Computational Network Pharmacology, Molecular Docking, and Molecular Dynamics to Decipher Natural Compounds of Alchornea laxiflora for Liver Cancer Chemotherapy
Abstract
1. Introduction
2. Results
2.1. Screened Bioactives of A. laxiflora
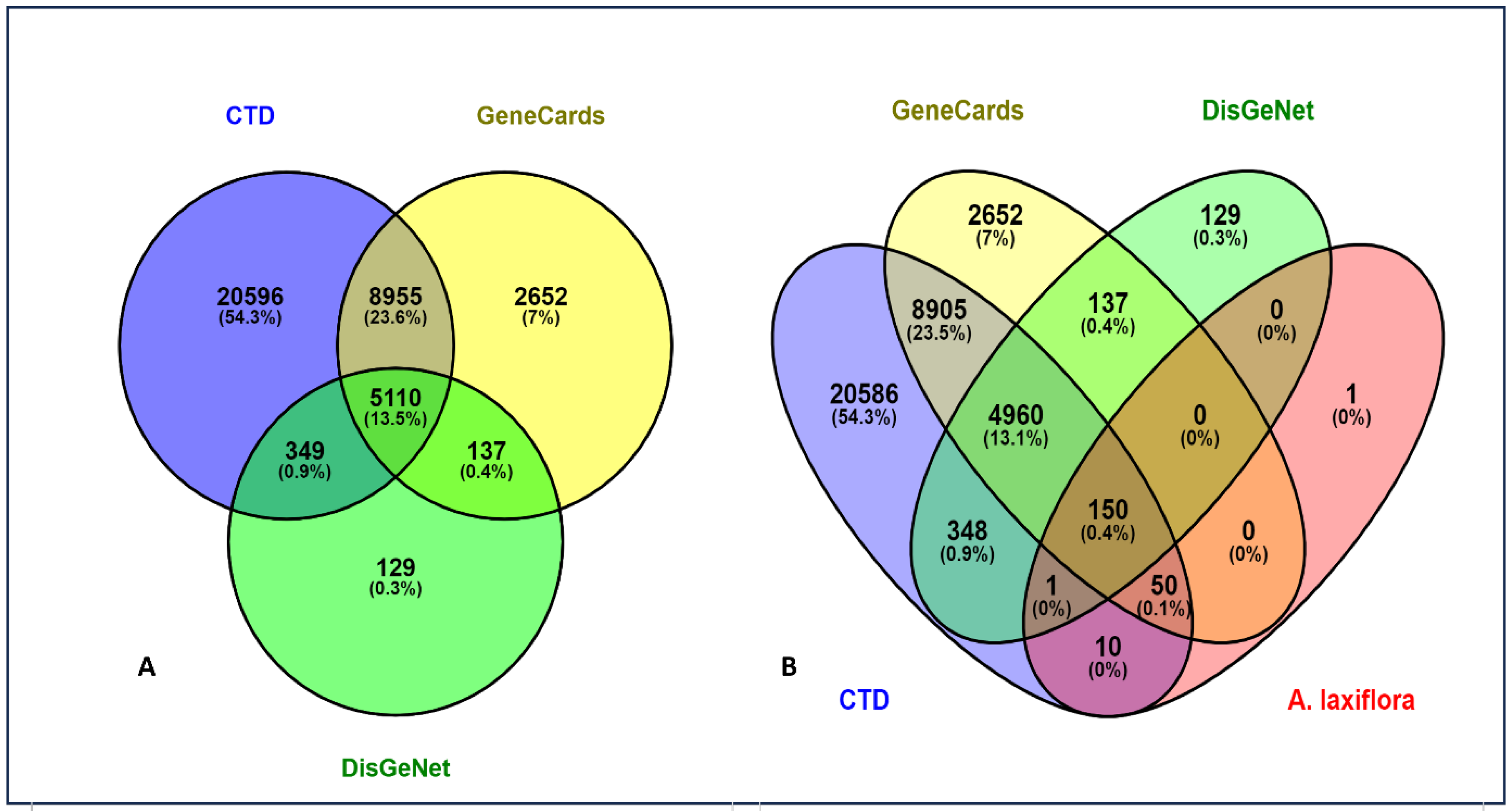
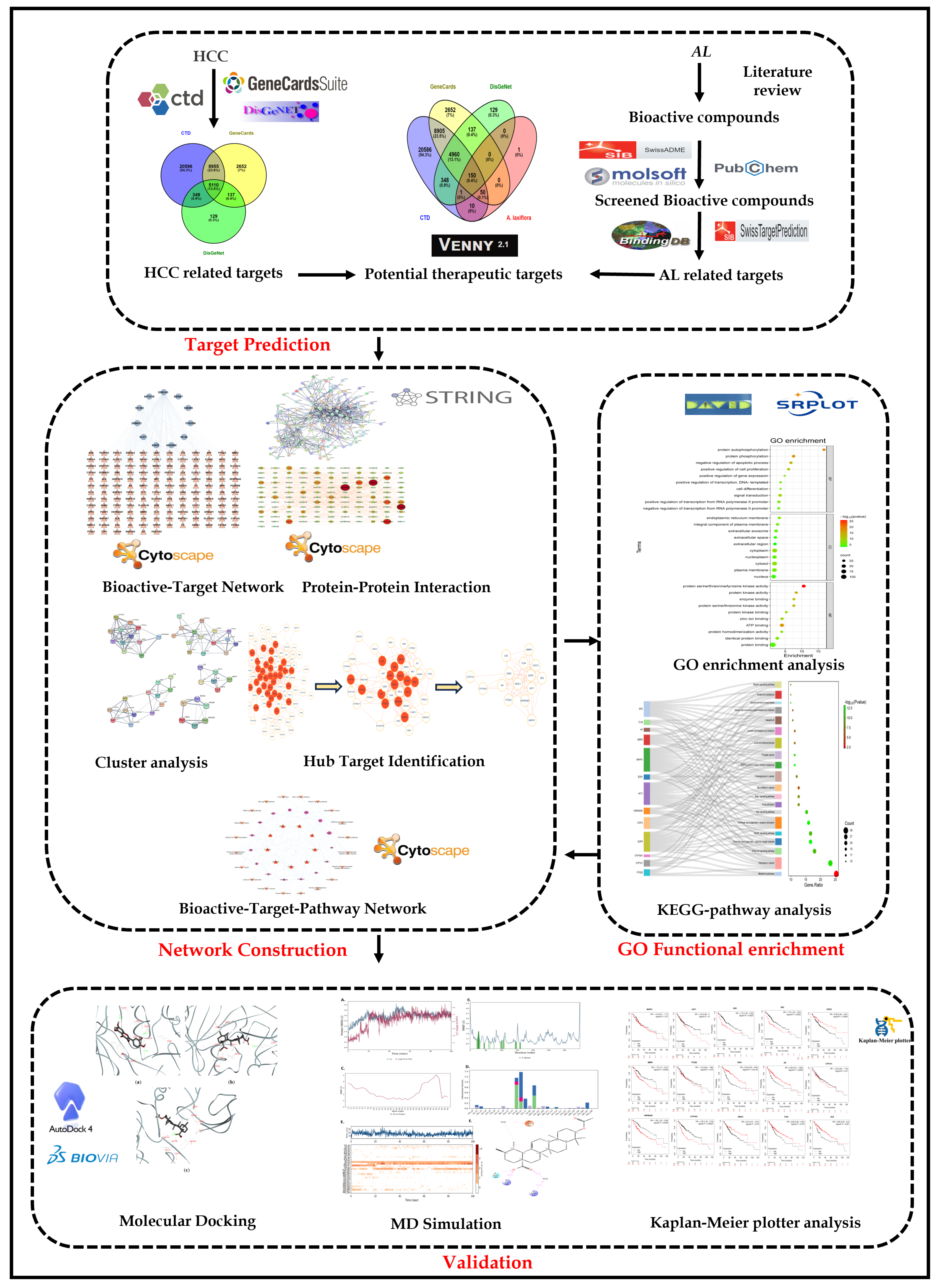
2.2. Potential Targets of A. laxiflora in the Treatment of Liver Cancer
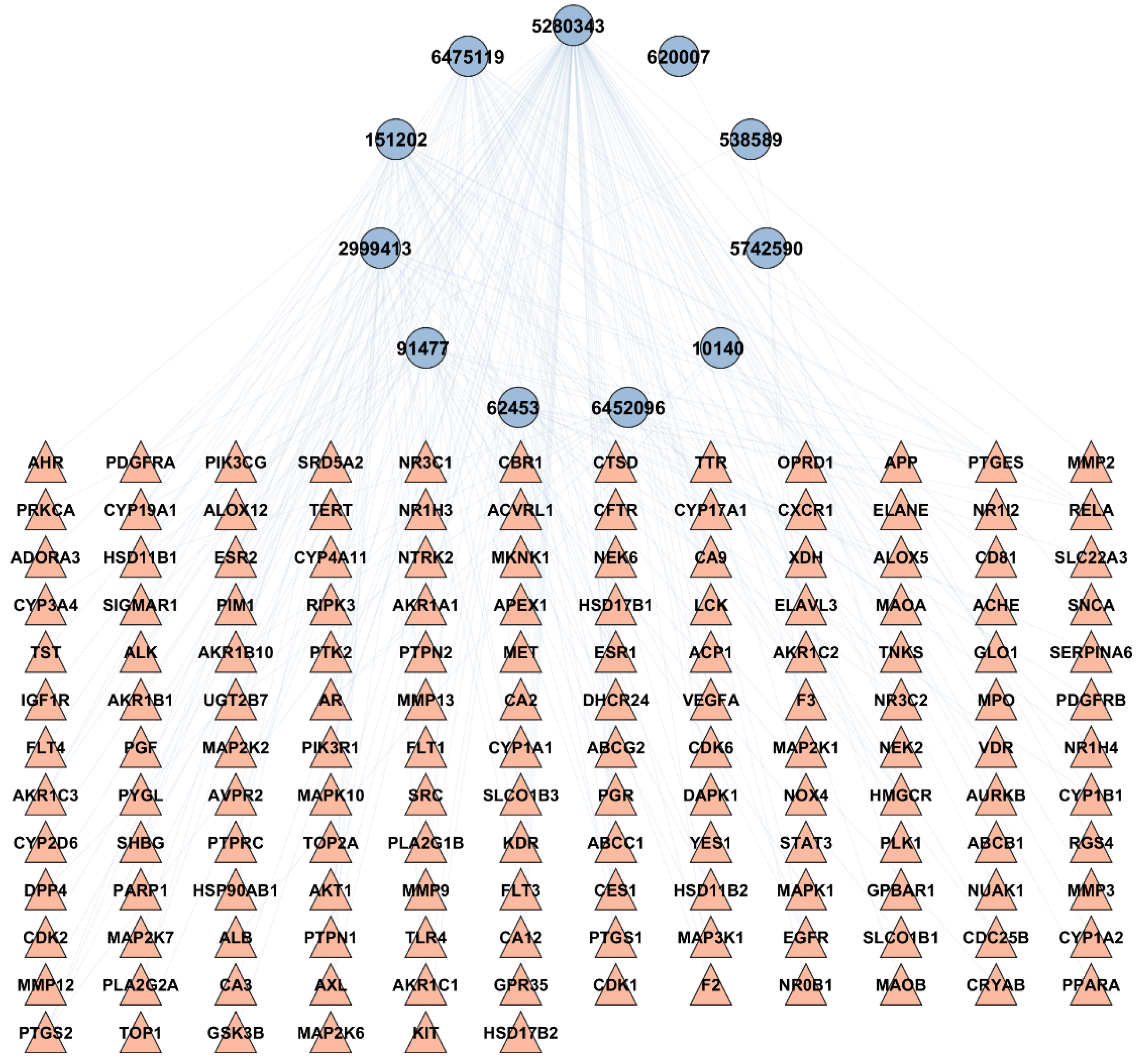
2.3. BA-TAR Network Construction
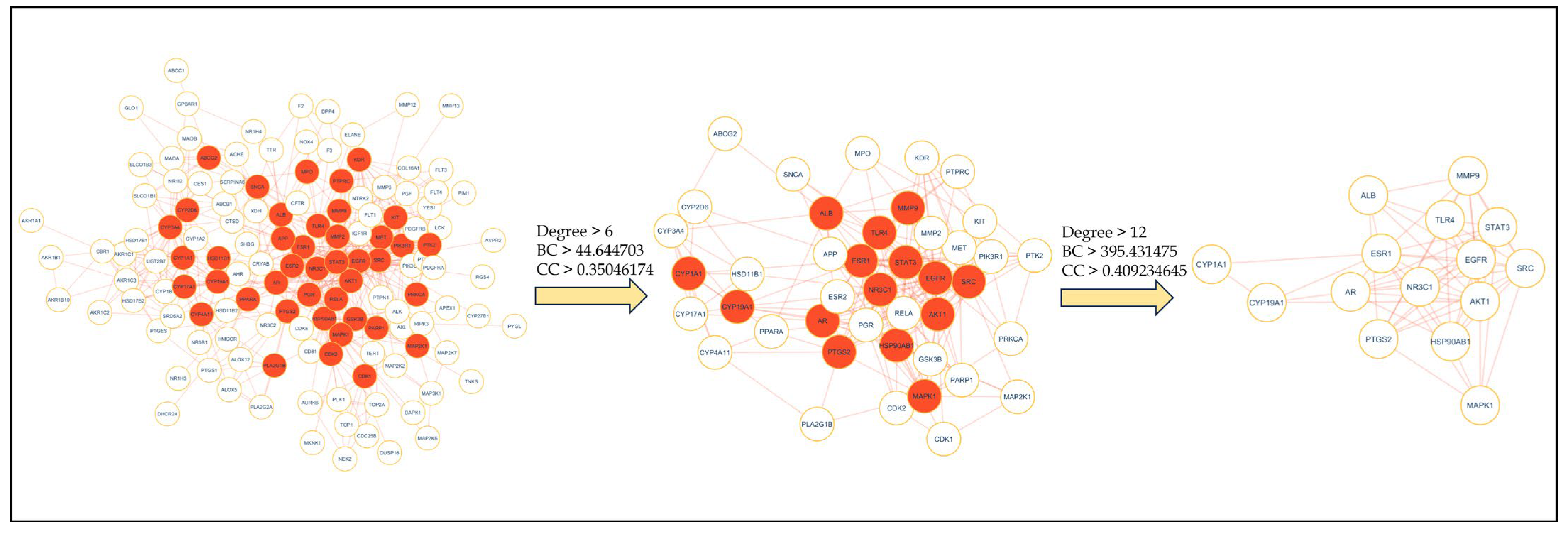
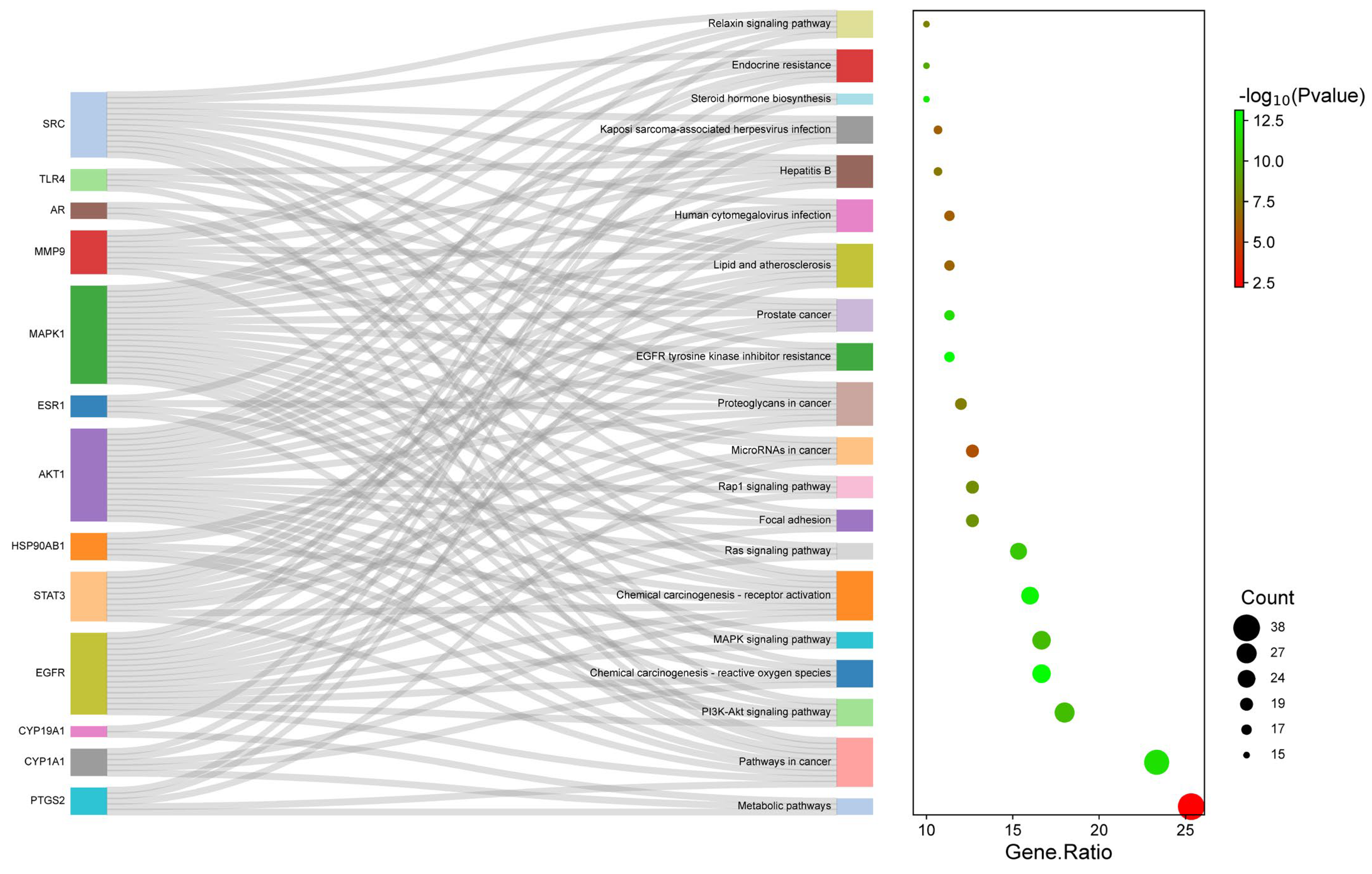
2.4. PPI Network Analysis
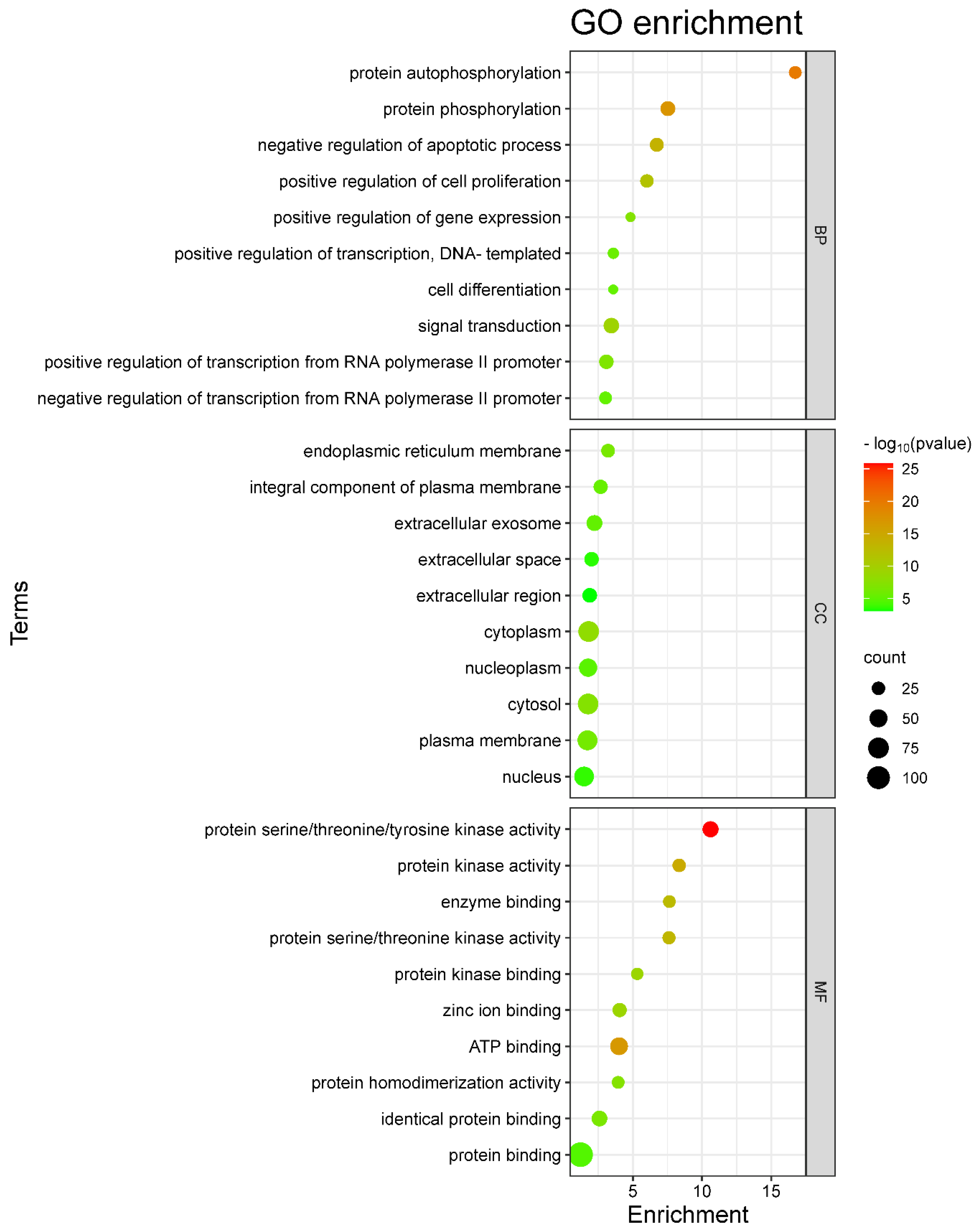
2.5. GO and KEGG Enrichment Analysis
2.6. BA-TAR-PATH Network Construction
2.7. Clusters Network Analysis
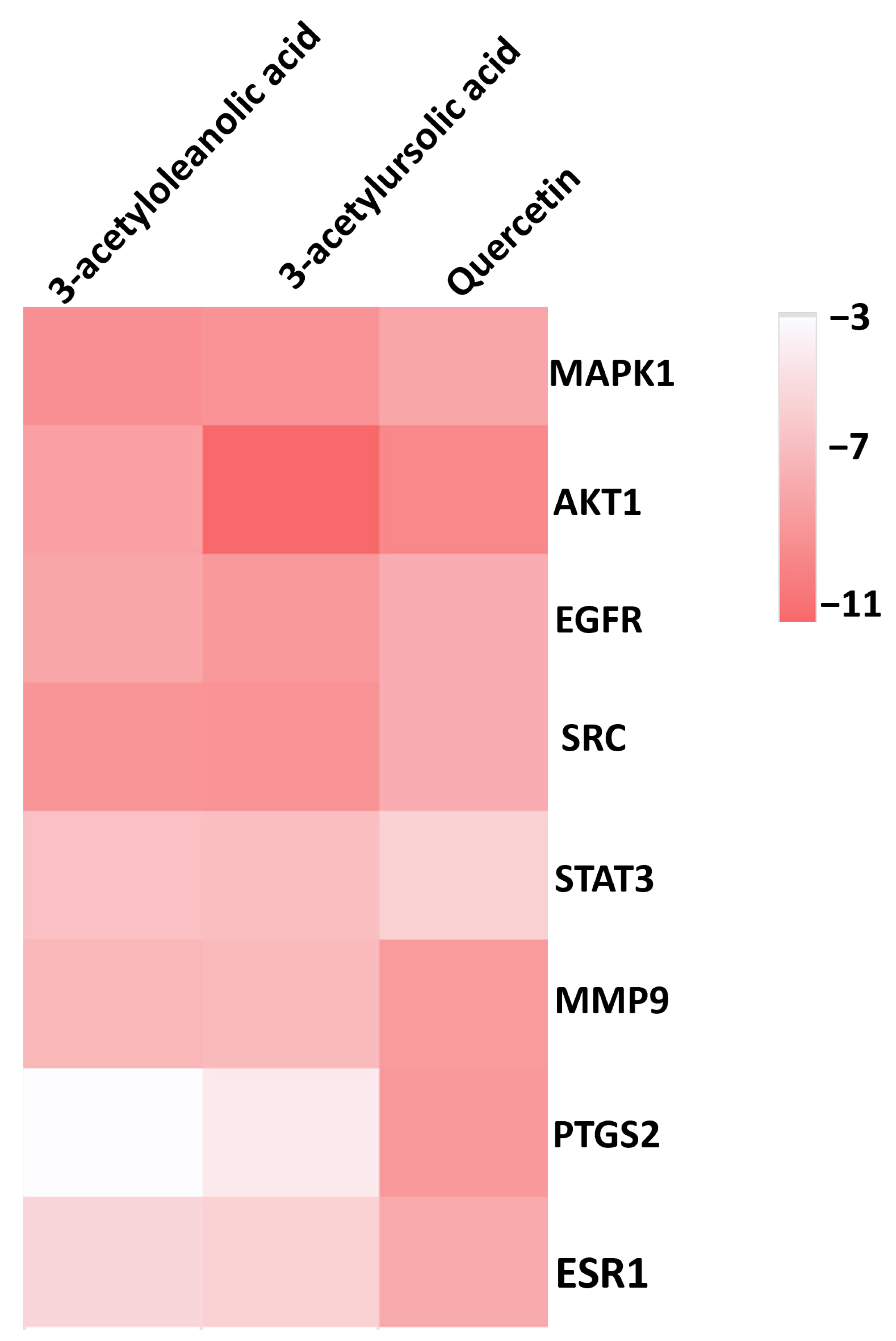
2.8. Molecular Docking
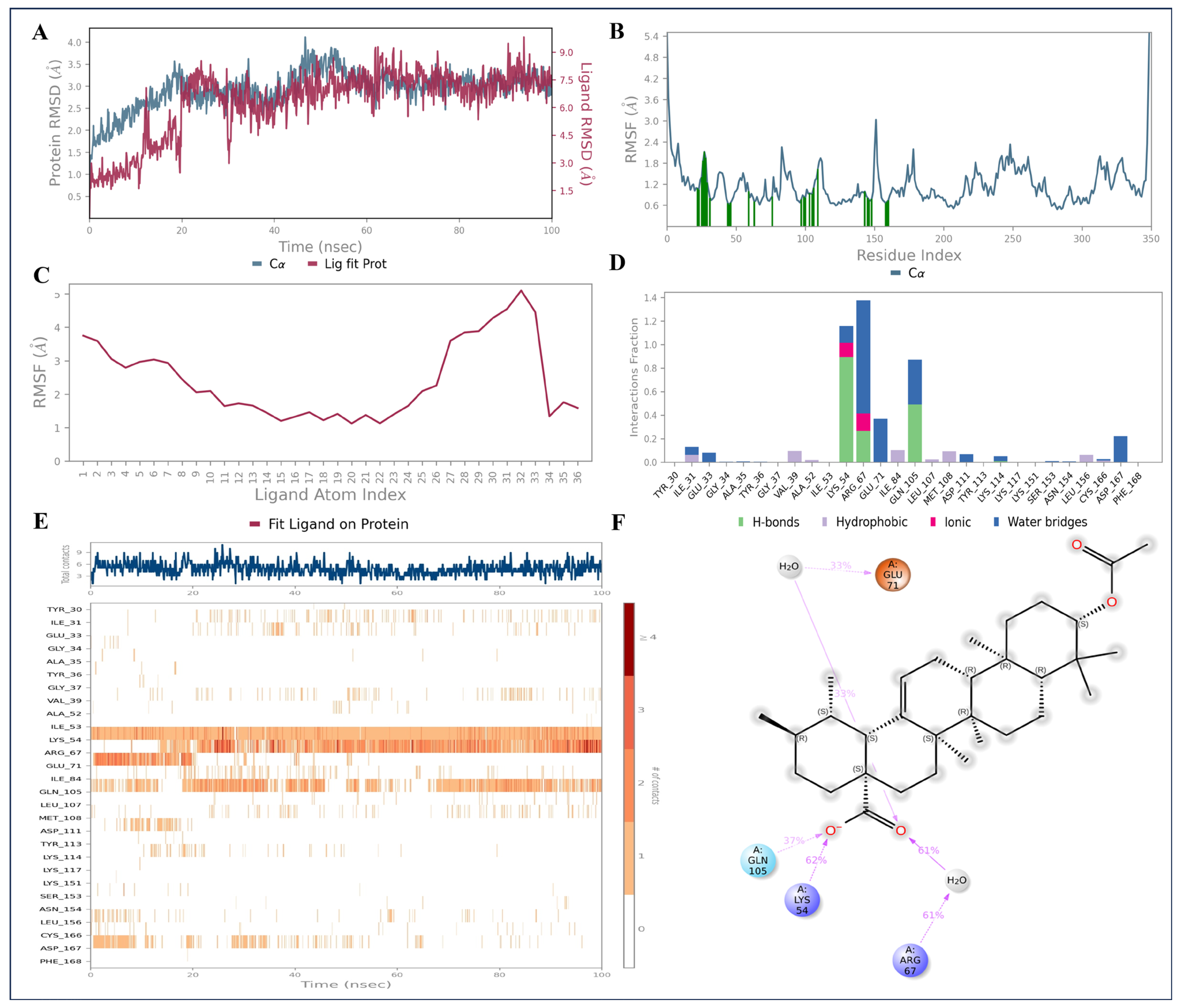
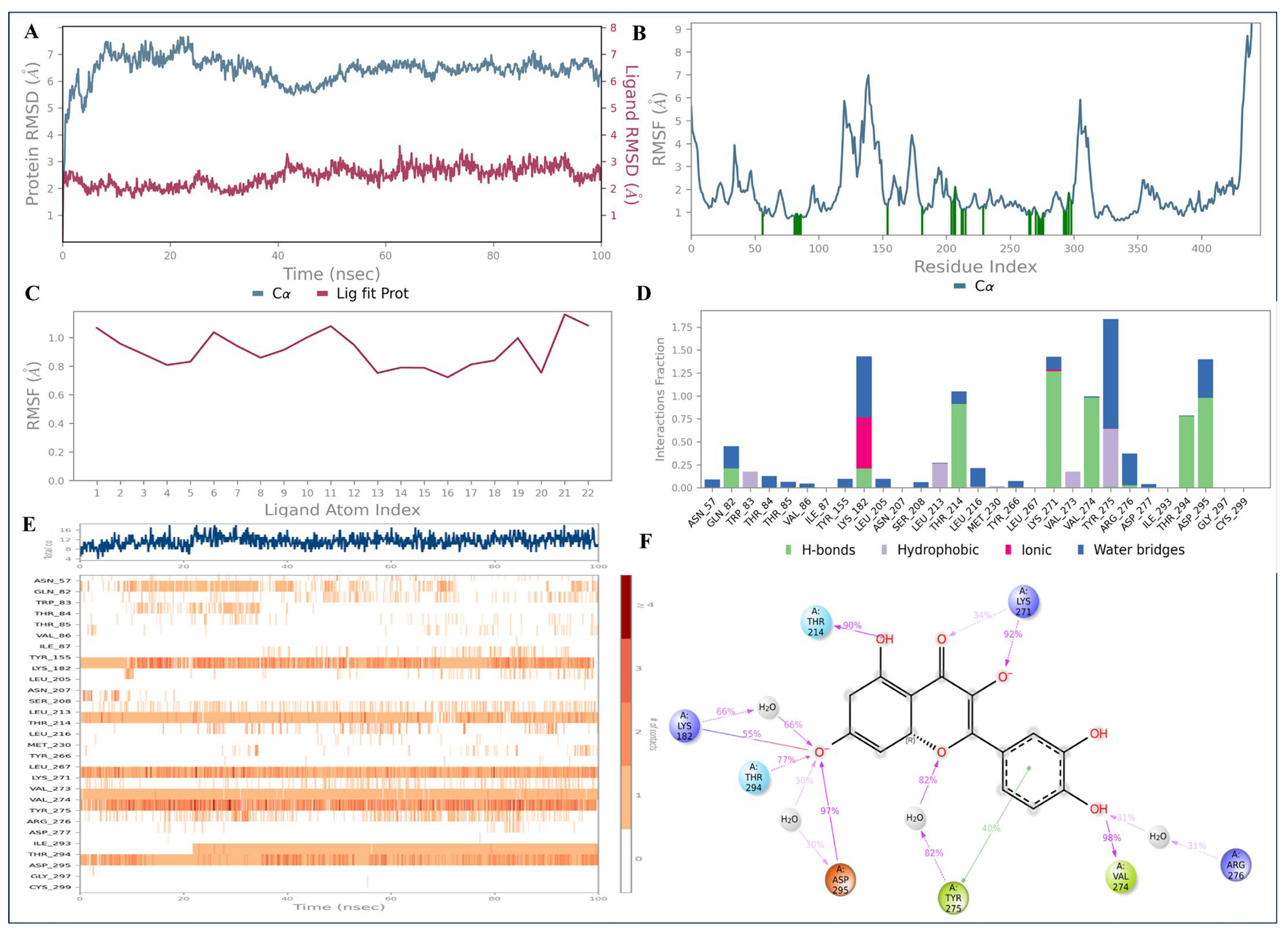
2.9. Molecular Dynamics Simulations
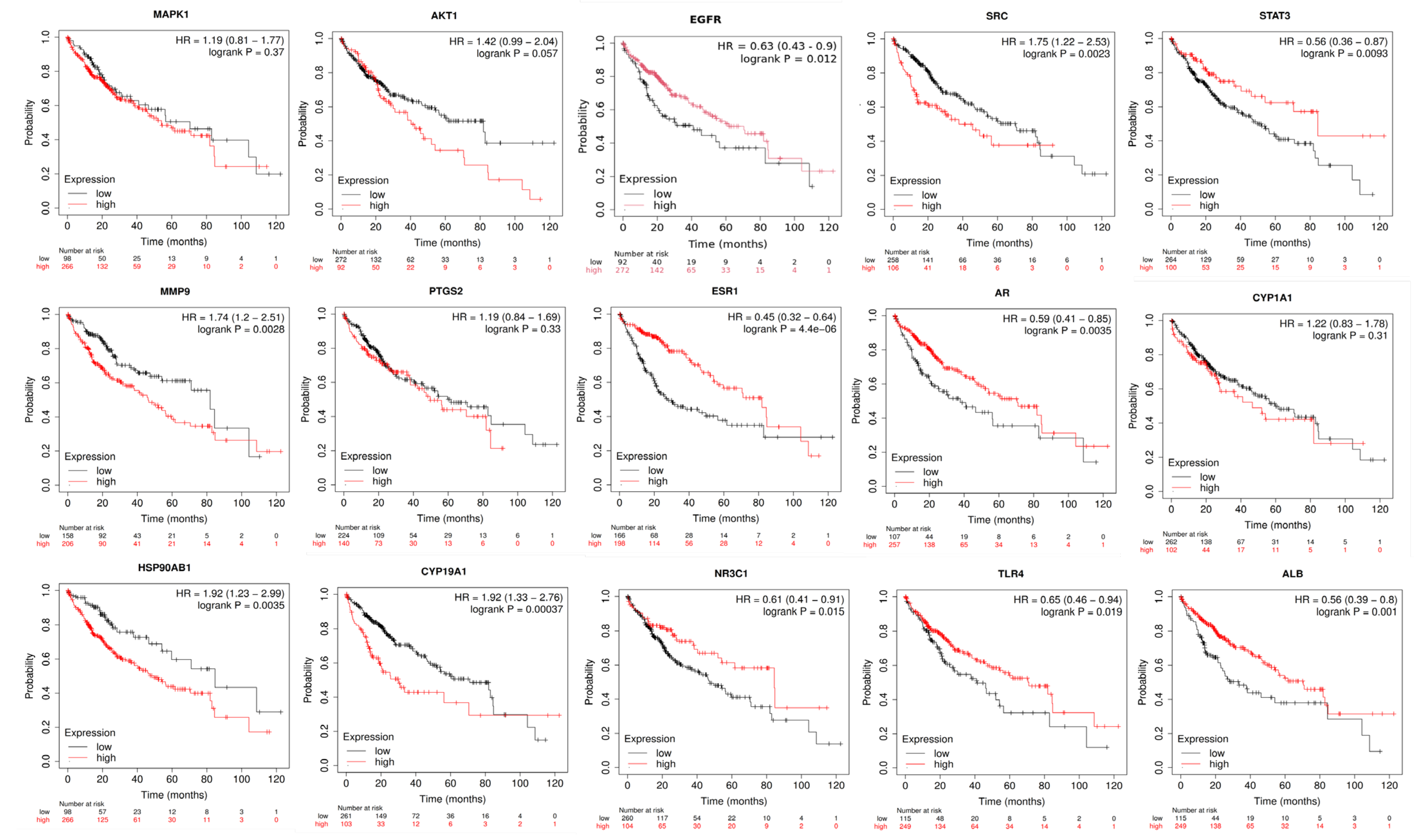
2.10. Anti-HCC Hub Gene Expression and Prognosis of Liver Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Collection of A. laxiflora Bioactive Compounds
4.2. Screened Bioactives’Target Prediction
4.3. Collection of Potential HCC-Related Protein Targets
4.4. Potential Anti-HCC Targets Retrieval
4.5. Bioactive-Target Network Construction
4.6. Protein–Protein Interactions (PPI) Network Construction and Hub Gene Identification
4.7. Gene Ontology (GO) and KEGG Pathway Enrichment Analyses
4.8. Bioactive-Target-Pathway Network Construction
4.9. Molecular Docking
4.10. Molecular Dynamics (MD) Simulations
4.11. Correlation Analysis of Hub Gene Expression and HCC Patient Prognosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A.; Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Calderaro, J.; Ziol, M.; Paradis, V.; Zucman-Rossi, J. Molecular and histological correlations in liver cancer. J. Hepatol. 2019, 71, 616–630. [Google Scholar] [CrossRef]
- Nelson, V.K.; Nuli, M.V.; Mastanaiah, J.; Saleem, T.M.; Birudala, G.; Jamous, Y.F.; Alshargi, O.; Kotha, K.K.; Sudhan, H.H.; Mani, R.R.; et al. Reactive oxygen species mediated apoptotic death of colon cancer cells: Therapeutic potential of plant derived alkaloids. Front. Endocrinol. 2023, 14, 1201198. [Google Scholar]
- Njayou, F.; Moundipa, P.; Tchana, A.; Ngadjui, B.; Tchouanguep, F. Inhibition Of Microsomal Lipid Peroxidation And Protein Oxidation By Extracts From Plants Used In Bamun Folk Medicine (Cameroon) Against Hepatitis. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 278–289. [Google Scholar] [CrossRef]
