Pharmacological Efficacy of Intravenous Magnesium in Attenuating Remifentanil-Induced Postoperative Hyperalgesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
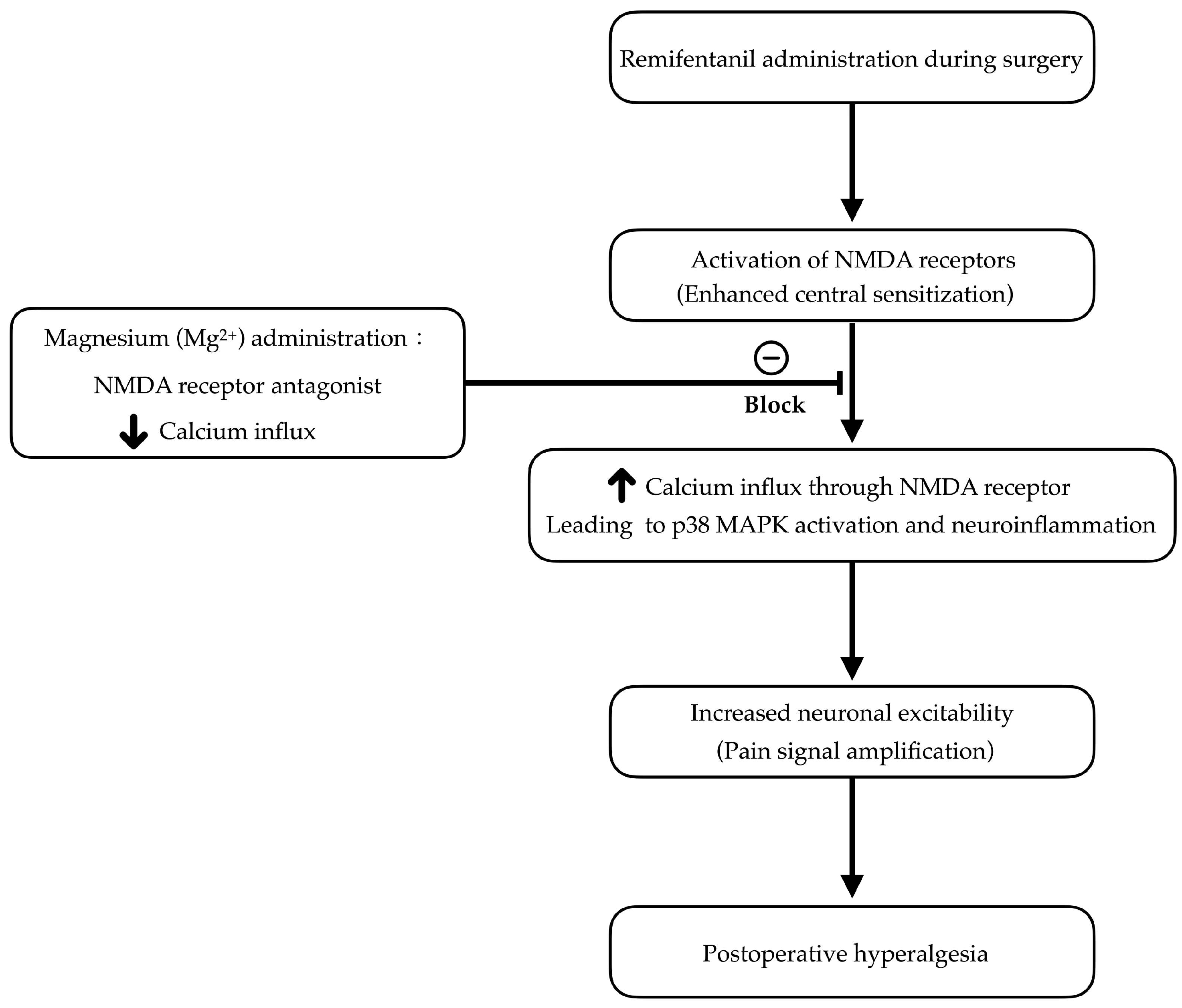
1. Introduction
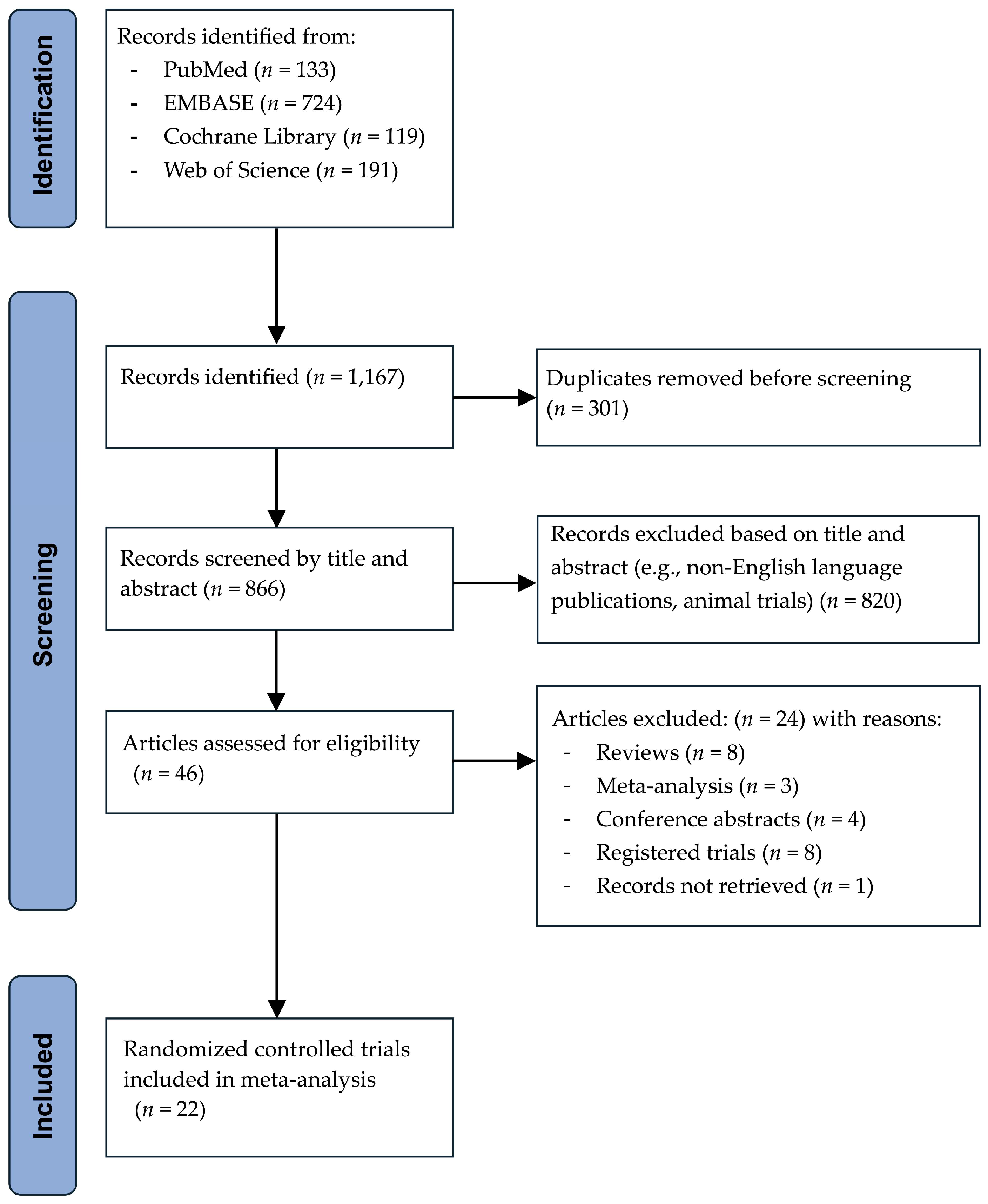
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment and Evidence Grading
2.5. Outcome Measures and Data Standardization
2.6. Data Synthesis and Analysis
2.7. Planned Subgroup Analyses
2.8. Assessment of Publication Bias
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. Primary Outcomes
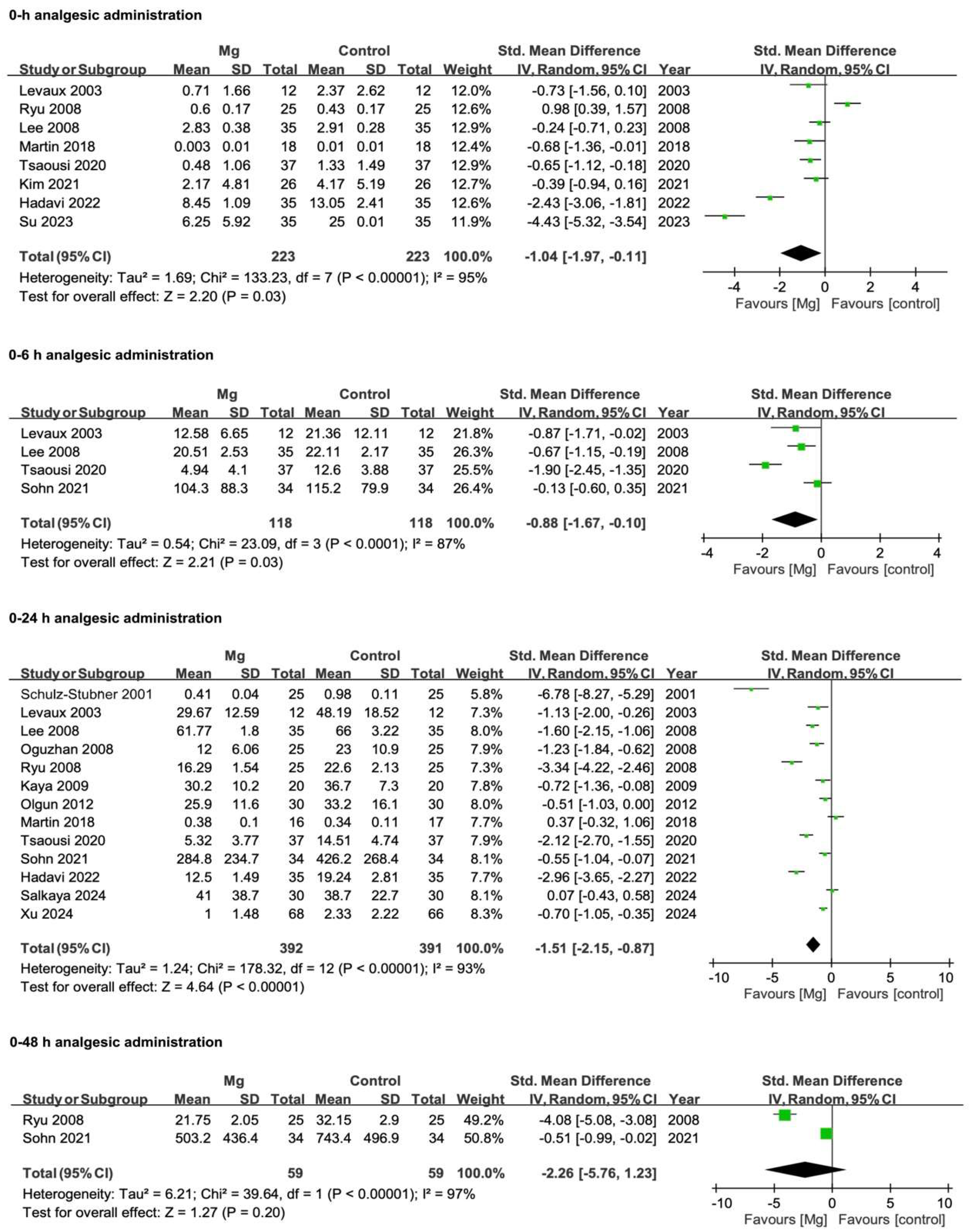
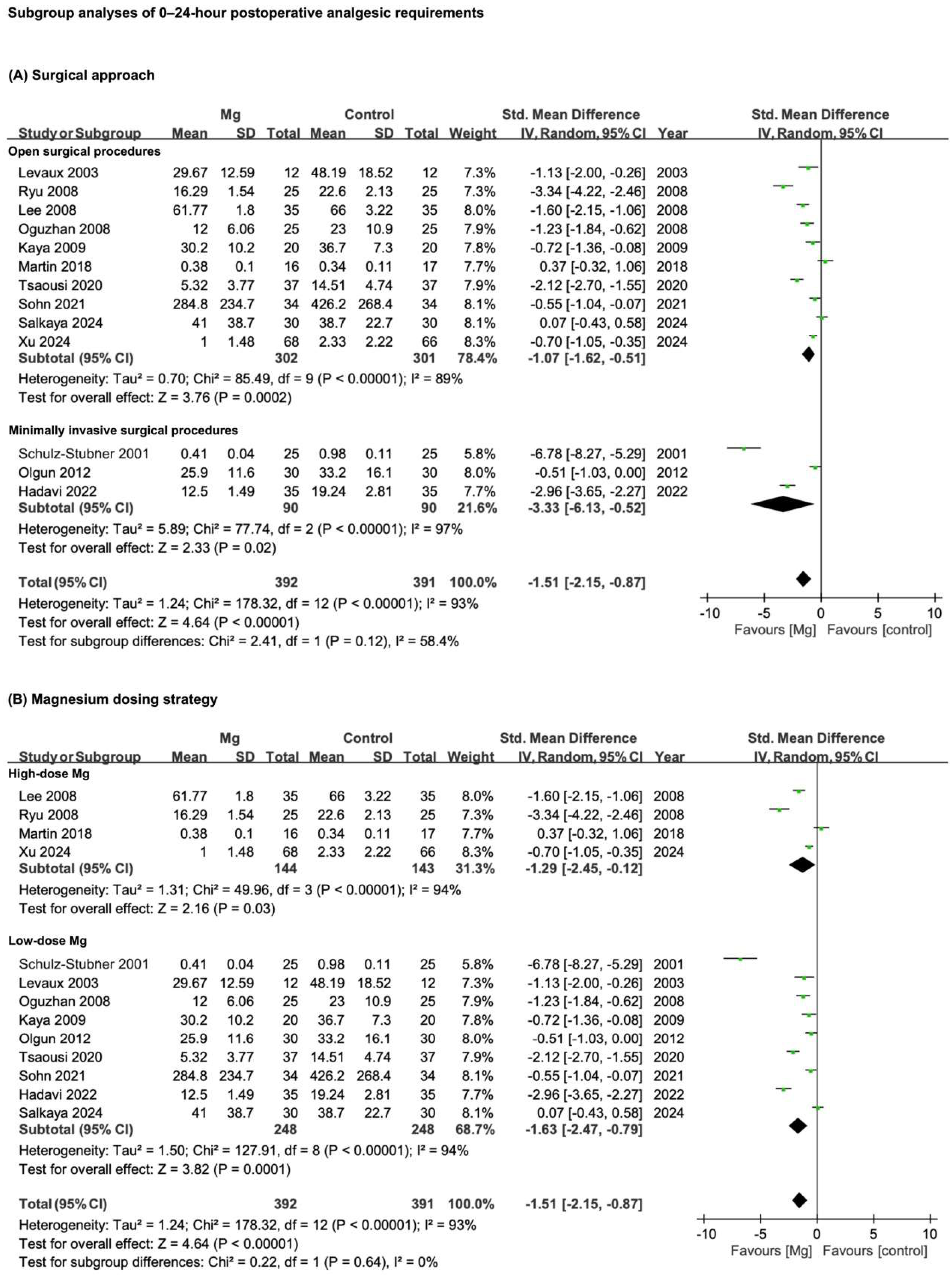
3.3.1. Postoperative Analgesic Requirements
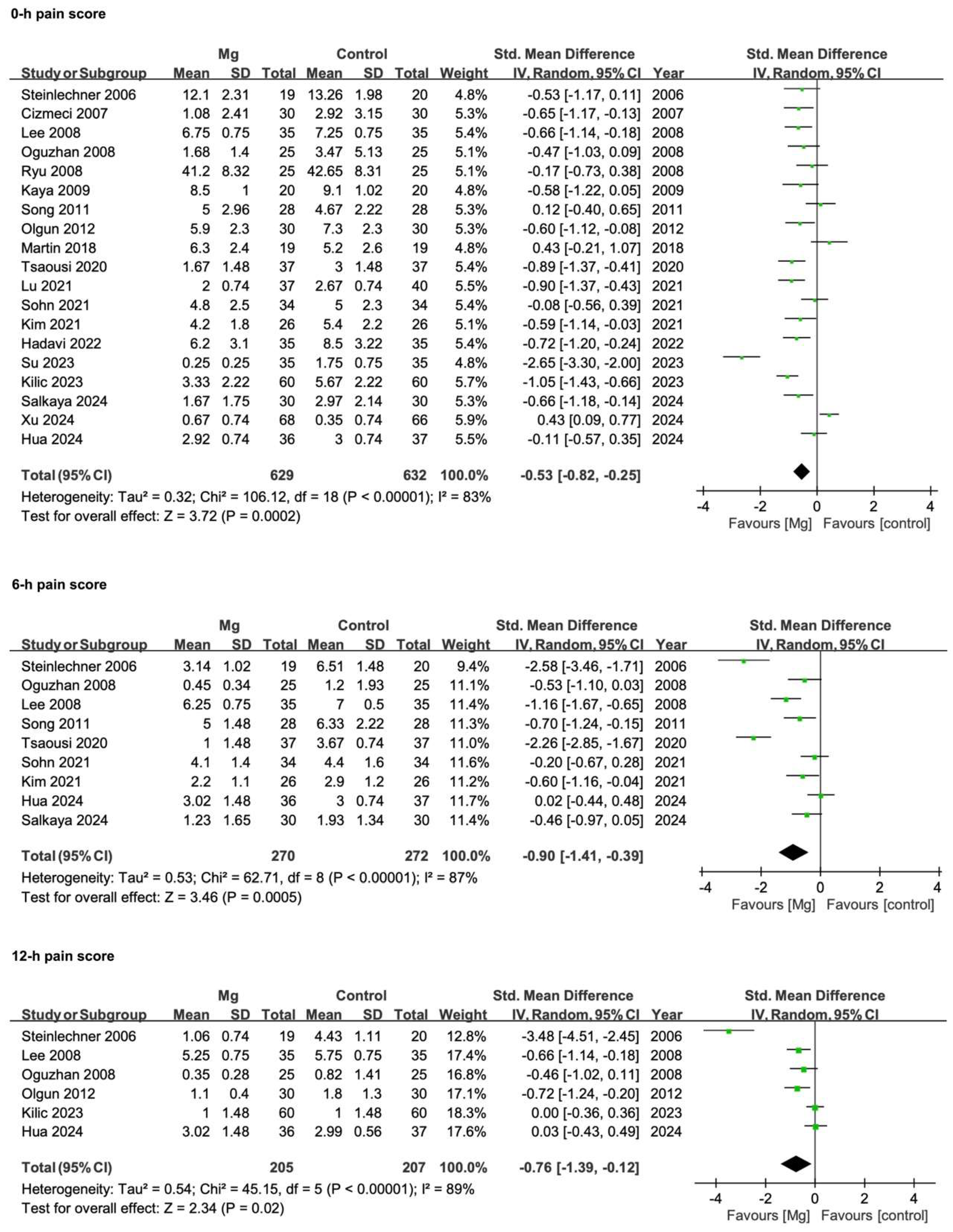
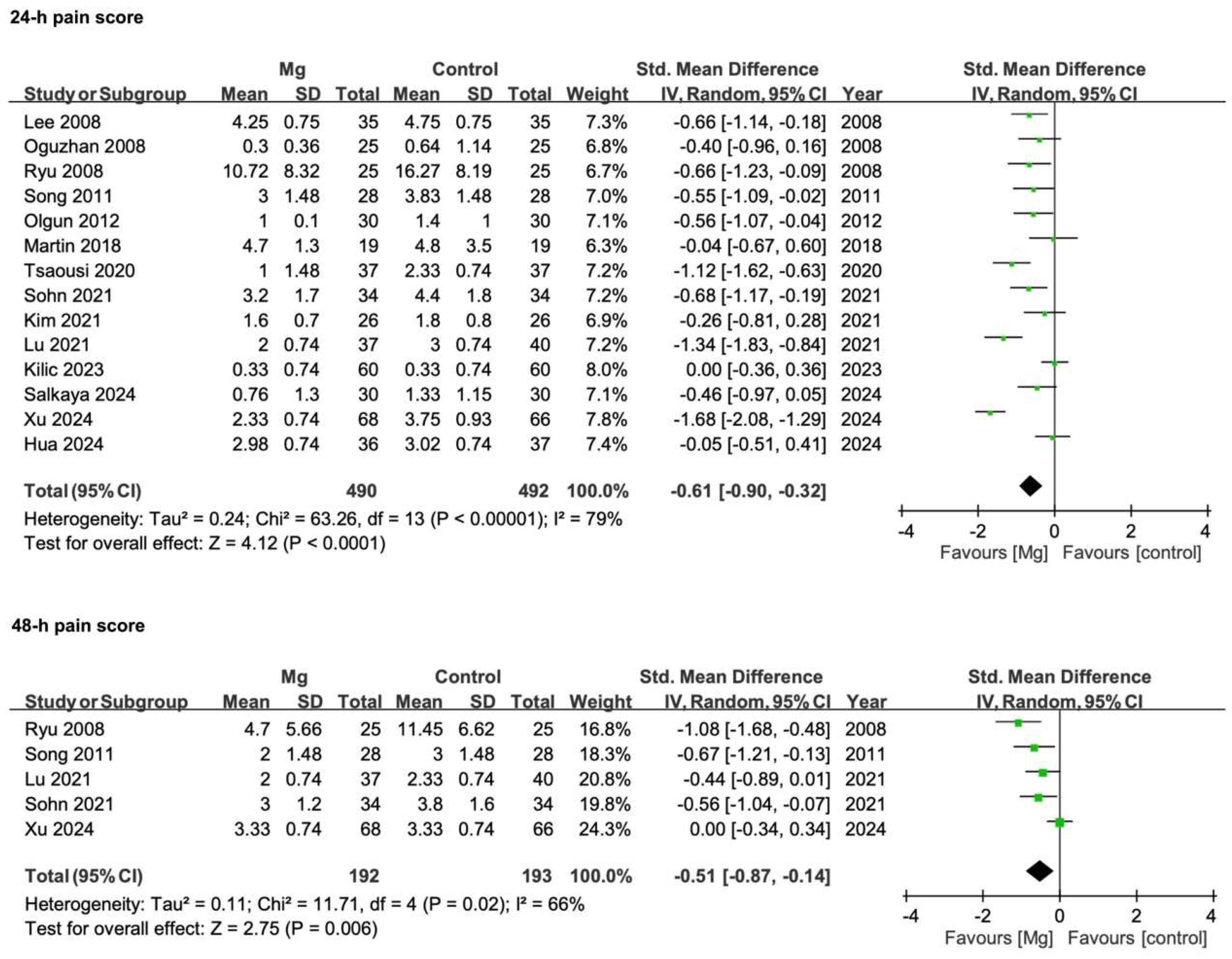
3.3.2. Postoperative Pain Scores
3.4. Secondary Outcomes
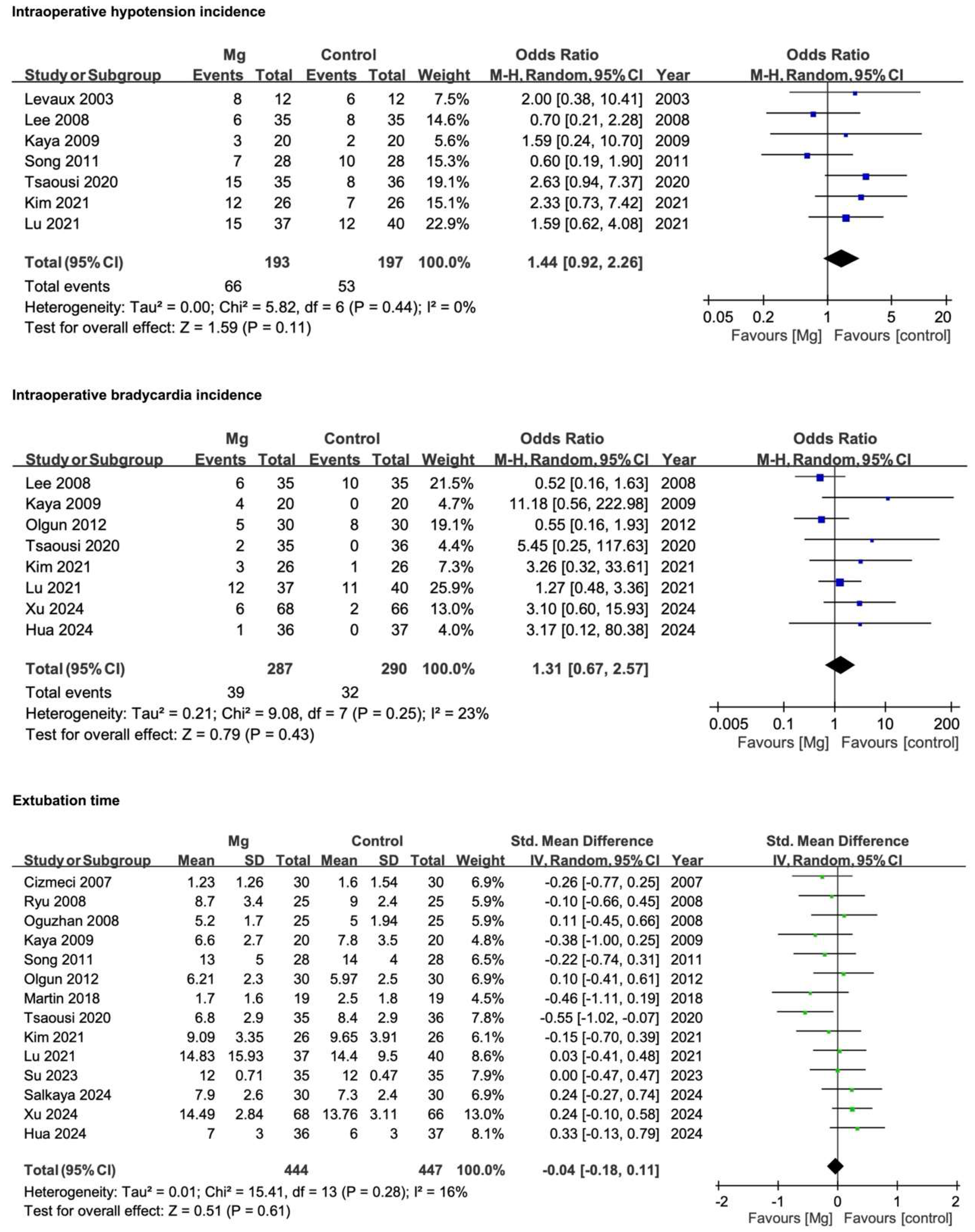
3.4.1. Intraoperative Hypotension Incidence
3.4.2. Intraoperative Bradycardia Incidence
3.4.3. Extubation Time
3.4.4. Number of Patients Requiring Rescue Analgesia
3.4.5. Incidence of Shivering
3.4.6. Incidence of PONV
3.4.7. Patient Satisfaction Scores
3.4.8. Intraoperative Remifentanil Consumption
3.5. Results of Subgroup Analyses
