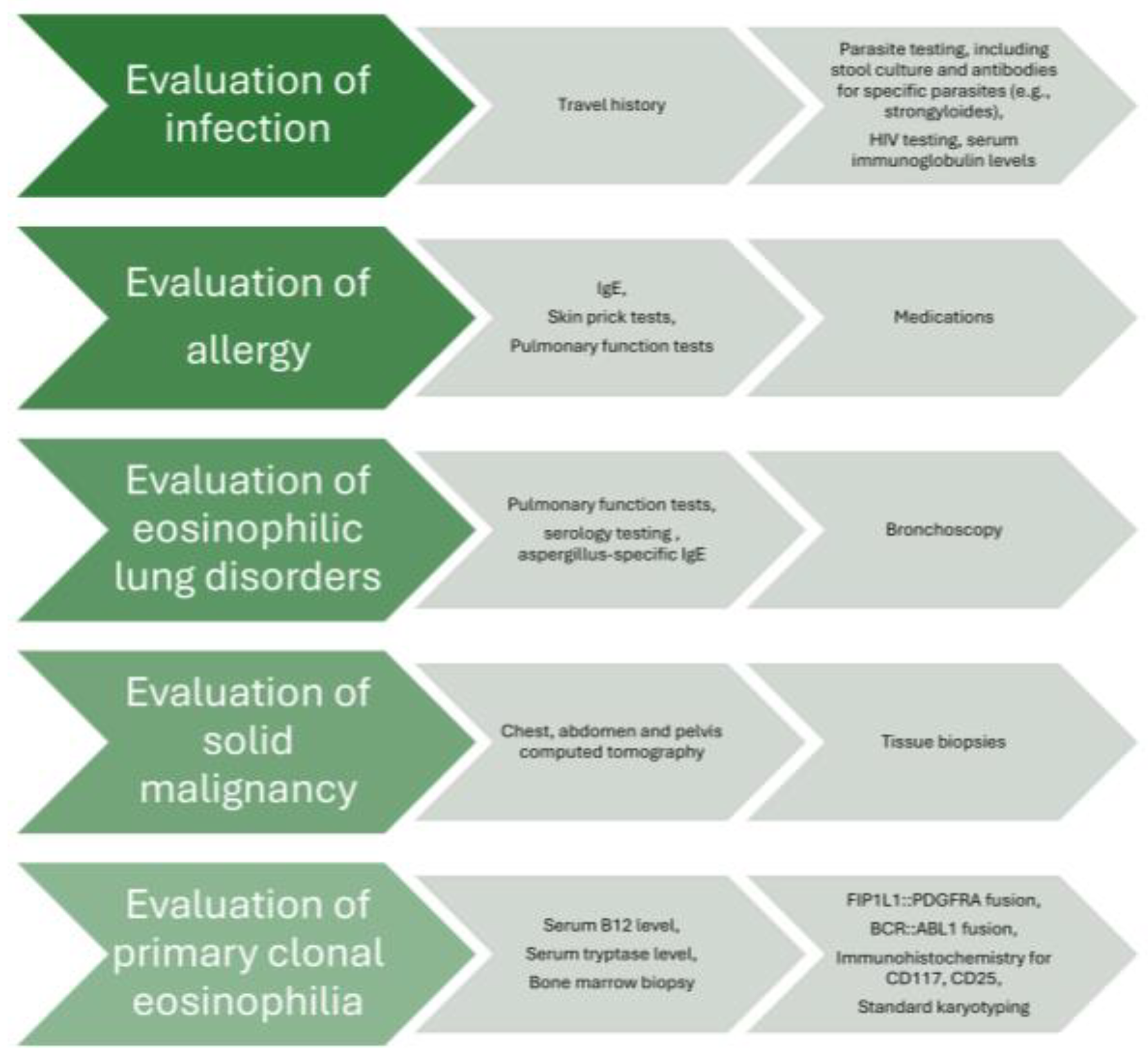
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valent, P.; Klion, A.D.; Roufosse, F.; Simon, D.; Metzgeroth, G.; Leiferman, K.M.; Schwaab, J.; Butterfield, J.H.; Sperr, W.R.; Sotlar, K.; et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy 2023, 78, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Klion, A.D.; Horny, H.-P.; Roufosse, F.; Gotlib, J.; Weller, P.F.; Hellmann, A.; Metzgeroth, G.; Leiferman, K.M.; Arock, M.; et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012, 130, 607–612.e9. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization and International Consensus Classification of eosinophilic disorders: 2024 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2024, 99, 946–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Orazi, A.; Gotlib, J.; Reiter, A.; Tzankov, A.; Hasserjian, R.P.; Arber, D.A.; Tefferi, A. The international consensus classification of eosinophilic disorders and systemic mastocytosis. Am. J. Hematol. 2023, 98, 1286–1306. [Google Scholar] [CrossRef]
- Klion, A.D.; Bochner, B.S.; Gleich, G.J.; Nutman, T.B.; Rothenberg, M.E.; Simon, H.U.; Wechsler, M.E.; Weller, P.F.; The Hypereosinophilic Syndromes Working Group. Approaches to the treatment of hypereosinophilic syndromes: A workshop summary report. J. Allergy Clin. Immunol. 2006, 117, 1292–1302. [Google Scholar] [CrossRef]
- Caminati, M.; Brussino, L.; Carlucci, M.; Carlucci, P.; Carpagnano, L.F.; Caruso, C.; Cosmi, L.; D’Amore, S.; Del Giacco, S.; Detoraki, A.; et al. Managing Patients with Hypereosinophilic Syndrome: A Statement from the Italian Society of Allergy, Asthma, and Clinical Immunology (SIAAIC). Cells 2024, 13, 1180. [Google Scholar] [CrossRef]
- Crane, M.M.; Chang, C.M.; Kobayashi, M.G.; Weller, P.F. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J. Allergy Clin. Immunol. 2010, 126, 179–181. [Google Scholar] [CrossRef]
- Weller, P.F.; Bubley, G.J. The idiopathic hypereosinophilic syndrome. Blood 1994, 83, 2759–2779. [Google Scholar] [CrossRef]