- Morah, F.N.I.; Uduagwu, D.N. Chemical composition, antioxidant and larvicidal activity of Alchornea laxiflora (Benth) leaf extracts. Edorium. J. Pharmacol. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Siwe-Noundou, X.; Ndinteh, D.T.; Olivier, D.K.; Mnkandhla, D.; Isaacs, M.; Muganza, F.M.; Mbafor, J.T.; Van Vuuren, S.F.; Patnala, S.; Hoppe, H.; et al. Biological activity of plant extracts and isolated compounds from Alchornea laxiflora: Anti-HIV, antibacterial and cytotoxicity evaluation. S. Afr. J. Bot. 2019, 122, 498–503. [Google Scholar] [CrossRef]
- Kuete, V.; Tchinda, C.F.; Mambe, F.T.; Beng, V.P.; Efferth, T. Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. BMC Complement. Altern. Med. 2016, 16, 267. [Google Scholar] [CrossRef]
- Oloyede, G.K.; Onocha, P.A.; Adaramoye, O.A.; Thonda, S.E. Hepatoprotective activity and flavonoids of Alchornea laxiflora leaf extract. Res. J. Phytochem. 2011, 5, 190–200. [Google Scholar] [CrossRef][Green Version]
- Khan, S.A.; Lee, T.K.W. Investigations of nitazoxanide molecular targets and pathways for the treatment of hepatocellular carcinoma using network pharmacology and molecular docking. Front. Pharmacol. 2022, 13, 968148. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Qamar, M.T.U.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Deng, J.; He, J.; Yin, L.; You, R.; Zhang, L.; Shen, J.; Han, Z.; Xie, F.; He, J.; et al. Integration of molecular docking, molecular dynamics and network pharmacology to explore the multi-target pharmacology of fenugreek against diabetes. J. Cell. Mol. Med. 2023, 27, 1959–1974. [Google Scholar] [CrossRef]
- Jain, N.K.; Tailang, M.; Kumar, S.; Chandrasekaran, B.; Alghazwani, Y.; Chandramoorthy, H.C.; Kumar, A.; Deshpande, H.; Wal, P.; Balamurugan, M.; et al. Appraising the therapeutical potentials of Alchornea laxiflora (Benth.) Pax & K. Hoffm. an underexplored medicinal herb: A systematic review. Front. Pharmacol. 2022, 13, 958453. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, M.; Zhao, Z.; Pan, J.; Huang, S. Network Pharmacology and Molecular Docking Analyses of Mechanisms Underlying Effects of the Cyperi Rhizoma-Chuanxiong Rhizoma Herb Pair on Depression. Evid. Based Complement. Alternat. Med. 2021, 2021, 5704578. [Google Scholar] [CrossRef]
- Gupta, M.; Sarwat, M. Protective effects of plant-derived natural products against hepatocellular carcinoma. In Herbal Medicines; Sarwat, M., Siddique, H.B.T.-H.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 609–627. [Google Scholar] [CrossRef]
- Basu, A.; Namporn, T.; Ruenraroengsak, P. Critical Review in Designing Plant-Based Anticancer Nanoparticles against Hepatocellular Carcinoma. Pharmaceutics 2023, 15, 1611. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Liu, T.; Li, S.; Feng, J.; Yu, Q.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019, 8, 4806–4820. [Google Scholar] [CrossRef]
- Ji, Y.; Li, L.; Ma, Y.-X.; Li, W.-T.; Li, L.; Zhu, H.-Z.; Wu, M.-H.; Zhou, J.-R. Quercetin inhibits growth of hepatocellular carcinoma by apoptosis induction in part via autophagy stimulation in mice. J. Nutr. Biochem. 2019, 69, 108–119. [Google Scholar] [CrossRef]
