Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review
Abstract
1. Introduction
2. Biodistribution: A Key Factor Influencing the Mechanisms of Action
2.1. Tumor Penetration
2.2. Retention Properties
2.3. Elimination from the Body and Safety
2.4. Modelling the Kinetics of Accumulation and Retention
2.5. Cell Internalization and Subcellular Localization
2.6. Cytotoxicity
3. Initial Interaction: Any Physical Increase in the Radiation Dose?
3.1. Interaction of Ionizing Radiation with Matter
3.2. Key Factors of Radioenhancement of High-Z NPs Beyond Limits
3.3. AGuIX Simulations with Different Models
3.4. Conclusions
4. Chemical Stage: Any Enhancement of ROS Production Under RT?
4.1. ROS Measurement in Biological Studies
4.2. ROS Production Under RT by High-Z Metal-Based NPs
4.3. ROS Production Under RT in the Presence of AGuIX
4.4. Conclusions
5. Biological Effects: Any Increase in DNA Damage or Tumor Cell Death?
5.1. Mechanisms Affecting DNA
5.1.1. DNA Damage Production
5.1.2. DNA Repair Mechanisms
5.1.3. Cell Cycle and DNA Replication
5.1.4. Conclusions
5.2. Mechanisms of Cell Death and Immune Response
5.2.1. Apoptosis
5.2.2. Autophagy
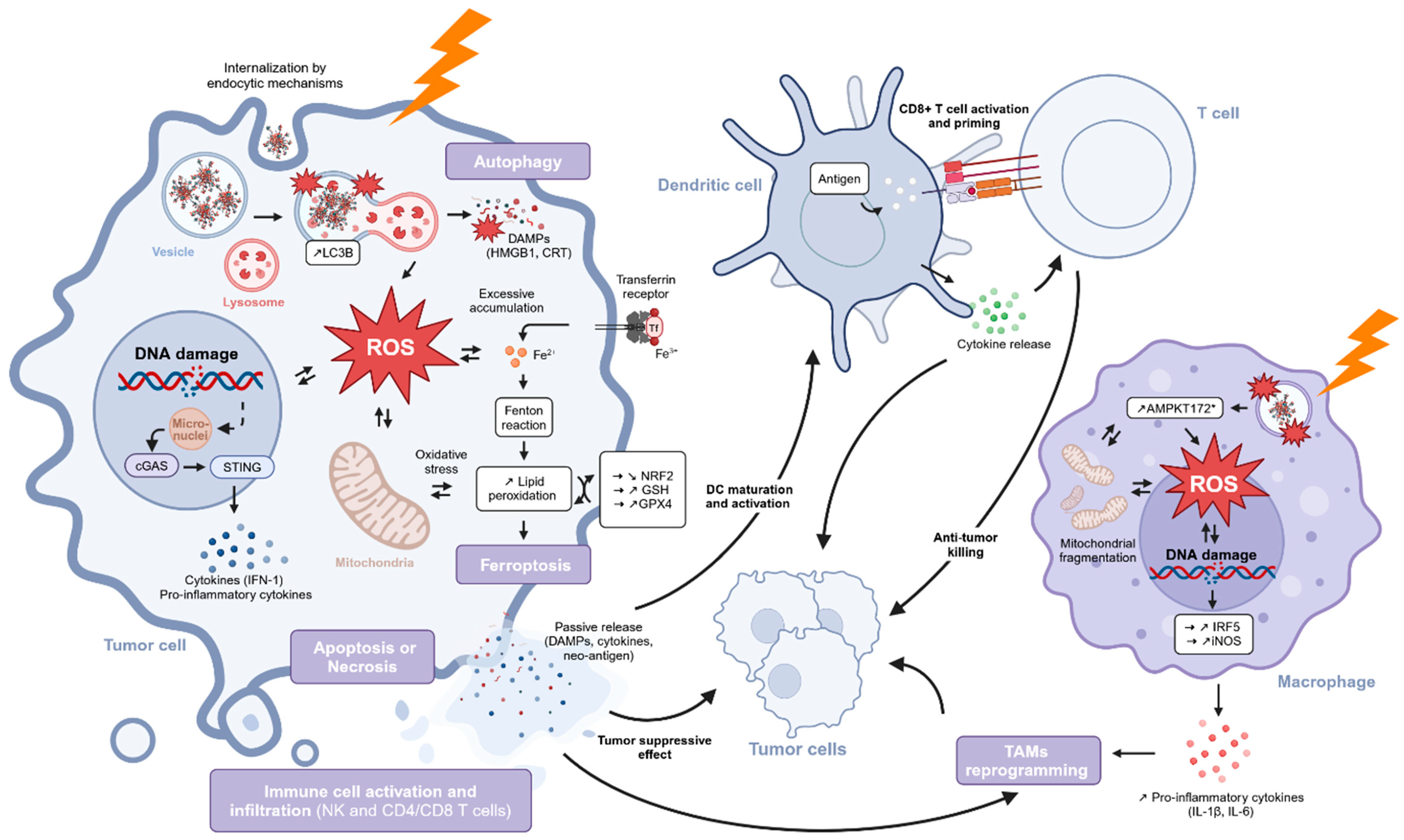
5.2.3. Ferroptosis
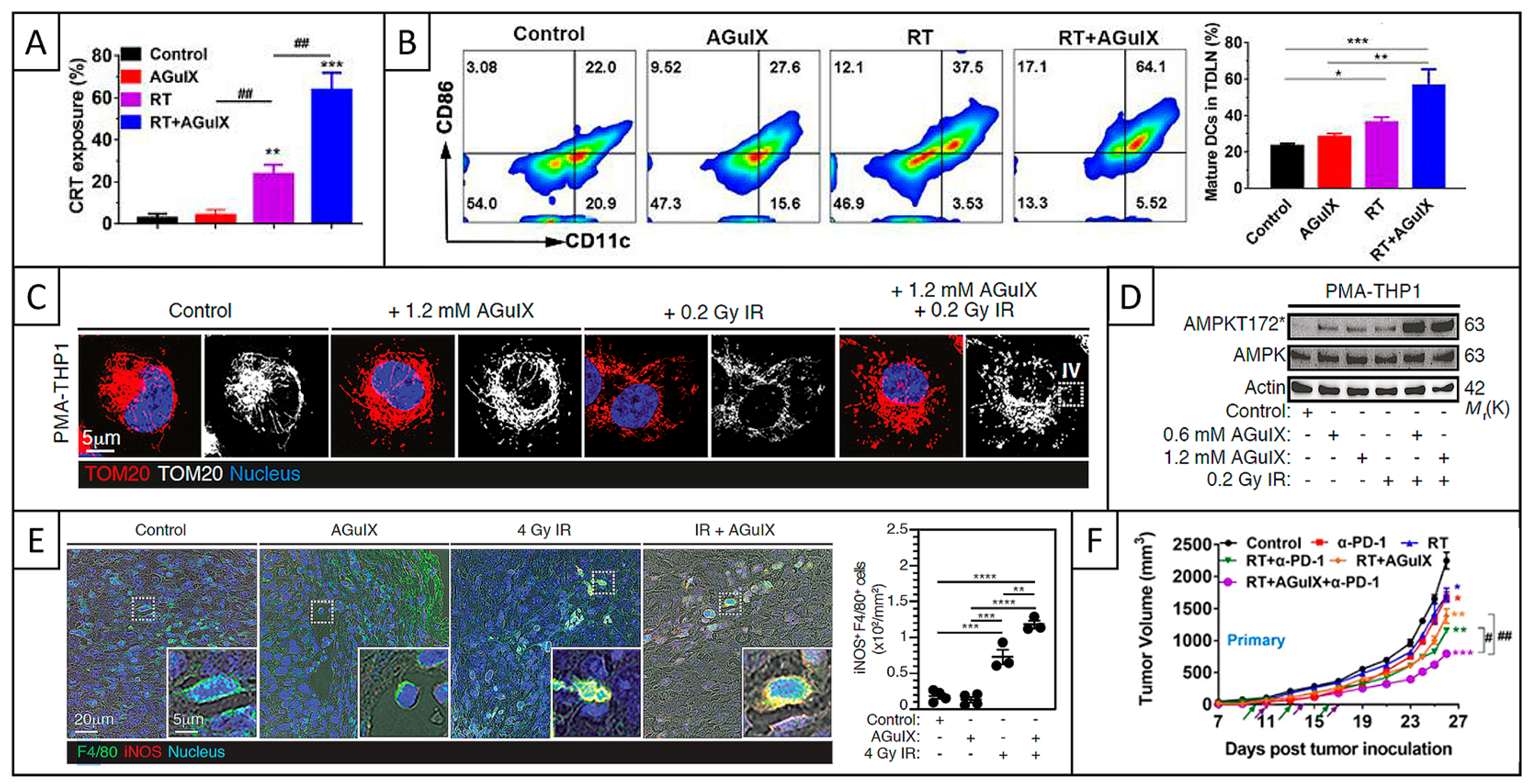
5.2.4. ICD and Immune Response
5.2.5. Conclusions
6. Conclusions
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EPR | Enhanced permeability and retention |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| WHO | World Health Organization |
| EBRT | External beam radiation therapy |
| LINACs | Linear particle accelerators |
| TRT | Targeted radionuclide therapy |
| SOCs | Standards of care |
| LET | Linear energy transfer |
| BNCT | Boron neutron capture therapy |
| OARs | Organs at risk |
| TME | Tumor microenvironment |
| SSBs | Single-strand breaks |
| DSBs | Double-strand breaks |
| PARP | Poly-ADP ribose polymerase |
| NP | Nanoparticle |
| AGuIX | Activation and Guidance of Irradiation by X-ray |
| GMP | Good manufacturing practice |
| MRI | Magnetic resonance imaging |
| WBRT | Whole brain radiation therapy |
| SE | Signal enhancement |
| EES | Extracellular extravascular space |
| % ID | Percentage of the injected dose |
| SD | Standard deviation |
| LIBS | Laser-induced breakdown spectroscopy |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| TEM | Transmission electron microscopy |
| FITC | Fluorescein isothiocyanate |
| Cy5.5 | Cyanine 5.5 |
| EEA1 | Early endosome antigen 1 |
| AIF | Apoptosis-inducing factor |
| LAMP-1 | Lysosome-associated membrane protein 1 |
| AF488 | Alexa Fluor 488 |
| IV | Intravenous |
| NIST | National Institute of Standards and Technology |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| LQ | Linear quadratic |
| SF | Survival fraction |
| SER | Sensitizing enhancement ratio |
| MC | Monte Carlo |
| FFF | Flattening filter free |
| RBE | Relative biological effectiveness |
| LEM | Local effect model |
| DEFs | Dose enhancement factors |
| TDRA | Theory of dual radiation action |
| DER | Dose enhancement ratio |
| DCFDA | 2′,7′-dichlorofluorescein diacetate |
| DHR123 | Dihydrorhodamine 123 |
| H2DCFDA or DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| M-H2DCFDA | 5-(and-6)-chloromethyl-H2DCFDA |
| mROS | Mitochondrial ROS |
| cROS | Cytosolic ROS |
| GSH | Glutathione |
| SEM | Standard error of the mean |
| ATM | Ataxia–telangiectasia mutated |
| ATR | Ataxia–telangiectasia and Rad3-related protein |
| DDR | DNA damage response |
| HR | Homologous recombination |
| NHEJ | Non-homologous end-joining |
| IF | Immunofluorescence |
| AGE | Agarose gel electrophoresis |
| FM | Fluorescence microscopy |
| cGAS | Cyclic guanosine monophosphate–adenosine monophosphate synthase |
| IFN | Interferon |
| STING | Stimulatory interferon genes |
| IHC | Immunohistochemistry |
| Unk | Unknown |
| BRCA1 | Breast cancer 1 |
| FACS | Fluorescence-activated cell sorting |
| PI | Propidium iodide |
| ANOVA | Analysis of variance |
| APC | Allophycocyanin |
| 7-AAD | 7-Aminoactinomycin D |
| SPECT/CT | Single-photon emission computed tomography/computed tomography |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| H-SCORE | Histochemistry score |
| LC3B | Microtubule-associated protein 1 light chain 3B |
| BODIPY | Boron-dipyrromethene |
| MDA | Malondialdehyde |
| 4-HNE | 4-hydroxynonenal |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| GPX4 | Glutathione peroxidase 4 |
| ns | Non-significant |
| ICD | Immunogenic cell death |
| DAMPs | Damage-associated molecular patterns |
| HMGB1 | High-mobility group box 1 |
| CRT | Calreticulin |
| DCs | Dendritic cells |
| TAMs | Tumor-associated macrophages |
| ICIs | Immune checkpoint inhibitors |
| PD-1 | Programmed cell death protein 1 |
| NK | Natural killer |
| PBMC | Peripheral blood mononuclear cells |
| NOX2 | NADPH oxidase 2 |
