1. Introduction
Hydrogen peroxide (H
2O
2) has been a staple in the field of neurosurgery for many decades. Its unique properties as a powerful oxidizing agent have made it an indispensable tool in surgical practice, achieving hemostasis and antiseptic effects [
1]. In recent years, research has explored the potential of hydrogen peroxide in the treatment of certain brain tumors as well [
2,
3,
4]. Despite its widespread use in neurosurgery, the mechanisms of action of H
2O
2 are not yet fully understood, and its use is not without controversy. Some studies have raised concerns about its potential toxicity to neural cells, particularly when used in high concentrations or for prolonged periods [
3,
5]. Further, there are several published cases in both spinal and cranial surgery reporting vulnerability to gas embolism with a rare case of clinically relevant morbidity [
1,
3,
6,
7,
8,
9]. Due to its two-sided characteristic, there are a variety of individual opinions about hydrogen peroxide in neurosurgical practice. Therefore, the aim of this study was first to perform a survey concerning the use of H
2O
2 in neurosurgery to gain clarity regarding current practice. According to the results of the survey, there was significant ambiguity concerning the pathophysiological effects of H
2O
2 on neuronal cells, which led us to the second aim of the study, namely, analyzing the penetration depth and damaging effects of H
2O
2 on neuronal cells in vitro and in vivo. In this context, the effect of bipolar coagulation as an inevitable hemostasis tool in neurosurgery was analyzed as well.
2. Results
2.1. Result of Online Survey
In total, 242 neurosurgeons practicing throughout Germany participated in the online survey. Of the respondents, 36.0% were consultants, 33.9% residents, 23,1% fellows, and 7% directors, respectively. Nearly half had more than 10 years of experience in neurosurgery. The use of H
2O
2 in neurosurgical practice was confirmed by 81% of participants, whereas 19% did not use H
2O
2. In particular, 62% of respondents confirmed the use of H
2O
2 in intracranial surgery; more than half of those (55.0%) used it up to as deep as the intradural tissue layer. In the case of H
2O
2 use up to intradural tissue, its use was similarly distributed in variety of surgical procedures, including tumor, abscess, traumatic brain injury, and intracerebral surgery. In the case of vascular surgery, the use of H
2O
2 was less frequent. The last question focused on reasons for the restrictive use of H
2O
2. Among all respondents, 28.6% assumed neuronal injury, 26.4% reported use based on departmental of internal regulations, and 24.7% did not know the reason. Interestingly, only 5.0% of the neurosurgeons had a literature-based knowledge of the pathophysiological mechanism of H
2O
2 concerning neuronal damage. Detailed results of survey are shown in
Figure 1.
2.2. Basic Demographics of the Participants
In total, four patients donated their brain/tumor tissue for the study’s purposes. The mean age of patients that donated brain and tumor tissue was 55.6 ± 6.0 years, and two patients were female (50%). Of the four patients, two (50%) had glioblastoma and the other two had metastasis (50%). None of the patients received a presurgical treatment like radiation or chemotherapy, and the surgical treatment was the first-line therapy according to the interdisciplinary neurooncologic conference.
2.3. Hydrogen Peroxide Exposure in Mouse and Human Brain Tissue
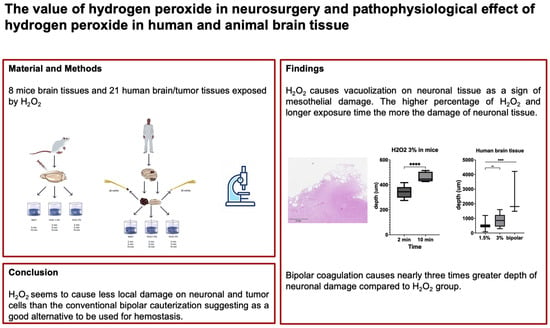
In total, eight mouse brain tissue samples, 21 human brain tissue samples, and seven human tumor tissue samples were processed and analyzed. For all slices of mouse and human brain tissues, the occurrence of vacuolization was independent of the status of intact arachnoidal/pial layer or subcortical tissue exposure.
The slices of mouse brain tissue after exposure to NaCl 0.9% and H
2O
2 in 1.5% and 3% concentrations divided by different time periods (
n = 8) are illustrated in
Figure S1. There was mesothelial damage visible with the occurrence of vacuolization in brain tissue exposed only to H
2O
2 3%. After 2 min exposure, the mean depth of the damage was 343.7 ± 39.7 μm, whereas after 10 min exposure, the mean depth of damage was significantly higher (460.1 ± 36.4 μm;
p < 0.0001; diff. 95% CI 75.5–157.3; effect size d
Cohen 3.1). In slices of mouse brain tissue after exposure to NaCl 0.9% across different time lines, there was no occurrence of vacuolization at all.
