Artemisinin and Its Derivatives: Promising Therapeutic Agents for Age-Related Macular Degeneration
Abstract
1. Introduction
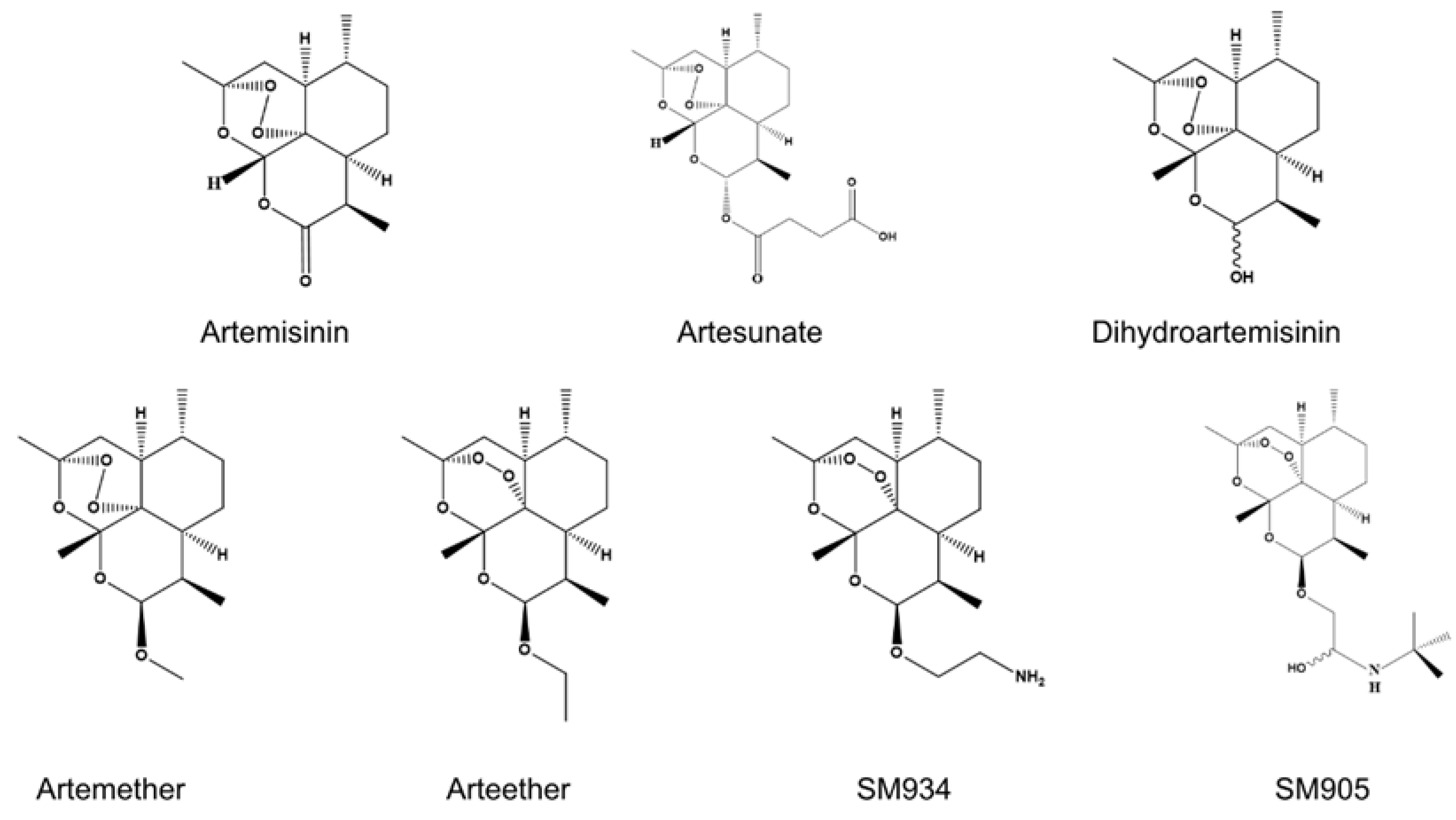
2. Overview of ART and Its Derivatives
3. Role of ART and Its Derivatives in AMD
3.1. ART and Its Derivatives Inhibit Inflammation
3.2. ART and Its Derivatives Against Neovascularization
3.3. ART and Its Derivatives Inhibit Oxidative Stress
3.4. ART and Its Derivatives Against Fibrosis
3.5. ART and Its Derivatives Maintain Mitochondrial Homeostasis
3.6. ART and Its Derivatives Regulate Lipid Metabolism
3.7. ART and Its Derivatives Modulate Immunity
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| VEGF | Vascular endothelial growth factor |
| ART | Artemisinin |
| CNV | Choroidal neovascularization |
| ARTS | Artesunate |
| DHA | Dihydroartemisinin |
| TNF-α | Tumor necrosis factor-α |
| IFNγ | Interferon γ |
| IL-1β | Interleukin-1β |
| NF-κB | Nuclear factor-κB |
| ROS | Reactive oxygen species |
| AMPK | Adenosine monophosphate-activated protein kinase |
| HDL | High-density lipoproteins |
| LDL | Low-density lipoproteins |
| TG | Triglycerides |
| TC | Total cholesterol |
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Chapman, N.A.; Jacobs, R.J.; Braakhuis, A.J. Role of diet and food intake in age-related macular degeneration: A systematic review. Clin. Exp. Ophthalmol. 2019, 47, 106–127. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Malek, G. Age-Related Macular Degeneration Revisited: From Pathology and Cellular Stress to Potential Therapies. Front. Cell Dev. Biol. 2020, 8, 612812. [Google Scholar] [CrossRef]
- Blasiak, J.; Chojnacki, J.; Szczepanska, J.; Fila, M.; Chojnacki, C.; Kaarniranta, K.; Pawlowska, E. Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration. Nutrients 2023, 15, 3358. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2022, 9, 62–79. [Google Scholar] [CrossRef]
- Apte, R.S. Age-Related Macular Degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.M.; Arepalli, S.; Ehlers, J.P. Current and Future Anti-VEGF Agents for Neovascular Age-Related Macular Degeneration. J. Exp. Pharmacol. 2021, 13, 905–912. [Google Scholar] [CrossRef]
- Song, D.; Liu, P.; Shang, K.; Ma, Y. Application and mechanism of anti-VEGF drugs in age-related macular degeneration. Front. Bioeng. Biotechnol. 2022, 10, 943915. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef]
- Casalino, G.; Stevenson, M.R.; Bandello, F.; Chakravarthy, U. Tomographic Biomarkers Predicting Progression to Fibrosis in Treated Neovascular Age-Related Macular Degeneration: A Multimodal Imaging Study. Ophthalmol. Retin. 2018, 2, 451–461. [Google Scholar] [CrossRef]
- Khoramnia, R.; Figueroa, M.S.; Hattenbach, L.O.; Pavesio, C.E.; Anderesi, M.; Schmouder, R.; Chen, Y.; de Smet, M.D. Manifestations of intraocular inflammation over time in patients on brolucizumab for neovascular AMD. Graefe Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2022, 260, 1843–1856. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Pintus, G.; Mohammed, S.A.; Orhan, I.E.; Fokou, P.V.T.; Sharopov, F.; Adetunji, C.O.; Gulsunoglu-Konuskan, Z.; Ydyrys, A.; et al. Medicinal and mechanistic overview of artemisinin in the treatment of human diseases. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 163, 114866. [Google Scholar] [CrossRef]
- Nabi, N.; Singh, S.; Saffeullah, P. An updated review on distribution, biosynthesis and pharmacological effects of artemisinin: A wonder drug. Phytochemistry 2023, 214, 113798. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xia, F.; Wang, Q.; Liao, F.; Guo, Q.; Xu, C.; Wang, J. Discovery and repurposing of artemisinin. Front. Med. 2022, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Palanichamy, R.; Velayutham, R.; Natesan, S. Development and Evaluation of Polyvinylpyrrolidone K90 and Poloxamer 407 Self-Assembled Nanomicelles: Enhanced Topical Ocular Delivery of Artemisinin. Polymers 2021, 13, 3038. [Google Scholar] [CrossRef]
- Chong, C.M.; Zheng, W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Navaratnam, V.; Mansor, S.M.; Sit, N.W.; Grace, J.; Li, Q.; Olliaro, P. Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 2000, 39, 255–270. [Google Scholar] [CrossRef]
- Kong, L.Y.; Tan, R.X. Artemisinin, a miracle of traditional Chinese medicine. Nat. Prod. Rep. 2015, 32, 1617–1621. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Chen, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Li, P.; Zhan, Y. Therapeutic potential of artemisinin and its derivatives in managing kidney diseases. Front. Pharmacol. 2023, 14, 1097206. [Google Scholar] [CrossRef]
- Tsui, K.H.; Wu, M.Y.; Lin, L.T.; Wen, Z.H.; Li, Y.H.; Chu, P.Y.; Li, C.J. Disruption of mitochondrial homeostasis with artemisinin unravels anti-angiogenesis effects via auto-paracrine mechanisms. Theranostics 2019, 9, 6631–6645. [Google Scholar] [CrossRef]
- Fu, W.; Ma, Y.; Li, L.; Liu, J.; Fu, L.; Guo, Y.; Zhang, Z.; Li, J.; Jiang, H. Artemether Regulates Metaflammation to Improve Glycolipid Metabolism in db/db Mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.; Weathers, P.; Dominko, T. Artemisinin and artemisinin derivatives as anti-fibrotic therapeutics. Acta Pharm. Sin. B 2021, 11, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Efferth, T. Repurposing of artemisinin-type drugs for the treatment of acute leukemia. Semin. Cancer Biol. 2021, 68, 291–312. [Google Scholar] [CrossRef]