| IRF5 | Interferon regulatory factor 5 |
| iNOS | Inducible nitric oxide synthase |
| TOM20 | Translocase of the outer mitochondrial membrane 20 |
| AMPK | Adenosine monophosphate activated protein kinase |
| AMPKT172* | Activated protein kinase on threonine 172 |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-derived suppressor cells |
| TDLNs | Tumor-draining lymph nodes |
| PMA | Phorbol-12-myristate 13-acetate |
| ROIs | Regions of interest |
| scRNA-seq | Single-cell RNA sequencing |
| IL | Interleukin |
| Tf | Transferrin |
References
- Chuang, Y.C.; Wu, P.H.; Shen, Y.A.; Kuo, C.C.; Wang, W.J.; Chen, Y.C.; Lee, H.L.; Chiou, J.F. Recent Advances in Metal-Based NanoEnhancers for Particle Therapy. Nanomaterials 2023, 13, 1011. [Google Scholar] [CrossRef]
- Gerken, L.R.H.; Gerdes, M.E.; Pruschy, M.; Herrmann, I.K. Prospects of nanoparticle-based radioenhancement for radiotherapy. Mater. Horizons 2023, 10, 4059–4082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.; Falconi, M.; Foglia, E.; Bartolomei, M.; Berruti, A.; D’Onofrio, M.; Ferone, D.; Giordano, A.; Grimaldi, F.; Milione, M.; et al. Optimising Radioligand Therapy for Patients with Gastro-Entero-Pancreatic Neuroendocrine Tumours: Expert Opinion from an Italian Multidisciplinary Group. Adv. Ther. 2024, 41, 113–129. [Google Scholar] [CrossRef]
- Jadvar, H. Targeted Radionuclide Therapy: An Evolution Toward Precision Cancer Treatment. AJR Am. J. Roentgenol. 2017, 209, 277. [Google Scholar] [CrossRef]
- Vozenin, M.C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 791–803. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef] [PubMed]
- Cho, B. Intensity-modulated radiation therapy: A review with a physics perspective. Radiat. Oncol. J. 2018, 36, 1. [Google Scholar] [CrossRef]
- Mijnheer, B.; Beddar, S.; Izewska, J.; Reft, C. In vivo dosimetry in external beam radiotherapy. Med. Phys. 2013, 40, 070903. [Google Scholar] [CrossRef]
- Jaffray, D.A. Image-guided radiotherapy: From current concept to future perspectives. Nat. Rev. Clin. Oncol. 2012, 9, 688–699. [Google Scholar] [CrossRef]
- Brock, K.K. Adaptive Radiotherapy: Moving Into the Future. Semin. Radiat. Oncol. 2019, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Noël, G.; Antoni, D. Organs at risk radiation dose constraints. Cancer Radiother. 2022, 26, 59–75. [Google Scholar] [CrossRef]
- Boustani, J.; Grapin, M.; Laurent, P.A.; Apetoh, L.; Mirjolet, C. The 6th R of Radiobiology: Reactivation of Anti-Tumor Immune Response. Cancers 2019, 11, 860. [Google Scholar] [CrossRef]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 109688. [Google Scholar] [CrossRef] [PubMed]
- Small, K.L.; Henthorn, N.T.; Angal-Kalinin, D.; Chadwick, A.L.; Santina, E.; Aitkenhead, A.; Kirkby, K.J.; Smith, R.J.; Surman, M.; Jones, J.; et al. Evaluating very high energy electron RBE from nanodosimetric pBR322 plasmid DNA damage. Sci. Rep. 2021, 11, 3341. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomedicine 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Pernin, V.; Mégnin-Chanet, F.; Pennaneach, V.; Fourquet, A.; Kirova, Y.; Hall, J. PARP inhibitors and radiotherapy: Rational and prospects for a clinical use. Cancer Radiother. 2014, 18, 790–798. [Google Scholar] [CrossRef]
- Sun, C.; Chu, A.; Song, R.; Liu, S.; Chai, T.; Wang, X.; Liu, Z. PARP inhibitors combined with radiotherapy: Are we ready? Front. Pharmacol. 2023, 14, 1234973. [Google Scholar] [CrossRef]
- Li, M.H.; Ito, D.; Sanada, M.; Odani, T.; Hatori, M.; Iwase, M.; Nagumo, M. Effect of 5-fluorouracil on G1 phase cell cycle regulation in oral cancer cell lines. Oral Oncol. 2004, 40, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.H.; Skibber, J.M.; Birnbaum, E.H.; Feig, B.W.; Singh, A.K.; Delclos, M.E.; Lin, E.H.; Fleshman, J.W.; Thames, H.D.; Kodner, I.J.; et al. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M.P. Cisplatin in cancer treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef]
- Douple, E.B.; Richmond, R.C.; O’Hara, J.A.; Coughlin, C.T. Carboplatin as a potentiator of radiation therapy. Cancer Treat. Rev. 1985, 12, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Mukherjee, A.G.; Wanjari, U.R.; Sukumar, A.; Murali, R.; Renu, K.; Vellingiri, B.; Dey, A.; Valsala Gopalakrishnan, A. Repurposing FDA-approved drugs against the toxicity of platinum-based anticancer drugs. Life Sci. 2022, 305, 120789. [Google Scholar] [CrossRef]
- Cartwright, B.M.; Corso, J.N.; Lightner, J.; Whitted, C.; Torrenegra, R.D.; Krishnan, K.; Palau, V.E. Achyrocline B (3,5 dihydroxy-6,7,8-trimethoxyflavone) synergizes with 5-fluorouracil allowing for dose reduction and reduced off-target toxicity in the treatment of colonic and pancreatic cancers. Biomed. Pharmacother. 2023, 167, 115546. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef]
- Penninckx, S.; Heuskin, A.C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef]
