Anti-Inflammatory Activity of Two Labdane Enantiomers from Gymnosperma glutinosum: An In Vivo, In Vitro, and In Silico Study
Abstract
1. Introduction
2. Results
2.1. Isolation and Structural Analysis of Gymglu Acid
2.1.1. Crystallographic Evaluation
2.1.2. FT-IR and NMR Evaluation
2.2. In Vivo and In Vitro Evaluation of Gymglu Acid
2.2.1. Anti-Inflammatory Activity in a Mouse Ear Edema Induced with TPA
2.2.2. Effect of Gymglu Acid on J774A.1 Macrophages Cell Viability
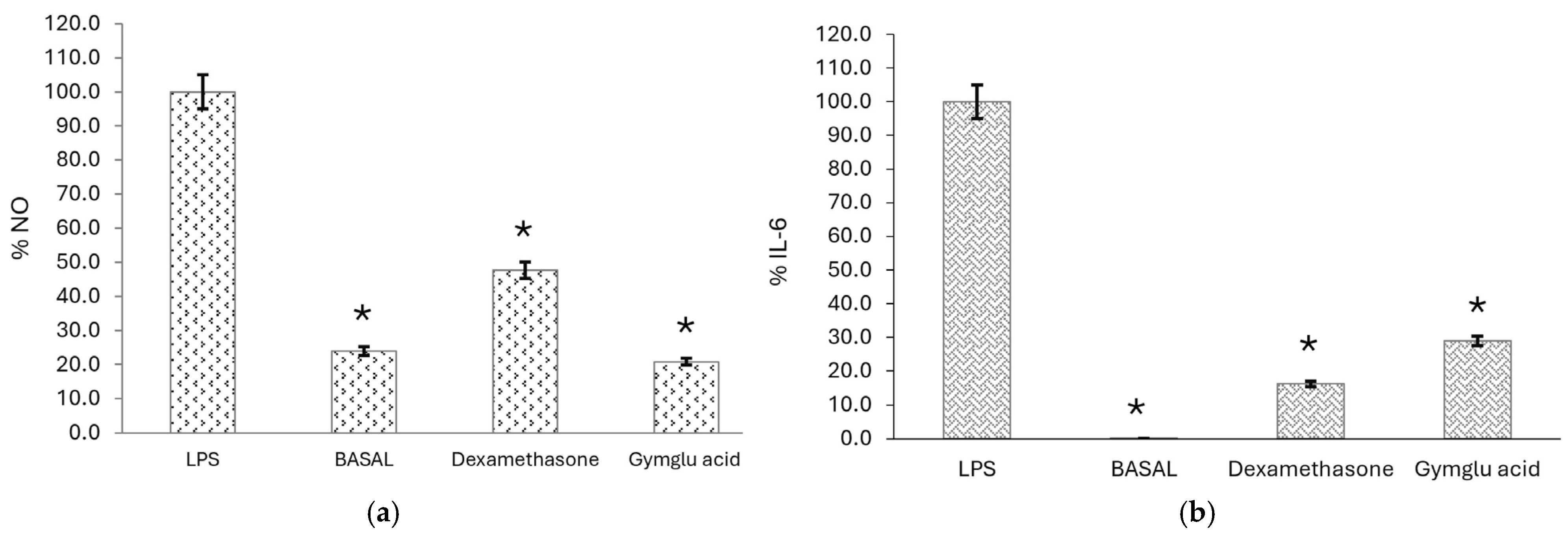
2.2.3. Measurement of Proinflammatory Mediator Levels
2.2.4. The Membrane Protective Effect of Gymglu Acid
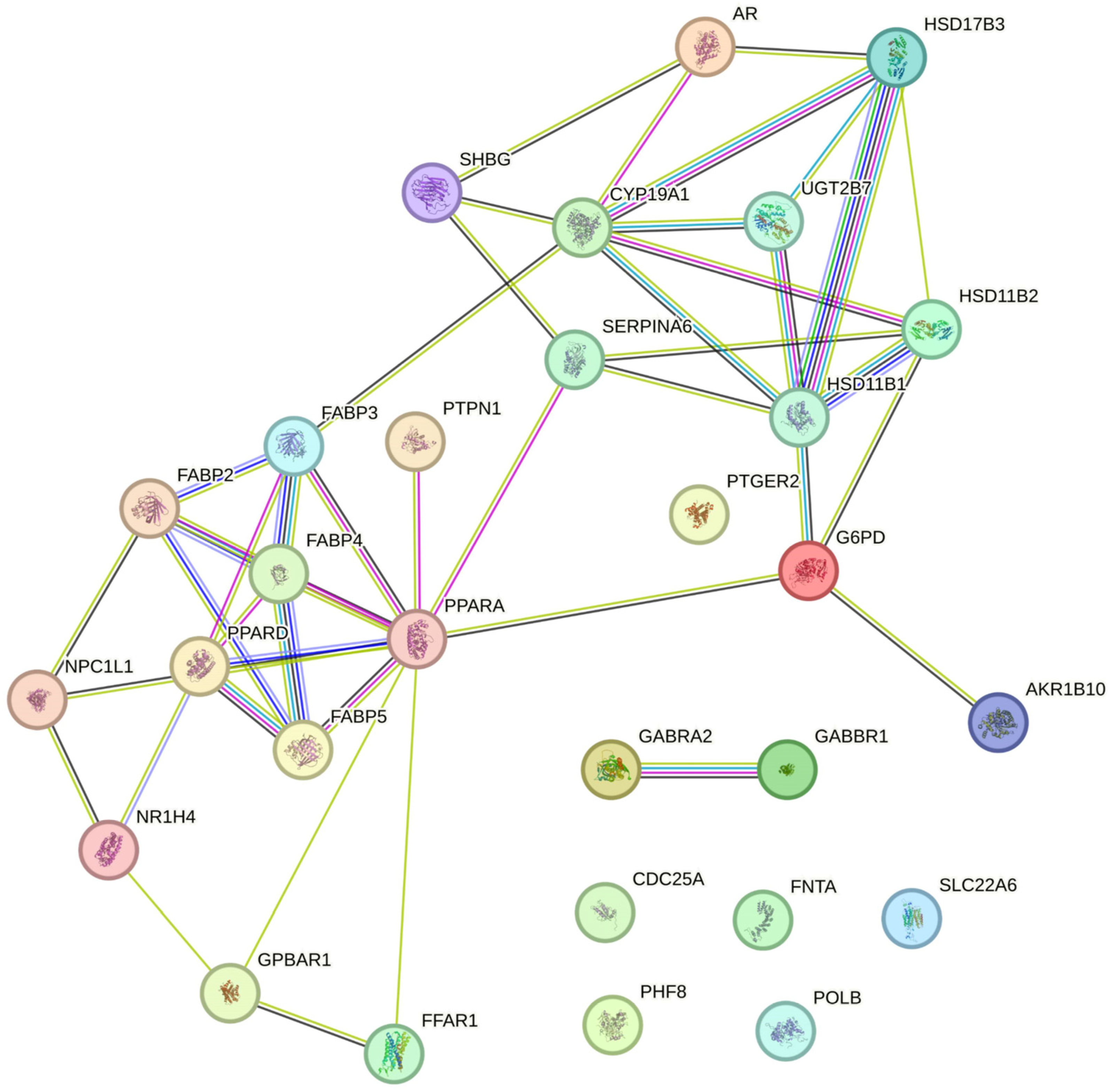
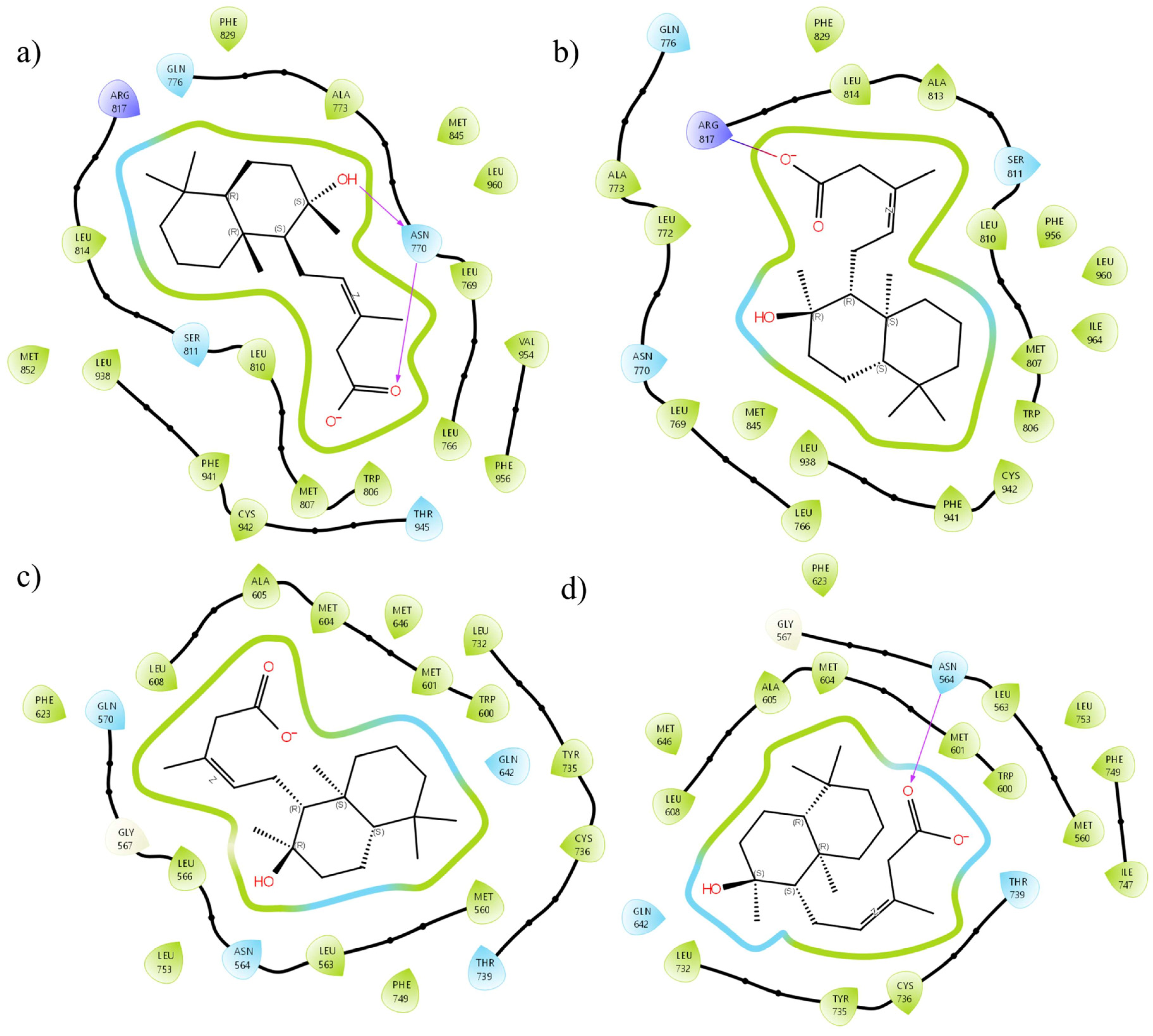
2.3. In Silico Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material
4.3. Obtaining Extracts
4.4. Isolation of a White Crystalline Solid: Gymglu Acid
4.5. Structural Analysis of the Solid Crystalline
4.6. Animals
4.7. Anti-Inflammatory Activity in Mouse Ear Edema Induced with TPA
4.8. Cell Viability Assay
4.9. Determination of NO and IL-6 Levels
4.10. Membrane Stabilization Assay
4.11. Statistical Analysis
4.12. Bioinformatics
4.12.1. Protein Preparation
4.12.2. Ligand Preparation
4.12.3. Molecular Docking
4.12.4. MMGBSA Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflectance |
| CDCl3 | Deuterated chloroform |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| eNOS, 3NOS | Endothelial nitric oxide synthase |
| FBS | Fetal bovine serum |
| FTIR | Fourier-transform infrared |
| GR, 4CSJ | Glucocorticoid receptor |