- Gotlib, J.; Cools, J.; Malone, J.M.; Schrier, S.L., 3rd; Gilliland, D.G.; Coutre, S.E. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: Implications for diagnosis, classification, and management. Blood 2004, 103, 2879–2891. [Google Scholar] [CrossRef]
- Wardlaw, A.J.; Wharin, S.; Aung, H.; Shaffu, S.; Siddiqui, S. The causes of a peripheral blood eosinophilia in a secondary care setting. Clin. Exp. Allergy 2021, 51, 902–914. [Google Scholar] [CrossRef]
- Klion, A.D.; Noel, P.; Akin, C.; Law, M.A.; Gilliland, D.G.; Cools, J.; Metcalfe, D.D.; Nutman, T.B. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 2003, 101, 4660–4666. [Google Scholar] [PubMed]
- Schwaab, J.; Jawhar, M.; Naumann, N.; Schmitt-Graeff, A.; Fabarius, A.; Horny, H.-P.; Cross, N.C.P.; Hofmann, W.-K.; Reiter, A.; Metzgeroth, G. Diagnostic challenges in the work up of hypereosinophilia: Pitfalls in bone marrow core biopsy interpretation. Ann. Hematol. 2016, 95, 557–562. [Google Scholar] [PubMed]
- Metzgeroth, G.; Walz, C.; Score, J.; Siebert, R.; Schnittger, S.; Haferlach, C.; Popp, H.; Haferlach, T.; Erben, P.; Mix, J.; et al. Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma. Leukemia 2007, 21, 1183–1188. [Google Scholar] [PubMed]
- Caminati, M.; Carpagnano, L.F.; Alberti, C.; Amaddeo, F.; Bixio, R.; Caldart, F.; De Franceschi, L.; Del Giglio, M.; Festi, G.; Friso, S.; et al. Idiopathic hypereosinophilic syndromes and rare dysimmune conditions associated with hyper-eosinophilia in practice: An innovative multidisciplinary approach. World Allergy Organ. J. 2024, 17, 100928. [Google Scholar]
- Curtis, C.; Ogbogu, P. Hypereosinophilic Syndrome. Clin. Rev. Allergy Immunol. 2016, 50, 240–251. [Google Scholar]
- Steinfeld, J.; Rofousse, F.; Kahn, J.-E.; Gleich, G.; Rothenberg, M.; Wardlaw, A.; Kirby, S.Y.; Gilson, M.; Bentley, J.; Bradford, E.; et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020, 146, 1397–1405. [Google Scholar]