- Xie, K.; Li, Z.; Zhang, Y.; Wu, H.; Zhang, T.; Wang, W. Artemisinin and its derivatives as promising therapies for autoimmune diseases. Heliyon 2024, 10, e27972. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Y.; Liu, G.; Xu, M. New clinical application prospects of artemisinin and its derivatives: A scoping review. Infect. Dis. Poverty 2023, 12, 115. [Google Scholar] [CrossRef]
- Tarning, J.; Hanboonkunupakarn, B.; Hoglund, R.M.; Chotivanich, K.; Mukaka, M.; Pukrittayakamee, S.; Day, N.P.J.; White, N.J.; Dondorp, A.M.; Jittamala, P. Safety and pharmacokinetic properties of a new formulation of parenteral artesunate in healthy Thai volunteers. Malar. J. 2024, 23, 296. [Google Scholar] [CrossRef]
- Sun, C.; Cao, Y.; Zhu, P.; Zhou, B. A mitochondria-targeting artemisinin derivative with sharply increased antitumor but depressed anti-yeast and anti-malaria activities. Sci. Rep. 2017, 7, 45665. [Google Scholar] [CrossRef]
- Aquino, I.; Perazzo, F.F.; Maistro, E.L. Genotoxicity assessment of the antimalarial compound artesunate in somatic cells of mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1335–1339. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, B. The antimalarial drug artemisinin induces an additional, Sod1-supressible anti-mitochondrial action in yeast. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Trincão-Marques, J.; Ayton, L.N.; Hickey, D.G.; Marques-Neves, C.; Guymer, R.H.; Edwards, T.L.; Sousa, D.C. Gene and cell therapy for age-related macular degeneration: A review. Surv. Ophthalmol. 2024, 69, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Bhumika; Bora, N.S.; Bora, P.S. Genetic Insights into Age-Related Macular Degeneration. Biomedicines 2024, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Corydon, T.J.; Bek, T. Multiple gene therapy as a tool for regulating the expression of molecules involved in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2025, 104, 101323. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef]
- Lambert, N.G.; ElShelmani, H.; Singh, M.K.; Mansergh, F.C.; Wride, M.A.; Padilla, M.; Keegan, D.; Hogg, R.E.; Ambati, B.K. Risk factors and biomarkers of age-related macular degeneration. Prog. Retin. Eye Res. 2016, 54, 64–102. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Park, Y.G.; Park, Y.S.; Kim, I.B. Complement System and Potential Therapeutics in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Logan, A.; Cerrada-Gimenez, M.; Fitzhenry, L.; Coffey, L.; Kaja, S.; Rani, S. Immune System, Inflammation and Autoantigens in Wet Age-Related Macular Degeneration: Pathological Significance and Therapeutic Importance. Life 2023, 13, 2236. [Google Scholar] [CrossRef]
- Terluk, M.R.; Ebeling, M.C.; Fisher, C.R.; Kapphahn, R.J.; Yuan, C.; Kartha, R.V.; Montezuma, S.R.; Ferrington, D.A. N-Acetyl-L-cysteine Protects Human Retinal Pigment Epithelial Cells from Oxidative Damage: Implications for Age-Related Macular Degeneration. Oxidative Med. Cell. Longev. 2019, 2019, 5174957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, R.; Ye, M.; Zhang, L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. Int. J. Mol. Med. 2019, 43, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Patel, M.; Chan, C.C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef]
- Edwards, M.; Lutty, G.A. Bruch’s Membrane and the Choroid in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2021, 1256, 89–119. [Google Scholar] [CrossRef]
- Curcio, C.A.; Zanzottera, E.C.; Ach, T.; Balaratnasingam, C.; Freund, K.B. Activated Retinal Pigment Epithelium, an Optical Coherence Tomography Biomarker for Progression in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, bio211–bio226. [Google Scholar] [CrossRef]
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, N.; Song, Y.S.; Farnoodian, M.; Inampudi, S.; Wang, S.; Darjatmoko, S.R.; Sorenson, C.M. Artesunate mitigates choroidal neovascularization and scar formation. Exp. Eye Res. 2023, 236, 109666. [Google Scholar] [CrossRef]
- Li, X.; Gao, S.; Zhang, Y.; Xin, M.; Zuo, C.; Yan, N.; Xia, Q.; Zhang, M. Dihydroartemisinin Inhibits Laser-Induced Choroidal Neovascularization in a Mouse Model of Neovascular AMD. Front. Pharmacol. 2022, 13, 838263. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Kandasamy, R.; Natesan, S. Design and development of artemisinin and dexamethasone loaded topical nanodispersion for the effective treatment of age-related macular degeneration. IET Nanobiotechnol. 2019, 13, 868–874. [Google Scholar] [CrossRef]
- Zong, Y.; Yuan, Y.; Qian, X.; Huang, Z.; Yang, W.; Lin, L.; Zheng, Q.; Li, Y.; He, H.; Gao, Q. Small Molecular-Sized Artesunate Attenuates Ocular Neovascularization via VEGFR2, PKCα, and PDGFR Targets. Sci. Rep. 2016, 6, 30843. [Google Scholar] [CrossRef]