3.5.1. Surgical Approach: Open vs. Minimally Invasive Procedures
3.5.2. Magnesium Dosing Strategy
3.6. Sensitivity Analyses
3.6.1. Excluding Kilic et al. (2023) [37]
3.6.2. Re-Analyzing Under a Fixed-Effects Model
3.7. Publication Bias Analyses Results
3.8. GRADE Assessment
3.9. Additional Subgroup and Meta-Regression Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OIH | Opioid-induced hyperalgesia |
| NMDA | N-methyl-D-aspartate |
| MAPK | Mitogen-activated protein kinase |
| RCT | Randomized controlled trial |
| SD | Standard deviation |
| SE | Standard error |
| CI | Confidence interval |
| IQR | Interquartile range |
| PONV | Postoperative nausea and vomiting |
| RoB | Risk of bias |
| GRADE | Grading of recommendations, assessment, development, and evaluation |
| PCA | Patient-controlled analgesia |
| VAS | Visual analogue scale |
| SMD | Standardized mean difference |
| OR | Odds ratio |
| ASA | American Society of Anesthesiologists |
| NAS | Numeric analogue scale |
| NRS | Numeric rating scale |
| VNRS | Verbal numeric rating scale |
| TCI | Target-controlled infusion |
References
- Fletcher, D.; Martinez, V. Opioid-Induced Hyperalgesia in Patients after Surgery: A Systematic Review and a Meta-Analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A Comprehensive Review of Opioid-Induced Hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [PubMed]