- Kuang, F.L.; Legrand, F.; Makiya, M.; Ware, J.; Wetzler, L.; Brown, T.; Magee, T.; Piligian, B.; Yoon, P.; Ellis, J.H.; et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N. Engl. J. Med. 2019, 380, 1336–1346. [Google Scholar] [CrossRef]
- Langton, D.; Politis, J.; Collyer, T.; Khung, S.W.; Bardin, P. Benralizumab and mepolizumab treatment outcomes in two severe asthma clinics. Respirology 2023, 28, 1117–1125. [Google Scholar]
- Steinfeld, J.; Gleich, G.; Roufosse, F.; Chupp, G.; Faguer, S.; Reiter, A.; Walz, B.; Bentley, J.; Bradford, E.; Yancey, S. Safety and Efficacy of Mepolizumab in Hypereosinophilic Syndrome: An Open-Label Extension Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 4431–4440.e1. [Google Scholar]
- Papaioannou, O.; Tsiri, P.; Sotiropoulou, V.; Christopoulos, I.; Komninos, D.; Koulousousa, E.; Theochari, E.; Tsirikos, G.; Sampsonas, F.; Tzouvelekis, A. Biologic agents in idiopathic hypereosinophilic syndrome: A 4-year-follow up in a case series. Eur. Respir. J. 2024, 64 (Suppl. S68), PA1772. [Google Scholar]
- Bacher, U.; Reiter, A.; Haferlach, T.; Mueller, L.; Schnittger, S.; Kern, W.; Schoch, C. A combination of cytomorphology, cytogenetic analysis, fluorescence in situ hybridization and reverse transcriptase polymerase chain reaction for establishing clonality in cases of persisting hypereosinophilia. Haematologica 2006, 91, 817–820. [Google Scholar] [PubMed]
- Haldar, P.; Brightling, C.E.; Singapuri, A.; Hargadon, B.; Gupta, S.; Monteiro, W.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; Ortega, H.; et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: A 12-month follow-up analysis. J. Allergy Clin. Immunol. 2014, 133, 921–923. [Google Scholar] [PubMed]
- Ortega, H.; Lemiere, C.; Llanos, J.-P.; Forshag, M.; Price, R.; Albers, F.; Yancey, S.; Castro, M. Outcomes following mepolizumab treatment discontinuation: Real-world experience from an open-label trial. Allergy Asthma Clin. Immunol. 2019, 15, 37. [Google Scholar] [PubMed]