- Li, S.; Chaudhary, S.C.; Zhao, X.; Gaur, U.; Fang, J.; Yan, F.; Zheng, W. Artemisinin Protects Human Retinal Pigmented Epithelial Cells Against Hydrogen Peroxide-induced Oxidative Damage by Enhancing the Activation of AMP-active Protein Kinase. Int. J. Biol. Sci. 2019, 15, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ge, L.; Yu, X.; Lazarovici, P.; Zheng, W. Artemisinin Confers Cytoprotection toward Hydrogen Peroxide-Induced Cell Apoptosis in Retinal Pigment Epithelial Cells in Correlation with the Increased Acetylation of Histone H4 at Lysine 8. Molecules 2024, 29, 1789. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wang, H.; Gao, Y.; Xu, J.; Zheng, W. Artemisinin Protects Retinal Neuronal Cells against Oxidative Stress and Restores Rat Retinal Physiological Function from Light Exposed Damage. ACS Chem. Neurosci. 2017, 8, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Huang, W.C.; JH, S.P.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1β-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- Leung, K.W.; Barnstable, C.J.; Tombran-Tink, J. Bacterial endotoxin activates retinal pigment epithelial cells and induces their degeneration through IL-6 and IL-8 autocrine signaling. Mol. Immunol. 2009, 46, 1374–1386. [Google Scholar] [CrossRef]
- Krogh Nielsen, M.; Subhi, Y.; Molbech, C.R.; Falk, M.K.; Nissen, M.H.; Sørensen, T.L. Systemic Levels of Interleukin-6 Correlate With Progression Rate of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 202–208. [Google Scholar] [CrossRef]
- Coughlin, B.; Schnabolk, G.; Joseph, K.; Raikwar, H.; Kunchithapautham, K.; Johnson, K.; Moore, K.; Wang, Y.; Rohrer, B. Connecting the innate and adaptive immune responses in mouse choroidal neovascularization via the anaphylatoxin C5a and γδT-cells. Sci. Rep. 2016, 6, 23794. [Google Scholar] [CrossRef]
- Ishikawa, K.; Kannan, R.; Hinton, D.R. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 2016, 142, 19–25. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef]
- Miraghazadeh, B.; Cook, M.C. Nuclear Factor-kappaB in Autoimmunity: Man and Mouse. Front. Immunol. 2018, 9, 613. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Han, J.; Meng, Q.; Xi, Q.; Wu, G.; Zhang, B. DCAF4L2 promotes colorectal cancer invasion and metastasis via mediating degradation of NFκb negative regulator PPM1B. Am. J. Transl. Res. 2016, 8, 405–418. [Google Scholar] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, X.; Yang, C.; Jiang, Y.; Zhou, W.; Zheng, W. Artemisinin Attenuates Amyloid-Induced Brain Inflammation and Memory Impairments by Modulating TLR4/NF-κB Signaling. Int. J. Mol. Sci. 2022, 23, 6354. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Wang, L.; Meng, S.; Fan, Y.; Chen, T.; Cao, J.; Jiang, R.; Wang, C. The anti-malarial artemisinin inhibits pro-inflammatory cytokines via the NF-κB canonical signaling pathway in PMA-induced THP-1 monocytes. Int. J. Mol. Med. 2011, 27, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Jiang, Y.; Shi, J.; Xu, C.; Liu, X.; Yang, T.; Fu, P.; Niu, T. Artemisinin inhibits the proliferation, migration, and inflammatory reaction induced by tumor necrosis factor-α in vascular smooth muscle cells through nuclear factor kappa B pathway. J. Surg. Res. 2015, 194, 667–678. [Google Scholar] [CrossRef]
- Wang, D.; Shi, J.; Lv, S.; Xu, W.; Li, J.; Ge, W.; Xiao, C.; Geng, D.; Liu, Y. Artesunate Attenuates Lipopolysaccharide-Stimulated Proinflammatory Responses by Suppressing TLR4, MyD88 Expression, and NF-κB Activation in Microglial Cells. Inflammation 2015, 38, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tian, Q.; Ai, X.; Qin, Y.; Cui, Z.; Li, M.; Yang, J.; Zhai, D.; Liu, Y.; Chen, S.; et al. Dihydroartemisinin attenuates autoimmune thyroiditis by inhibiting the CXCR3/PI3K/AKT/NF-κB signaling pathway. Oncotarget 2017, 8, 115028–115040. [Google Scholar] [CrossRef]
- Wang, J.X.; Hou, L.F.; Yang, Y.; Tang, W.; Li, Y.; Zuo, J.P. SM905, an artemisinin derivative, inhibited NO and pro-inflammatory cytokine production by suppressing MAPK and NF-kappaB pathways in RAW 264.7 macrophages. Acta Pharmacol. Sin. 2009, 30, 1428–1435. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kolosova, N.G. P38 MAPK Signaling in the Retina: Effects of Aging and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 11586. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Yang, Q.; Chen, K.; Ye, H.; Wang, X.; Xia, J.; Chen, X.; Wang, X.; Shen, Y.; et al. Senescent retinal pigment epithelial cells promote angiogenesis in choroidal neovascularization via the TAK1/p38 MAPK pathway. Exp. Eye Res. 2025, 251, 110232. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Li, J.; Yin, S.; Wu, Y.; Wang, A. Antimalarial agent artesunate protects Concanavalin A-induced autoimmune hepatitis in mice by inhibiting inflammatory responses. Chem.-Biol. Interact. 2017, 274, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.; Fan, Y.; Xie, Y.; Xu, Z.; Yin, Z.; Gao, L.; Wang, C. Artemisinin inhibits monocyte adhesion to HUVECs through the NF-κB and MAPK pathways in vitro. Int. J. Mol. Med. 2016, 37, 1567–1575. [Google Scholar] [CrossRef]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Q.; Yang, J.; Wang, C.; Cao, J.; Sun, J.; Fan, Z.; Fu, L. Artemisinin suppresses myocardial ischemia-reperfusion injury via NLRP3 inflammasome mechanism. Mol. Cell. Biochem. 2020, 474, 171–180. [Google Scholar] [CrossRef]
- Liang, R.; Chen, W.; Fan, H.; Chen, X.; Zhang, J.; Zhu, J.S. Dihydroartemisinin prevents dextran sodium sulphate-induced colitisthrough inhibition of the activation of NLRP3 inflammasome and p38 MAPK signaling. Int. Immunopharmacol. 2020, 88, 106949. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.M.; Fan, D.; Yang, X.Q.; Zhu, F.H.; Shao, M.J.; Li, Q.; Liu, Y.T.; Lin, Z.M.; Cao, S.Q.; Tang, W.; et al. The artemisinin analog SM934 alleviates dry eye disease in rodent models by regulating TLR4/NF-κB/NLRP3 signaling. Acta Pharmacol. Sin. 2021, 42, 593–603. [Google Scholar] [CrossRef]