| HRBC | Suspension of erythrocytes with isosaline solution. |
| HSD11B, 2RBE | Hydroxysteroid 11-beta dehydrogenase 1 |
| IL-1β | Interleukin one beta |
| IL-6 | Interleukin six |
| IND | Indomethacin |
| iNOS, 4NOS | Inducible nitric oxide synthase |
| J774A.1 | Cells murine macrophage |
| MR | Mineralocorticoid receptor |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| NMR | Nuclear magnetic resonance |
| nNOS, 4EUX | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| LPS | Lipopolysaccharide |
| PBS | Phosphate-buffered saline |
| PPARA, 6GHK | Peroxisome proliferator-activated receptor Alpha |
| PVP | Polyvinylpyrrolidone |
| SMILES | Simplified Molecular Input Line Entry System |
| TNF-α | Tumor Necrosis Factor Alpha |
| TPA | 12-O-tetradecanoyl phorbol-13-acetate |
| 3VHU | Mineralocorticoid receptor ligand-binding domain with spironolactone |
References
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for Evaluation of Chronic Inflammatory Status in Ageing Research: Reliability and Phenotypic Characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Torres, A.G.; López-Castillo, G.N.; Marín-Torres, J.L.; Portillo-Reyes, R.; Luna, F.; Baca, B.E.; Sandoval-Ramírez, J.; Carrasco-Carballo, A. Cymbopogon Citratus Essential Oil: Extraction, GC–MS, Phytochemical Analysis, Antioxidant Activity, and In Silico Molecular Docking for Protein Targets Related to CNS. Curr. Issues Mol. Biol. 2023, 45, 5164–5179. [Google Scholar] [CrossRef]