- Hwang-Bo, J.; Bae, M.G.; Park, J.-H.; Chung, I.S. 3-O-Acetyloleanolic acid inhibits VEGF-A-induced lymphangiogenesis and lymph node metastasis in an oral cancer sentinel lymph node animal model. BMC Cancer 2018, 18, 714. [Google Scholar] [CrossRef]
- Ou-Yang, Q.; Xuan, C.-X.; Wang, X.; Luo, H.-Q.; Liu, J.-E.; Wang, L.-L.; Li, T.-T.; Chen, Y.-P.; Liu, J. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol. Sin. 2018, 39, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- AlQathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, X.; Wang, L.; Zhu, B.; Wang, X.; Xia, J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J. Cell. Mol. Med. 2014, 18, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, E.; Housset, C.; Cacheux, W.; Wendum, D.; Desbois-Mouthon, C.; Rey, C.; Clergue, F.; Poupon, R.; Barbu, V.; Rosmorduc, O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 2005, 41, 307–314. [Google Scholar] [CrossRef]
- Mroweh, M.; Roth, G.; Decaens, T.; Marche, P.N.; Lerat, H.; Jílková, Z.M. Targeting Akt in Hepatocellular Carcinoma and Its Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 1794. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Zhang, L.; Zhu, A.X.; Bernards, R.; Qin, W.; Wang, C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 203–222. [Google Scholar] [CrossRef]
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef]
- Nwosu, Z.C.; Piorońska, W.; Battello, N.; Zimmer, A.D.; Dewidar, B.; Han, M.; Pereira, S.; Blagojevic, B.; Castven, D.; Charlestin, V.; et al. Severe metabolic alterations in liver cancer lead to ERK pathway activation and drug resistance. eBioMedicine 2020, 54, 102699. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, Y.; Wang, T.; Zhang, Y.; Kong, D.; Zhang, L.; Li, X.; Wang, G.; Jin, Y.; Jin, X.; et al. Elevated Src expression associated with hepatocellular carcinoma metastasis in northern Chinese patients. Oncol. Lett. 2015, 10, 3026–3034. [Google Scholar] [CrossRef]
- Ren, H.; Fang, J.; Ding, X.; Chen, Q. Role and inhibition of Src signaling in the progression of liver cancer. Open Life Sci. 2016, 11, 513–518. [Google Scholar] [CrossRef]
- Li, Y.; Song, Z.; Han, Q.; Zhao, H.; Pan, Z.; Lei, Z.; Zhang, J. Targeted inhibition of STAT3 induces immunogenic cell death of hepatocellular carcinoma cells via glycolysis. Mol. Oncol. 2022, 16, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Yi, D.-H. CDK1-SRC Interaction-Dependent Transcriptional Activation of HSP90AB1 Promotes Antitumor Immunity in Hepatocellular Carcinoma. J. Proteome Res. 2023, 22, 3714–3729. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Yan, J.; Huang, X.; Jiang, T.; Zhang, S.; Zhang, G. Dihydrotanshinone I targets ESR1 to induce DNA double-strand breaks and proliferation inhibition in hepatocellular carcinoma. Phytomedicine 2024, 130, 155767. [Google Scholar] [CrossRef]