- Kanazawa, H. VEGF, angiopoietin-1 and -2 in bronchial asthma: New molecular targets in airway angiogenesis and microvascular remodeling. Recent Pat. Inflamm. Allergy Drug Discov. 2007, 1, 1–8. [Google Scholar] [CrossRef]
- Lu, M.; Adamis, A.P. Molecular biology of choroidal neovascularization. Ophthalmol. Clin. N. Am. 2006, 19, 323–334. [Google Scholar] [CrossRef]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef]
- Meng, L.B.; Zhang, Y.M.; Shan, M.J.; Qiu, Y.; Zhang, T.J.; Gong, T. Pivotal micro factors associated with endothelial cells. Chin. Med. J. 2019, 132, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Abou-Khalil, R.; Mounier, R.; Chazaud, B. Regulation of myogenic stem cell behavior by vessel cells: The “ménage à trois” of satellite cells, periendothelial cells and endothelial cells. Cell Cycle 2010, 9, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Jeong, H.W.; Stehling, M.; Dinh, V.V.; Zhou, B.; Adams, R.H. Apelin(+) Endothelial Niche Cells Control Hematopoiesis and Mediate Vascular Regeneration after Myeloablative Injury. Cell Stem Cell 2019, 25, 768–783.e766. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.D.; Teran, M.; Nugent, M.A. Extracellular Matrix Stiffness Controls VEGF Signaling and Processing in Endothelial Cells. J. Cell. Physiol. 2016, 231, 2026–2039. [Google Scholar] [CrossRef]
- Russo, T.A.; Banuth, A.M.M.; Nader, H.B.; Dreyfuss, J.L. Altered shear stress on endothelial cells leads to remodeling of extracellular matrix and induction of angiogenesis. PLoS ONE 2020, 15, e0241040. [Google Scholar] [CrossRef]
- Riddell, J.R.; Maier, P.; Sass, S.N.; Moser, M.T.; Foster, B.A.; Gollnick, S.O. Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF-1α. PLoS ONE 2012, 7, e50394. [Google Scholar] [CrossRef]
- Zhu, X.X.; Yang, L.; Li, Y.J.; Zhang, D.; Chen, Y.; Kostecká, P.; Kmoníčková, E.; Zídek, Z. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharmacol. Rep. PR 2013, 65, 410–420. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhou, H.J.; Wu, G.D.; Lou, X.E. Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology 2004, 71, 1–9. [Google Scholar] [CrossRef]
- Verma, S.; Das, P.; Kumar, V.L. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem.-Biol. Interact. 2017, 278, 84–91. [Google Scholar] [CrossRef]
- Guo, L.; Dong, F.; Hou, Y.; Cai, W.; Zhou, X.; Huang, A.L.; Yang, M.; Allen, T.D.; Liu, J. Dihydroartemisinin inhibits vascular endothelial growth factor-induced endothelial cell migration by a p38 mitogen-activated protein kinase-independent pathway. Exp. Ther. Med. 2014, 8, 1707–1712. [Google Scholar] [CrossRef]
- Dong, F.; Zhou, X.; Li, C.; Yan, S.; Deng, X.; Cao, Z.; Li, L.; Tang, B.; Allen, T.D.; Liu, J. Dihydroartemisinin targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis. Cancer Biol. Ther. 2014, 15, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, X.; Wang, H.; Zhou, Q.; Jin, Z.; Zhang, Y.; Wang, Y.; Yue, F.; Zhou, S.; Yang, J. Integrating network pharmacology and experimental models to investigate the mechanisms of dihydroartemisinin in preventing NSCLC progression via mTOR/HIF-1α signaling. Eur. J. Pharmacol. 2021, 909, 174411. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhou, H.J.; Fang, X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol. Res. 2003, 48, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.O.; Monteleone, G.; Pozharitskiy, N.; Molfetta, T.; Boscia, G.; Alessio, G.; Boscia, F. SEVERE VISUAL LOSS DURING ANTI-VEGF INTRAVITREAL INJECTIONS IN NEOVASCULAR AGE-RELATED MACULAR DEGENERATION: TIMING, PROGNOSIS, AND OPTICAL COHERENCE TOMOGRAPHY FINDINGS. Retina 2023, 43, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Nichani, P.A.H.; Popovic, M.M.; Dhoot, A.S.; Pathak, A.; Muni, R.H.; Kertes, P.J. Treat-and-extend dosing of intravitreal anti-VEGF agents in neovascular age-related macular degeneration: A meta-analysis. Eye 2023, 37, 2855–2863. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef]
- Mitter, S.K.; Rao, H.V.; Qi, X.; Cai, J.; Sugrue, A.; Dunn, W.A., Jr.; Grant, M.B.; Boulton, M.E. Autophagy in the retina: A potential role in age-related macular degeneration. Adv. Exp. Med. Biol. 2012, 723, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Ji, X.; Ivanchenko, M.V.; Chung, M.; Piper, M.; Rana, P.; Wang, S.K.; Xue, Y.; West, E.; Zhao, S.R.; et al. Nrf2 overexpression rescues the RPE in mouse models of retinitis pigmentosa. JCI Insight 2021, 6, e145029. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Chae, J.B.; Jang, H.; Son, C.; Park, C.W.; Choi, H.; Jin, S.; Lee, H.Y.; Lee, H.; Ryu, J.H.; Kim, N.; et al. Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. GeroScience 2021, 43, 2809–2833. [Google Scholar] [CrossRef]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Hytti, M.; Piippo, N.; Salminen, A.; Honkakoski, P.; Kaarniranta, K.; Kauppinen, A. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells. Exp. Eye Res. 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, X.; Zhou, W.; Li, Q.; Lazarovici, P.; Zheng, W. Artemisinin conferred cytoprotection to human retinal pigment epithelial cells exposed to amiodarone-induced oxidative insult by activating the CaMKK2/AMPK/Nrf2 pathway. J. Transl. Med. 2024, 22, 844. [Google Scholar] [CrossRef]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015, 19, 21–27. [Google Scholar] [CrossRef]
