Synthesis and Evaluation of a Chitosan-Based Cationic Hydrogel with Strong Antifungal and Antibiofilm Activities Against Clinical Isolates of Candida auris
Abstract
1. Introduction
2. Results
2.1. Culture, Identification, and ASFT of Isolates
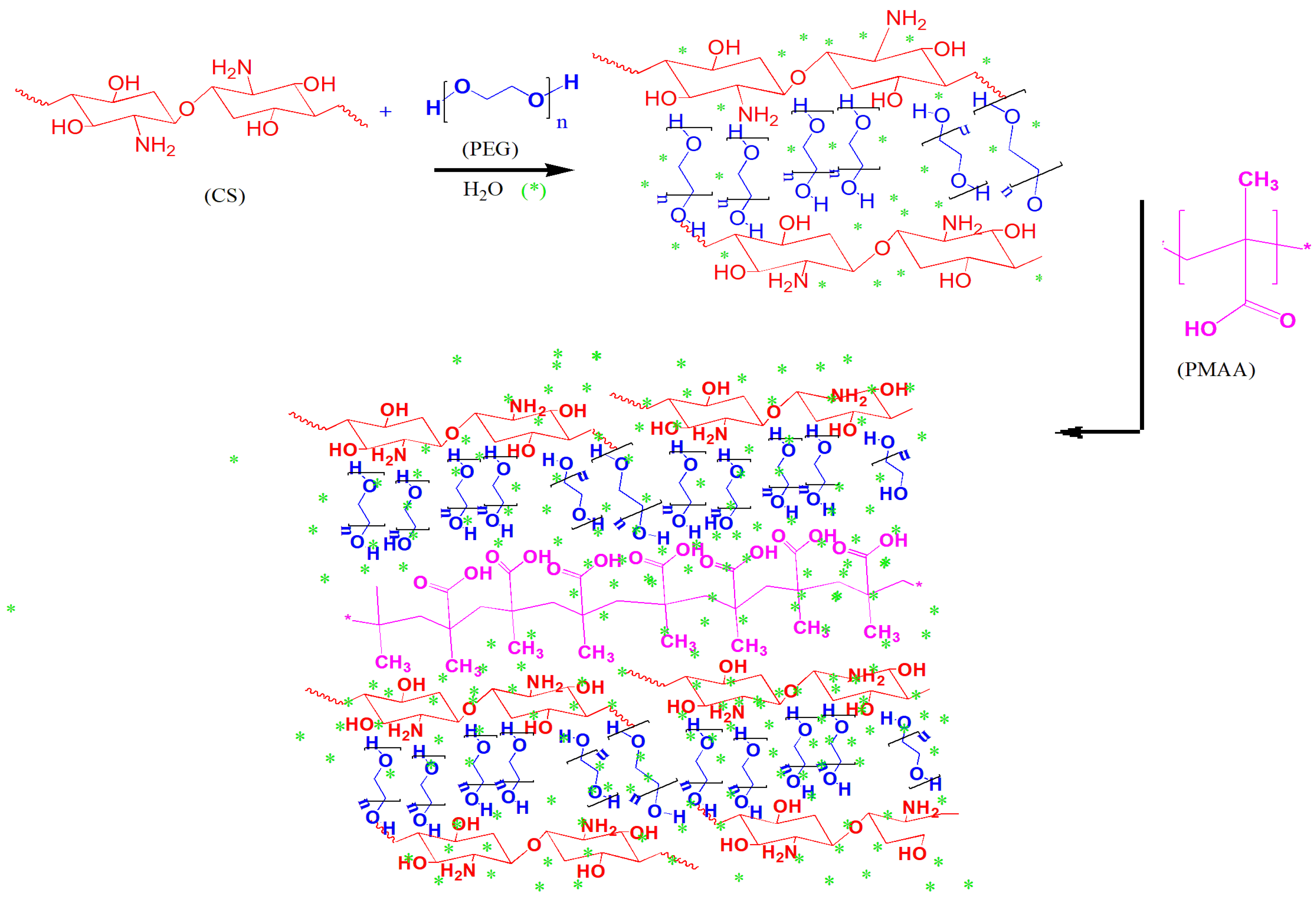
2.2. Synthesis of Hydrogel
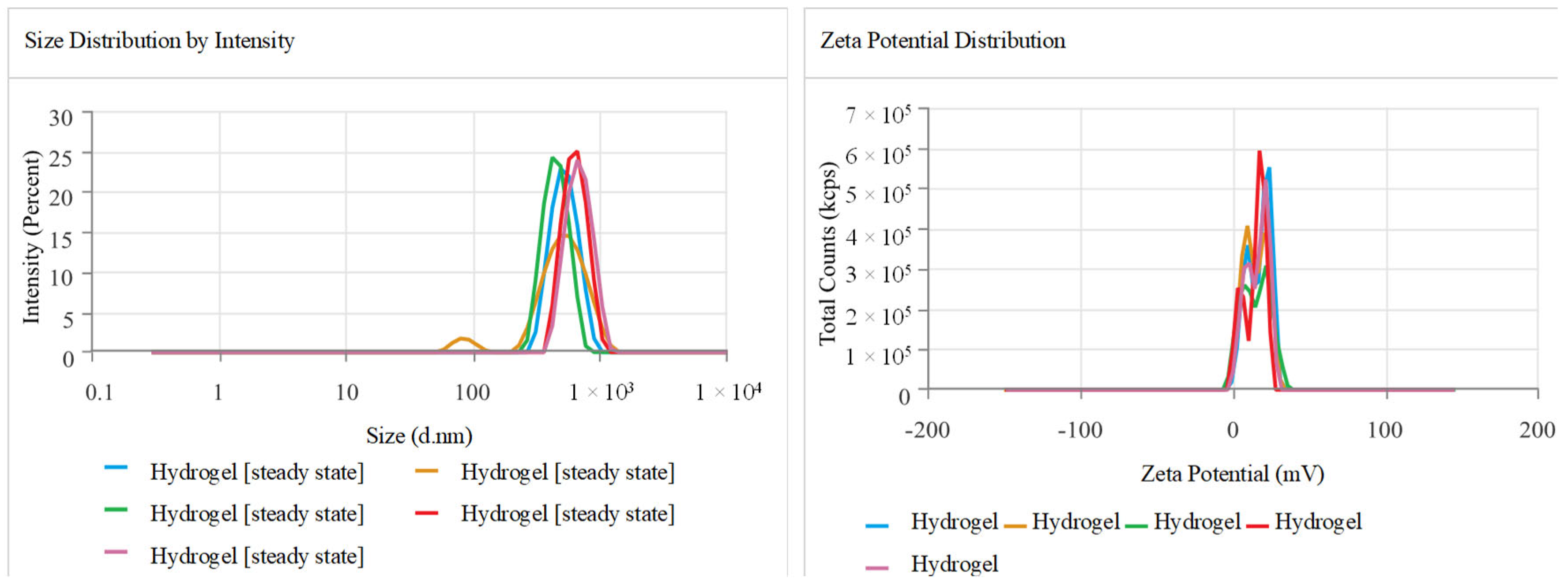
2.3. Zeta Size, Potential, and Polydispersity Index (PDI)
2.4. Scanning Electron Microscope (SEM)
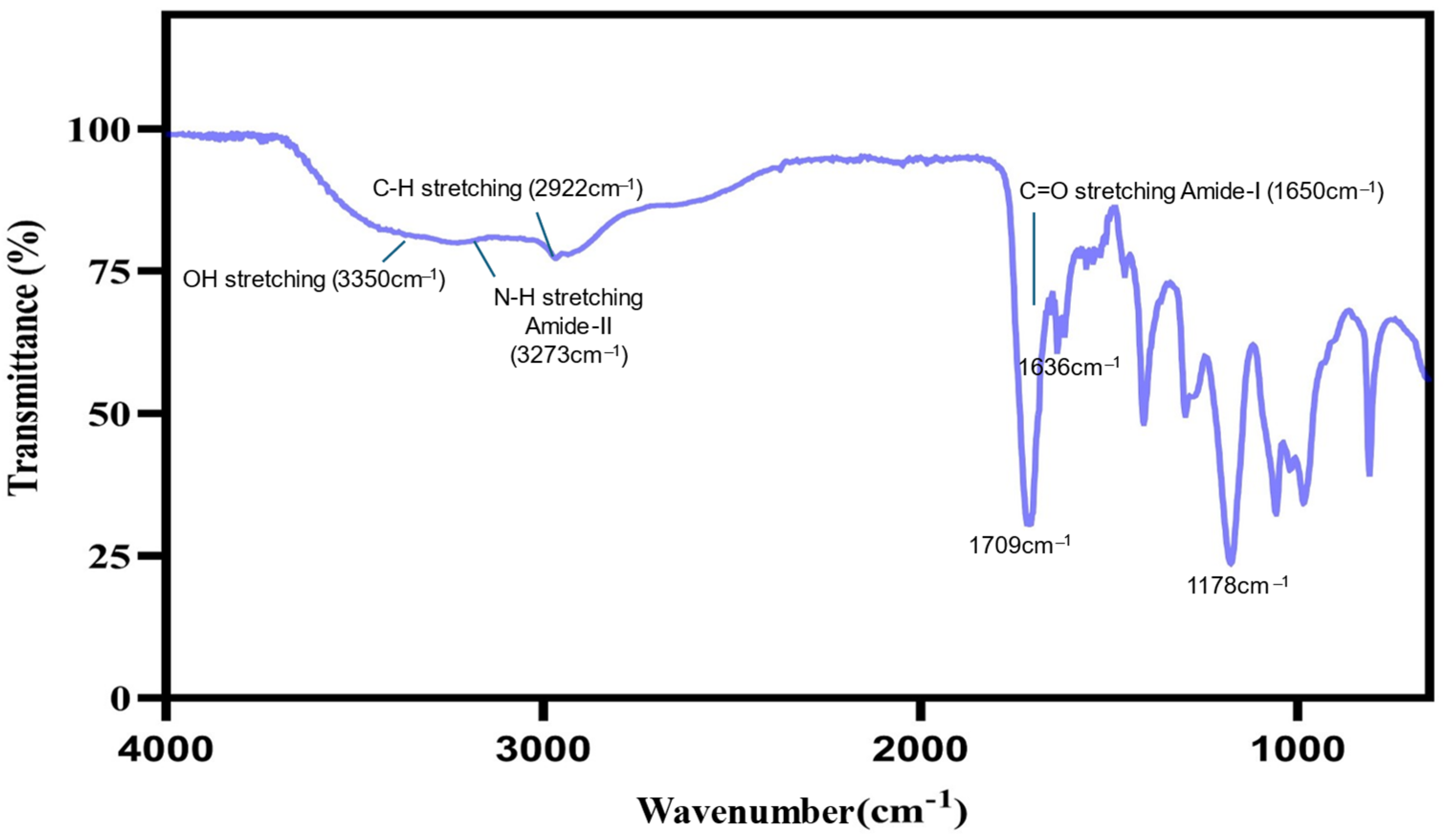
2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
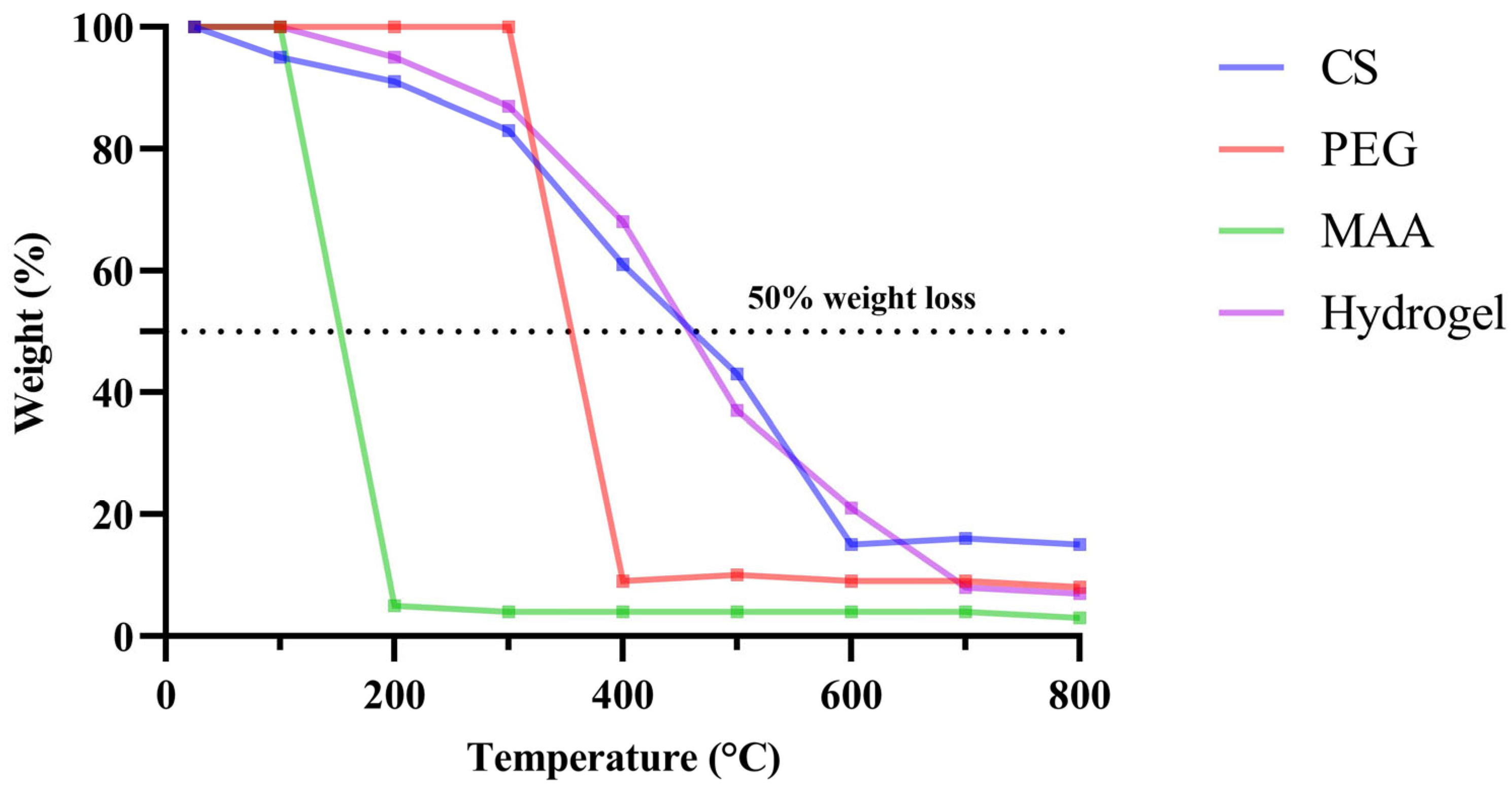
2.6. Thermogravimetric Analysis (TGA)
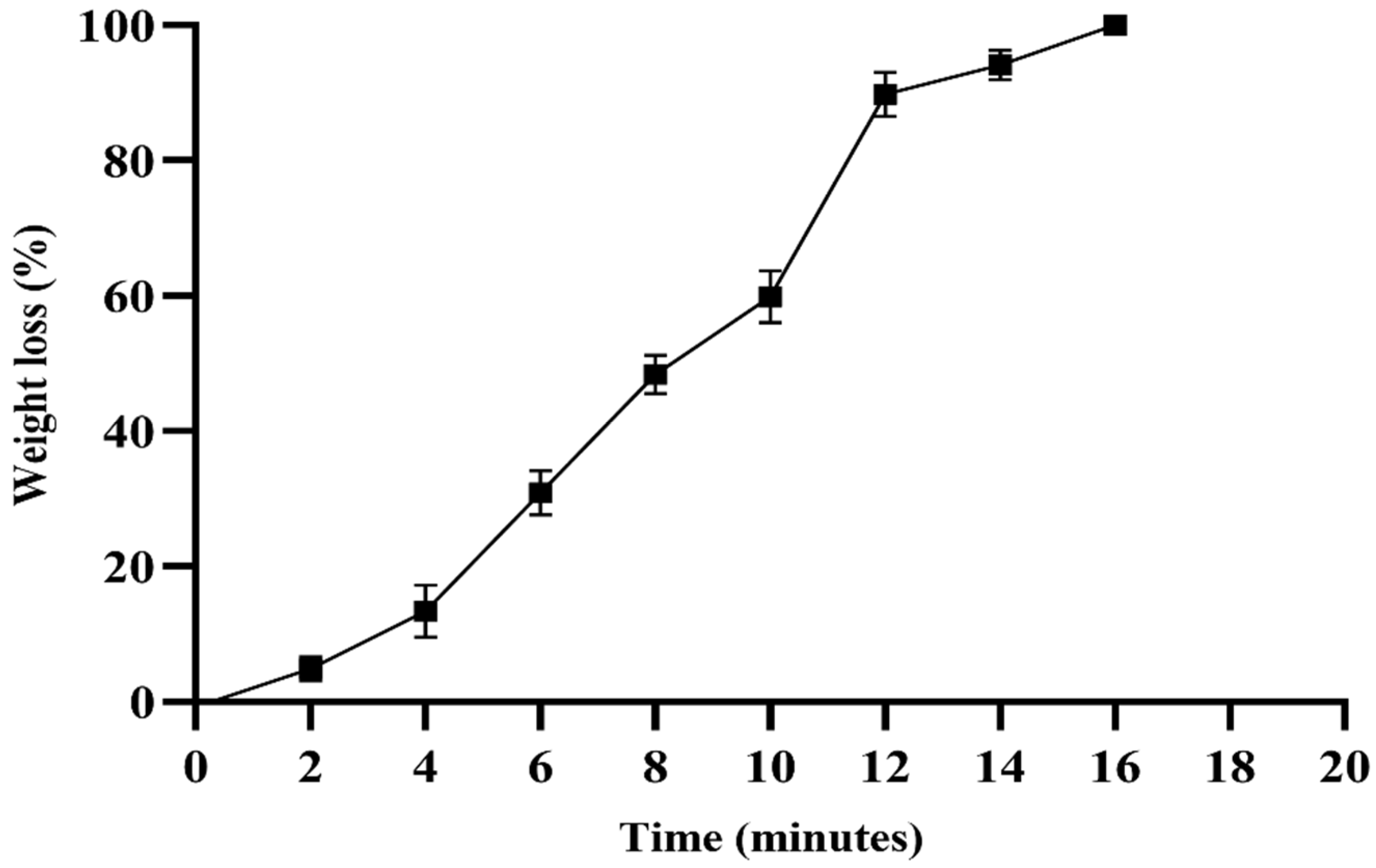
2.7. Swelling and Degradation Assay of Hydrogel
2.8. Drug Entrapment Efficiency (%DEE) and Release Kinetics
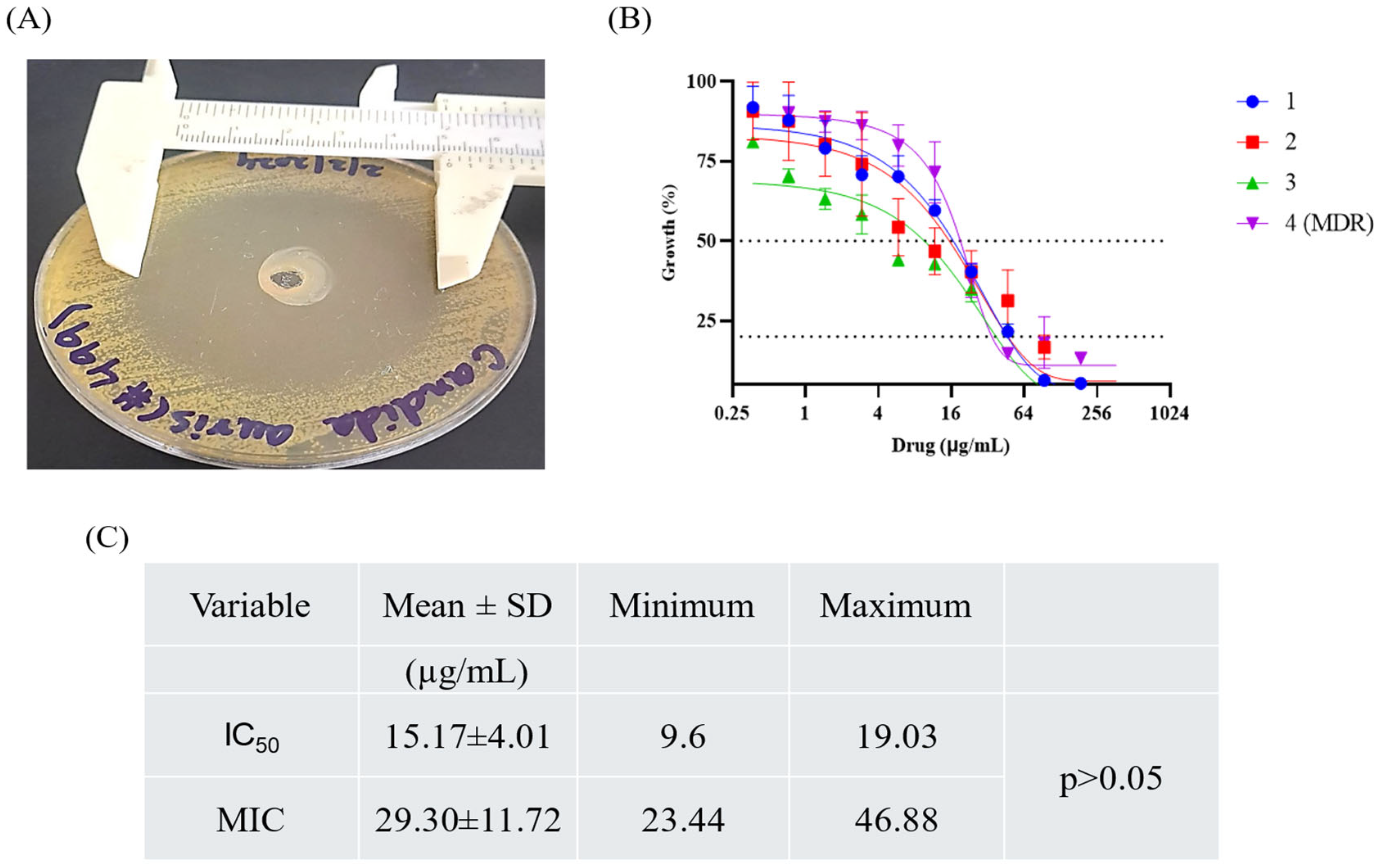
2.9. Antifungal Activity of Hydrogel
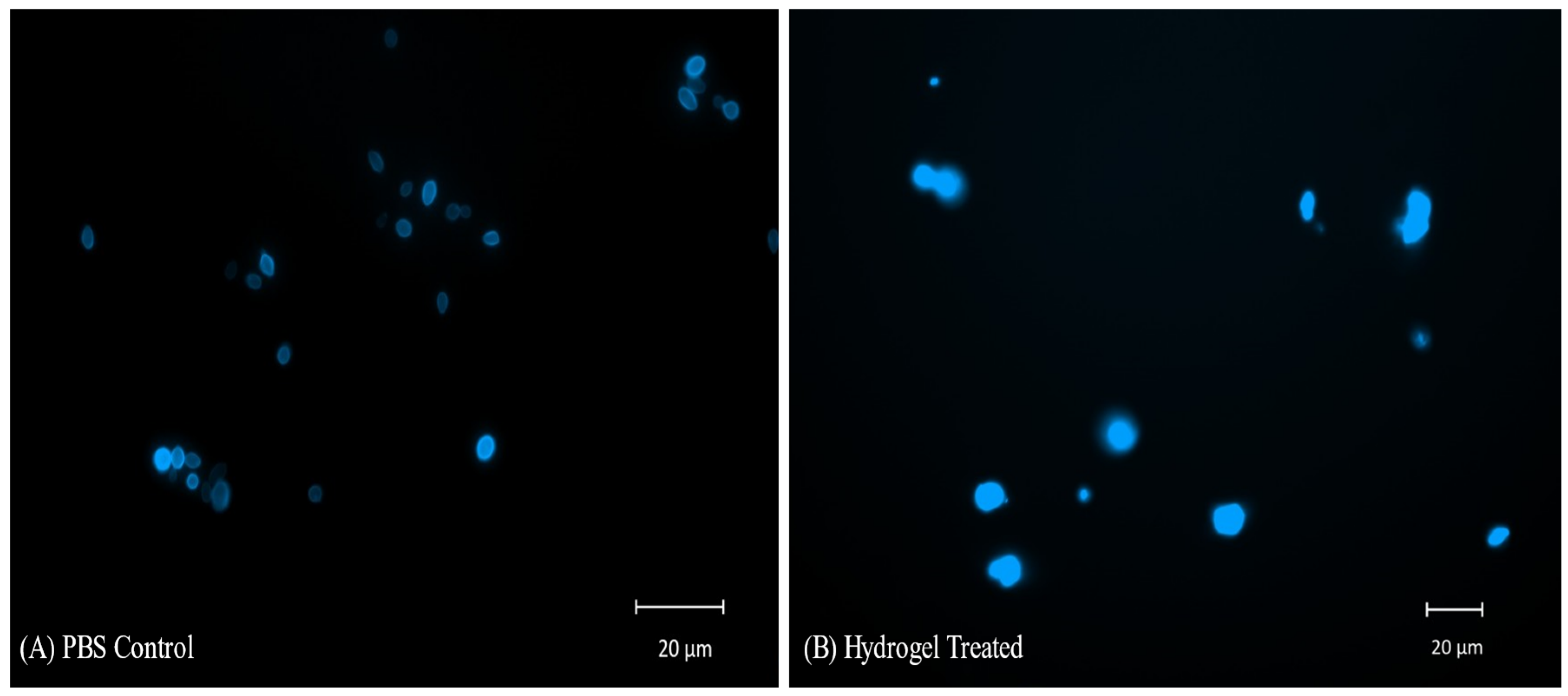
2.10. CFW Staining and Fluorescent Microscopy of C. auris Cells Treated with Hydrogel
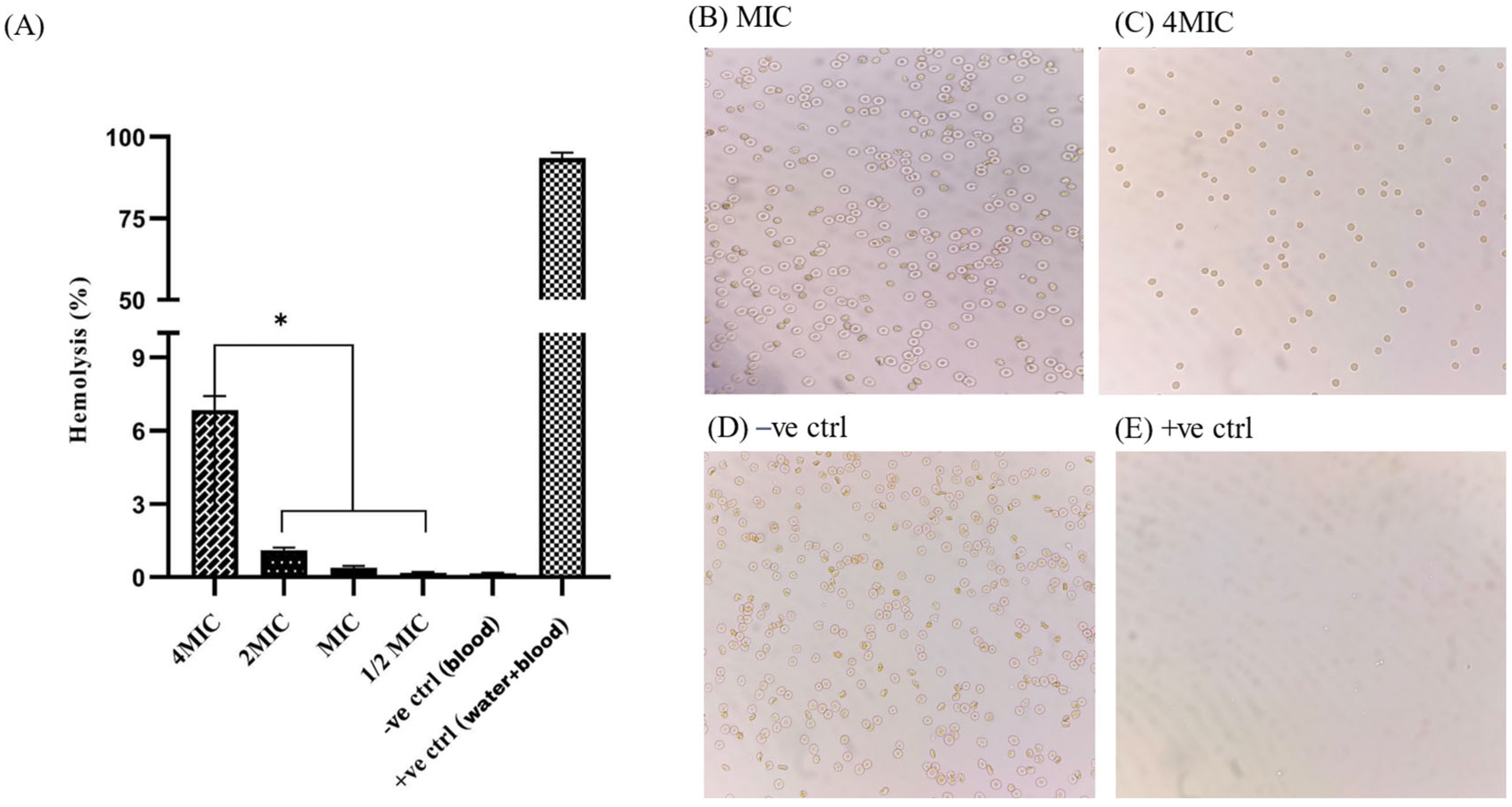
2.11. Hemocompatibility Assay
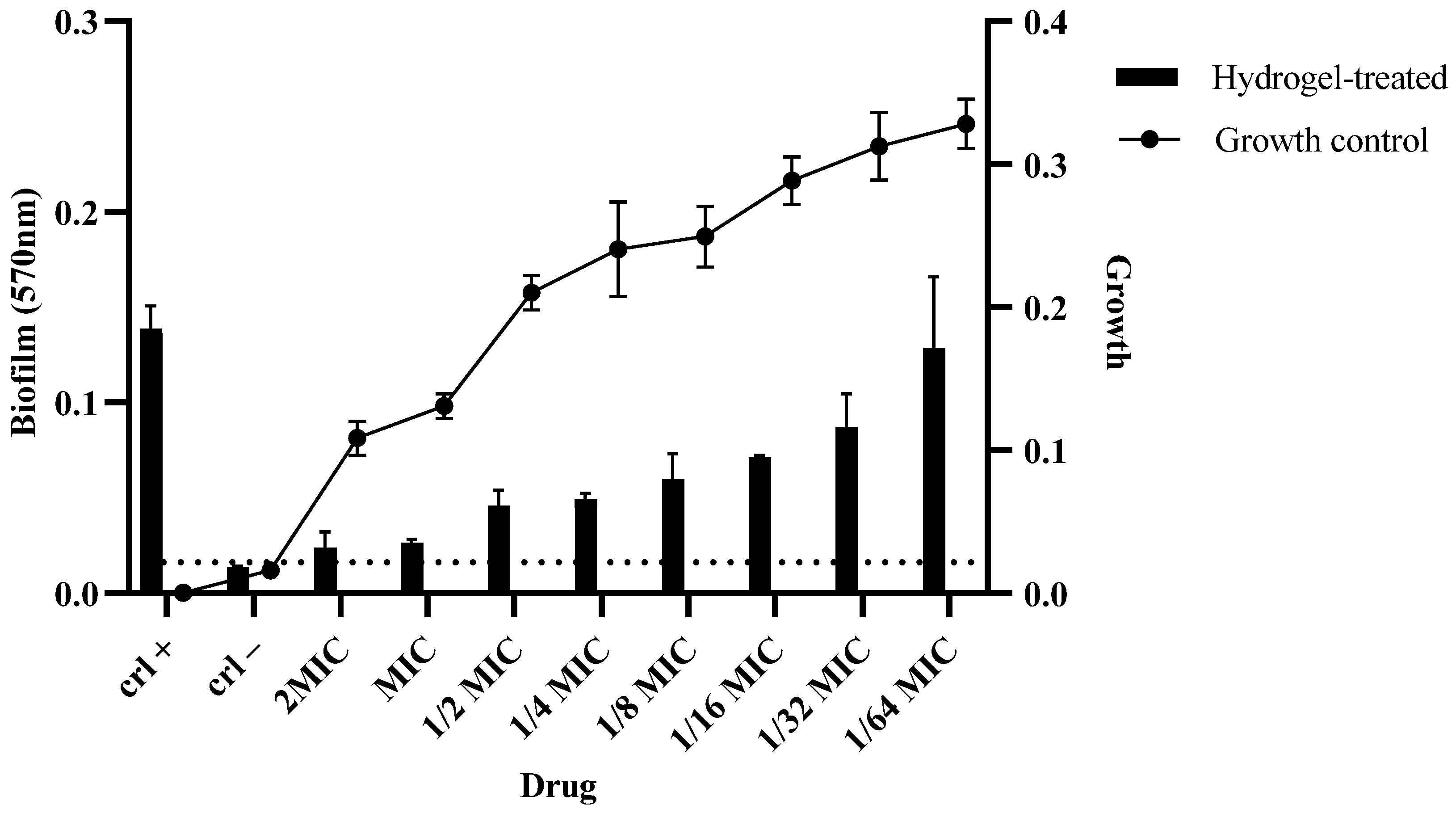
2.12. Biofilm Assay
2.13. In Silico Analysis
2.13.1. Molecular Properties and Drug Likeness
2.13.2. In Silico Interaction with Cytochrome Enzymes
2.13.3. Toxicity Prediction
2.13.4. Nuclear Receptor Signaling Pathway Toxicity Prediction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation and Identification of C. auris
4.3. Antifungal Susceptibility Testing
4.4. Synthesis of Hydrogel
4.5. Characterization of Hydrogel
4.5.1. Zeta Potential and PDI Measurement
4.5.2. Morphological Studies
4.5.3. Fourier Transform Infrared (FT-IR) Spectroscopy
4.5.4. Thermogravimetric Analysis
4.6. Swelling and Degradation Assay of Hydrogel
4.7. Drug Entrapment Efficiency (%DEE) of Hydrogel
4.8. Antifungal Activity of Hydrogel
4.9. Calcofluor White Staining and Fluorescent Microscopy of C. auris Cells
4.10. Hemocompatibility Assay of Hydrogel
4.11. Biofilm Assay
4.12. In Silico Analysis
4.13. Data Analysis
5. Conclusions and Future Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alp, Ş.; Arıkan Akdağlı, S. Candida auris and Mechanisms of Antifungal Drug Resisstance. Mikrobiyol. Bul. 2021, 55, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Antinori, S. The WHO fungal priority pathogens list: A crucial reappraisal to review the prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef] [PubMed]
- García, C.S.; Palop, N.T.; Bayona, J.V.M.; García, M.M.; Rodríguez, D.N.; Álvarez, M.B.; Serrano, M.D.R.G.; Cardona, C.G. Candida auris: Report of an outbreak. Enfermedades Infecc. Microbiol. Clin. 2020, 38 (Suppl. S1), 39–44. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.T.T.; Pharkjaksu, S.; Chongtrakool, P.; Suwannakarn, K.; Ngamskulrungroj, P. A predominance of clade 17 Candida albicans isolated from hemocultures in a tertiary care hospital in Thailand. Front. Microbiol. 2019, 10, 1194. [Google Scholar] [CrossRef]