- de Lima, F.O.; Lauria, P.S.S.; do Espirito-Santo, R.F.; Evangelista, A.F.; Nogueira, T.M.O.; Araldi, D.; Soares, M.B.P.; Villarreal, C.F. Unveiling Targets for Treating Postoperative Pain: The Role of the TNF-Alpha/p38 MAPK/NF-KappaB/Nav1.8 and Nav1.9 Pathways in the Mouse Model of Incisional Pain. Int. J. Mol. Sci. 2022, 23, 11630. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kang, M.H.; Park, K.S.; Do, S.H. Effects of Magnesium Sulphate on Intraoperative Anaesthetic Requirements and Postoperative Analgesia in Gynaecology Patients Receiving Total Intravenous Anaesthesia. Br. J. Anaesth. 2008, 100, 397–403. [Google Scholar] [CrossRef]
- Fawcett, W.J.; Haxby, E.J.; Male, D.A. Magnesium: Physiology and Pharmacology. Br. J. Anaesth. 1999, 83, 302–320. [Google Scholar] [CrossRef]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. A Narrative Review on the Role of Magnesium in Immune Regulation, Inflammation, Infectious Diseases, and Cancer. J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef]
- Lysakowski, C.; Dumont, L.; Czarnetzki, C.; Tramer, M.R. Magnesium as an Adjuvant to Postoperative Analgesia: A Systematic Review of Randomized Trials. Anesth. Analg. 2007, 104, 1532–1539. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Gu, X.; Ma, Z. The Efficacy of NMDA Receptor Antagonists for Preventing Remifentanil-Induced Increase in Postoperative Pain and Analgesic Requirement: A Meta-Analysis. Minerva Anestesiol. 2012, 78, 653–667. [Google Scholar]
- Wu, L.; Huang, X.; Sun, L. The Efficacy of N-Methyl-D-Aspartate Receptor Antagonists on Improving the Postoperative Pain Intensity and Satisfaction after Remifentanil-Based Anesthesia in Adults: A Meta-Analysis. J. Clin. Anesth. 2015, 27, 311–324. [Google Scholar] [CrossRef]
- Yue, L.; Lin, Z.M.; Mu, G.Z.; Sun, H.L. Impact of Intraoperative Intravenous Magnesium on Spine Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. EClinicalMedicine 2022, 43, 101246. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Cochrane: London, UK, 2024; Available online: https://www.training.cochrane.org/handbook (accessed on 1 December 2024).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading the Quality of Evidence and the Strength of Recommendations, Updated October; 2024. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 1 December 2024).