- Domínguez, X.A.; Torre, B. Two Pentamethoxylated Flavonoids from Gymnosperma Glutinosum. Phytochemistry 1974, 13, 1624–1625. [Google Scholar] [CrossRef]
- Canales, M.; Hernández, T.; Serrano, R.; Hernández, L.B.; Duran, A.; Ríos, V.; Sigrist, S.; Hernández, H.L.H.; Garcia, A.M.; Angeles-López, O.; et al. Antimicrobial and General Toxicity Activities of Gymnosperma Glutinosum: A Comparative Study. J. Ethnopharmacol. 2007, 110, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Licea, R.; Morado-Castillo, R.; Gomez-Flores, R.; Laatsch, H.; Verde-Star, M.J.; Hernández-Martínez, H.; Tamez-Guerra, P.; Tamez-Guerra, R.; Rodríguez-Padilla, C. Bioassay-Guided Isolation and Identification of Cytotoxic Compounds from Gymnosperma Glutinosum Leaves. Molecules 2012, 17, 11229–11241. [Google Scholar] [CrossRef]
- Serrano, R.; Hernández, T.; Canales, M.; García-Bores, A.M.; Romo De Vivar, A.; Céspedes, C.L.; Avila, J.G. Ent-Labdane Type Diterpene with Antifungal Activity from Gymnosperma Glutinosum (Spreng.) Less. (Asteraceae). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2009, 8, 412–418. [Google Scholar]
- Maldonado, E.; Segura-Correa, R.; Ortega, A.; Calderón, J.S.; Fronczek, F.R. Ent-Labdane and Neo-Clerodane Diterpenes from Gymnosperma Glutinosum. Phytochemistry 1994, 35, 721–724. [Google Scholar] [CrossRef]
- González-Chávez, M.M.; Arana-Argáez, V.; Zapata-Morales, J.R.; Ávila-Venegas, A.K.; Alonso-Castro, A.J.; Isiordia-Espinoza, M.; Martínez, R. Pharmacological Evaluation of 2-Angeloyl Ent-Dihydrotucumanoic Acid. Pharm. Biol. 2017, 55, 873–879. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; González-Chávez, M.M.; Zapata-Morales, J.R.; Verdinez-Portales, A.K.; Sánchez-Recillas, A.; Ortiz-Andrade, R.; Isiordia-Espinoza, M.; Martínez-Gutiérrez, F.; Ramírez-Morales, M.A.; Domínguez, F.; et al. Antinociceptive Activity of Ent-Dihydrotucumanoic Acid Isolated from Gymnosperma Glutinosum Spreng Less. Drug Dev. Res. 2017, 78, 340–348. [Google Scholar] [CrossRef]
- Salas-Balgañón, K.; Pérez.-Ramos, J.; Serrano-Vega, J.R.; Campos-Xolalpa, N.; Pérez-Gutiérrez, S. Evaluación Antiinflamatoria Del Extracto Metanólico y Clorofórmico de Gymnosperma Glutinosum. In Proceedings of the 16a Reunión Internacional de Investigación en Productos Naturales; Garduño-Ramírez María, L., Rivas-Galindo Verónica, M., Antunez Mojica Mayra, Y., Robles Zepeda Ramón, E., Eds.; AMIPRONAT: Zacatecas, Mexico, 2021; p. 33. [Google Scholar]
- Agilent CrysAlisPro Software System; Technologies UK Ltd.: Yarnton, UK, 2014; Volume 44.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Streek, J.V.D. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar]
- Hiraganahalli Bhaskarmurthy, D.; Evan Prince, S. Effect of Baricitinib on TPA-Induced Psoriasis like Skin Inflammation. Life Sci. 2021, 279, 119655. [Google Scholar] [CrossRef]
- Hamaguchi, K.; Kuwata, H.; Yoshihara, K.; Masuda, S.; Shimbara, S.; Oh-Ishi, S.; Murakami, M.; Kudo, I. Induction of Distinct Sets of Secretory Phospholipase A2 in Rodents during Inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1635, 37–47. [Google Scholar] [CrossRef]