- Guruprasad, B.; Chaudhary, P.; Choedon, T.; Kumar, V.L. Artesunate Ameliorates Functional Limitations in Freund’s Complete Adjuvant-Induced Monoarthritis in Rat by Maintaining Oxidative Homeostasis and Inhibiting COX-2 Expression. Inflammation 2015, 38, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, T.; Rauber, S.; Uebe, S.; Luber, M.; Soare, A.; Ekici, A.; Weber, S.; Matei, A.E.; Chen, C.W.; Maier, C.; et al. PU.1 controls fibroblast polarization and tissue fibrosis. Nature 2019, 566, 344–349. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L.; Aubert, J.D.; Mikulic, J.; Golshayan, D. Fibrogenic Disorders in Human Diseases: From Inflammation to Organ Dysfunction. J. Med. Chem. 2018, 61, 9811–9840. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.X.; Cheung, C.M.G.; Teo, K.Y.C. Review of Fibrosis in Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2023, 246, 192–222. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Little, K.; Llorián-Salvador, M.; Tang, M.; Du, X.; Marry, S.; Chen, M.; Xu, H. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J. Neuroinflamm. 2020, 17, 355. [Google Scholar] [CrossRef]
- Patel, P.J.; Villavicencio, P.; Hanumunthadu, D. Systematic Review of Neovascular Age-Related Macular Degeneration Disease Activity Criteria Use to Shorten, Maintain or Extend Treatment Intervals with Anti-VEGF in Clinical Trials: Implications for Clinical Practice. Ophthalmol. Ther. 2023, 12, 2323–2346. [Google Scholar] [CrossRef]
- Chandra, S.; Arpa, C.; Menon, D.; Khalid, H.; Hamilton, R.; Nicholson, L.; Pal, B.; Fasolo, S.; Hykin, P.; Keane, P.A.; et al. Ten-year outcomes of antivascular endothelial growth factor therapy in neovascular age-related macular degeneration. Eye 2020, 34, 1888–1896. [Google Scholar] [CrossRef]
- Roberts, P.K.; Zotter, S.; Montuoro, A.; Pircher, M.; Baumann, B.; Ritter, M.; Hitzenberger, C.K.; Schmidt-Erfurth, U. Identification and Quantification of the Angiofibrotic Switch in Neovascular AMD. Investig. Ophthalmol. Vis. Sci. 2019, 60, 304–311. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; You, F.; Xue, J. Novel use for old drugs: The emerging role of artemisinin and its derivatives in fibrosis. Pharmacol. Res. 2020, 157, 104829. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Liu, X.M.; Ying, J.; Zu, T.; Jiang, J.; Liu, M.M.; Jin, J. Targeted inhibition of transforming growth factor-β type I receptor by AZ12601011 improves paraquat poisoning-induced multiple organ fibrosis. Pestic. Biochem. Physiol. 2024, 200, 105831. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hwang, Y.J.; Jung, G.S. Alantolactone Attenuates Renal Fibrosis via Inhibition of Transforming Growth Factor β/Smad3 Signaling Pathway. Diabetes Metab. J. 2024, 48, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J.; et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Park, J.H.; Park, B.; Park, K.K. Suppression of Hepatic Epithelial-to-Mesenchymal Transition by Melittin via Blocking of TGFβ/Smad and MAPK-JNK Signaling Pathways. Toxins 2017, 9, 138. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Li, X.; Sun, L.; Guo, J.W. Therapeutic effect of dihydroartemisinin on pulmonary fibrosis in rats with dust. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi = Zhonghua Laodong Weisheng Zhiyebing Zazhi = Chin. J. Ind. Hyg. Occup. Dis. 2019, 37, 96–103. [Google Scholar] [CrossRef]
- Nong, X.; Rajbanshi, G.; Chen, L.; Li, J.; Li, Z.; Liu, T.; Chen, S.; Wei, G.; Li, J. Effect of artesunate and relation with TGF-β1 and SMAD3 signaling on experimental hypertrophic scar model in rabbit ear. Arch. Dermatol. Res. 2019, 311, 761–772. [Google Scholar] [CrossRef]
- Li, H.X.; Liu, H.; Wang, C.M.; Wang, H.J.; Chen, J. Artesunate restraining MAPK passage by smad7 to resist pulmonary fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3199–3204. [Google Scholar]
- Wang, Z.Y.; Zhang, Y.; Wu, L.D.; Chen, J.; Chen, M.L.; Chen, C.M.; Xu, Q.H. Artesunate inhibits proliferation and migration of RPE cells and TGF-β2 mediated epithelial mesenchymal transition by suppressing PI3K/AKT pathway. Int. J. Ophthalmol. 2022, 15, 197–204. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, G.; Mo, B.; Wang, C. Artesunate modulates expression of matrix metalloproteinases and their inhibitors as well as collagen-IV to attenuate pulmonary fibrosis in rats. Genet. Mol. Res. GMR 2016, 15, 12. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, W.; Fang, B.; Gao, S.; Yan, J. Artesunate ameliorates hepatic fibrosis induced by bovine serum albumin in rats through regulating matrix metalloproteinases. Eur. J. Pharmacol. 2014, 744, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bai, R.; Wang, L.; Gao, J.; Zhang, H. Artesunate may inhibit liver fibrosis via the FAK/Akt/β-catenin pathway in LX-2 cells. BMC Pharmacol. Toxicol. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, Z.; Tong, B.; Wang, C.; Yang, J.; Zou, J.; Jiang, J.; Zhang, L.; Jiang, B. Artesunate protects against ocular fibrosis by suppressing fibroblast activation and inducing mitochondria-dependent ferroptosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2023, 37, e22954. [Google Scholar] [CrossRef]