- Schunemann, H.J.; Neumann, I.; Hultcrantz, M.; Brignardello-Petersen, R.; Zeng, L.; Murad, M.H.; Izcovich, A.; Morgano, G.P.; Baldeh, T.; Santesso, N.; et al. GRADE Guidance 35: Update on Rating Imprecision for Assessing Contextualized Certainty of Evidence and Making Decisions. J. Clin. Epidemiol. 2022, 150, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- McGrath, S.; Zhao, X.; Steele, R.; Thombs, B.D.; Benedetti, A.; DESD Collaboration. Estimating the Sample Mean and Standard Deviation from Commonly Reported Quantiles in Meta-Analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Schulz-Stübner, S.; Wettmann, G.; Reyle-Hahn, S.M.; Rossaint, R. Magnesium as Part of Balanced General Anaesthesia with Propofol, Remifentanil and Mivacurium: A Double-Blind, Randomized Prospective Study in 50 Patients. Eur. J. Anaesthesiol. 2001, 18, 723–729. [Google Scholar] [CrossRef]
- Levaux, C.; Bonhomme, V.; Dewandre, P.Y.; Brichant, J.F.; Hans, P. Effect of Intra-Operative Magnesium Sulphate on Pain Relief and Patient Comfort after Major Lumbar Orthopaedic Surgery. Anaesthesia 2003, 58, 131–135. [Google Scholar] [CrossRef]
- Steinlechner, B.; Dworschak, M.; Birkenberg, B.; Grubhofer, G.; Weigl, M.; Schiferer, A.; Lang, T.; Rajek, A. Magnesium Moderately Decreases Remifentanil Dosage Required for Pain Management after Cardiac Surgery. Br. J. Anaesth. 2006, 96, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Cizmeci, P.; Ozkose, Z. Magnesium Sulphate as an Adjuvant to Total Intravenous Anesthesia in Septorhinoplasty: A Randomized Controlled Study. Aesthetic Plast. Surg. 2007, 31, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jang, M.S.; Song, Y.K.; Seri, O.; Moon, S.Y.; Kang, D.B. The Effect of Magnesium Sulfate on Postoperative Pain in Patients Undergoing Major Abdominal Surgery under Remifentanil-Based Anesthesia. Korean J. Anesthesiol. 2008, 55, 286–290. [Google Scholar] [CrossRef]
- Oguzhan, N.; Gunday, I.; Turan, A. Effect of Magnesium Sulfate Infusion on Sevoflurane Consumption, Hemodynamics, and Perioperative Opioid Consumption in Lumbar Disc Surgery. J. Opioid Manag. 2008, 4, 105–110. [Google Scholar] [CrossRef]
- Kaya, S.; Kararmaz, A.; Gedik, R.; Turhanoglu, S. Magnesium Sulfate Reduces Postoperative Morphine Requirement after Remifentanil-Based Anesthesia. Med. Sci. Monit. 2009, 15, PI15–PI19. [Google Scholar]
- Song, J.W.; Lee, Y.W.; Yoon, K.B.; Park, S.J.; Shim, Y.H. Magnesium Sulfate Prevents Remifentanil-Induced Postoperative Hyperalgesia in Patients Undergoing Thyroidectomy. Anesth. Analg. 2011, 113, 390–397. [Google Scholar] [CrossRef]
- Olgun, B.; Oǧuz, G.O.; Kaya, M.; Şalvi, S.; Eskiçirak, H.E.; Güney, I.; Kadioǧullari, N. The Effects of Magnesium Sulphate on Desflurane Requirement, Early Recovery and Postoperative Analgesia in Laparascopic Cholecystectomy. Magnes. Res. 2012, 25, 72–78. [Google Scholar] [CrossRef]
- Asadollah, S.; Vahdat, M.; Yazdkhasti, P.; Nikravan, N. The Effect of Magnesium Sulphate on Postoperative Analgesia Requirements in Gynecological Surgeries. Turk. J. Obstet. Gynecol. 2015, 12, 34–37. [Google Scholar] [CrossRef]
- Martin, D.P.; Samora, W.P., 3rd; Beebe, A.C.; Klamar, J.; Gill, L.; Bhalla, T.; Veneziano, G.; Thung, A.; Tumin, D.; Barry, N.; et al. Analgesic Effects of Methadone and Magnesium Following Posterior Spinal Fusion for Idiopathic Scoliosis in Adolescents: A Randomized Controlled Trial. J. Anesth. 2018, 32, 702–708. [Google Scholar] [CrossRef]
- Tsaousi, G.; Nikopoulou, A.; Pezikoglou, I.; Birba, V.; Grosomanidis, V. Implementation of Magnesium Sulphate as an Adjunct to Multimodal Analgesic Approach for Perioperative Pain Control in Lumbar Laminectomy Surgery: A Randomized Placebo-Controlled Clinical Trial. Clin. Neurol. Neurosurg. 2020, 197, 106091. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, S.Y.; Lee, H.S.; Jun, B.K.; Choi, J.B.; Kim, J.E. Beneficial Effects of Intravenous Magnesium Administration during Robotic Radical Prostatectomy: A Randomized Controlled Trial. Adv. Ther. 2021, 38, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, J.F.; Guo, C.L.; Yin, Q.; Cheng, W.; Qian, B. Intravenously Injected Lidocaine or Magnesium Improves the Quality of Early Recovery after Laparoscopic Cholecystectomy: A Randomised Controlled Trial. Eur. J. Anaesthesiol. 2021, 38, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.M.; Kim, B.Y.; Bae, Y.K.; Seo, W.S.; Jeon, Y.T. Magnesium Sulfate Enables Patient Immobilization during Moderate Block and Ameliorates the Pain and Analgesic Requirements in Spine Surgery, Which Cannot Be Achieved with Opioid-Only Protocol: A Randomized Double-Blind Placebo-Controlled Study. J. Clin. Med. 2021, 10, 4289. [Google Scholar] [CrossRef]
- Hadavi, S.M.R.; Eghbal, M.H.; Kaboodkhani, R.; Alizadeh, N.; Sahmeddini, M.A. Comparison of Pregabalin with Magnesium Sulfate in the Prevention of Remifentanil-Induced Hyperalgesia in Patients Undergoing Rhinoplasty: A Randomized Clinical Trial. Laryngoscope Investig. Otolaryngol. 2022, 7, 1360–1366. [Google Scholar] [CrossRef]
- Kilic, K.; Sakat, M.S.; Sahin, A.; Ahiskalioglu, E.O.; Altunok, H. Efficacy of Intravenous Magnesium Sulfate Infusion on Postoperative Pain and Quality of Recovery for Septorhinoplasty: A Randomized Controlled Study. Acta Oto-Laryngol. 2023, 143, 979–983. [Google Scholar] [CrossRef]