- Soulountsi, V.; Schizodimos, T.; Kotoulas, S.C. Deciphering the epidemiology of invasive candidiasis in the intensive care unit: Is it possible? Infection 2021, 49, 1107–1131. [Google Scholar] [CrossRef]
- Mamali, V.; Siopi, M.; Charpantidis, S.; Samonis, G.; Tsakris, A.; Vrioni, G.; Network, C.-C. Increasing incidence and shifting epidemiology of candidemia in Greece: Results from the first nationwide 10-year survey. J. Fungi 2022, 8, 116. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Mokaddas, E.; Ahmad, S.; Abdullah, A.A.; De Groot, T.; Meis, J.F.; Shetty, S.A. Molecular characterisation of Candida auris isolates from immunocompromised patients in a tertiary-care hospital in Kuwait reveals a novel mutation in FKS1 conferring reduced susceptibility to echinocandins. Mycoses 2022, 65, 331–343. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
- Arita, G.S.; Faria, D.R.; Capoci, I.R.; Kioshima, E.S.; Bonfim-Mendonça, P.S.; Svidzinski, T.I. Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans. Microbiol. Res. 2022, 258, 126996. [Google Scholar] [CrossRef]
- Burrack, L.S.; Todd, R.T.; Soisangwan, N.; Wiederhold, N.P.; Selmecki, A. Genomic Diversity across Candida auris Clinical Isolates Shapes Rapid Development of Antifungal Resistance In Vitro and In Vivo. mBio 2022, 13, e00842-22. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Fontaine, T.; Kanneganti, T.-D.; Gow, N.A.; Papon, N. Fungal cell wall components modulate our immune system. Cell Surf. 2021, 7, 100067. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Jain, K.; Chauhan, N. Candida auris Genetics and Emergence. Annu. Rev. Microbiol. 2023, 77, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Rossow, J.; Ostrowsky, B.; Adams, E.; Greenko, J.; McDonald, R.; Vallabhaneni, S.; Forsberg, K.; Perez, S.; Lucas, T.; Alroy, K.A. Factors associated with Candida auris colonization and transmission in skilled nursing facilities with ventilator units, New York, 2016–2018. Clin. Infect. Dis. 2021, 72, e753–e760. [Google Scholar] [CrossRef]
- Azevedo, M.I.G.; Souza, P.F.N.; Monteiro Júnior, J.E.; Grangeiro, T.B. Chitosan and Chitooligosaccharides: Antifungal Potential and Structural Insights. Chem. Biodivers. 2024, 21, e202400044. [Google Scholar] [CrossRef]
- Muangsawat, S. Effects of Efflux Pumps on Antifungal Activity of Chitosan Against Candida albicans. J. Oral Microbiol. 2024, 16, 2357976. [Google Scholar] [CrossRef]
- Aftab, M.; Javed, F.; Haider, S.; Khan, R.; Khan, S.U.; Alam, K.; Amir, A.; Ullah, F.; Shah, N.A. Design and Characterization of Chitosan-Based Smart Injectable Hydrogel for Improved Sustained Release of Antinarcotics. Pharmaceuticals 2024, 17, 749. [Google Scholar] [CrossRef]