- Larson, S.A.; Dolivo, D.M.; Dominko, T. Artesunate inhibits myofibroblast formation via induction of apoptosis and antagonism of pro-fibrotic gene expression in human dermal fibroblasts. Cell Biol. Int. 2019, 43, 1317–1322. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Miceli, M.V.; Liles, M.R.; Newsome, D.A. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp. Cell Res. 1994, 214, 242–249. [Google Scholar] [CrossRef]
- King, A.; Gottlieb, E.; Brooks, D.G.; Murphy, M.P.; Dunaief, J.L. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem. Photobiol. 2004, 79, 470–475. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- La Cunza, N.; Tan, L.X.; Thamban, T.; Germer, C.J.; Rathnasamy, G.; Toops, K.A.; Lakkaraju, A. Mitochondria-dependent phase separation of disease-relevant proteins drives pathological features of age-related macular degeneration. JCI Insight 2021, 6, e142254. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Romagnoli, A.; Refolo, G.; Vescovo, T.; Ciccosanti, F.; Zuchegna, C.; Lozzi, F.; Occhigrossi, L.; Piacentini, M.; Fimia, G.M. Role of AMBRA1 in mitophagy regulation: Emerging evidence in aging-related diseases. Autophagy 2024, 20, 2602–2615. [Google Scholar] [CrossRef]
- Dieguez, H.H.; Romeo, H.E.; Alaimo, A.; Bernal Aguirre, N.A.; Calanni, J.S.; Adán Aréan, J.S.; Alvarez, S.; Sciurano, R.; Rosenstein, R.E.; Dorfman, D. Mitochondrial quality control in non-exudative age-related macular degeneration: From molecular mechanisms to structural and functional recovery. Free Radic. Biol. Med. 2024, 219, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Ebeling, M.C.; Kapphahn, R.J.; Terluk, M.R.; Fisher, C.R.; Polanco, J.R.; Roehrich, H.; Leary, M.M.; Geng, Z.; Dutton, J.R.; et al. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol. 2017, 13, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide H2O2-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.R.; Ma, C.Q.; Jiang, J.H.; Wang, D.P.; Zhang, Q.Q.; Liu, M.R.; Zhao, H.R.; Fang, Q.; Liu, Y. Artesunate restores mitochondrial fusion-fission dynamics and alleviates neuronal injury in Alzheimer’s disease models. J. Neurochem. 2022, 162, 290–304. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, P.; Weng, W.; Sun, Z.; Liu, H.; Chen, Y.; Cai, Y.; Yu, X.; Wang, T.; Shao, M.; et al. Artemether attenuates renal tubular injury by targeting mitochondria in adriamycin nephropathy mice. Am. J. Transl. Res. 2022, 14, 2002–2012. [Google Scholar]
- Iacovelli, J.; Rowe, G.C.; Khadka, A.; Diaz-Aguilar, D.; Spencer, C.; Arany, Z.; Saint-Geniez, M. PGC-1α Induces Human RPE Oxidative Metabolism and Antioxidant Capacity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1038–1051. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Wang, M.; Weng, W.; Zhan, H.; Yu, X.; Yuan, C.; Shao, M.; Sun, H. Artemether improves type 1 diabetic kidney disease by regulating mitochondrial function. Am. J. Transl. Res. 2019, 11, 3879–3889. [Google Scholar]
- Han, P.; Cai, Y.; Wang, Y.; Weng, W.; Chen, Y.; Wang, M.; Zhan, H.; Yu, X.; Wang, T.; Shao, M.; et al. Artemether ameliorates kidney injury by restoring redox imbalance and improving mitochondrial function in Adriamycin nephropathy in mice. Sci. Rep. 2021, 11, 1266. [Google Scholar] [CrossRef]
- Schachar, I.H.; Zahid, S.; Comer, G.M.; Stem, M.; Schachar, A.G.; Saxe, S.J.; Gardner, T.W.; Elner, V.M.; Jayasundera, T. Quantification of fundus autofluorescence to detect disease severity in nonexudative age-related macular degeneration. JAMA Ophthalmol. 2013, 131, 1009–1015. [Google Scholar] [CrossRef]
- Waldstein, S.M.; Vogl, W.D.; Bogunovic, H.; Sadeghipour, A.; Riedl, S.; Schmidt-Erfurth, U. Characterization of Drusen and Hyperreflective Foci as Biomarkers for Disease Progression in Age-Related Macular Degeneration Using Artificial Intelligence in Optical Coherence Tomography. JAMA Ophthalmol. 2020, 138, 740–747. [Google Scholar] [CrossRef]
- van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.E.; Klein, R.; VandenLangenberg, G.M.; Klein, B.E.; Palta, M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995, 113, 743–748. [Google Scholar] [CrossRef]
- Jun, S.; Datta, S.; Wang, L.; Pegany, R.; Cano, M.; Handa, J.T. The impact of lipids, lipid oxidation, and inflammation on AMD, and the potential role of miRNAs on lipid metabolism in the RPE. Exp. Eye Res. 2019, 181, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Gabrielle, P.H. Lipid metabolism and retinal diseases. Acta Ophthalmol. 2022, 100 (Suppl 269), 3–43. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Wei, P.; He, M.; Jia, L.; Su, Q.; Yang, X.; Hao, R. Role of plasma fatty acid in age-related macular degeneration: Insights from a mendelian randomization analysis. Lipids Health Dis. 2024, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.; Kreis, A.J.; Wong, T.Y.; Simpson, J.A.; Guymer, R.H. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: A systematic review and meta-analysis. Arch. Ophthalmol. 2008, 126, 826–833. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Zhang, X.; Zhang, Q.; Nie, J.; Zhang, M.; Liu, X.; Ma, L. The Association between the Lipids Levels in Blood and Risk of Age-Related Macular Degeneration. Nutrients 2016, 8, 663. [Google Scholar] [CrossRef]