- Moore, W.C.; Kornmann, O.; Humbert, M.; Poirier, C.; Bel, E.H.; Kaneko, N.; Smith, S.G.; Martin, N.; Gilson, M.J.; Price, R.G.; et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur. Respir. J. 2022, 59, 2100396. [Google Scholar]
- Soendergaard, M.B.; Bjerrum, A.S.; Rasmussen, L.M.; Lock-Johansson, S.; Hilberg, O.; Hansen, S.; von Bulow, A.; Porsbjerg, C. Titration of anti-IL-5 biologics in severe asthma: An open-label randomised controlled trial (the OPTIMAL study). Eur. Respir. J. 2024, 64, 2400404. [Google Scholar]
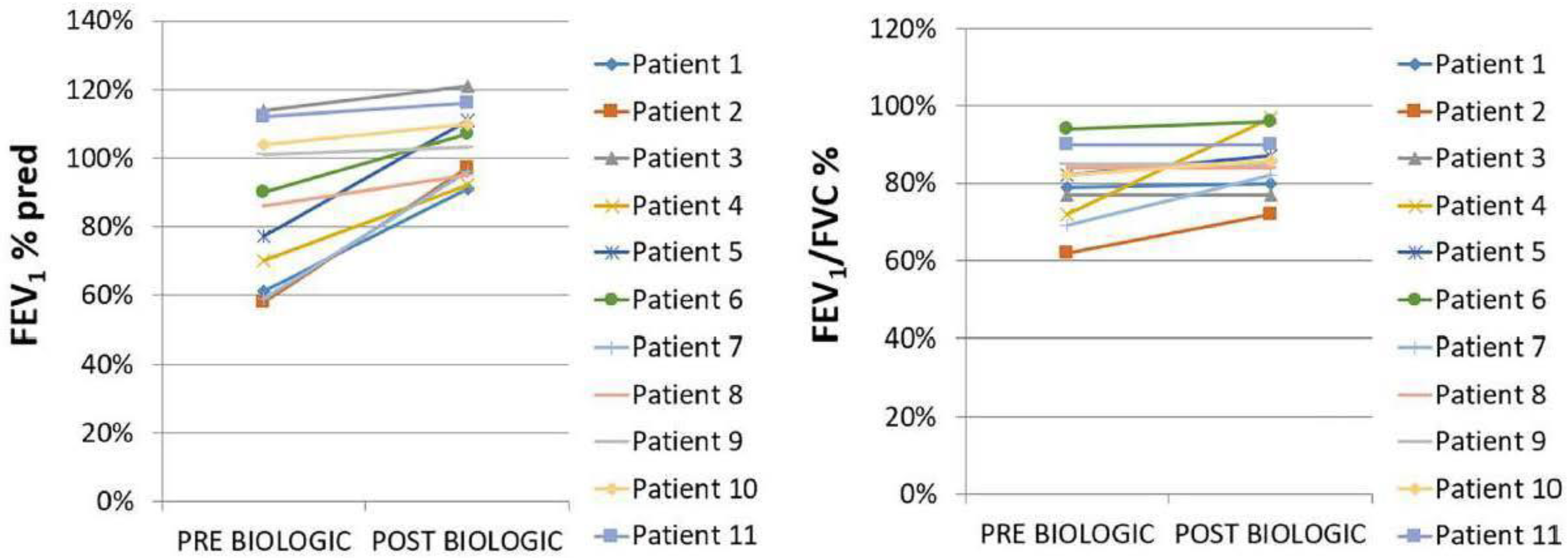
| Characteristics | Pre-BT | Post BT |
|---|---|---|
| Oral corticosteroids: prednisone (longitudinal use) | 11 (100%)–20 mg/d | 2 (18%)–7.5 mg/d |
| Median value of eosinophils (K/μL) (95%CI) | 3000 (2172 to 11,365) | 50 (3 to 190) |
| HES flares | 11 (100%) | 0 (0%) |
| Asthma control (ACQ < 0.75) | 0 * (0%) | 8 * (100%) |
| ΔFEV1 ± SD | NA | 857 ± 594 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, O.; Sampsonas, F.; Tsiri, P.; Sotiropoulou, V.; Christopoulos, I.; Komninos, D.; Tzouvelekis, A. Biologic Agents in Idiopathic Hypereosinophilic Syndrome. Pharmaceuticals 2025, 18, 543. https://doi.org/10.3390/ph18040543
Papaioannou O, Sampsonas F, Tsiri P, Sotiropoulou V, Christopoulos I, Komninos D, Tzouvelekis A. Biologic Agents in Idiopathic Hypereosinophilic Syndrome. Pharmaceuticals. 2025; 18(4):543. https://doi.org/10.3390/ph18040543
Chicago/Turabian StylePapaioannou, Ourania, Fotios Sampsonas, Panagiota Tsiri, Vasilina Sotiropoulou, Ioannis Christopoulos, Dimitrios Komninos, and Argyrios Tzouvelekis. 2025. "Biologic Agents in Idiopathic Hypereosinophilic Syndrome" Pharmaceuticals 18, no. 4: 543. https://doi.org/10.3390/ph18040543
APA StylePapaioannou, O., Sampsonas, F., Tsiri, P., Sotiropoulou, V., Christopoulos, I., Komninos, D., & Tzouvelekis, A. (2025). Biologic Agents in Idiopathic Hypereosinophilic Syndrome. Pharmaceuticals, 18(4), 543. https://doi.org/10.3390/ph18040543