- Kurzyna, J.M.; Kopiasz, R.J.; Paul, M.; Flont, M.; Baranowska, P.; Mierzejewska, J.; Drężek, K.; Tomaszewski, W.; Jastrzębska, E.; Jańczewski, D. Unlocking the Potential: PEGylation and Molecular Weight Reduction of Ionenes for Enhanced Antifungal Activity and Biocompatibility. Macromol. Biosci. 2024, 24, e2400032. [Google Scholar] [CrossRef]
- de Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.; Chowdhary, A.; Meis, J.F. Antifungal activity of a medical-grade honey formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef]
- Fuchs, F.; Hof, H.; Hofmann, S.; Kurzai, O.; Meis, J.F.; Hamprecht, A. Antifungal activity of nitroxoline against Candida auris isolates. Clin. Microbiol. Infect. 2021, 27, 1697-e7. [Google Scholar] [CrossRef]
- Marquez, L. Potent Antifungal Activity of Penta-O-Galloyl-B-d-Glucose Against Drug-Resistant Candida albicans, Candida auris, and Other Non-albicans Candida Species. Acs Infect. Dis. 2023, 9, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Thienngern, P.; Panichuttra, A.; Ratisoontorn, C.; Aumnate, C.; Matangkasombut, O. Efficacy of Chitosan Paste as Intracanal Medication Against Enterococcus Faecalis and Candida Albicans Biofilm Compared with Calcium Hydroxide in an in Vitro Root Canal Infection Model. BMC Oral Health 2022, 22, 354. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Singha, I.; Basu, A. Chitosan based injectable hydrogels for smart drug delivery applications. Sens. Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Ullah, F.; Javed, F.; Mushtaq, I.; Rahman, L.U.; Ahmed, N.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Ahmad, A.; Bakht, M.A.; et al. Development of highly-reproducible hydrogel based bioink for regeneration of skin-tissues via 3-D bioprinting technology. Int. J. Biol. Macromol. 2023, 230, 123131. [Google Scholar]
- Acosta, L.D.; Pérez-Camacho, O.; Acosta, R.; Escobar, D.M.; Gallardo, C.A.; Sánchez-Vargas, L.O. Reduction of Candida albicans biofilm formation by coating polymethyl methacrylate denture bases with a photopolymerized film. J. Prosthet. Dent. 2020, 124, 605–613. [Google Scholar] [CrossRef]
- Ahmad, A.; Spencer, J.E.; Lockhart, S.R.; Singleton, S.; Petway, D.J.; Bagarozzi, D.A.; Herzegh, O.T. A high-throughput and rapid method for accurate identification of emerging multidrug-resistant Candida auris. Mycoses 2019, 62, 513–518. [Google Scholar] [CrossRef]
- Takara, E.A.; Marchese, J.; Ochoa, N.A. NaOH treatment of chitosan films: Impact on macromolecular structure and film properties. Carbohydr. Polym. 2015, 132, 25–30. [Google Scholar] [CrossRef]
- Magalhães, S.; Goodfellow, B.J.; Nunes, A. FTIR spectroscopy in biomedical research: How to get the most out of its potential. Appl. Spectrosc. Rev. 2021, 56, 869–907. [Google Scholar] [CrossRef]