- Che, L.; Wu, J.-S.; Du, Z.-B.; He, Y.-Q.; Yang, L.; Lin, J.-X.; Lei, Z.; Chen, X.-X.; Guo, D.-B.; Li, W.-G.; et al. Targeting Mitochondrial COX-2 Enhances Chemosensitivity via Drp1-Dependent Remodeling of Mitochondrial Dynamics in Hepatocellular Carcinoma. Cancers 2022, 14, 821. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.; Yang, Y.; Huang, J.; Yao, M.; Li, J.; Huang, Y.; Cai, X.; Geller, D.A.; Yan, Y. MICA+ Tumor Cell Upregulated Macrophage-Secreted MMP9 via PROS1-AXL Axis to Induce Tumor Immune Escape in Advanced Hepatocellular Carcinoma (HCC). Cancers 2024, 16, 269. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Lee, C.-W. Protein phosphatases regulate the liver microenvironment in the development of hepatocellular carcinoma. Exp. Mol. Med. 2022, 54, 1799–1813. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Ghadyani, F.; Hashemi, M.; Abbaspour, A.; Zabolian, A.; Javanshir, S.; Razzazan, M.; Mirzaei, S.; Entezari, M.; Goharrizi, M.A.S.B.; et al. Biological impact and therapeutic perspective of targeting PI3K/Akt signaling in hepatocellular carcinoma: Promises and Challenges. Pharmacol. Res. 2023, 187, 106553. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Qu, Z.; Luo, Y.; Chen, R.; Wei, S.; Yang, X.; Wang, Q. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma. Am. J. Transl. Res. 2019, 11, 6952–6964. [Google Scholar]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Z.; Xie, Z.; Li, X.; Zhang, H.; Chen, Y.; Wang, Y.; Lin, Z.; Li, C.; Liu, H.; et al. HIGD2A silencing impairs hepatocellular carcinoma growth via inhibiting mitochondrial function and the MAPK/ERK pathway. J. Transl. Med. 2023, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Hu, Q.; Wang, J.; Hai, L.; Nie, X.; Zhao, Q. Butorphanol inhibits angiogenesis and migration of hepatocellular carcinoma and regulates MAPK pathway. J. Antibiot. 2022, 75, 626–634. [Google Scholar] [CrossRef]
- Newell, P.; Toffanin, S.; Villanueva, A.; Chiang, D.Y.; Minguez, B.; Cabellos, L.; Savic, R.; Hoshida, Y.; Lim, K.H.; Melgar-Lesmes, P.; et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J. Hepatol. 2009, 51, 725–733. [Google Scholar] [CrossRef]
- Batool, S.; Javed, M.R.; Aslam, S.; Noor, F.; Javed, H.M.F.; Seemab, R.; Rehman, A.; Aslam, M.F.; Paray, B.A.; Gulnaz, A. Network Pharmacology and Bioinformatics Approach Reveals the Multi-Target Pharmacological Mechanism of Fumaria indica in the Treatment of Liver Cancer. Pharmaceuticals 2022, 15, 654. [Google Scholar] [CrossRef]
- Guo, W.; Huang, J.; Wang, N.; Tan, H.-Y.; Cheung, F.; Chen, F.; Feng, Y. Integrating Network Pharmacology and Pharmacological Evaluation for Deciphering the Action Mechanism of Herbal Formula Zuojin Pill in Suppressing Hepatocellular Carcinoma. Front. Pharmacol. 2019, 10, 1185. [Google Scholar]