- Penninckx, S.; Martinive, P.; Mirjolet, C. Radiation-activated nanoparticles: Which combination to optimize radiosensitization? Cancer/Radiothérapie 2023, 27, 494–498. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2023, 13, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.L.; Broekgaarden, M.; Chaput, F.; Baisamy, V.; Garrevoet, J.; Busser, B.; Brueckner, D.; Youssef, A.; Ravanat, J.L.; Dujardin, C.; et al. Radiation Dose-Enhancement Is a Potent Radiotherapeutic Effect of Rare-Earth Composite Nanoscintillators in Preclinical Models of Glioblastoma. Adv. Sci. 2020, 7, 2001675. [Google Scholar] [CrossRef]
- Aubrun Fulbert, C.; Chaput, F.; Stelse-Masson, S.; Henry, M.; Chovelon, B.; Bohic, S.; Brueckner, D.; Garrevoet, J.; Moriscot, C.; Gallet, B.; et al. Nanoscintillator Coating: A Key Parameter That Strongly Impacts Internalization, Biocompatibility, and Therapeutic Efficacy in Pancreatic Cancer Models. Small Sci. 2024, 4, 2400041. [Google Scholar] [CrossRef]
- Le Duc, G.; Miladi, I.; Alric, C.; Mowat, P.; Bräuer-Krisch, E.; Bouchet, A.; Khalil, E.; Billotey, C.; Janier, M.; Lux, F.; et al. Toward an image-guided microbeam radiation therapy using gadolinium-based nanoparticles. ACS Nano 2011, 5, 9566–9574. [Google Scholar] [CrossRef] [PubMed]
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S. AGuIX ® from bench to bedside—Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2018, 92, 20180365. [Google Scholar] [CrossRef]
- Zhang, P.; Marill, J.; Darmon, A.; Anesary, N.M.; Lu, B.; Paris, S. NBTXR3 Radiotherapy-Activated Functionalized Hafnium Oxide Nanoparticles Show Efficient Antitumor Effects Across a Large Panel of Human Cancer Models. Int. J. Nanomed. 2021, 16, 2761–2773. [Google Scholar] [CrossRef]
- Li, Y.; Yun, K.H.; Lee, H.; Goh, S.H.; Suh, Y.G.; Choi, Y. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 2019, 197, 12–19. [Google Scholar] [CrossRef]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Scher, N.; Bonvalot, S.; Le Tourneau, C.; Chajon, E.; Verry, C.; Thariat, J.; Calugaru, V. Review of clinical applications of radiation-enhancing nanoparticles. Biotechnol. Rep. 2020, 28, e00548. [Google Scholar] [CrossRef]
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res. 2017, 23, 908–917. [Google Scholar] [CrossRef]
- Bonvalot, S.; Rutkowski, P.L.; Thariat, J.; Carrère, S.; Ducassou, A.; Sunyach, M.P.; Agoston, P.; Hong, A.; Mervoyer, A.; Rastrelli, M.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Mignot, A.; Truillet, C.; Lux, F.; Sancey, L.; Louis, C.; Denat, F.; Boschetti, F.; Bocher, L.; Gloter, A.; Stéphan, O.; et al. A Top-Down Synthesis Route to Ultrasmall Multifunctional Gd-Based Silica Nanoparticles for Theranostic Applications. Chem.–A Eur. J. 2013, 19, 6122–6136. [Google Scholar] [CrossRef]
- Rocchi, P. Conception de Nanoparticules de Seconde Génération Issues d’ AGuIX ® Pour une Application en Oncologie. Ph.D. Thesis, Université Claude Bernard—Lyon I, Villeurbanne, France, 2022. [Google Scholar]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 2020, 6, eaay5279. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.; Quesada, J.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Chargari, C.; Maury, P.; Texier, M.; Genestie, C.; Morice, P.; Bockel, S.; Gouy, S.; Ba, M.; Achkar, S.; Lux, F.; et al. Theragnostic gadolinium-based nanoparticles safely augment X-ray radiation effects in patients with cervical cancer. ACS Nano 2024, 18, 16516–16529. [Google Scholar] [CrossRef]
- Du, Y.; Sun, H.; Lux, F.; Xie, Y.; Du, L.; Xu, C.; Zhang, H.; He, N.; Wang, J.; Liu, Y.; et al. Radiosensitization Effect of AGuIX, a Gadolinium-Based Nanoparticle, in Nonsmall Cell Lung Cancer. ACS Appl. Mater. Interfaces 2020, 12, 56874–56885. [Google Scholar] [CrossRef]
- Aloy, M.T.; Boumedine, J.S.; Deville, A.; Kryza, D.; Gauthier, A.; Brichart-Vernos, D.; Ollier, G.; La Padula, V.; Lux, F.; Tillement, O.; et al. Proof of Concept of the Radiosensitizing Effect of Gadolinium Oxide Nanoparticles in Cell Spheroids and a Tumor-Implanted Murine Model of Chondrosarcoma. Int. J. Nanomed. 2022, 17, 6655–6673. [Google Scholar] [CrossRef]
- Diaz Garcia-Prada, C.; Carmes, L.; Atis, S.; Parach, A.; Bertolet, A.; Jarlier, M.; Poty, S.; Garcia, D.S.; Shin, W.G.; Du Manoir, S.; et al. Gadolinium-Based Nanoparticles Sensitize Ovarian Peritoneal Carcinomatosis to Targeted Radionuclide Therapy. J. Nucl. Med. 2023, 64, 1956–1964. [Google Scholar] [CrossRef]
- Kotb, S.; Detappe, A.; Lux, F.; Appaix, F.; Barbier, E.L.; Tran, V.L.; Plissonneau, M.; Gehan, H.; Lefranc, F.; Rodriguez-Lafrasse, C.; et al. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: Proof of concept before phase I trial. Theranostics 2016, 6, 418–427. [Google Scholar] [CrossRef]