Slices of human healthy brain tissue after exposure to NaCl and H
2O
2 1.5%/3% and bipolar cauterization with 20 mA (
n = 19) are illustrated in
Figure 2. Vacuolization in human brain tissue could be observed in 50% of the cases after exposure to 1.5% as well as 3% H
2O
2. The depth of damage was significantly different among those groups: 543.8 ± 304.5 μm in H
2O
2 1.5%, 859.0 ± 379 μm in H
2O
2 3%, and 2504 ± 1490 μm in the bipolar cauterization group (H
2O
2 1.5% vs. bipolar:
p < 0.001; diff. 95% CI 1368–2553; effect size d
Cohen 1.8; H
2O
2 3% vs. bipolar:
p < 0.001; diff. 95% CI 994.5–2296; effect size d
Cohen 1.5; H
2O
2 1.5% vs. H
2O
2 3%;
p = 0.003, diff. 95% CI 137.5–492.9; effect size d
Cohen 0.9; respectively). Of note, the depth of damage was nearly three times greater in the bipolar cauterization group compared to the H
2O
2 groups. Concerning the duration of H
2O
2 exposure, there was no significant difference among the groups (
Figure 3). Similarly to the mouse brain slices, there was no occurrence of vacuolization or mesothelial damage across different time lines in human brain slices exposed to NaCl 0.9%.
In the case of tumor tissue, vacuolization was only visible in 66.7% of cases in the group with H
2O
2 3% exposure (
Figure S2). Comparing the depth of damage between healthy brain tissue and tumor tissue, there was no statistical difference after exposure to H
2O
2 3% (767.9 ± 387.9 μm vs. 1041 ± 300.0 μm;
p > 0.05; diff. 95% CI −250.4–171; effect size d
Cohen 0.8) (
Figure 3).
2.4. Characterization of Mesothelial Vacuolization in Mouse and Human Brain Tissue
The number of vacuoles, percentage of vacuole areas, and the total size of the vacuoles are illustrated in
Figure 4.
In mouse brain tissues, the number of vacuoles, the percentage of vacuole areas, and the total size of vacuoles were higher after longer exposure to H2O2 (n = 38 versus 62; 1.0% versus 3.4%; 0.01 ± 0.01 mm2 versus 0.03 ± 0.03 mm2, p < 0.001; diff. 95% CI 0.006–0.024; effect size dCohen 0.9).
In human brain tissues, the results were inconsistent. The number of vacuoles and vacuole sizes in terms of area were not different between the different concentrations of H2O2; however, the sizes of vacuoles were significantly larger in tissues exposed to H2O2 3% compared to H2O2 1.5% (0.02 ± 0.03 mm2 versus 0.03 ± 0.06 mm2; p = 0.001; diff. 95% CI 0.004–0.025; effect size dCohen 0.2). There was a trend toward higher numbers and larger vacuole areas after longer exposure to H2O2 1.5%; however, surprisingly, the numbers of vacuoles and their areas were lower and smaller, respectively, after longer exposure to 3% H2O2.
3. Discussion
In this study, we investigated the pathophysiological effects of H2O2 in neuronal tissues with a focus on its use as a potential hemostatic and tumor treating tool in neurosurgery. Our results showed that neuronal damage induced by H2O2 was dependent on concentration and duration and independent of intact arachnoidal/pial layer. The depth of neuronal damage was limited to up to 1 mm, which was 2.5 times less than that caused by bipolar coagulation. The effect was visible both in healthy brain and tumor tissues.
To date, H
2O
2 has been continuously used in a variety of different fields, including plastic, orthopedic, and general surgery, as well as neurosurgery [
1]. The primary concern surrounding the use of H
2O
2 in neurosurgery is its potential neurotoxicity, as evidenced by the current survey, which revealed that 20% of respondents do not utilize it, and when it is used, it is more frequently employed in spinal surgery. However, when asked about their reason for H
2O
2 restriction, less than 5% of respondents could support it with literature-based evidence; therefore, we felt a responsibility to pursue this matter with a preclinical and clinical study.