- Mester, L.; Govyadinov, A.A.; Chen, S.; Goikoetxea, M.; Hillenbrand, R. Subsurface chemical nanoidentification by nano-FTIR spectroscopy. Nat. Commun. 2020, 11, 3359. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Muñoz, J.F.; Subotić, A.; Cruz, R.B.; Cuomo, C.A.; Dijck, P.V. Genome-Wide Analysis of Experimentally Evolved Candida Auris Reveals Multiple Novel Mechanisms of Multidrug Resistance. Mbio 2021, 12, e03333-20. [Google Scholar] [CrossRef] [PubMed]
- Negi, D.; Singh, Y. Gallium Oxide Nanoparticle-Loaded, Quaternized Chitosan-Oxidized Sodium Alginate Hydrogels for Treatment of Bacteria-Infected Wounds. Acs Appl. Nano Mater. 2023, 6, 13616–13628. [Google Scholar] [CrossRef]
- Jang, K.; Lee, W.; Park, S.; Han, J.; Kim, J.E.; Kim, B.M.; Chung, J.H. Sulfur(VI) Fluoride Exchange (SuFEx)-Mediated Synthesis of the Chitosan-Peg Conjugate and Its Supramolecular Hydrogels for Protein Delivery. Nanomaterials 2021, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Ofokansi, K.C.; Kenechukwu, F.C.; Ezugwu, R.O.; Attama, A.A. Improved dissolution and anti-inflammatory activity of ibuprofen-polyethylene glycol 8000 solid dispersion systems. Int. J. Pharm. Investig. 2016, 6, 139. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of swelling behavior and mechanical properties of a pH-sensitive superporous hydrogel composite. Iran. J. Pharm. Res. 2012, 11, 481. [Google Scholar]
- Chen, H.; Fei, F.; Li, X.; Nie, Z.; Zhou, D.; Liu, L.; Zhang, J.; Zhang, H.; Fei, Z.; Xu, T. A Structure-Supporting, Self-Healing, and High Permeating Hydrogel Bioink for Establishment of Diverse Homogeneous Tissue-Like Constructs. Bioact. Mater. 2021, 6, 3580–3595. [Google Scholar] [CrossRef]
- Keklikian, A.; de Barros, N.R.; Rashad, A.; Chen, Y.; Tan, J.L.; Sheng, R.; Sun, D.; Liu, H.; Thankam, F.G. Chitosan–Polyethylene Glycol Inspired Polyelectrolyte Complex Hydrogel Templates Favoring NEO-Tissue Formation for Cardiac Tissue Engineering. Gels 2024, 10, 46. [Google Scholar] [CrossRef]
- Jiang, S.; Mohanty, M. Synthesis and Characterization of a Novel Biocompatible, Non-Toxic, and Fast-Gelling Chitosan-Peg Hydrogel Based on Michael Addition. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Ayach, J.; Duma, L.; Badran, A.; Hijazi, A.; Martinez, A.; Bechelany, M.; Baydoun, E.; Hamad, H. Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes. Materials 2024, 17, 2724. [Google Scholar] [CrossRef]
- Prigyai, N.; Bunchuay, T.; Ruengsuk, A.; Yoshinari, N.; Manissorn, J.; Pumirat, P.; Sapudom, J.; Kosiyachinda, P.; Thongnuek, P. Photo-Controlled Reversible Uptake and Release of a Modified Sulfamethoxazole Antibiotic Drug From a Pillar[5]arene Cross-Linked Gelatin Hydrogel. Acs Appl. Mater. Interfaces 2024, 16, 8250–8265. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lü, Y.; Li, L.; Shi, C.; Zhang, X.; Li, X.; Niu, Y.; Liu, F.; Wang, L.; Xu, W. Electrostatic Induced Peptide Hydrogels for pH-Controllable Doxorubicin Release and Antitumor Activity. Chemistryselect 2022, 7, e202202284. [Google Scholar] [CrossRef]