- López-Luque, J.; Bertran, E.; Crosas-Molist, E.; Maiques, O.; Malfettone, A.; Caja, L.; Serrano, T.; Ramos, E.; Sanz-Moreno, V.; Fabregat, I. Downregulation of Epidermal Growth Factor Receptor in hepatocellular carcinoma facilitates Transforming Growth Factor-β-induced epithelial to amoeboid transition. Cancer Lett. 2019, 464, 15–24. [Google Scholar] [CrossRef]
- Jain, N.K.; Tailang, M.; Chandrasekaran, B.; Khazaleh, N.; Thangavel, N.; Makeen, H.A.; Albratty, M.; Najmi, A.; Alhazmi, H.A.; Zoghebi, K.; et al. Integrating network pharmacology with molecular docking to rationalize the ethnomedicinal use of Alchornea laxiflora (Benth.) Pax & K. Hoffm. for efficient treatment of depression. Front. Pharmacol. 2024, 15, 1290398. [Google Scholar] [CrossRef]
- Cheng, T.; Pan, Y.; Hao, M.; Wang, Y.; Bryant, S.H. PubChem applications in drug discovery: A bibliometric analysis. Drug Discov. Today 2014, 19, 1751–1756. [Google Scholar] [CrossRef]
- James, T.; Hsieh, M.-L.; Knipling, L.; Hinton, D. Determining the Architecture of a Protein-DNA Complex by Combining FeBABE Cleavage Analyses, 3-D Printed Structures, and the ICM Molsoft Program. Methods Mol. Biol. 2015, 1334, 29–40. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ibrahim, R.S.; El-Banna, A.A. Network pharmacology-based analysis for unraveling potential cancer-related molecular targets of Egyptian propolis phytoconstituents accompanied with molecular docking and in vitro studies. RSC Adv. 2021, 11, 11610–11626. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2023. Nucleic Acids Res. 2023, 51, D1257–D1262. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Y.; Shu, X.; Lu, C.; Shao, S.; Liu, X.; Yang, C.; Luo, J.; Du, Q. Using Network Pharmacology and Molecular Docking to Explore the Mechanism of Shan Ci Gu (Cremastra appendiculata) Against Non-Small Cell Lung Cancer. Front. Chem. 2021, 9, 682862. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Jiao, X.; Sherman, B.T.; Huang, D.W.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics 2012, 28, 1805–1806. [Google Scholar] [CrossRef]
- He, J.; Han, D.; Jia, C.; Xie, J.; Zhu, F.; Wei, J.; Li, D.; Wei, D.; Li, Y.; Tang, L.; et al. Molecular Docking and Pharmacological Evaluation for Exploring the Polyrhachis vicina Rogers in Ameliorating Depression. Drug Des. Devel. Ther. 2023, 17, 717–735. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Agrawal, A.; Kulkarni, G.T.; Tailang, M. Molecular docking study on phytoconstituents of traditional ayurvedic drug tulsi (Ocimum sanctum linn.) against COVID-19 Mpro enzyme: An in silico study. Int. J. Pharm. Pharm. Sci. 2022, 14, 44–50. [Google Scholar] [CrossRef]