- Detappe, A.; Kunjachan, S.; Sancey, L.; Motto-Ros, V.; Biancur, D.; Drane, P.; Guieze, R.; Makrigiorgos, G.M.; Tillement, O.; Langer, R.; et al. Advanced multimodal nanoparticles delay tumor progression with clinical radiation therapy. J. Control. Release 2016, 238, 103–113. [Google Scholar] [CrossRef]
- Detappe, A.; Kunjachan, S.; Drané, P.; Kotb, S.; Myronakis, M.; Biancur, D.E.; Ireland, T.; Wagar, M.; Lux, F.; Tillement, O.; et al. Key clinical beam parameters for nanoparticle-mediated radiation dose amplification. Sci. Rep. 2016, 6, 34040. [Google Scholar] [CrossRef]
- Hu, P.; Fu, Z.; Liu, G.; Tan, H.; Xiao, J.; Shi, H.; Cheng, D. Gadolinium-Based Nanoparticles for Theranostic MRI-Guided Radiosensitization in Hepatocellular Carcinoma. Front. Bioeng. Biotechnol. 2019, 7, 368. [Google Scholar] [CrossRef]
- Hu, P.; Cheng, D.; Huang, T.; Banizs, A.B.; Xiao, J.; Liu, G.; Chen, Q.; Wang, Y.; He, J.; Shi, H. Evaluation of Novel 64Cu-Labeled Theranostic Gadolinium-Based Nanoprobes in HepG2 Tumor-Bearing Nude Mice. Nanoscale Res. Lett. 2017, 12, 8–13. [Google Scholar] [CrossRef]
- Dufort, S.; Le Duc, G.; Salomé, M.; Bentivegna, V.; Sancey, L.; Bräuer-Krisch, E.; Requardt, H.; Lux, F.; Coll, J.L.; Perriat, P.; et al. The High Radiosensitizing Efficiency of a Trace of Gadolinium-Based Nanoparticles in Tumors. Sci. Rep. 2016, 6, 29678. [Google Scholar] [CrossRef]
- Sancey, L.; Kotb, S.; Truillet, C.; Appaix, F.; Marais, A.; Thomas, E.; Van Der Sanden, B.; Klein, J.P.; Laurent, B.; Cottier, M.; et al. Long-term in Vivo clearance of gadolinium-based AGuIX nanoparticles and their biocompatibility after systemic injection. ACS Nano 2015, 9, 2477–2488. [Google Scholar] [CrossRef]
- Bennett, S.; Verry, C.; Kaza, E.; Miao, X.; Dufort, S.; Boux, F.; Crémillieux, Y.; de Beaumont, O.; Le Duc, G.; Berbeco, R.; et al. Quantifying gadolinium-based nanoparticle uptake distributions in brain metastases via magnetic resonance imaging. Sci. Rep. 2024, 14, 11959. [Google Scholar] [CrossRef]
- Biau, J.; Durando, X.; Boux, F.; Molnar, I.; Moreau, J.; Leyrat, B.; Guillemin, F.; Lavielle, A.; Cremillieux, Y.; Seddik, K.; et al. NANO-GBM trial of AGuIX nanoparticles with radiotherapy and temozolomide in the treatment of newly diagnosed Glioblastoma: Phase 1b outcomes and MRI-based biodistribution. Clin. Transl. Radiat. Oncol. 2024, 48, 100833. [Google Scholar] [CrossRef]
- Le Duc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F.; et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Tillement, O.; Lux, F.; Dufort, S.; Verry, C.; LeDuc, G. Methods for Treating Tumors. WO 2021/019268. 4 February 2021. Available online: https://hal.science/hal-04753043 (accessed on 11 February 2025).
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; De Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1663–1701. [Google Scholar] [CrossRef]
- Epple, M.; Rotello, V.M.; Dawson, K. The Why and How of Ultrasmall Nanoparticles. Acc. Chem. Res. 2023, 56, 3369–3378. [Google Scholar] [CrossRef]
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.W.; Lee, T.-Y.; Mayr, N.A.; Parker, G.J.M.; et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Detappe, A.; Kunjachan, S.; Rottmann, J.; Robar, J.; Tsiamas, P.; Korideck, H.; Tillement, O.; Berbeco, R. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Štefanciková, L.; Lacombe, S.; Salado, D.; Porcel, E.; Pagáčová, E.; Tillement, O.; Lux, F.; Depeš, D.; Kozubek, S.; Falk, M. Effect of gadolinium-based nanoparticles on nuclear DNA damage and repair in glioblastoma tumor cells. J. Nanobiotechnol. 2016, 14, 63. [Google Scholar] [CrossRef]
- Simonet, S.; Rodriguez-Lafrasse, C.; Beal, D.; Gerbaud, S.; Malesys, C.; Tillement, O.; Lux, F.; Fayyad-Kazan, H.; Rachidi, W.; Ardail, D. Gadolinium-Based Nanoparticles Can Overcome the Radioresistance of Head and Neck Squamous Cell Carcinoma Through the Induction of Autophagy. J. Biomed. Nanotechnol. 2020, 16, 111–124. [Google Scholar] [CrossRef]
- Goodarzi, S.; Prunet, A.; Rossetti, F.; Bort, G.; Tillement, O.; Porcel, E.; Lacombe, S.; Wu, T.-D.; Guerquin-Kern, J.L.; Delanoë-Ayari, H.; et al. Quantifying nanotherapeutic penetration using a hydrogel-based microsystem as a new 3D in vitro platform. Lab Chip 2021, 21, 2495–2510. [Google Scholar] [CrossRef]
- Maury, P.; Porcel, E.; Mau, A.; Lux, F.; Tillement, O.; Mahou, P.; Schanne-Klein, M.C.; Lacombe, S. Rapid Evaluation of Novel Therapeutic Strategies Using a 3D Collagen-Based Tissue-Like Model. Front. Bioeng. Biotechnol. 2021, 9, 574035. [Google Scholar] [CrossRef]
- Ahmad, R.; Schettino, G.; Royle, G.; Barry, M.; Pankhurst, Q.A.; Tillement, O.; Russell, B.; Ricketts, K. Radiobiological Implications of Nanoparticles Following Radiation Treatment. Part. Part. Syst. Charact. 2020, 37, 1900411. [Google Scholar] [CrossRef]
- Liu, W.; Deacon, J.; Yan, H.; Sun, B.; Liu, Y.; Hegan, D.; Li, Q.; Coman, D.; Parent, M.; Hyder, F.; et al. Tumor-targeted pH-low insertion peptide delivery of theranostic gadolinium nanoparticles for image-guided nanoparticle-enhanced radiation therapy. Transl. Oncol. 2020, 13, 100839. [Google Scholar] [CrossRef]