- Duceac, I.A.; Vereștiuc, L.; Dimitriu, D.C.; Maier, V.P.; Coseri, S. Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications. Polymers 2020, 12, 1473. [Google Scholar] [CrossRef] [PubMed]
- Sareethammanuwat, M.; Boonyuen, S.; Arpornmaeklong, P. Effects of Beta-tricalcium Phosphate Nanoparticles on the Properties of a Thermosensitive Chitosan/Collagen Hydrogel and Controlled Release of Quercetin. J. Biomed. Mater. Res. A 2020, 109, 1147–1159. [Google Scholar] [CrossRef]
- Yu, C.; Chen, X.; Zhu, W.; Li, L.; Peng, M.; Zhong, Y.; Naeem, A.; Zang, Z.; Guan, Y. Synthesis of Gallic Acid-Loaded Chitosan-Grafted-2-Acrylamido-2-Methylpropane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery: In Vitro Biodegradation, Antioxidant, and Antibacterial Effects. Gels 2022, 8, 806. [Google Scholar] [CrossRef]
- Ehtermi, A.; Kolarijani, N.R.; Nazarnezhad, S.; Alizadeh, M.; Masoudi, A.; Salehi, M. Peripheral Nerve Regeneration by Thiolated Chitosan Hydrogel Containing Taurine: In Vitro and in Vivo Study. J. Bioact. Compat. Polym. 2022, 37, 85–97. [Google Scholar] [CrossRef]
- Flores-Espinoza, A.I. Gelatin–Chitosan Hydrogel Biological, Antimicrobial and Mechanical Properties for Dental Applications. Biomimetics 2023, 8, 575. [Google Scholar] [CrossRef]
- Thongchai, K.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Integration of Collagen Into Chitosan Blend Film Composites: Physicochemical Property Aspects for Pharmaceutical Materials. SN Appl. Sci. 2020, 2, 255. [Google Scholar] [CrossRef]
- Arikibe, J.E.; Lata, R.A.; Kuboyama, K.; Ougizawa, T.; Rohindra, D. pH-Responsive Studies of Bacterial Cellulose/Chitosan Hydrogels Crosslinked with Genipin: Swelling and Drug Release Behaviour. ChemistrySelect 2019, 4, 9915–9926. [Google Scholar] [CrossRef]
- Tenório, F.S.; do Montanheiro, T.L.; Isaias Santos, A.M.; dos Santos Silva, M.; Lemes, A.P.; Tada, D.B. Chitosan Hydrogel Covalently Crosslinked by Gold Nanoparticle: Eliminating the Use of Toxic Crosslinkers. J. Appl. Polym. Sci. 2020, 138, 49819. [Google Scholar] [CrossRef]
- Rueda, C.; Cuenca-Estrella, M.; Zaragoza, O. Paradoxical Growth of Candida albicans in the Presence of Caspofungin Is Associated with Multiple Cell Wall Rearrangements and Decreased Virulence. Antimicrob. Agents Chemother. 