- Mooers, B.H.M. Shortcuts for faster image creation in PyMOL. Protein Sci. 2020, 29, 268–276. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- He, Q.; Chen, X.; Liu, J.; Li, C.; Xing, H.; Shi, Y.; Tang, Q. Combining Network Pharmacology with Molecular Docking for Mechanistic Research on Thyroid Dysfunction Caused by Polybrominated Diphenyl Ethers and Their Metabolites. BioMed Res. Int. 2021, 2021, 2961747. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE Conference Supercomput, Tampa, FL, USA, 28 June–1 July 2006; Association for Computing Machinery: New York, NY, USA; p. 84-es. [Google Scholar] [CrossRef]
- Jha, V.; Holmelin, F.L.; Eriksson, L.A. Binding Analysis and Structure-Based Design of Tricyclic Coumarin-Derived MTHFD2 Inhibitors as Anticancer Agents: Insights from Computational Modeling. ACS Omega 2023, 8, 14440–14458. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Wentzcovitch, R.M. Invariant molecular-dynamics approach to structural phase transitions. Phys. Rev. B 1991, 44, 2358–2361. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]




| PubChem ID | Bioactive | SMILES | BA | DL |
|---|---|---|---|---|
| 5280343 | Quercetin | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O | 0.55 | 0.52 |
| 62453 | 4-Vinylphenol | C=CC1=CC=C(C=C1)O | 0.55 | 0.29 |
| 2999413 | Zeranol | C[C@H]1CCC[C@@H](CCCCCC2=C(C(=CC(=C2)O)O)C(=O)O1)O | 0.55 | 0.5 |
| 151202 | 3-Acetyloleanolic acid | CC(=O)O[C@H]1CC[C@]2([C@H](C1(C)C)CC[C@@]3([C@@H]2CC=C4[C@]3(CC[C@@]5([C@H]4CC(CC5)(C)C)C(=O)O)C)C)C | 0.85 | 0.57 |
| 6475119 | 3-Acetoxyursolic acid | C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OC(=O)C)C)C)[C@@H]2[C@H]1C)C)C(=O)O | 0.85 | 0.84 |
| 91477 | Cholest-4-en-3-one | C[C@H](CCCC(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@]34C)C | 0.55 | 0.62 |
| 5742590 | β-Sitosterol-3-O-β-D-glucopyranoside | CC[C@H](CC[C@@H](C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O)O)O)C)C)C(C)C | 0.55 | 0.5 |
| 10140 | Glycocholic acid | C[C@H](CCC(=O)NCC(=O)O)[C@H]1CC[C@@H]2[C@@]1([C@H](C[C@H]3[C@H]2[C@@H](C[C@H]4[C@@]3(CC[C@H](C4)O)C)O)O)C | 0.56 | 0.29 |
| 6452096 | Ethyl iso-allocholate | CCOC(=O)CC[C@@H](C)[C@H]1CC[C@@H]2[C@@]1([C@H](C[C@H]3[C@H]2[C@@H](C[C@H]4[C@@]3(CC[C@H](C4)O)C)O)O)C | 0.55 | 0.39 |
| 538589 | 2H-Pyran-2-one, tetrahydro-4-hydroxy-6-pentyl- | CCCCCC1CC(CC(=O)O1)O | 0.55 | 0.29 |
| 620007 | 4-Fluoro-2-nitroaniline, 5-[4-(pyrrolidin-1-yl)carbonylmethylpiperazin1-yl]- | C1CCN(C1)C(=O)CN2CCN(CC2)C3=C(C=C(C(=C3)N)[N+](=O)[O-])F | 0.55 | 0.46 |
| 253193 | Phaeophorbide A | CCC1=C(C2=NC1=CC3=C(C4=C([C@@H](C(=C5[C@H]([C@@H](C(=CC6=NC(=C2)C(=C6C)C=C)N5)C)CCC(=O)O)C4=N3)C(=O)OC)O)C)C | 0.56 | 0.6 |
| 14135395 | Byzantionoside B | CC1=CC(=O)CC([C@H]1CC[C@@H](C)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)CO)O)O)O)(C)C | 0.55 | 0.6 |