- Varzandeh, M.; Labbaf, S.; Varshosaz, J.; Laurent, S. An overview of the intracellular localization of high-Z nanoradiosensitizers. Prog. Biophys. Mol. Biol. 2022, 175, 14–30. [Google Scholar] [CrossRef]
- Štefančíková, L.; Porcel, E.; Eustache, P.; Li, S.; Salado, D.; Marco, S.; Guerquin-Kern, J.L.; Réfrégiers, M.; Tillement, O.; Lux, F.; et al. Cell localisation of gadolinium-based nanoparticles and related radiosensitising efficacy in glioblastoma cells. Cancer Nanotechnol. 2014, 5, 6. [Google Scholar] [CrossRef]
- Rima, W.; Sancey, L.; Aloy, M.T.; Armandy, E.; Alcantara, G.B.; Epicier, T.; Malchère, A.; Joly-Pottuz, L.; Mowat, P.; Lux, F.; et al. Internalization pathways into cancer cells of gadolinium-based radiosensitizing nanoparticles. Biomaterials 2013, 34, 181–195. [Google Scholar] [CrossRef]
- Yousef, I.; Seksek, O.; Gil, S.; Prezado, Y.; Sulé-Suso, J.; Martínez-Rovira, I. Study of the biochemical effects induced by X-ray irradiations in combination with gadolinium nanoparticles in F98 glioma cells: First FTIR studies at the Emira laboratory of the SESAME synchrotron. Analyst 2016, 141, 2238–2249. [Google Scholar] [CrossRef]
- Sun, H.; Cai, H.; Xu, C.; Zhai, H.; Lux, F.; Xie, Y.; Feng, L.; Du, L.; Liu, Y.; Sun, X.; et al. AGuIX nanoparticles enhance ionizing radiation-induced ferroptosis on tumor cells by targeting the NRF2-GPX4 signaling pathway. J. Nanobiotechnol. 2022, 20, 449. [Google Scholar] [CrossRef]
- Song, H.; Sun, H.; He, N.; Xu, C.; Wang, Y.; Du, L.; Liu, Y.; Wang, Q.; Ji, K.; Wang, J.; et al. Gadolinium-based ultra-small nanoparticles augment radiotherapy-induced T-cell response to synergize with checkpoint blockade immunotherapy. Nanoscale 2022, 14, 11429–11442. [Google Scholar] [CrossRef]
- Carter, J.D.; Cheng, N.N.; Qu, Y.; Suarez, G.D.; Guo, T. Nanoscale energy deposition by X-ray absorbing nanostructures. J. Phys. Chem. B 2007, 111, 11622–11625. [Google Scholar] [CrossRef]
- Yan, H.; Carlson, D.J.; Abolfath, R.; Liu, W. Microdosimetric investigation and a novel model of radiosensitization in the presence of metallic nanoparticles. Pharmaceutics 2021, 13, 2191. [Google Scholar] [CrossRef]
- Mott, J.H.L.; Daniel, J.M. Interactions of Electromagnetic Radiation and Subatomic Particles with Matter—Part 1. Clin. Oncol. 2021, 33, 455–460. [Google Scholar] [CrossRef]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. Optimising element choice for nanoparticle radiosensitisers. Nanoscale 2016, 8, 581–589. [Google Scholar] [CrossRef]
- Lux, F.; Detappe, A.; Dufort, S.; Sancey, L.; Louis, C.; Carme, S.; Tillement, O. Ultrasmall nanoparticles for radiotherapy: AGuIX. Cancer/Radiotherapie 2015, 19, 508–514. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Liang, Y.; Chen, T.; Quan, E.; Wang, L. Biological modeling of gadolinium-based nanoparticles radio-enhancement for kilovoltage photons: A Monte Carlo study. Cancer Nanotechnol. 2023, 14, 47. [Google Scholar] [CrossRef]
- Ge, Y.; Ji, X.; Zhang, R.; Li, K.; Chen, G.H. K-edge energy-based calibration method for photon counting detectors. Phys. Med. Biol. 2017, 63, 015022. [Google Scholar] [CrossRef]
- Maury, P.; Mondini, M.; Chargari, C.; Darricau, A.; Shahin, M.; Ammari, S.; Bockel, S.; Genestie, C.; Wu, T.-D.; Lux, F.; et al. Clinical transfer of AGuIX®-based radiation treatments for locally advanced cervical cancer: MR quantification and in vitro insights in the NANOCOL clinical trial framework. Nanomed. Nanotechnol. Biol. Med. 2023, 50, 102676. [Google Scholar] [CrossRef]
- Delorme, R.; Taupin, F.; Flaender, M.; Ravanat, J.L.; Champion, C.; Agelou, M.; Elleaume, H. Comparison of gadolinium nanoparticles and molecular contrast agents for radiation therapy-enhancement. Med. Phys. 2017, 44, 5949–5960. [Google Scholar] [CrossRef]
- Cho, S.H. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Phys. Med. Biol. 2005, 50, N163–N173. [Google Scholar] [CrossRef]
- Mesbahi, A.; Jamali, F.; Gharehaghaji, N. Effect of photon beam energy, gold nanoparticle size and concentration on the dose enhancement in radiation therapy. BioImpacts 2013, 3, 29–35. [Google Scholar] [CrossRef]
- Subiel, A.; Ashmore, R.; Schettino, G. Standards and Methodologies for Characterizing Radiobiological Impact of High-Z Nanoparticles. Theranostics 2016, 6, 1651. [Google Scholar] [CrossRef]
- Hernández Millares, R.; Bae, C.; Kim, S.J.; Kim, T.; Park, S.Y.; Lee, K.; Ye, S.J. Clonogenic assay and computational modeling using real cell images to study physical enhancement and cellular sensitization induced by metal nanoparticles under MV and kV X-ray irradiation. Nanoscale 2024, 16, 7110–7122. [Google Scholar] [CrossRef]