2014, 58, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Srimaneepong, V.; Thanamee, T.; Wattanasirmkit, K.; Muangsawat, S.; Matangkasombut, O. Efficacy of Low-molecular Weight Chitosan Against Candida albicans Biofilm on Polymethyl Methacrylate Resin. Aust. Dent. J. 2021, 66, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Tarek, A. Fighting Emerging Caspofungin-Resistant Candida Species: Mitigating Fks1-Mediated Resistance and Enhancing Caspofungin Efficacy by Chitosan. Antibiotics 2024, 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C. Synergistic Antifungal Activity of Chitosan with Fluconazole Against Candida Albicans, Candida Tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Saraf, N.S. Formulation and Evaluation of Antifungal Agent in a Hydrogel Containing Nanoparticle of Low Molecular Weight Chitosan. Int. J. Res. Pharm. Sci. 2020, 11, 247–259. [Google Scholar]
- Ailincai, D.; Roșca, I. New Hydrogels and Formulations Based on Piperonyl-Imino-Chitosan Derivatives. Polymers 2023, 15, 753. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef]
- Chatzimoschou, A.; Giampani, A.; Meis, J.F.; Roilides, E. Activities of Nine Antifungal Agents Against Candida auris Biofilms. Mycoses 2020, 64, 381–384. [Google Scholar] [CrossRef]
- Munir, S.; Shah, A.A.; Shahid, M.; Manzoor, I.; Aslam, B.; Rasool, M.H.; Saeed, M.; Ayaz, S.; Khurshid, M. Quorum Sensing Interfering Strategies and Their Implications in the Management of Biofilm-Associated Bacterial Infections. Braz. Arch. Biol. Technol. 2020, 63, e20190555. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, P.; Sarker, R.K.; Chatterjee, A.; Maiti, D.; Das, A.; Mandal, S.; Bhattacharjee, S.; Dastidar, D.G.; Tribedi, P. Tryptophan Interferes with the Quorum Sensing and Cell Surface Hydrophobicity of Staphylococcus Aureus: A Promising Approach to Inhibit the Biofilm Development. 3 Biotech 2021, 11, 376. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef] [PubMed]
- Rasib, S.Z.; Ahmad, Z.; Khan, A.; Akil, H.M.; Othman, M.B.; Hamid, Z.A.; Ullah, F. Synthesis and evaluation on pH-and temperature-responsive chitosan-p (MAA-co-NIPAM) hydrogels. Int. J. Biol. Macromol. 2018, 108, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Shami, A.; Rubina, M.S.; Abramchuk, S.S.; Shtykova, E.V.; Vasil’kov, A.Y. Copper-Chitosan Nanocomposite Hydrogels Against Aflatoxigenic Aspergillus Flavus From Dairy Cattle Feed. J. Fungi 2020, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, D.; Qin, S. Preparation and Performance of Antibacterial Polyvinyl Alcohol/Polyethylene Glycol/Chitosan Hydrogels Containing Silver Chloride Nanoparticles via One-Step Method. Nanomaterials 2019, 9, 972. [Google Scholar] [CrossRef]
- Wang, X.; Song, R.; Johnson, M.; Sigen, A.; He, Z.; Milne, C.; Wang, X.; Lara-Sáez, I.; Xu, Q.; Wang, W. An Injectable Chitosan-Based Self-Healable Hydrogel System as an Antibacterial Wound Dressing. Materials 2021, 14, 5956. [Google Scholar] [CrossRef]
- Salehi, M.; Zamiri, S.; Samadian, H.; Ai, J.; Foroutani, L.; Ai, A.; Khanmohammadi, M. Chitosan Hydrogel Loaded with Aloe vera gel and Tetrasodium Ethylenediaminetetraacetic Acid (EDTA) as the Wound Healing Material: In Vitro and In Vivo Study. J. Appl. Polym. Sci. 2020, 138, 50225. [Google Scholar] [CrossRef]