- Green, S.J.; Mellouk, S.; Hoffman, S.L.; Meltzer, M.S.; Nacy, C.A. Cellular Mechanisms of Nonspecific Immunity to Intracellular Infection: Cytokine-Induced Synthesis of Toxic Nitrogen Oxides from l-Arginine by Macrophages and Hepatocytes. Immunol. Lett. 1990, 25, 15–19. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Hu, W.; Ren, L.; Jiang, P.; Margolskee, R.F.; Wang, H.; Feng, S. Lipopolysaccharide-Induced Inflammation Increases Nitric Oxide Production in Taste Buds. Brain Behav. Immun. 2022, 103, 145–153. [Google Scholar] [CrossRef]
- Jin, J.O.; Han, X.; Yu, Q. Interleukin-6 Induces the Generation of IL-10-Producing Tr1 Cells and Suppresses Autoimmune Tissue Inflammation. J. Autoimmun. 2013, 40, 28–44. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane Diterpenoids as Potential Anti-Inflammatory Agents. Pharmacol. Res. 2017, 124, 43–63. [Google Scholar] [CrossRef]
- Chetehouna, S.; Derouiche, S.; Boulaares, I.; Réggami, Y. Phytochemical Profile, Anti-Inflammatory Analysis and Cytotoxic Activity of SmE-SeNPs against Breast (MCF-7) Cancer Cells. Biocatal. Agric. Biotechnol. 2024, 57, 103122. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Obeagu, G.U. Exploring the Intersection: Peptic Ulcers and Hemolysis—Unraveling the Complex Relationship. Medicine 2024, 103, e37565. [Google Scholar] [CrossRef]
- Especificaciones Técnicas para la Producción, Cuidado y Uso de los Animales de Laboratorio (NOM-062-ZOO-1999). Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 31 March 2025).