- Jia, S.; Ge, S.; Fan, X.; Leong, K.W.; Ruan, J. Promoting reactive oxygen species generation: A key strategy in nanosensitizer-mediated radiotherapy. Nanomedicine 2021, 16, 759–778. [Google Scholar] [CrossRef]
- Howard, D.; Sebastian, S.; Le, Q.V.C.; Thierry, B.; Kempson, I. Chemical mechanisms of nanoparticle radiosensitization and radioprotection: A review of structure-function relationships influencing reactive oxygen species. Int. J. Mol. Sci. 2020, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Shintoku, R.; Kubota, C.; Yaegashi, M.; Torii, R.; Sasaki, M.; Suzuki, T.; Mori, M.; Yoshimoto, Y.; Takeuchi, T.; et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J. 2016, 473, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Brandsma, I.; Gent, D.C. Pathway choice in DNA double strand break repair: Observations of a balancing act. Genome Integr. 2012, 3, 9. [Google Scholar] [CrossRef]
- Baatout, S. Radiobiology Textbook; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Bin Cho, S.; Im, H.-J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Wozny, A.S.; Aloy, M.T.; Alphonse, G.; Magné, N.; Janier, M.; Tillement, O.; Lux, F.; Beuve, M.; Rodriguez-Lafrasse, C. Gadolinium-based nanoparticles as sensitizing agents to carbon ions in head and neck tumor cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2655–2660. [Google Scholar] [CrossRef]
- Detappe, A.; Mathieu, C.; Jin, C.; Agius, M.P.; Diringer, M.C.; Tran, V.L.; Pivot, X.; Lux, F.; Tillement, O.; Kufe, D.; et al. Anti-MUC1-C Antibody–Conjugated Nanoparticles Potentiate the Efficacy of Fractionated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1380–1389. [Google Scholar] [CrossRef]
- Tannous, D. The Combination of Gadolinium-Based Nanoparticles, Radiotherapy, and Immune Checkpoint Inhibitors: A Novel Therapeutic Opportunity for Cancer Treatment. PhD Thesis, Université Paris-Saclay, Orsay, France, 2022. [Google Scholar]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, R.J.; Chiu, H.W.; Yang, L.X.; Wang, Y.L.; Chen, Y.Y.; Yeh, Y.L.; Liao, M.Y.; Wang, Y.J. Nanoparticles augment the therapeutic window of RT and immunotherapy for treating cancers: Pivotal role of autophagy. Theranostics 2023, 13, 40–58. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, X. HMGB1 Signaling-Mediated Tumor Immunity in Cancer Progress. Front. Biosci. 2023, 28, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, J.; Liu, J.; Bai, X.; Zeng, X.; Zhang, Z.; Liu, F. Calreticulin as a marker and therapeutic target for cancer. Clin. Exp. Med. 2022, 23, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.-P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Paoletti, A.; Leteur, C.; Mirjolet, C.; Martins, I.; Voisin, L.; Law, F.; Dakhli, H.; Mintet, E.; et al. NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy. Cell Death Differ. 2017, 24, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The Abscopal Effect of Radiation Therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Penninckx, S.; Thariat, J.; Mirjolet, C. Radiation therapy-activated nanoparticle and immunotherapy: The next milestone in oncology? Int. Rev. Cell Mol. Biol. 2023, 378, 157–200. [Google Scholar] [CrossRef]
- Muradova, Z.; Tannous, D.; Mostefa-Kara, A.; Cao-Pham, T.T.; Lamy, C.; Broutin, S.; Paci, A.; Dufort, S.; Doussineau, T.; Lux, F.; et al. Gadolinium-based nanoparticles AGuIX and their combination with ionizing radiation trigger AMPK-dependent proinflammatory reprogramming of tumor-associated macrophages. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mittelheisser, V.; Lefebvre, O.; Banerjee, M.; Ghosh, S.; Dupas, A.; Diringer, M.-C.; Blumberger, J.; Bochler, L.; Harlepp, S.; Larnicol, A.; et al. Nanomaterials trigger functional responses in primary human immune cells. bioRxiv 2024, 1–47. [Google Scholar] [CrossRef]

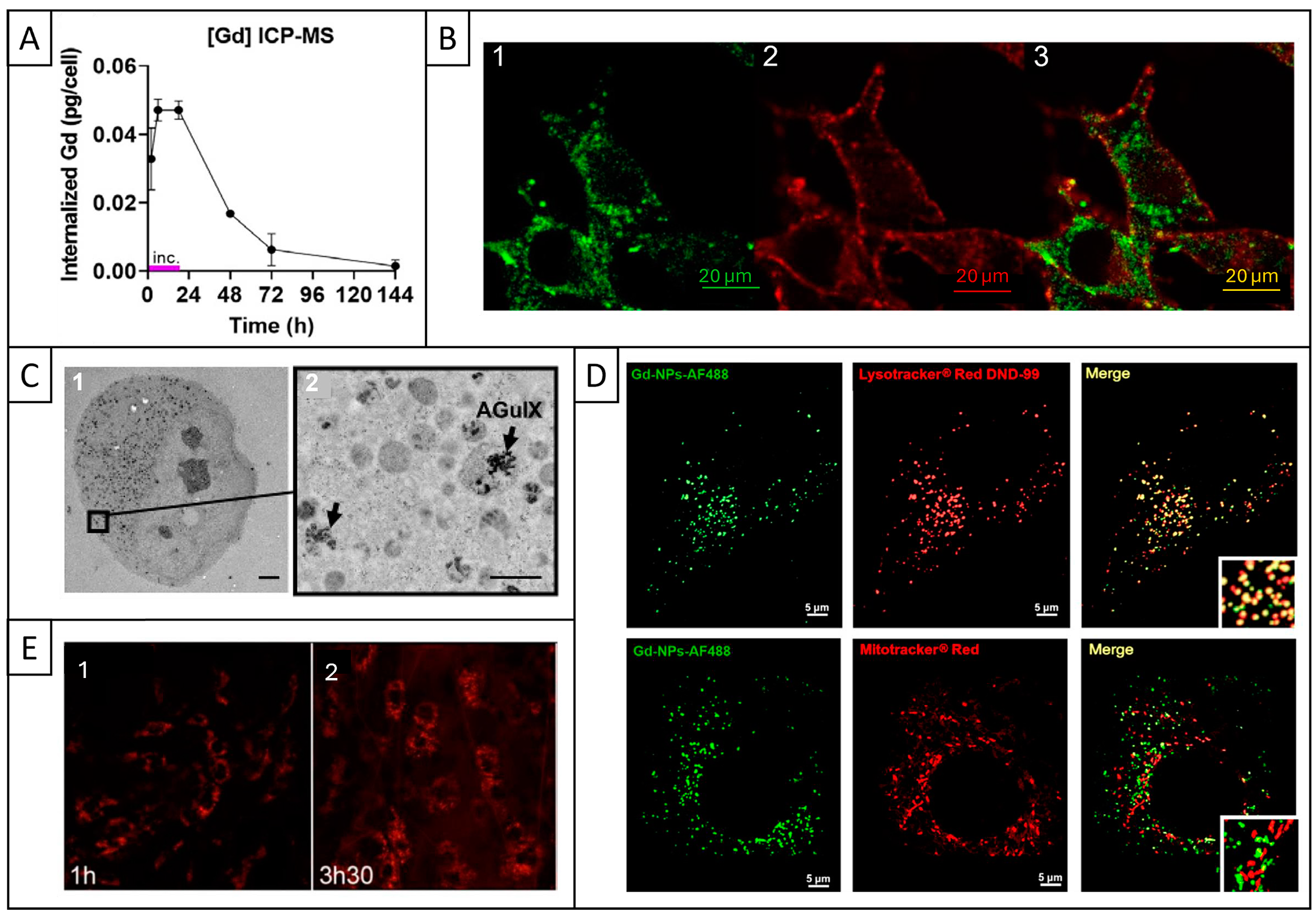
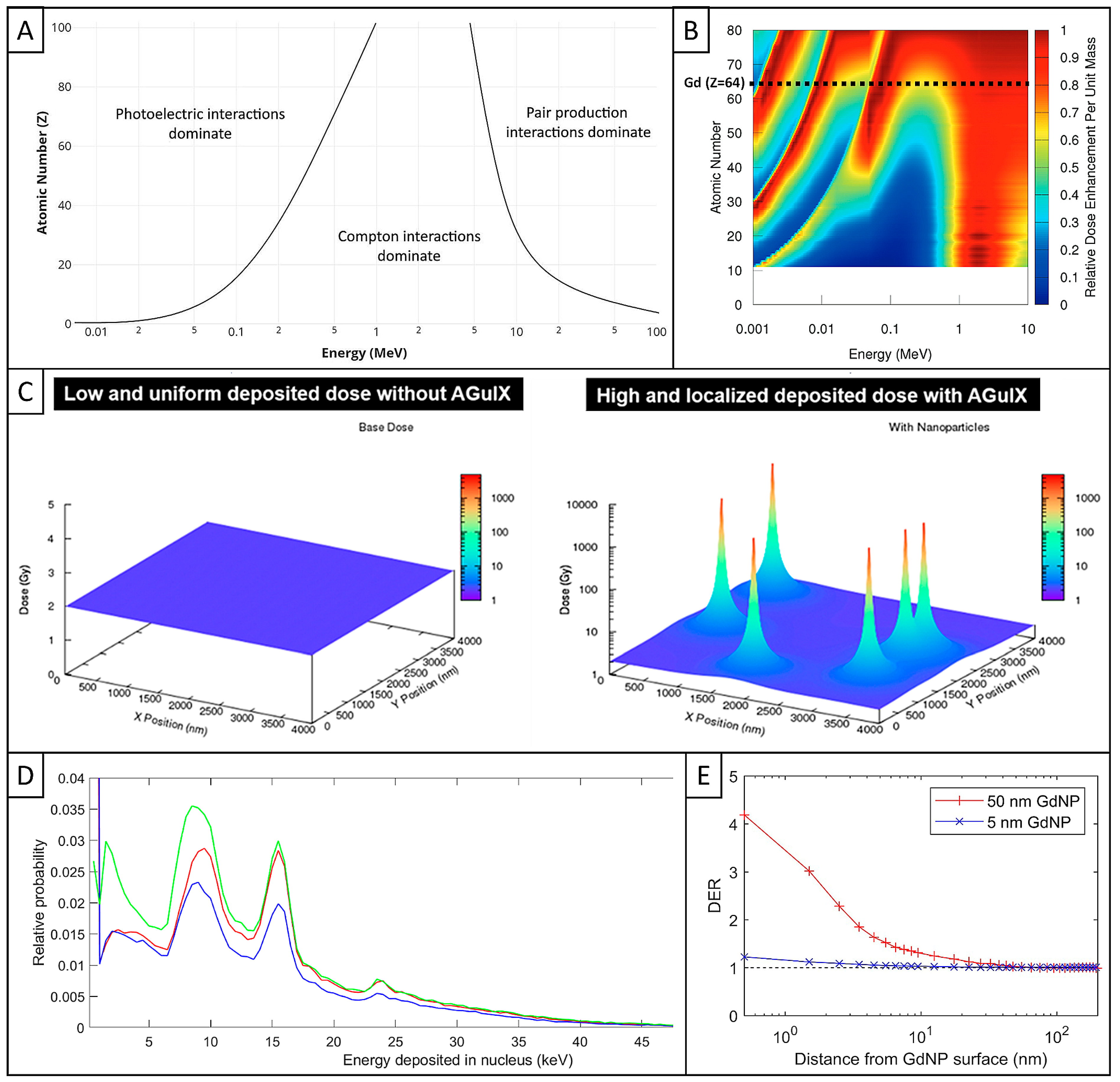


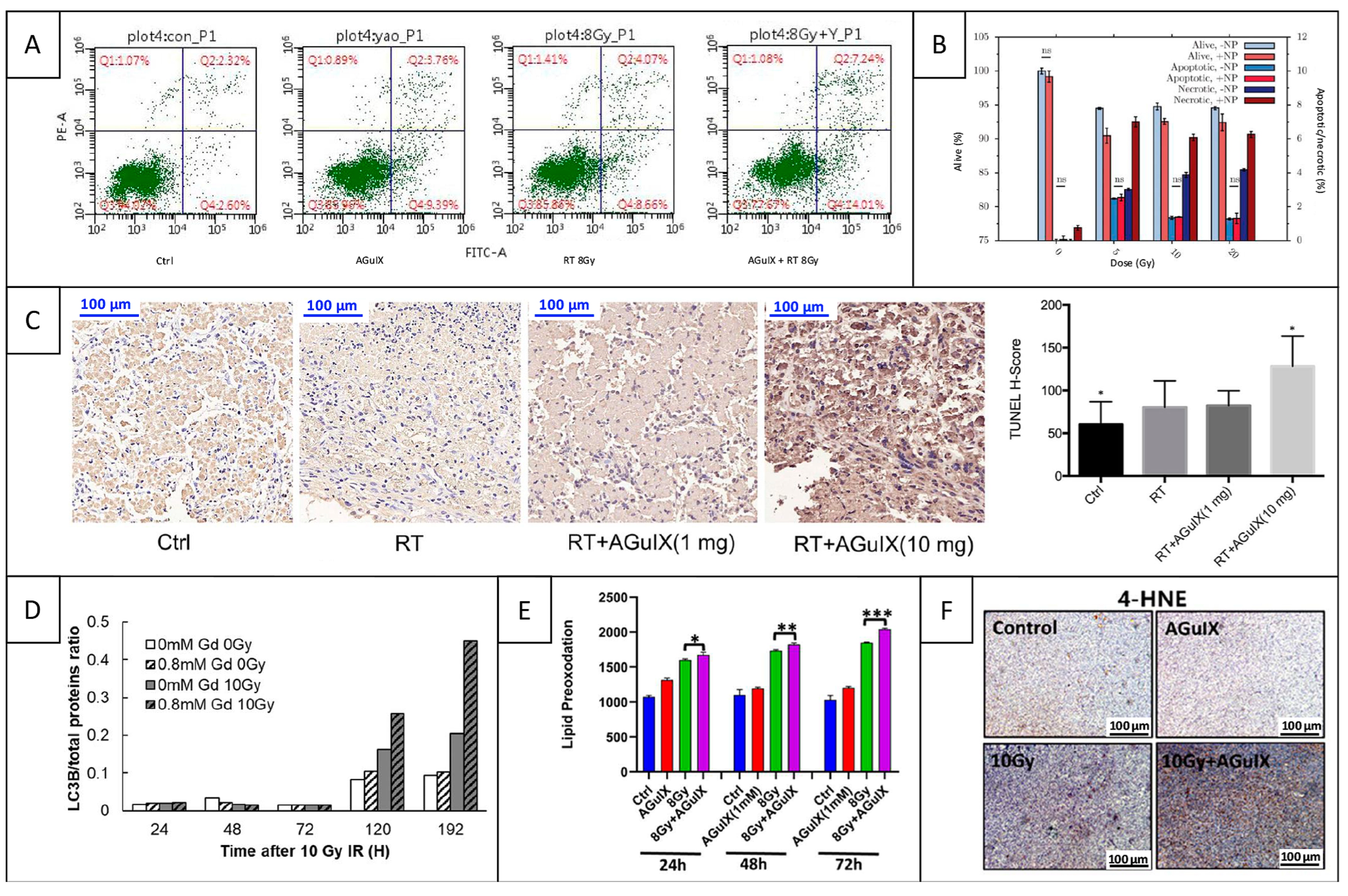
| Cell Line (Species and Cancer Type) | NP Incubation | Method | Reference |
|---|---|---|---|
| PANC-1 (human pancreatic adenocarcinoma) | 30 min, 1, 3, 6, 24 and 48 h at 0.5 mM | MRI scanner and ICP-MS | Detappe, 2015 [66] |
| SQ20B (human larynx carcinoma) | 1, 4, 12 and 24 h at 0.8 mM | ICP-OES | Simonet, 2020 [68] |
| U87 (human glioblastoma) and MCF7 (human breast adenocarcinoma) | 24 h at 0.5 mg/mL | ICP-MS | Ahmad, 2020 [71] |
| A549 (human lung adenocarcinoma) | 2 h at 0.5 mM Gd | ICP-MS | Liu, 2020 [72] |
| HCT-116 (human colon carcinoma; cell monolayers and spheroids) | 24 h at 0.8, 1.5 and 2 mM Gd, AGuIX-Cy5.5 | ICP-MS | Goodarzi, 2021 [69] |
| HeLa (human cervix carcinoma) | 4 h at 1 mM of Gd | ICP-MS | Maury, 2021 [70] |
| SK-OV-3-luc (human ovarian adenocarcinoma) | 18 h at 1.15 mM Gd | ICP-MS | Diaz Garcia-Prada, 2023 [50] |
| Cell Line (Species and Cancer Type) | NP Incubation | Method and Reagent | Reference |
|---|---|---|---|