- Su, Y.H.; Luo, D.C.; Pang, Y. Effects of Intraoperative Magnesium Sulfate Infusion on Emergency Agitation during General Anesthesia in Patients Undergoing Radical Mastectomy: A Randomized Controlled Study. BMC Anesthesiol. 2023, 23, 326. [Google Scholar] [CrossRef]
- Hua, X.; Chen, Y.; Wu, Z.; Zheng, G.; Yang, D.; Li, J.; Wu, Q.; Fan, W. Effects of Intra-Operative Magnesium Sulfate Infusion on Orthognathic Surgery: A Prospective and Randomized Controlled Trial. Heliyon 2024, 10, e30342. [Google Scholar] [CrossRef]
- Salkaya, A.; Oba, S.; Altınay, M.; Türk, H.Ş.; Kılınç, L.; Yılmaz, A. The Effects of Perioperative Low-Dose Magnesium Sulfate Infusion on Postoperative Pain in Lumbar Surgery. Signa Vitae 2024, 20, 50–56. [Google Scholar] [CrossRef]
- Xu, H.; Hao, C.; Wang, X.; Du, J.; Zhang, T.; Zhang, X. Effect of Intraoperative Infusion Magnesium Sulfate Infusion on Postoperative Quality of Recovery in Patients Undergoing Total Knee Arthroplasty: A Prospective, Double-Blind, Randomized Controlled Trial. Drug Des. Dev. Ther. 2024, 18, 919–929. [Google Scholar] [CrossRef]
- Egelund, T.A.; Wassil, S.K.; Edwards, E.M.; Linden, S.; Irazuzta, J.E. High-Dose Magnesium Sulfate Infusion Protocol for Status Asthmaticus: A Safety and Pharmacokinetics Cohort Study. Intensive Care Med. 2013, 39, 117–122. [Google Scholar] [CrossRef]
- Bouida, W.; Beltaief, K.; Msolli, M.A.; Azaiez, N.; Ben Soltane, H.; Sekma, A.; Trabelsi, I.; Boubaker, H.; Grissa, M.H.; Methemem, M.; et al. Low-Dose Magnesium Sulfate Versus High Dose in the Early Management of Rapid Atrial Fibrillation: Randomized Controlled Double-Blind Study (LOMAGHI Study). Acad. Emerg. Med. 2019, 26, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, T.O.; Tugrul, M.; Sungur, M.O.; Kayacan, S.; Telci, L.; Pembeci, K.; Akpir, K. Effects of Three Different Dose Regimens of Magnesium on Propofol Requirements, Haemodynamic Variables and Postoperative Pain Relief in Gynaecological Surgery. Br. J. Anaesth. 2006, 96, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, R.; Turan, A.M.; Orhan-Sungur, M.; McGuire, J.; Radke, O.C.; Apfel, C.C. Remifentanil for General Anaesthesia: A Systematic Review. Anaesthesia 2007, 62, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Tremble, S.M.; Chan, S.L.; Moseley, J.; LaMarca, B.; Nagle, K.J.; Cipolla, M.J. Magnesium Sulfate Treatment Reverses Seizure Susceptibility and Decreases Neuroinflammation in a Rat Model of Severe Preeclampsia. PLoS ONE 2014, 9, e113670. [Google Scholar] [CrossRef]
- Campos, J.; Bas, J.L.; Campos, C.; Mariscal, G.; Bas, T.; Bas, P. Efficacy and Safety of Intravenous Magnesium Sulfate in Spinal Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3122. [Google Scholar] [CrossRef]
- Guo, B.L.; Lin, Y.; Hu, W.; Zhen, C.X.; Bao-Cheng, Z.; Wu, H.H.; Kaye, A.D.; Duan, J.H.; Qu, Y. Effects of Systemic Magnesium on Post-operative Analgesia: Is the Current Evidence Strong Enough? Pain Physician 2015, 18, 405–418. [Google Scholar]
- Xie, W.J.; Hong, J.S.; Feng, C.F.; Chen, H.F.; Li, W.; Li, Y.C. Pharmacological Interventions for Preventing Opioid-Induced Hyperalgesia in Adults after Opioid-Based Anesthesia: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 2023, 14, 1199794. [Google Scholar] [CrossRef]
- Carron, M.; Tamburini, E.; Linassi, F.; Pettenuzzo, T.; Boscolo, A.; Navalesi, P. Efficacy of Nonopioid Analgesics and Adjuvants in Multimodal Analgesia for Reducing Postoperative Opioid Consumption and Complications in Obesity: A Systematic Review and Network Meta-Analysis. Br. J. Anaesth. 2024, 133, 1234–1249. [Google Scholar] [CrossRef]




| Study (Author/Year) | Country | Participants | Gender (M/F) | Mean Age ± SD | ASA | Type of Surgery | Magnesium Intervention | Remifentanil Infusion Rate | Premedication | Anaesthesia Maintenance | Postoperative Analgesia | Trial Registration ID |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schulz-Stübner 2001 [21] | Germany | Mg: 25 Control: 25 | Mg: 12/13 Control: 15/10 | Mg: 60.76 ± 14.9 Control: 51.76 ± 17.7 | I–III | Pars plana vitrectomy | Magnesium sulfate 50 mg kg−1 IV | 0.1 mcg kg−1min−1 (both groups) | Midazolam 3.75–7.5 mg orally | Propofol Remifentanil Mivacurium | Metamizol 10 mg kg−1 IV + Nalbuphine for VRS > 3 | Not reported |
| Levaux 2003 [22] | Belgium | Mg: 12 Control: 12 | Mg: 4/8 Control: 7/5 | Mg: 55 ± 16 Control: 46 ± 19 | I–II | Major lumbar orthopedic surgery | Magnesium sulfate 50 mg kg−1 IV | 0.25 mcg kg−1min−1 (both groups) | Alprazolam 1 mg Atropine 0.5 mg orally | Sevoflurane Remifentanil Nitrous oxide Rocuronium | PCA with piritramide | Not reported |
| Steinlechner 2006 [23] | Austria | Mg: 19 Control: 20 | Mg: 15/4 Control: 16/4 | Mg: 59 (23–79) Control: 61 (33–77) * Mean (range) | I–IV | Cardiac surgery | Magnesium gluconate 86.5 mg kg−1 IV followed by continuous infusion of 13.8 mg kg−1h−1 for 12 h after extubation | 0.15–0.5 mcg kg−1min−1 (both groups) | Midazolam 7.5 mg orally | Sevoflurane Remifentanil Cisatracurium | Remifentanil infusion (titrated) followed by piritramide and paracetamol | Not reported |
| Cizmeci 2007 [24] | Turkey | Mg: 30 Control: 30 | Mg: 10/20 Control: 12/18 | Mg: 25.8 ± 7.6 Control: 26.5 ± 7.9 | I–II | Septorhinoplasty | Magnesium sulfate 50 mg kg−1 IV + 8 mg kg−1h−1 continuous infusion | 0.1 mcg kg−1min−1 (both groups) | None | Propofol Remifentanil Vecuronium | Meperidine 1 mg kg−1 IM for VRS > 4 | Not reported |