The first scientific study to evaluate the in vitro effects of H
2O
2 on neuronal tissue was conducted by Hansson and Vallfors, published in the 1980s [
10]. Therein, there was thrombosis of leptomeningeal vessels and extensive blood–brain barrier dysfunction, with cell disintegration observed by exposure to H
2O
2 3%. In a more recent study, by Mesiwala et al., the damage to the surface was limited to the arachnoid surface, and up to 1 mm of tissue beyond the resection margin was characterized by vacuolization and degeneration of neurons, astrocytes, and microglia [
3]. Our study was able to show results similar to those of the previous study, with development of stomal vacuolization of up to 1 mm, whereas the lower concentration of H
2O
2 (1.5%) showed significantly less invasiveness, with smaller vacuole sizes and numbers. Otherwise, the duration of exposure was not a significant factor for increased damage in human brain tissue compared to mouse brain tissue. Of note, bipolar coagulation showed significantly increased invasive damage to the neuronal cells and connective tissue compared to the use of H
2O
2.
There have been several H
2O
2-related cases reported with adverse effects [
2,
5,
6,
7,
8,
9]. The most dreaded complication is gas embolism in the vessel, which might cause, albeit rarely, severe ischemic and systematic sequalae. This effect underlies the extremely efficient decomposition of H
2O
2 into water and O
2 by exposure to the family of catalase enzymes (200,000 reactions per second), which are found in all cells [
11]. Usually, the cascade is physiological to avoid the production of cell-damaging hydroxyl radicals. The microbubbles induce mechanical removal of tissue debris, thus exhibiting antimicrobial properties [
12]. On the other hand, 1 mL of H
2O
2 3% produces 10 mL of O
2. In the case of an excess amount of H
2O
2, the sudden high O
2 burden can result in oxygen embolization, with systemic embolism in coronary or cerebral arteries [
5,
13]. Further, H
2O
2 has the property of inducing vasoconstriction, whereas there are some inconsistencies depending on the vascular segment and species as well [
12]. The risk of gas embolism related to the use of H
2O
2 is not restricted to intracranial procedures, but occurs also in other types of surgery as well, such as in spinal, traumatic, and orthopedic procedures. In a recent review, some risk factors for vulnerability to gas embolism were identified, like open dural wounds with close relationships to major vessels and the seated position of the patients [
1]. In our clinical experience of using H
2O
2 in over 50 cranial surgeries (not illustrated in this study), all postoperative MRI scans performed within 3 months after surgical treatment were evaluated and showed no radiological signs of embolic ischemic complications nor abnormalities in the diffusion-weighted sequence in the resection margin of the brain tumors or intracerebral hemorrhage. Of note, we do not perform intracranial surgery with patients in the seated position anymore due to the risk of gas embolism independent of the use of H
2O
2, and restrict the use of H
2O
2 in closed cavities. In a previous study, there was one case of embolic complication reported in over 800 surgical cases, resulting in a complication rate of 0.1% [
12]. While there are still concerns surrounding the use of H
2O
2 in neurosurgery, our study suggests that its cautious use (e.g., H
2O
2 in a diluted concentration of 1.5% with an exposure time of less than 2 min), with respect to the correct positioning of patients and local application in the resection cavity, might be beneficial for hemostasis in cases of diffuse bleeding or marginal tumor treatment with less local damage compared to the conventional bipolar coagulation and low risk for gas embolism. However, it is again important to be alert to the possibility of gas embolism.
Limitations
While this study provides valuable insights into the effects of hydrogen peroxide on neural tissue, there are several limitations that should be mentioned.
Firstly, the study only examined the effects of H2O2 on neural tissue, and did not investigate the effects of other oxidative stressors or antioxidants. Further, the effect of H2O2 on cellular or molecular areas with its pathophysiological mechanism is still elusive. This limits the generalizability of the findings and highlights the need for further research in this area. Secondly, the study used a relatively small sample size, which may not be representative of the larger population. This may limit the statistical power of the study and increase the risk of type II errors. Finally, the study did not investigate the long-term effects of hydrogen peroxide on neural tissue. In our point of view, there are many confounding factors that might influence the effect on neural tissue like postoperative adjuvant radiochemotherapy, tumor progress, sequalae of traumatic brain injury or intracerebral hemorrhage, or infection, resulting in some limitations to the focus solely on the effect of hydrogen peroxide. However, this is an important area for future research, as the long-term effects of oxidative stress on neural tissue are not well understood. Lastly, the survey was performed using an online CME platform. This might not fully represent the broader neurosurgical community and may suffer from response or selection bias.