| PANC-1 (human pancreatic adenocarcinoma) | 1 h at 0.5 mM | TEM | Detappe, 2015 [66] |
| B16F10 (mouse melanoma) | 1 h at 0.6 mg/mL, AGuIX-FITC | Confocal microscopy | Kotb, 2016 [51] |
| 18 h at 0.6 mg/mL | TEM | ||
| U87 (human glioblastoma) | 1, 6, 16 h at 1 mM, AGuIX-Cy5.5 | Confocal microscopy | Štefanciková, 2016 [67] |
| HepG2 (human hepatocellular carcinoma) | 1 h at 0.5 mM | TEM | Hu, 2017 [55] |
| SQ20B (human larynx carcinoma) | 24 h at 0.8 mM, AGuIX-Cy5.5 | Confocal microscopy, MitoTracker green, LysoTracker green (Thermo Fisher Scientific, Saint-Aubin, France) | Simonet, 2020 [68] |
| H1299 (human lung carcinoma) | 1 h at 1 mM, AGuIX-FITC | Inverted microscopy | Du, 2020 [48] |
| HCT-116 (human colon carcinoma) | 24 h at 0.8, 1.5, 2 mM Gd, AGuIX-Cy5.5 | Confocal microscopy, EEA1, AIF, LAMP-1 (Cell Signaling Technology, #3288, #5318, #9091). | Goodarzi, 2021 [69] |
| HeLa (human cervix carcinoma) | 4 h at 1 mM Gd | Confocal microscopy, Cell Mask Deep Red Actin (Invitrogen) | Maury, 2021 [70] |
| SK-OV-3-luc (human ovarian adenocarcinoma) | 18 h, AGuIX-AF488 | Inverted microscopy, MitoTracker red (M7513, Thermo Fisher Scientific), LysoTracker red (L7528, Thermo Fisher Scientific) | Diaz Garcia-Prada, 2023 [50] |
| Animal Model | NP Injection | Method | Reference |
| Mice with B16F10 tumors (subcutaneous) | 10 mg, intravenous | Intravital two-photon microscopy | Kotb, 2016 [51] |
| Cell Line (Species and Cancer Type) | NP Incubation | Irradiation | Fluorescence Analysis, ROS Probe | Reference | ||
|---|---|---|---|---|---|---|
| Dose | Energy | Type | ||||
| Capan-1 (human pancreatic adenocarcinoma) | 30 min at 0.43 to 1 mg/mL | 4 Gy | 6 MV | X-rays | Plate reader, DHR123 | Detappe, 2016 [53] |
| SQ20B (human larynx carcinoma) | 24 h at 0.8 mM | 10 Gy | 250 kV | X-rays | Flow cytometry, M-H2DCFDA or MitoSOX™ (Thermo Fisher Scientific) | Simonet, 2020 [68] |
| H1299 and A549 (human lung carcinoma) | 1 h at 1 mM | 4 Gy | 662 keV | Cs137 γ-rays | Flow cytometry, ROS kit (DCFH-DA, Thermo Fisher Scientific, USA) | Du, 2020 [48] |
| B16 (mouse melanoma) | 1 h at 1 mM | 6 Gy | 662 keV | Cs137 γ-rays | Flow cytometry, ROS kit (DCFH-DA, Thermo Fisher Scientific, MA, USA) | Song, 2022 [78] |
| MDA-MB-231 and MDA-MB-468 (human breast cancer) | 1 h at 1 mM | 8 Gy | 662 keV | Cs137 γ-rays | Flow cytometry, carboxy-H2DCFDA (Thermo Fisher Scientific, 88-5930-74) | Sun, 2022 [77] |
| Cell Line (Species and Cancer Type) | NP Incubation | Irradiation | Method and Assay | Reference | ||
|---|---|---|---|---|---|---|
| Dose | Energy | Type | ||||
| Capan-1 (human pancreatic adenocarcinoma) | 15 min at 0.43 mg/mL | 4 and 10 Gy | 220 kVp | X-rays | IF, proportion of positive cells (>10 foci); 53BP1 (H-300, Santacruz, USA) | Detappe, 2016 [52] |
| Capan-1 (human pancreatic adenocarcinoma) | 15 min at 0.43 mg/mL | 4 Gy | 6 MV | X-rays | IF, proportion of positive cells (>10 foci); 53BP1 (H-300, Santacruz, USA) | Detappe, 2016 [53] |
| B16F10 (mouse melanoma) | 1 h at 0.6 mg/L | 2 Gy | 220 kV | X-rays | IF, number of foci per nucleus, γ-H2AX (Merck Millipore) | Kotb, 2016 [51] |
| U87 (human glioblastoma) | 1, 6 and 24 h at 1 mM | 1 and 4 Gy | 1.173 & 1.332 MeV | 60Co γ-rays | IF, number of foci per nucleus, γ-H2AX (Upstate Biotechnology) and 53BP1 (Cell Signaling Technology) | Štefanciková, 2016 [67] |
| SQ20B (human larynx carcinoma) | 1 h at 0.8 mg/mL | 1 Gy | 75 MeV/n | 13C6+ ions | IF, number of foci per nucleus, γ-H2AX | Wozny, 2017 [100] |
| 2 Gy | 250 kV | X-rays | ||||
| MCF-7 (human breast adenocarcinoma), U87 (human glioblastoma) | 24 h at 0.5 mg/mL | 1 Gy | 6 MV | X-rays | IF, number of foci per nucleus, 53BP1 (Novus Biologicals, USA) | Ahmad, 2020 [71] |
| E0771 (human breast carcinoma) | 30 min at 0.4 mg/mL | 2 Gy | 220 kVp | X-rays | IF, number of foci per nucleus, γ-H2AX (Merck Millipore) | Detappe, 2020 [101] |
| H1299 and A549 (human lung carcinoma) | 1 h at 1 mM | 4 Gy | 662 keV | Cs137 γ-rays | IF, proportion of positive cells (>10 foci), γ-H2AX (Merck Millipore, Belford, MA, USA) and 53BP1 (Abcam, Cambridge, UK) | Du, 2020 [48] |
| AGE, olive tail moment, Comet assay | ||||||
| SQ20B (human larynx carcinoma) | 24 h at 0.8 mM | 2 Gy | 250 kV | X-rays | IF, number of foci per nucleus, γ-H2AX | Simonet, 2020 [68] |
| 4 Gy | AGE, tail intensity, Comet assay | |||||
| B16 (mouse melanoma) | 1 h at 1 mM | 6 Gy | 662 keV | Cs137 γ-rays | IF, number of foci per nucleus, γ-H2AX | Song, 2022 [78] |
| MDA-MB-231 and MDA-MB-468 (human breast adenocarcinoma) | 1 h at 1 mM | 4 Gy | 662 keV | Cs137 γ-rays | IF, proportion of positive cells (>10 foci), γ-H2AX (Millipore, Belford, MA, USA) | Sun, 2022 [77] |
| 8 Gy | AGE, olive tail moment, Comet assay | |||||
| Caco-2 (human colon adenocarcinoma) | 1 h at 0.6 or 1.2 mM | 6 Gy | 200 keV | X-rays | IF, proportion of positive cells, γ-H2AX (Sigma, 05-636) | Tannous, 2022 [102] |