| Lee 2008 [25] | South Korea | Mg: 35 Control: 35 | Mg: 16/19 Control: 18/17 | Mg: 62.1 ± 3.8 Control: 61.4 ± 4.2 | I–II | Major abdominal surgery | Magnesium sulfate 50 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | 0.25 mcg kg−1min−1 (both groups) | None | Sevoflurane Remifentanil Rocuronium | PCA with morphine and ketorolac | Not reported |
| Oguzhan 2008 [26] | Turkey | Mg: 25 Control: 25 | Mg: 13/12 Control: 14/11 | Mg: 44 (41–48) Control: 42 (38–46) * Mean (95%CI) | I–II | Lumbar disc surgery | Magnesium sulfate 30 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | 1.67 mcg kg−1min−1 (both groups) | Not reported | Sevoflurane Remifentanil Nitrous oxide Atracurium | PCA with morphine | Not reported |
| Ryu 2008 [4] | South Korea | Mg: 25 Control: 25 | All female | Mg: 41.1 Control: 43.7 | I–II | Total abdominal hysterectomy | Magnesium sulfate 50 mg kg−1 IV + 15 mg kg−1h−1 continuous infusion | TCI 4.0 ng mL−1 (both groups) | Not reported | Propofol Remifentanil Rocuronium | PCA with morphine and ketorolac | Not reported |
| Kaya 2009 [27] | Turkey | Mg: 20 Control: 20 | All female | Mg: 50 ± 10.7 Control: 50 ± 7.5 | I–II | Total abdominal hysterectomy | Magnesium sulfate 30 mg kg−1 IV + 500 mg h−1 continuous infusion | 0.25 mcg kg−1min−1 (both groups) | Diazepam 10 mg orally | Sevoflurane Remifentanil Cisatracurium | PCA with morphine | Not reported |
| Song 2011 [28] | South Korea | Mg: 28 HI: 28 (High-dose remifentanil) LO: 28 (Low-dose remifentanil) | Mg: 6/22 HI: 5/23 LO: 4/24 | Mg: 45 ± 10 HI: 47 ± 11 LO: 47 ± 10 | I–II | Thyroidectomy | Magnesium sulfate 30 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | Mg/HI: 0.20 mcg kg−1min−1 LO: 0.05 mcg kg−1min−1 | Midazolam 0.05 mg kg−1 IM | Sevoflurane Remifentanil Rocuronium | PACU: Fentanyl IV for VNRS > 4 General ward: Tramadol 37.5 mg + acetaminophen 325 mg orally | NCT01025245 |
| Olgun 2012 [29] | Turkey | Mg: 30 Control: 30 | Mg: 5/25 Control: 5/25 | Mg: 47.7 ± 11.4 Control: 45.4 ± 12.4 | I–II | Laparoscopic cholecystectomy | Magnesium sulfate 40 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | 0.25 mcg kg−1min−1 to 0.125 mcg kg−1min−1 10 min after intubation (both groups) | Midazolam 0.07 mg kg−1 IM | Desflurane Nitrous oxide Remifentanil Vecuronium | PCA with morphine | Not reported |
| Asadollah 2015 [30] | Iran | Mg: 15 Control: 15 | All female | Mg: 48.6 ± 4.91 Control: 49.1 ± 4.8 | I–II | Lower abdominal laparotomy | Magnesium sulfate 50 mg kg−1 IV + 8 mg kg−1h−1 continuous infusion | 0.40 mcg kg−1min−1 (both groups) | Midazolam 0.2 mg kg−1 IV | Propofol Remifentanil Atracurium | Fentanyl 1 mcg kg−1 + Meperidine 30 mg IV for VRS > 4 | Not reported |
| Martin 2018 [31] | United States | Mg: 19 (Remifentanil + Mg) MET: 22 (Remifentanil + methadone) REMI: 19 (Remifentanil alone) | Mg: 3/16 MET: 5/17 REMI: 3/16 | Mg: 15.3 ± 1.9 MET: 15.4 ± 1.2 REMI: 14.2 ± 1.4 | I–III | Posterior spinal fusion for idiopathic scoliosis | Mg: Magnesium sulfate 50 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | 0.05–0.3 mcg kg−1min−1 (all groups) | Midazolam 20 mg orally | Desflurane Nitrous oxide Remifentanil Rocuronium | PCA with hydromorphone + acetaminophen orally and ketorolac IV | NCT01795495 |
| Tsaousi 2020 [32] | Greece | Mg: 35 Control: 36 | Mg: 13/22 Control: 15/21 | Mg: 55.9 ± 10.8 Control: 49.0 ± 15.0 | I–III | Lumbar laminectomy | Magnesium sulfate 20 mg kg−1 IV + 20 mg kg−1h−1 continuous infusion | TCI mode: Plasma concentration 1.5–3.0 ng mL−1 | Diazepam 5–10 mg orally | Desflurane Remifentanil Cisatracurium | Paracetamol 1 g IV Lornoxicam 8 mg orally Morphine 3 mg IV | NCT04161729 |
| Kim 2021 [33] | South Korea | Mg: 26 Control: 26 | All male | Mg: 63 ± 7 Control: 65 ± 7 | I–II | Robotic radical prostatectomy | Magnesium sulfate 50 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | TCI 0.5–4.0 ng mL−1 | Not reported | Sevoflurane Remifentanil Rocuronium | PCA with fentanyl + nefopam and ibuprofen IV + acetaminophen orally | NCT02833038 |
| Lu 2021 [34] | China | Mg: 37 Lidocaine: 37 Control: 40 | Mg: 8/29 Lidocaine: 8/29 Control: 11/29 | Mg: 46.8 ± 13.3 Lidocaine: 42.2 ± 10.5 Control: 45.4 ± 11.7 | I–II | Laparoscopic cholecystectomy | Magnesium sulfate 20 mg kg−1 IV + 20 mg kg−1h−1 continuous infusion | TCI 4.7–8 ng mL−1 | Not reported | Propofol Remifentanil Cisatracurium | Flurbiprofen 100 mg IV + Fentanyl 50 mcg IV for NRS > 3 | ChiCTR1800019092 |
| Sohn 2021 [35] | South Korea | Mg: 34 Control: 34 | Mg:14/20 Control: 17/17 | Mg: 56.5 ± 13.7 Control: 56.5 ± 14.7 | I–III | Spine surgery | Magnesium sulfate 30 mg kg−1 IV + 15 mg kg−1h−1 continuous infusion | TCI 1.0–5.0 ng mL−1 | Not reported | Propofol Remifentanil Rocuronium | PCA with fentanyl + rescue analgesics depending on patients’ need (morphine, propacetamol, meperidine, fentanyl, acetaminophen, ketorolac, and nefopam) | KCT0004173 |
| Hadavi 2022 [36] | Iran | Mg: 35 Pregabalin: 35 Control: 35 | Mg: 8/27 Pregabalin: 4/31 Control: 12/23 | Mg: 29.31 ± 7.94 Pregabalin: 28.12 ± 6.57 Control: 25.47 ± 4.81 | Not reported | Rhinoplasty | Magnesium sulfate 30 mg kg−1 IV | 0.3 mcg kg−1min−1 (All groups) | Not reported | Isoflurane Nitrous oxide Remifentanil Atracurium | Morphine 1-2 mg every 5 min until NRS < 4 | IRCT20121204011662N12 |