- Colijn, J.M.; den Hollander, A.I.; Demirkan, A.; Cougnard-Grégoire, A.; Verzijden, T.; Kersten, E.; Meester-Smoor, M.A.; Merle, B.M.J.; Papageorgiou, G.; Ahmad, S.; et al. Increased High-Density Lipoprotein Levels Associated with Age-Related Macular Degeneration: Evidence from the EYE-RISK and European Eye Epidemiology Consortia. Ophthalmology 2019, 126, 393–406. [Google Scholar] [CrossRef]
- Landowski, M.; Bowes Rickman, C. Targeting Lipid Metabolism for the Treatment of Age-Related Macular Degeneration: Insights from Preclinical Mouse Models. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2022, 38, 3–32. [Google Scholar] [CrossRef]
- Jang, B.C. Artesunate inhibits adipogeneis in 3T3-L1 preadipocytes by reducing the expression and/or phosphorylation levels of C/EBP-α, PPAR-γ, FAS, perilipin A, and STAT-3. Biochem. Biophys. Res. Commun. 2016, 474, 220–225. [Google Scholar] [CrossRef]
- Zhang, G.; Li, N.; Tong, Y.; Li, P.; Han, H.; Song, Q.; Yang, B.; Cui, L. Artemisinin derivatives inhibit adipogenic differentiation of 3T3-L1 preadipocytes through upregulation of CHOP. Biochem. Biophys. Res. Commun. 2021, 557, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cheng, Y.; Li, H.; Guan, H.; Xiao, H.; Li, Y. The Potential of Artemisinins as Novel Treatment for Thyroid Eye Disease by Inhibiting Adipogenesis in Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Gao, J.H.; Yu, X.H.; Wen, F.J.; Luo, J.J.; Qin, Y.S.; Chen, M.X.; Zhang, D.W.; Wang, Z.B.; Tang, C.K. Artesunate inhibits atherosclerosis by upregulating vascular smooth muscle cells-derived LPL expression via the KLF2/NRF2/TCF7L2 pathway. Eur. J. Pharmacol. 2020, 884, 173408. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Liu, Y.; Liang, S.; Liu, P.; Qian, H.; Wu, Q.; Wang, Y. The Metabolic Changes of Artesunate and Ursolic Acid on Syrian Golden Hamsters Fed with the High-Fat Diet. Molecules 2020, 25, 1392. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Tian, K.; Bai, X.; Wang, Y.; Wang, Y. Artesunate inhibits macrophage-like phenotype switching of vascular smooth muscle cells and attenuates vascular inflammatory injury in atherosclerosis via NLRP3. Biomed. Pharmacother. = Biomed. Pharmacother. 2024, 172, 116255. [Google Scholar] [CrossRef]
- Fu, W.; Hongwei, J.; Li, J. Artemether treatment improves islet function and metabolic homeostasis in diabetic nonhuman primates. J. Diabetes 2023, 15, 76–80. [Google Scholar] [CrossRef]
- Lei, Z.; Wu, H.; Yang, Y.; Hu, Q.; Lei, Y.; Liu, W.; Nie, Y.; Yang, L.; Zhang, X.; Yang, C.; et al. Dihydroartemisinin improves hypercholesterolemia in ovariectomized mice via enhancing vectorial transport of cholesterol and bile acids from blood to bile. Bioorg. Med. Chem. 2022, 53, 116520. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood-retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004, 78, 715–721. [Google Scholar] [CrossRef]
- Caspi, R.R. Ocular autoimmunity: The price of privilege? Immunol. Rev. 2006, 213, 23–35. [Google Scholar] [CrossRef]
- Allingham, M.J.; Loksztejn, A.; Cousins, S.W.; Mettu, P.S. Immunological Aspects of Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2021, 1256, 143–189. [Google Scholar] [CrossRef]
- Moir, J.; Hyman, M.J.; Wang, J.; Shah, A.; Maatouk, C.; Flores, A.; Skondra, D. Associations Between Autoimmune Disease and the Development of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2023, 64, 45. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, K.; Goodwin, A.M.; Ohbayashi, M.; Ono, S.J. Autoimmunity in retinal degeneration: Autoimmune retinopathy and age-related macular degeneration. J. Autoimmun. 2009, 33, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Ohbayashi, M.; Nugent, A.K.; Ramchand, K.; Toda, M.; Chau, K.Y.; Bunce, C.; Webster, A.; Bird, A.C.; Ono, S.J.; et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology 2005, 115, 422–430. [Google Scholar] [CrossRef]
- Fletcher, E.L. Contribution of microglia and monocytes to the development and progression of age related macular degeneration. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. 2020, 40, 128–139. [Google Scholar] [CrossRef]
- Gu, J.; Pauer, G.J.; Yue, X.; Narendra, U.; Sturgill, G.M.; Bena, J.; Gu, X.; Peachey, N.S.; Salomon, R.G.; Hagstrom, S.A.; et al. Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Mol. Cell. Proteom. MCP 2009, 8, 1338–1349. [Google Scholar] [CrossRef]
- Lin, J.B.; Santeford, A.; Usmani, D.; Shah, A.V.; Ruzycki, P.A.; Apte, R.S. Cell-specific Systemic Immune Signatures Associated with Treatment Burden in Neovascular Age-related Macular Degeneration. Ophthalmol. Sci. 2024, 4, 100410. [Google Scholar] [CrossRef]
- Shakir, L.; Hussain, M.; Javeed, A.; Ashraf, M.; Riaz, A. Artemisinins and immune system. Eur. J. Pharmacol. 2011, 668, 6–14. [Google Scholar] [CrossRef]
- Morad, H.O.J.; Luqman, S.; Pinto, L.G.; Cunningham, K.P.; Vilar, B.; Clayton, G.; Shankar-Hari, M.; McNaughton, P.A. Artemisinin inhibits neutrophil and macrophage chemotaxis, cytokine production and NET release. Sci. Rep. 2022, 12, 11078. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, T.; Wang, W.; Yang, B.; Cha, X. Dihydroartemisinin attenuated the symptoms of mice model of systemic lupus erythematosus by restoring the Treg/Th17 balance. Clin. Exp. Pharmacol. Physiol. 2021, 48, 626–633. [Google Scholar] [CrossRef]
- Li, T.; Chen, H.; Yang, Z.; Liu, X.G.; Zhang, L.M.; Wang, H. Evaluation of the immunosuppressive activity of artesunate in vitro and in vivo. Int. Immunopharmacol. 2013, 16, 306–312. [Google Scholar] [CrossRef]