- Guo, W. Recent Advances of Chitosan-Based Hydrogels for Skin-Wound Dressings. Gels 2024, 10, 175. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Vanaraj, R.; Ulagesan, S.; Moorthy, M.S.; Raj, V.; Krishnakumar, G.S.; Vy Phan, T.T.; Kim, S.-C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2022, 9, 35. [Google Scholar] [CrossRef]
- Che, X. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef]
- Maevskaia, E.N.; Burkova, N.V.; Kuznetzov, S.I.; Dresvyanina, E.N.; Yudin, V.V.; Morganti, P. Hemocompatible Chitin-Chitosan Composite Fibers. Cosmetics 2020, 7, 28. [Google Scholar] [CrossRef]
- Deshkar, S. Polyelectrolyte-Complex-Based Hydrogel Inserts for Vaginal Delivery of Posaconazole and Probiotics. Gels 2023, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, L.R.; Klein, J.M.; Sandri, I.G.; Brandalise, R.N. Este Trabajo Se Centró en El Desarrollo De Envases Activos Biodegradables Con Mezclas De Poli (Ácido Láctico) (PLA), Poli (Etileno-Co-Acetato De Vinilo) (EVA), Polietilenglicol (PEG) Y Quitosano (QUI). Se Investigaron Las Características Morfológicas Térmicas Y Mecánicas De Las Mezclas, Así Como, Al Mismo Tiempo, La Actividad Antifúngica Del Envase. Para Evaluar La Actividad Antimicrobiana De Las Mezclas PLA/EVA/PEG/QUI, Las Muestras Se Insertaron Entre Rebanadas De Pan Sin Conservantes Para Evaluar Su Vid. Res. Soc. Dev. 2021, 10, e50010916964. [Google Scholar] [CrossRef]
- Shahid, F.; Javed, F.; Gul, H.; Kaleem, M.; Aftab, M.; Ahmed, N.; Shahid, F.; Toor, S.A.; Ullah, F. Classification of Oral Ulcers and its Treatment by focusing on Exosome Loaded Hydrogel: A Comprehensive Review. J. Health Rehabil. Res. 2024, 4, 820–830. [Google Scholar]
- Saha, S.; Gilliam, M.S.; Wang, Q.H.; Green, A.A. Eradication of Fungi Using MoSe2/Chitosan Nanosheets. Acs Appl. Nano Mater. 2022, 5, 133–148. [Google Scholar] [CrossRef]

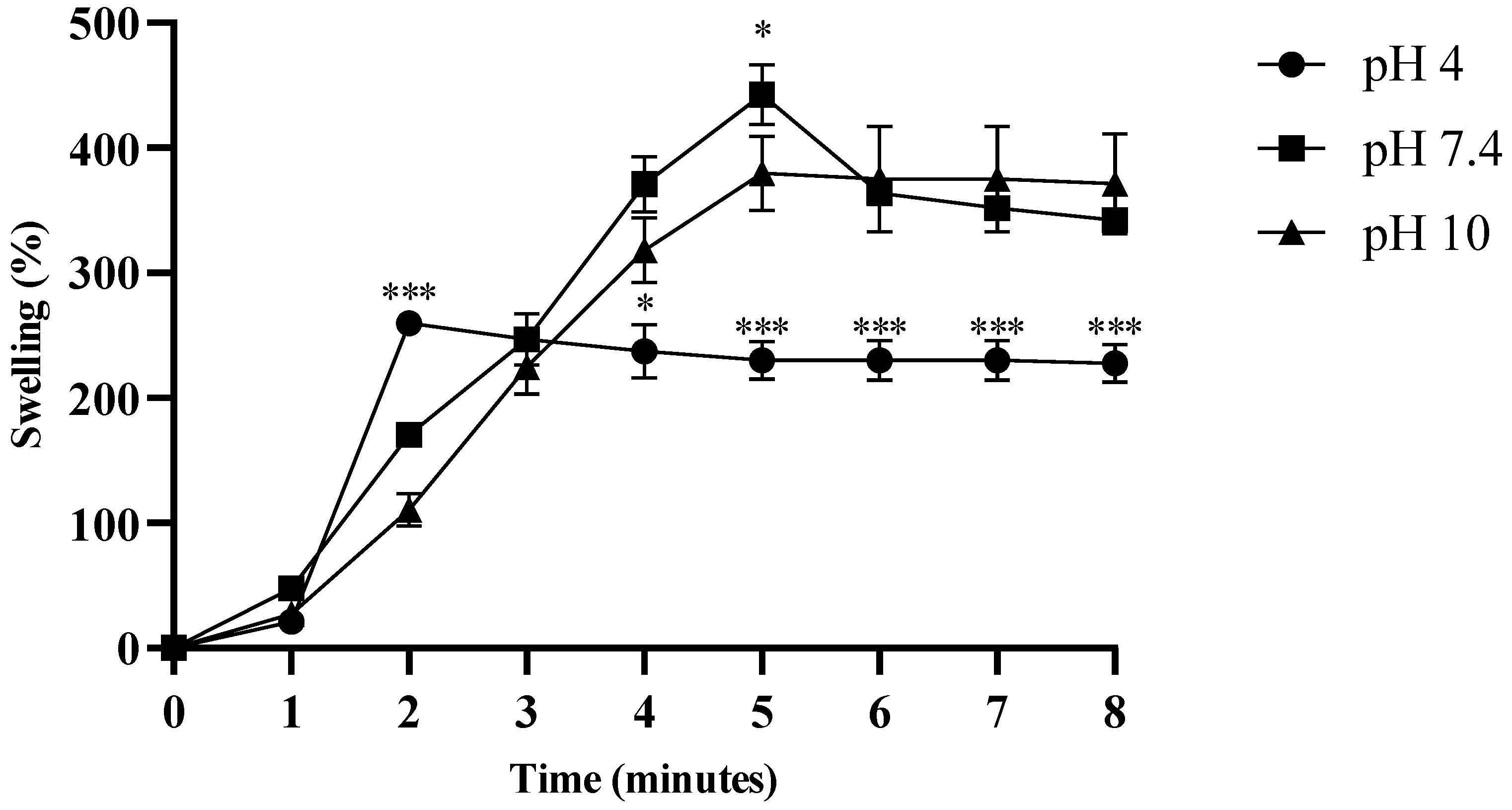
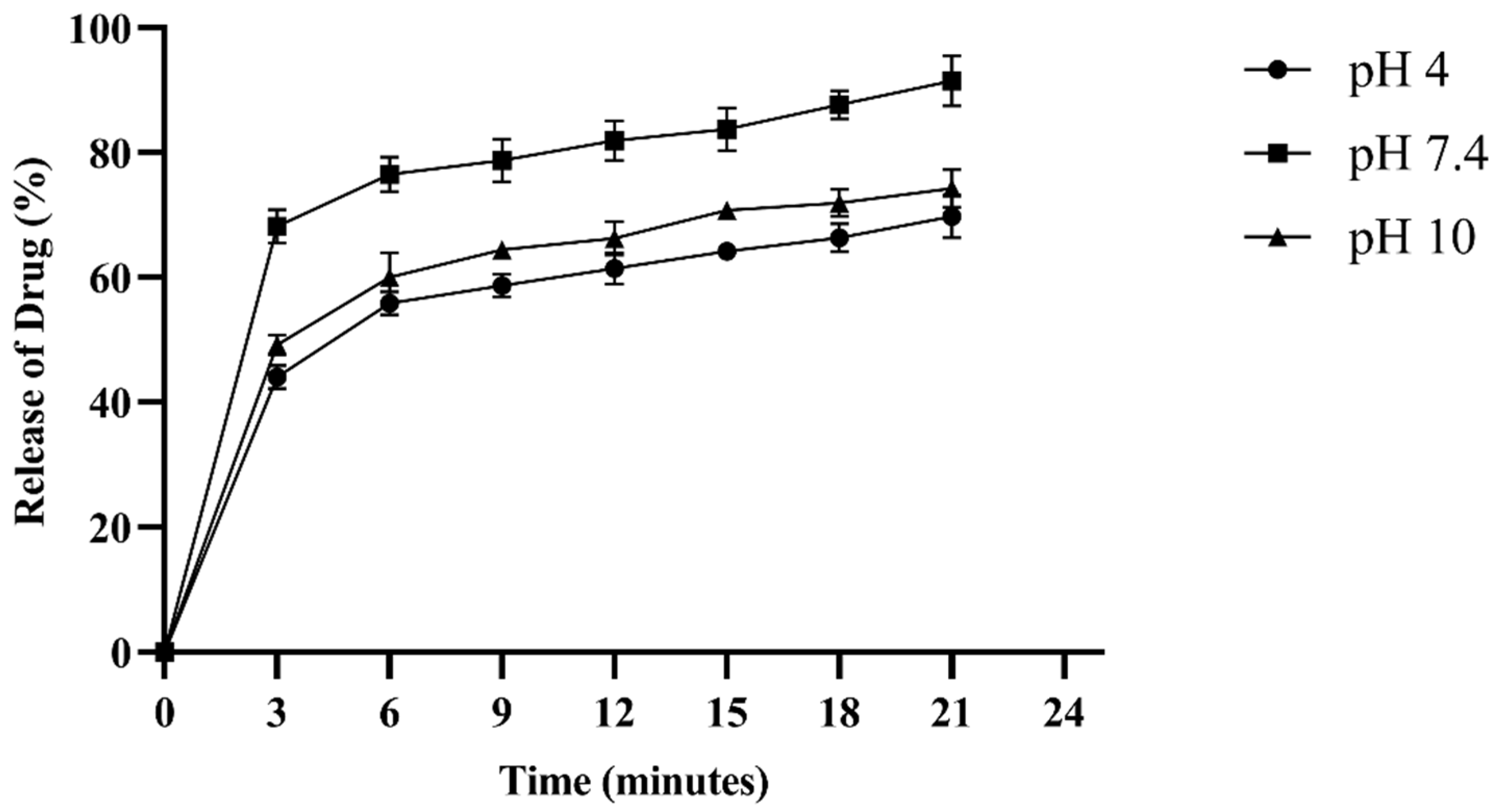
| pH | Release Medium | Zero-Order Kinetics | First-Order Kinetics | Korsemeyer–Peppas Model | Mechanism of Drug Release | |||
|---|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | n | |||
| 4.0 | Acetate buffer | 0.894 | 0.120 | 0.846 | 0.009 | 0.967 | 0.54 | Non-Fickian diffusion |
| 7.4 | Phosphate-buffered saline (PBS) | 0.958 | 0.113 | 0.942 | 0.006 | 0.974 | 0.75 | Non-Fickian diffusion |
| 10 | Borate buffer | 0.894 | 0.122 | 0.854 | 0.008 | 0.978 | 0.59 | Non-Fickian diffusion |
| Tested Items | CS | PEG | MAA |
|---|---|---|---|
| Log P | 0.88 | −1.03 | 0.65 |
| TPSA (Å) | 56.79 | 40.46 | 37.30 |
| HBDs | 1 | 2 | 1 |
| HBAs | 5 | 2 | 2 |
| MW (g/mol) | 184.1 | 62.07 | 86.1 |
| nRT | 4 | 1 | 1 |
| PAINS | 0 | 0 | 0 |
| Bioavailability score | 0.55 | 0.55 | 0.85 |
| Synthetic accessibility | 3.14 | 1.00 | 1.01 |
| Lepinski rule | Yes (0) | Yes (0) | Yes (0) |