| 551497 | D-galactitol, 3,6-anhydro-1,2,4,5-tetra-o-methyl- | COCC(C1C(C(CO1)OC)OC)OC | 0.55 | 0.29 |
| PubChem ID | Bioactive Compound | Degree |
|---|---|---|
| 5280343 | Quercetin | 94 |
| 6475119 | 3-Acetoxyursolic acid | 35 |
| 151202 | 3-Acetyloleanolic acid | 34 |
| 2999413 | Zeranol | 24 |
| 91477 | Cholest-4-en-3-one | 23 |
| 62453 | 4-Vinylphenol | 12 |
| 6452096 | Ethyl iso-allocholate | 8 |
| 10140 | Glycocholic acid | 4 |
| 538589 | 2H-Pyran-2-one, tetrahydro-4-hydroxy-6-pentyl- | 2 |
| 5742590 | β-Sitosterol-3-O-β-D-glucopyranoside | 2 |
| 620007 | 4-Fluoro-2-nitroaniline, 5-[4-(pyrrolidin-1-yl)carbonylmethylpiperazin1-yl]- | 1 |
| 551497 | D-galactitol, 3,6-anhydro-1,2,4,5-tetra-o-methyl- | 0 |
| Hub Genes | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|
| EGFR | 38 | 2456.533 | 0.513514 |
| SRC | 37 | 1730.981 | 0.485401 |
| STAT3 | 35 | 1978.653 | 0.496269 |
| HSP90AB1 | 28 | 1993.469 | 0.475 |
| AKT1 | 25 | 1173.136 | 0.5 |
| ESR1 | 25 | 2190.166 | 0.503788 |
| PTGS2 | 19 | 1503.855 | 0.457045 |
| MAPK1 | 19 | 1499.365 | 0.434641 |
| ALB | 19 | 1962.353 | 0.471631 |
| TLR4 | 18 | 1014.664 | 0.455479 |
| MMP9 | 18 | 651.2776 | 0.446309 |
| CYP1A1 | 18 | 725.4012 | 0.413043 |
| CYP19A1 | 16 | 848.6445 | 0.410494 |
| AR | 15 | 760.8222 | 0.438944 |
| NR3C1 | 13 | 407.2651 | 0.415625 |
| Pathway ID | Pathway Name | p-Value | Gene Count | Hub Gene Count | Enriched Gene IDs |
|---|---|---|---|---|---|
| hsa01100 | Metabolic pathways | 0.005864 | 38 | 3 | MAOB, PLA2G1B, MAOA, GLO1, AKR1B1, ALOX12, PYGL, HMGCR, CYP3A4, PTGS2, CYP19A1, PIK3CG, PTGS1, CYP17A1, HSD11B1, HSD11B2, CA3, CA2, HSD17B1, ALOX5, HSD17B2, CA9, XDH, ACP1, CA12, CBR1, SRD5A2, AKR1C1, PLA2G2A, AKR1C3, AKR1A1, DHCR24, AKR1B10, TST, CYP1A2, CYP1A1, UGT2B7, PTGES |
| hsa05200 | Pathways in cancer | 1.46 × 10−12 | 35 | 8 | ALK, GSK3B, HSP90AB1, FLT3, FLT4, PIK3R1, PTGS2, RELA, EGFR, IGF1R, TERT, PIM1, AKT1, MAPK1, PDGFRB, PDGFRA, MAP2K1, MAP2K2, DAPK1, MMP2, STAT3, PRKCA, F2, MMP9, ESR1, PGF, PTK2, ESR2, VEGFA, MAPK10, AR, CDK6, KIT, CDK2, MET |
| hsa04151 | PI3K-Akt signaling pathway | 4.93 × 10−11 | 27 | 5 | GSK3B, FLT1, HSP90AB1, FLT3, FLT4, PIK3R1, RELA, EGFR, PIK3CG, IGF1R, KDR, AKT1, MAPK1, PDGFRB, PDGFRA, NTRK2, MAP2K1, MAP2K2, PRKCA, PGF, PTK2, VEGFA, CDK6, KIT, CDK2, MET, TLR4 |
| hsa05208 | Chemical carcinogenesis-reactive oxygen species | 8.13 × 10−14 | 25 | 5 | SRC, AHR, PIK3R1, RELA, EGFR, CYP1B1, AKT1, MAPK1, MAP2K7, ACP1, PTPN1, CBR1, MAP2K1, MAP2K2, AKR1C1, AKR1C3, AKR1A1, AKR1C2, PTK2, VEGFA, MAPK10, CYP1A2, CYP1A1, NOX4, MET |
| hsa04010 | MAPK signaling pathway | 5.90 × 10−11 | 25 | 3 | FLT1, FLT3, FLT4, RELA, EGFR, IGF1R, MKNK1, KDR, AKT1, MAPK1, MAP2K7, MAP2K6, PDGFRB, PDGFRA, NTRK2, MAP2K1, MAP2K2, MAP3K1, PRKCA, PGF, CDC25B, VEGFA, MAPK10, KIT, MET |