- De Young, L.M.; Kheifets, J.B.; Ballaron, S.J.; Young, J.M. Edema and Cell Infiltration in the Phorbol Ester-Treated Mouse Ear Are Temporally Separate and Can Be Differentially Modulated by Pharmacologic Agents. Agents Actions 1989, 26, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Parameswari, P.; Devika, R.; Vijayaraghavan, P. In Vitro Anti-Inflammatory and Antimicrobial Potential of Leaf Extract from Artemisia Nilagirica (Clarke) Pamp. Saudi J. Biol. Sci. 2019, 26, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A Web Server for Target Prediction of Bioactive Small Molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Schrödinger. Protein Preparation Wizard; Version 2023-2; Schrödinger: Cambridge, MA, USA, 2023. [Google Scholar]
- Carrasco-Carballo, A.; Mendoza-Lara, D.F.; Rojas-Morales, J.A.; Alatriste, V.; Merino-Montiel, P.; Luna, F.; Sandoval-Ramirez, J. In Silico Study of Coumarins Derivatives With Potential Use in Systemic Diseases. Biointerface Res. Appl. Chem. 2023, 13, 240. [Google Scholar] [CrossRef]
- Schrödinger. Schrödinger Release 2022-1: Glide; Schrödinger, LLC: New York, NY, USA, 2022. [Google Scholar]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Rosales-López, A.; López-Castillo, G.; Sandoval-Ramírez, J.; Terán, J.; Carrasco-Carballo, A. Correlation between Molecular Docking and the Stabilizing Interaction of HOMO-LUMO: Spirostans in CHK1 and CHK2, an In Silico Cancer Approach. Int. J. Mol. Sci. 2024, 25, 8588. [Google Scholar] [CrossRef]
- Release Notes: Release 2024-2. Available online: https://www.schrodinger.com/life-science/download/release-notes/release-2024-2/ (accessed on 11 December 2024).
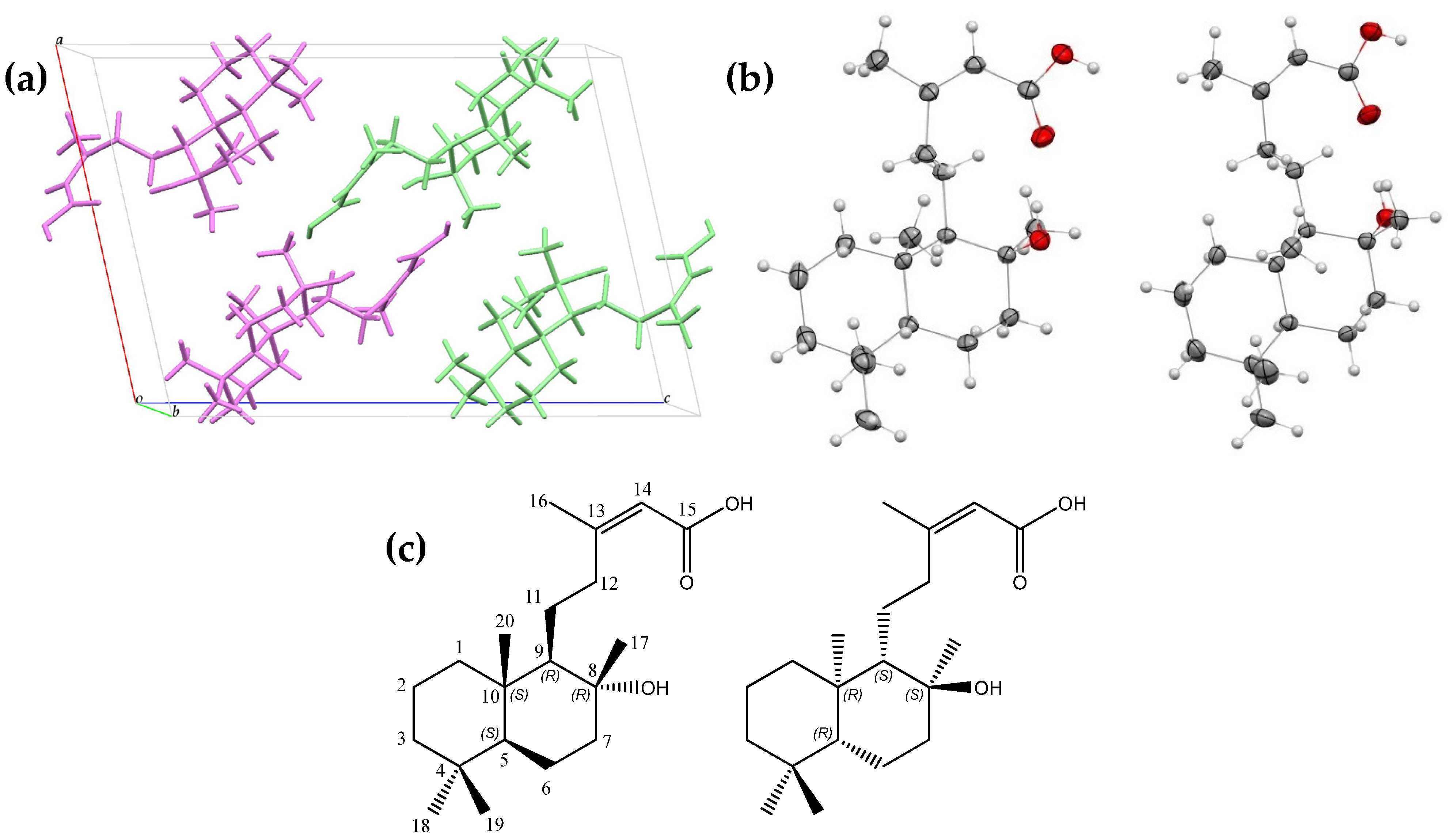
| Carbon | HSQC Correlations | HMBC Correlations |
|---|---|---|
| 1 | 39.17: 1.96, 0.93 | C1, H20 |
| 2 | 18.45: 1.60, 1.43 | C2, H3′ |
| 3 | 41.94: 1.37, 1.14 | C3, H18; C3, H19 |
| 4 | 33.24 | C4, H18; C4, H19 |
| 5 | 55.97: 0.92 | C5, H20; C5, H19; C5, H18 |