| Veber rule | Yes | Yes | Yes |
| Tested Items | CS | PEG | MAA |
|---|---|---|---|
| CYP2C19 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | No | No |
| CYP1A2 inhibitor | No | No | No |
| CYP2C9 inhibitor | No | No | No |
| CYP2D6 inhibitor | No | No | No |
| Tested Items | CS | PEG | MAA |
|---|---|---|---|
| Predicted LD50 (mg/kg) | 1000 | 4700 | 118 |
| Toxicity class | 4 | 5 | 3 |
| Neurotoxicity | Inactive (0.50) | Inactive (0.90) | Inactive (0.62) |
| Hepatotoxicity | Inactive (0.77) | Inactive (0.80) | Inactive (0.65) |
| Carcinogenicity | Inactive (0.58) | Inactive (0.79) | Inactive (0.78) |
| Respiratory toxicity | Active (0.57) | Inactive (0.57) | Inactive (0.87) |
| Mutagenicity | Inactive (0.64) | Inactive (0.89) | Inactive (0.89) |
| Tested Items | CS | PEG | MAA |
|---|---|---|---|
| AhR | Inactive (0.96) | Inactive (0.97) | Inactive (1.0) |
| AR | Inactive (0.98) | Inactive (0.98) | Inactive (1.0) |
| AR-LBD | Inactive (0.98) | Inactive (0.96) | Inactive (0.99) |
| Aromatase | Inactive (0.94) | Inactive (1.0) | Inactive (1.0) |
| ER | Inactive (0.89) | Inactive (0.91) | Inactive (0.90) |
| ER-LBD | Inactive 0.97) | Inactive (0.98) | Inactive (1.0) |
| PPAR-Gamma | Inactive (0.95) | Inactive (0.95) | Inactive (0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, M.; Aftab, M.; Amir, A.; Javed, F.; Latif, A.Q.; Saldera, K.A.; Ahad, A.; Jardan, Y.A.B.; Walker, L.A.; Nisa, K.; et al. Synthesis and Evaluation of a Chitosan-Based Cationic Hydrogel with Strong Antifungal and Antibiofilm Activities Against Clinical Isolates of Candida auris. Pharmaceuticals 2025, 18, 506. https://doi.org/10.3390/ph18040506
Kamran M, Aftab M, Amir A, Javed F, Latif AQ, Saldera KA, Ahad A, Jardan YAB, Walker LA, Nisa K, et al. Synthesis and Evaluation of a Chitosan-Based Cationic Hydrogel with Strong Antifungal and Antibiofilm Activities Against Clinical Isolates of Candida auris. Pharmaceuticals. 2025; 18(4):506. https://doi.org/10.3390/ph18040506
Chicago/Turabian StyleKamran, Muhammad, Maryam Aftab, Afreenish Amir, Fatima Javed, Amtul Quddos Latif, Kausar Abbas Saldera, Abdul Ahad, Yousef A. Bin Jardan, Louise Ann Walker, Kiran Nisa, and et al. 2025. "Synthesis and Evaluation of a Chitosan-Based Cationic Hydrogel with Strong Antifungal and Antibiofilm Activities Against Clinical Isolates of Candida auris" Pharmaceuticals 18, no. 4: 506. https://doi.org/10.3390/ph18040506
APA StyleKamran, M., Aftab, M., Amir, A., Javed, F., Latif, A. Q., Saldera, K. A., Ahad, A., Jardan, Y. A. B., Walker, L. A., Nisa, K., Ullah, F., & Shah, N. A. (2025). Synthesis and Evaluation of a Chitosan-Based Cationic Hydrogel with Strong Antifungal and Antibiofilm Activities Against Clinical Isolates of Candida auris. Pharmaceuticals, 18(4), 506. https://doi.org/10.3390/ph18040506