| hsa05207 | Chemical carcinogenesis-receptor activation | 2.39 × 10−13 | 24 | 9 | MAP2K1, MAP2K2, HSP90AB1, SRC, VDR, STAT3, PRKCA, AHR, PIK3R1, CYP3A4, ESR1, EGFR, RELA, ESR2, VEGFA, AR, CYP1A2, CYP1A1, CYP1B1, AKT1, MAPK1, PGR, PPARA, UGT2B7 |
| hsa04014 | Ras signaling pathway | 1.81 × 10−11 | 23 | 3 | PDGFRB, PDGFRA, NTRK2, MAP2K1, MAP2K2, FLT1, PLA2G1B, FLT3, FLT4, PLA2G2A, PRKCA, PIK3R1, EGFR, PGF, RELA, IGF1R, VEGFA, MAPK10, KIT, KDR, AKT1, MAPK1, MET |
| hsa04510 | Focal adhesion | 3.29 × 10−9 | 19 | 4 | PDGFRB, PDGFRA, GSK3B, MAP2K1, FLT1, SRC, FLT4, PRKCA, PIK3R1, EGFR, PGF, PTK2, IGF1R, VEGFA, MAPK10, KDR, AKT1, MAPK1, MET |
| hsa04015 | Rap1 signaling pathway | 5.66 × 10−9 | 19 | 4 | PDGFRB, PDGFRA, MAP2K1, MAP2K2, FLT1, SRC, FLT4, PRKCA, PIK3R1, EGFR, PGF, IGF1R, VEGFA, KIT, KDR, AKT1, MAPK1, MET, MAP2K6 |
| hsa05206 | MicroRNAs in cancer | 2.07 × 10−6 | 19 | 5 | PDGFRB, PDGFRA, MAP2K1, ABCC1, MAP2K2, ABCB1, STAT3, PRKCA, PIK3R1, PTGS2, MMP9, EGFR, CDC25B, VEGFA, CDK6, PIM1, CYP1B1, MAPK1, MET |
| Ligand (PubChem ID) | MAPK1 | AKT1 | EGFR | SRC | STAT3 | MMP9 | PTGS2 | ESR1 |
|---|---|---|---|---|---|---|---|---|
| Quercetin (5280343) | −8.2 * | −9.8 * | −7.8 * | −7.8 * | −5.9 * | −8.8 | −8.9 | −8.0 |
| 3-Acetylursolic acid (6475119) | −9.2 * | −11.5 * | −8.9 * | −9.2 * | −6.9 * | −7.1 | −4.6 | −5.9 |
| 3-Acetyloleanolic acid (151202) | −9.4 * | −8.5 * | −8.2 * | −9.1 * | −6.8 * | −7.3 | −3.6 | −5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, N.K.; Chandrasekaran, B.; Khazaleh, N.; Jain, H.K.; Lal, M.; Joshi, G.; Jha, V. Computational Network Pharmacology, Molecular Docking, and Molecular Dynamics to Decipher Natural Compounds of Alchornea laxiflora for Liver Cancer Chemotherapy. Pharmaceuticals 2025, 18, 508. https://doi.org/10.3390/ph18040508
Jain NK, Chandrasekaran B, Khazaleh N, Jain HK, Lal M, Joshi G, Jha V. Computational Network Pharmacology, Molecular Docking, and Molecular Dynamics to Decipher Natural Compounds of Alchornea laxiflora for Liver Cancer Chemotherapy. Pharmaceuticals. 2025; 18(4):508. https://doi.org/10.3390/ph18040508
Chicago/Turabian StyleJain, Nem Kumar, Balakumar Chandrasekaran, Nasha’t Khazaleh, Hemant Kumar Jain, Moti Lal, Gaurav Joshi, and Vibhu Jha. 2025. "Computational Network Pharmacology, Molecular Docking, and Molecular Dynamics to Decipher Natural Compounds of Alchornea laxiflora for Liver Cancer Chemotherapy" Pharmaceuticals 18, no. 4: 508. https://doi.org/10.3390/ph18040508
APA StyleJain, N. K., Chandrasekaran, B., Khazaleh, N., Jain, H. K., Lal, M., Joshi, G., & Jha, V. (2025). Computational Network Pharmacology, Molecular Docking, and Molecular Dynamics to Decipher Natural Compounds of Alchornea laxiflora for Liver Cancer Chemotherapy. Pharmaceuticals, 18(4), 508. https://doi.org/10.3390/ph18040508