- Shi, X.; Liao, T.; Chen, Y.; Chen, J.; Liu, Y.; Zhao, J.; Dang, J.; Sun, Q.; Pan, Y. Dihydroartemisinin inhibits follicular helper T and B cells: Implications for systemic lupus erythematosus treatment. Arch. Pharmacal Res. 2024, 47, 632–644. [Google Scholar] [CrossRef]
- Tong, X.; Chen, L.; He, S.J.; Zuo, J.P. Artemisinin derivative SM934 in the treatment of autoimmune and inflammatory diseases: Therapeutic effects and molecular mechanisms. Acta Pharmacol. Sin. 2022, 43, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.M.; Yang, X.Q.; Zhu, F.H.; He, S.J.; Tang, W.; Zuo, J.P. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells. Sci. Rep. 2016, 6, 38115. [Google Scholar] [CrossRef]
- Chen, K.; Tang, L.; Nong, X. Artesunate targets cellular metabolism to regulate the Th17/Treg cell balance. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2023, 72, 1037–1050. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Fu, X.; Huang, X.; Zhang, S.; Zhao, N.; Ma, X.; Saiding, Q.; Yang, M.; Tao, W.; et al. Innovative Nanotechnology in Drug Delivery Systems for Advanced Treatment of Posterior Segment Ocular Diseases. Adv. Sci. 2024, 11, e2403399. [Google Scholar] [CrossRef]
| Artemisinins | Vivo or Vitro | Model | Dosage and Duration | Administration Route | Curative Effects | Potential Mechanism | References |
|---|---|---|---|---|---|---|---|
| Dihydroartemisinin | in vivo (mice) | laser-induced CNV | 200 mg/kg/d for 12 days | Oral (intragastric administration) | Inhibited CNV formation | Suppresses the classic NF-κB signaling pathway and downregulates the expression of VEGFR-2 and VEGF | [48] |
| Artemisinin | in vitro | CAM assay | Artemisinin 0.025% and dexamethasone 0.025% for 8 h | - | Anti-angiogenic | Produces remarkable good anti-angiogenic effect by its improved solubility and enhanced permeability | [49] |
| Artemisinin (loaded nanomicelles) | in vitro | CAM assay | Artemisinin 0.05% for 24 h | - | Anti-angiogenic | Increases solubility, promotes corneal penetration and affects drug release | [16] |
| Artesunate | in vitro/ in vivo (mice) | ChEC cell/ laser-induced CNV | 10 μM for 24 h/ 8 μg (4 μg per dose, once weekly) | -/ Intravitreal injection | Inhibited CNV and the accompanying fibrosis | Reduces inflammatory factors, downregulates fibrotic factors and inhibits MP recruitment | [47] |
| Artesunate | in vivo (rabbit/ monkey) | ocular neovascularization | A single dose of 1 μg/ A single dose of 20 μg | intravitreal injection | Attenuated ocular neovascularization and macular edema | Downregulates VEGFR-2, PKCα, and PDGFR expression | [50] |
| Artemisinin | in vitro | D407 cells | 3–100 μM for 2 h | - | Reduce oxidative stress | Inhibits the generation of intracellular ROS, modulates △ψm and caspase 3/7 dependent pathway, and activates ERK1/2 signaling | [17] |
| Artemisinin | in vitro | D407 and primary cultured RPE cells | 3.125–100 μM for 2 h | - | Reduce oxidative stress | Reduces intracellular ROS generation and oxidative stress, decreases LDH release and the loss of mitochondrial membrane potential, and enhances the activation of AMPK | [51] |
| Artemisinin | in vitro | D407 and ARPE19 cell line | 20 μM for 1 h | - | Reduce oxidative stress | Increase Acetyl-H4 (Lys 8) level | [52] |
| Artemisinin | in vitro/ in vivo (SD rats) | RGC-5 cells/ light-exposed retinal damage | 6.25–100 μM for 24 h/ 30, 100, 300 μg/mL | -/ Intravitreous injection | Inhibit oxidative damage | Decreases the production of intracellular ROS, increases mitochondrial membrane potential, decreases cell apoptosis and upregulates the phosphorylation of p38 and ERK1/2 | [53] |
| Mitochondrial Process | Role in AMD Pathogenesis | ART’s Therapeutic Action | References |
|---|---|---|---|
| Energy production | ROS induces RPE oxidative damage | Inhibits ROS via ERK1/2 and p38 signaling pathways | [53] |
| Fusion-fission dynamics | Fragmentation impairs metabolic efficiency | Enhances fusion kinetics and delays fragmentation | [144] |
| Membrane potential stability | Loss of ΔΨm triggers apoptosis | Activates AMPK to stabilize ΔΨm and inhibit caspase-3 | [51,143] |
| Biosynthesis (PGC-1α) | Impaired biosynthesis reduces antioxidant capacity | Upregulates PGC-1α and regulates pyruvate metabolism | [145,146,147] |
| Redox homeostasis | Oxidative imbalance disrupts Bruch’s membrane | Restores balance via H2O2 regulation and Nrf2 activation | [109,148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, X.; Duan, J. Artemisinin and Its Derivatives: Promising Therapeutic Agents for Age-Related Macular Degeneration. Pharmaceuticals 2025, 18, 535. https://doi.org/10.3390/ph18040535
Liu C, Liu X, Duan J. Artemisinin and Its Derivatives: Promising Therapeutic Agents for Age-Related Macular Degeneration. Pharmaceuticals. 2025; 18(4):535. https://doi.org/10.3390/ph18040535
Chicago/Turabian StyleLiu, Chun, Xiaoqin Liu, and Junguo Duan. 2025. "Artemisinin and Its Derivatives: Promising Therapeutic Agents for Age-Related Macular Degeneration" Pharmaceuticals 18, no. 4: 535. https://doi.org/10.3390/ph18040535
APA StyleLiu, C., Liu, X., & Duan, J. (2025). Artemisinin and Its Derivatives: Promising Therapeutic Agents for Age-Related Macular Degeneration. Pharmaceuticals, 18(4), 535. https://doi.org/10.3390/ph18040535