| 6 | 20.18: 1.64, 1.25 | C6, H5; C6, H7′ |
| 7 | 42.96: 1.83, 1.44 | C7, H5; C7, H17 |
| 8 | 74.06 | C8, H17; C8, H9; C8, H7; C8, H7′; C8, H11 |
| 9 | 61.02: 1.17 | C9, H12; C9, H12′; C9, H7; C9, H7′; C9, H17; C9, H20; C9, H5; C9, H11 |
| 10 | 38.73 | C10, H5; C10, H20 |
| 11 | 24.23: 1.48 | C11, H12; C11, H12′ |
| 12 | 37.27: 2.95, 2.19 | C12, H14; C12, H16; C12, H11 |
| 13 | 161.98 | C13, H12; C13, H12′; C13, H19; C13, H11; C13, H14 |
| 14 | 115.86: 5.68 | C14, H12; C14, H12′; C14, H16 |
| 15 | 168.70 | C15, H14 |
| 16 | 25.85: 1.91 | C16, H14; C16, H12; C16, H12′ |
| 17 | 24.21: 1.17 | C17, H12; C17, H12′; C17, H9; C17, H7; C17, H7′ |
| 18 | 33.34: 0.87 | C18, H19; C19, H5; C18, H3′ |
| 19 | 21.44: 0.78 | C19, H18; C19, H5; C19, H3′ |
| 20 | 15.48: 0.75 | C20, H5; C20, H9 |
| Group | Percent Decrease in Inflammation | Weight Differences (mg) |
|---|---|---|
| Methanol extract (2 mg/ear) | 41.96 ± 6.5 * | 6.14 ± 0.69 # |
| Dichloromethane extract (2 mg/ear) | 52.83 ± 5.28 | 4.99 ± 0.56 # |
| Indomethacin (2 mg/ear) | 49.74 ± 4.25 | 5.31 ± 0.48 # |
| Negative | ------ | 10.58 ± 0.41 |
| Group | Percent Decrease in Inflammation | Weight Differences (mg) |
|---|---|---|
| Gymglu acid (1 mg/ear) | 36.07 ± 0.84 * | 7.57 ± 0.19 # |
| Gymglu acid (2 mg/ear) | 41.99 ± 3.57 * | 6.7 ± 0.44 # |
| Indomethacin (2 mg/ear) | 50.27 ± 4.46 | 5.7 ± 0.52 # |
| Negative | ------ | 11.55 ± 0.36 |
| µM | % Cell Viability |
|---|---|
| 0 | 100 ± 3.32 |
| 3.11 | 92.93 ± 2.86 |
| 15.53 | 83.79 ± 1.93 |
| 31.06 | 81.92 ± 1.75 |
| 77.64 | 73.33 ± 2.13 |
| 155.28 | 74.27 ± 1.86 |
| 232.92 | 71.24 ± 1.82 |
| 310.56 | 57.03 ± 2.05 |
| Concentration (μg/mL) | % Erythrocyte Membrane Protection | |
|---|---|---|
| Diclofenac | Gymglu Acid | |
| 200 | 63.04 ± 3.98 | 56.43 ± 0.95 |
| 100 | 61.30 ± 0.54 | 52.46 ± 0.59 |
| 50 | 51.15 ± 3.05 | 30.24 ± 0.48 |
| 25 | 45.96 ± 4.81 | 17.69 ± 1.69 |
| 12.5 | 31.39 ± 5.17 | 8.57 ± 1.35 |
| 0 | 100.00 ± 0.59 | 100.00 ± 0.58 |