| Caco-2 and CT26 (murine colon carcinoma) | FM, proportion of cells with micronuclei | |||||
| Animal Model | NP Injection | Irradiation | Method and Assay | Reference | ||
|---|---|---|---|---|---|---|
| Dose | Energy | Type | ||||
| Nude mice with Capan-1 tumors (subcutaneous) | 250 mg/kg, 15 min prior RT, intravenous | 10 Gy | 220 kVp | X-rays | IHC, proportion of positive nuclei (brown), γ-H2AX (Abcam, ab26350) | Detappe, 2016 [52] |
| Nude mice with Capan-1 tumors (subcutaneous) | 250 mg/kg, 15 min prior RT, intravenous | unk. | 6 MV | X-rays | IHC, proportion of positive nuclei (brown), γ-H2AX (Abcam, ab11174) | Detappe, 2016 [53] |
| Nude mice with H1299 tumors (subcutaneous) | 420 mg/kg, 15 min prior RT, intravenous | 10 Gy | 662 keV | Cs137 γ-rays | IHC, proportion of positive nuclei (brown), γ-H2AX (Millipore, Belford, MA, USA) | Du, 2020 [48] |
| Cell Line (Species and Cancer Type) | NP Incubation | Irradiation | Method and Reagent | Reference | ||
|---|---|---|---|---|---|---|
| Dose | Energy | Type | ||||
| H1299 (human lung carcinoma) | 1 h at 1 mM | 4 and 8 Gy | 662 keV | Cs137 γ-rays | FACS, PI (Solarbio, Beijing, China) | Du, 2020 [48] |
| MDA-MB-231 and MDA-MB-468 (human breast adenocarcinoma) | 1 h at 1 mM | 4 Gy | 662 keV | Cs137 γ-rays | FACS, PI (Solarbio, Beijing, China) | Sun, 2022 [77] |
| Caco-2 (human colon adenocarcinoma) | 24 h at 0.6 or 1.2 mM | 6 Gy | 200 keV | X-rays | FACS, PI (Sigma, #P4864) | Tannous, 2022 [102] |
| Cell Line (Species and Cancer Type) | NP Incubation | Irradiation | Method and Reagent | Reference | ||
|---|---|---|---|---|---|---|
| Dose | Energy | Type | ||||
| Capan-1 (human pancreatic adenocarcinoma) | 15 min at 0.43 mg/mL | 4 Gy | 220 kVp | X-rays | FACS, Annexin V-APC/7-AAD (BioLegend, USA) | Detappe, 2016 [52] |
| F98 (rat glioma) | 6 h at 1 mM | 5, 10, 20 Gy | 90 keV | X-rays | FACS, Annexin V-AF488/PI (Invitrogen) | Yousef, 2016 [76] |
| H1299 and A549 (human lung carcinoma) | 1 h at 1 mM | 4 and 8 Gy | 662 keV | Cs137 γ-ray | FACS, Annexin V-FITC (BD Pharmingen, San Diego, CA, USA) | Du, 2020 [48] |
| SQ20B (human larynx carcinoma) | 24 h at 0.8 mM | 10 Gy | 250 kV | X-rays | FACS, Annexin V-FITC/PI (Life Technologies, Courtaboeuf, France) and CaspACE™ FITC-VAD-FMK marker (Promega, Charbonnières Les Bains, France) | Simonet, 2020 [68] |
| B16 (mouse melanoma) | 1 h at 1 mM | 6 Gy | 662 keV | Cs137 γ-rays | FACS, Annexin V-FITC/PI (BD Pharmingen, San Diego, CA, USA) | Song, 2022 [78] |
| MDA-MB-231 and MDA-MB-468 (human breast adenocarcinoma) | 1 h at 1 mM | 8 Gy | 662 keV | Cs137 γ-rays | FACS, Annexin V-FITC/PI (BD Pharmingen, San Diego, CA, USA) | Sun, 2022 [77] |
| Animal Model | NP Injection | Irradiation | Method and Assay | Reference | ||
|---|---|---|---|---|---|---|
| Dose | Energy | Type | ||||
| Mice with HepG2 tumors (subcutaneous) | 1 or 10 mg, intravenous, 1 h prior RT | 6 Gy | 300 kV | X-rays | SPECT/CT imaging; 99mTc-duramycin IHC, TUNEL (Roche Diagnostics, Indianapolis, IN, USA) | Hu, 2019 [54] |
| Nude mice with H1299 tumors (subcutaneous) | 420 mg/kg, intravenous, 15 min prior RT | 10 Gy | 662 keV | Cs137 γ-rays | IHC, TUNEL | Du, 2020 [48] |
| Nude mice with HEMC-SS tumors (subcutaneous) | 100 mM Gd, intratumoral, 5 min prior RT | 4 Gy | 320 kV | X-rays | IHC, TUNEL (Promega, Madison, WI, USA) | Aloy, 2022 [49] |
| Mice with B16 tumors (subcutaneous) | 1 mM Gd, intravenous, 4 h prior RT | 4 Gy | 662 keV | Cs137 γ-rays | IHC, TUNEL | Song, 2022 [78] |
| Nude mice with MDA-MB-231 tumors (subcutaneous) | 420 mg/kg, intravenous, 30 min prior RT | 10 Gy | 662 keV | Cs137 γ-rays | IHC, TUNEL | Sun, 2022 [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubrun, C.; Doussineau, T.; Carmès, L.; Meyzaud, A.; Boux, F.; Dufort, S.; Delfour, A.; De Beaumont, O.; Mirjolet, C.; Le Duc, G. Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review. Pharmaceuticals 2025, 18, 519. https://doi.org/10.3390/ph18040519
Aubrun C, Doussineau T, Carmès L, Meyzaud A, Boux F, Dufort S, Delfour A, De Beaumont O, Mirjolet C, Le Duc G. Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review. Pharmaceuticals. 2025; 18(4):519. https://doi.org/10.3390/ph18040519
Chicago/Turabian StyleAubrun, Clémentine, Tristan Doussineau, Léna Carmès, Aurélien Meyzaud, Fabien Boux, Sandrine Dufort, Adeline Delfour, Olivier De Beaumont, Céline Mirjolet, and Géraldine Le Duc. 2025. "Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review" Pharmaceuticals 18, no. 4: 519. https://doi.org/10.3390/ph18040519
APA StyleAubrun, C., Doussineau, T., Carmès, L., Meyzaud, A., Boux, F., Dufort, S., Delfour, A., De Beaumont, O., Mirjolet, C., & Le Duc, G. (2025). Mechanisms of Action of AGuIX as a Pan-Cancer Nano-Radiosensitizer: A Comprehensive Review. Pharmaceuticals, 18(4), 519. https://doi.org/10.3390/ph18040519