| Kilic 2023 [37] | Turkey | Mg: 60 Control: 60 | Mg:20/40 Control: 17/43 | Ages 18–45 (both groups) | Not reported | Septorhinoplasty | Magnesium sulfate 30 mg kg−1 IV + 9 mg kg−1h−1 continuous infusion | 0.25–1 mcg kg−1min−1 (both groups) | Not reported | Sevoflurane Remifentanil Rocuronium | Dexketoprofen 25 mg IV + Tramadol 1 mg kg−1 IV for NRS > 4 | Not reported |
| Su 2023 [38] | China | Mg: 35 Control: 35 | All female | Mg: 52 (48–57) Control: 54 (48–59) * Median (range) | I–II | Radical mastectomy | Magnesium sulfate 30 mg kg−1 IV + 10 mg kg−1h−1 continuous infusion | 0.1 mcg kg−1min−1 (both groups) | None | Sevoflurane Remifentanil Cisatracurium | Fentanyl 1 mcg kg−1 + Tramadol 25 mg IV for emergency agitation | ChiCTR2300070595 |
| Hua 2024 [39] | China | Mg: 36 Control: 37 | Mg:12/24 Control: 13/24 | Mg: 24 ± 4 Control: 24 ± 5 | I–II | Orthognathic surgery | Magnesium sulfate 50 mg kg−1 IV + 15 mg kg−1h−1 continuous infusion | 0.1 mcg kg−1min−1 (both groups) | None | Sevoflurane Dexmedetomidine Remifentanil Cisatracurium | PACU: Fentanyl 20-30 mcg IV for NRS > 3 Ward: Celecoxib orally + tramadol IM | ChiCTR2100045981 |
| Salkaya 2024 [40] | Turkey | Mg: 30 Control: 30 | Mg:13/17 Control: 12/18 | Mg: 58.4 ± 9.5 (range 44–80) Control: 60.3 ± 11 (range 36–82) | I–II | Lumbar spine surgery | Magnesium sulfate 10 mg kg−1h−1 continuous infusion | Not reported | None | Sevoflurane Remifentanil Rocuronium | PCA with meperidine + diclofenac sodium for VAS > 4 | Not reported |
| Xu 2024 [41] | China | Mg: 68 Control: 66 | Mg:14/54 Control: 16/50 | Mg: 67.00 (64.00–70.00) Control: 66.00 (62.00–71.00) * Median (IQR) | II–III | Total knee arthroplasty | Magnesium sulfate 40 mg kg−1 IV + 15 mg kg−1h−1 continuous infusion | 0.1–0.3 mcg kg−1min−1 (both groups) | Not reported | Propofol Remifentanil Cisatracurium | PCA with fentanyl + femoral nerve block with 20 mL of 0.25% ropivacaine | ChiCTR2200065940 |
| Certainty Assessment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | No. of Trials | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other | No. of Patients (n) | Effect Estimate (95% CI) | Quality of Evidence | |
| Mg | Control | ||||||||||
| Postoperative analgesic requirements—0–24 h | 13 | RCTs | Serious * | Serious ** | Not serious | Not serious | Publication bias | 393 | 392 | SMD −1.51 (−2.15 to −0.87) | ⊕○○○ |
| Very low | |||||||||||
| Postoperative pain scores—24 h | 14 | RCTs | Serious * | Serious ** | Not serious | Not serious | None | 488 | 491 | SMD −0.61 (−0.90 to −0.32) | ♁♁○○ |
| Low | |||||||||||
| Intraoperative hypotension incidence | 7 | RCTs | Serious * | Not serious | Not serious | Serious *** | None | 193 | 197 | SMD 1.44 (0.92 to 2.26) | ♁♁○○ |
| Low | |||||||||||
| Intraoperative bradycardia incidence | 8 | RCTs | Serious * | Not serious | Not serious | Serious *** | None | 287 | 290 | SMD 1.31 (0.67 to 2.57) | ♁♁○○ |
| Low | |||||||||||
| Extubation time | 14 | RCTs | Serious * | Not serious | Not serious | Serious *** | Publication bias | 444 | 447 | SMD −0.04 (−0.18 to 0.11) | ♁○○○ |
| Very low | |||||||||||
| Number of patients requiring rescue analgesia | 8 | RCTs | Serious * | Serious ** | Not serious | Not serious | None | 253 | 251 | SMD 0.32 (0.15 to 0.70) | ♁♁○○ |
| Low | |||||||||||
| Incidence of shivering | 8 | RCTs | Serious * | Not serious | Not serious | Not serious | None | 248 | 248 | SMD 0.25 (0.12 to 0.52) | ♁♁♁○ |
| Moderate | |||||||||||
| Incidence of PONV | 17 | RCTs | Serious * | Not serious | Not serious | Not serious | None | 525 | 529 | SMD 0.66 (0.44 to 0.98) | ♁♁♁○ |
| Moderate | |||||||||||
| Patient satisfaction scores | 3 | RCTs | Serious * | Not serious | Not serious | Serious *** | None | 72 | 73 | SMD 1.04 (0.53 to 1.56) | ♁♁○○ |
| Low | |||||||||||
| Intraoperative remifentanil consumption | 16 | RCTs | Serious * | Serious ** | Not serious | Not serious | None | 493 | 497 | SMD −0.52 (−0.86 to −0.18) | ♁♁○○ |
| Low | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, E.-B.; Wu, K.-L.; Hsu, W.-T.; Yuan, W.-C.; Chen, K.-B. Pharmacological Efficacy of Intravenous Magnesium in Attenuating Remifentanil-Induced Postoperative Hyperalgesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceuticals 2025, 18, 518. https://doi.org/10.3390/ph18040518
Wu E-B, Wu K-L, Hsu W-T, Yuan W-C, Chen K-B. Pharmacological Efficacy of Intravenous Magnesium in Attenuating Remifentanil-Induced Postoperative Hyperalgesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceuticals. 2025; 18(4):518. https://doi.org/10.3390/ph18040518
Chicago/Turabian StyleWu, En-Bo, Kuen-Lin Wu, Wei-Ti Hsu, Wei-Chin Yuan, and Kuen-Bao Chen. 2025. "Pharmacological Efficacy of Intravenous Magnesium in Attenuating Remifentanil-Induced Postoperative Hyperalgesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Pharmaceuticals 18, no. 4: 518. https://doi.org/10.3390/ph18040518
APA StyleWu, E.-B., Wu, K.-L., Hsu, W.-T., Yuan, W.-C., & Chen, K.-B. (2025). Pharmacological Efficacy of Intravenous Magnesium in Attenuating Remifentanil-Induced Postoperative Hyperalgesia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmaceuticals, 18(4), 518. https://doi.org/10.3390/ph18040518