| IC50 (µg/mL) | 38.806 | 94.47 |
| Protein | a-Gymglu Acid | b-ent-Gymglu Acid | Reference |
|---|---|---|---|
| PPARA | NI/NI | −6.229/−55.19 | −4.287/−43.56 (thiazolidinediones) |
| MR | −7.245/−83.98 | −6.016/−67.30 | −9.324/−93.64 (dexamethasone) |
| GR | −7.867/−19.98 | −7.019/−17.37 | −10.258/−31.02 (dexamethasone) |
| HSD11B1 | −6.095/−36.87 | −5.064/−39.36 | −6.038/−37.26 (glycyrrhizic acid) |
| iNOS | −4.025/−9.96 | −4.318/−12.38 | −3.876/−11.38 (L-NMMA) |
| eNOS | −4.128/−10.12 | −3.975/−11.52 | −3.957/−11.45 (L-NMMA) |
| nNOS | −3.017/−9.35 | −3.358/−10.98 | −3.797/−11.92 (L-NMMA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Gutiérrez, S.; Campos-Xolalpa, N.; Estrada-Barajas, S.A.; Carrasco-Carballo, A.; Mendoza, A.; Sánchez-Mendoza, E. Anti-Inflammatory Activity of Two Labdane Enantiomers from Gymnosperma glutinosum: An In Vivo, In Vitro, and In Silico Study. Pharmaceuticals 2025, 18, 516. https://doi.org/10.3390/ph18040516
Pérez-Gutiérrez S, Campos-Xolalpa N, Estrada-Barajas SA, Carrasco-Carballo A, Mendoza A, Sánchez-Mendoza E. Anti-Inflammatory Activity of Two Labdane Enantiomers from Gymnosperma glutinosum: An In Vivo, In Vitro, and In Silico Study. Pharmaceuticals. 2025; 18(4):516. https://doi.org/10.3390/ph18040516
Chicago/Turabian StylePérez-Gutiérrez, Salud, Nimsi Campos-Xolalpa, Sofía A. Estrada-Barajas, Alan Carrasco-Carballo, Angel Mendoza, and Ernesto Sánchez-Mendoza. 2025. "Anti-Inflammatory Activity of Two Labdane Enantiomers from Gymnosperma glutinosum: An In Vivo, In Vitro, and In Silico Study" Pharmaceuticals 18, no. 4: 516. https://doi.org/10.3390/ph18040516
APA StylePérez-Gutiérrez, S., Campos-Xolalpa, N., Estrada-Barajas, S. A., Carrasco-Carballo, A., Mendoza, A., & Sánchez-Mendoza, E. (2025). Anti-Inflammatory Activity of Two Labdane Enantiomers from Gymnosperma glutinosum: An In Vivo, In Vitro, and In Silico Study. Pharmaceuticals, 18(4), 516. https://doi.org/10.3390/ph18040516










