Development and Validation of an HPLC–PDA Method for Quality Control of Jwagwieum, an Herbal Medicine Prescription: Simultaneous Analysis of Nine Marker Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Profiling of Six Herbal Medicines Constituting JGE and Selection of Marker Compounds
2.2. Optimization of Operating Conditions for HPLC–PDA Simultaneous Analysis
2.3. Method Validation of the Developed HPLC–PDA Assay
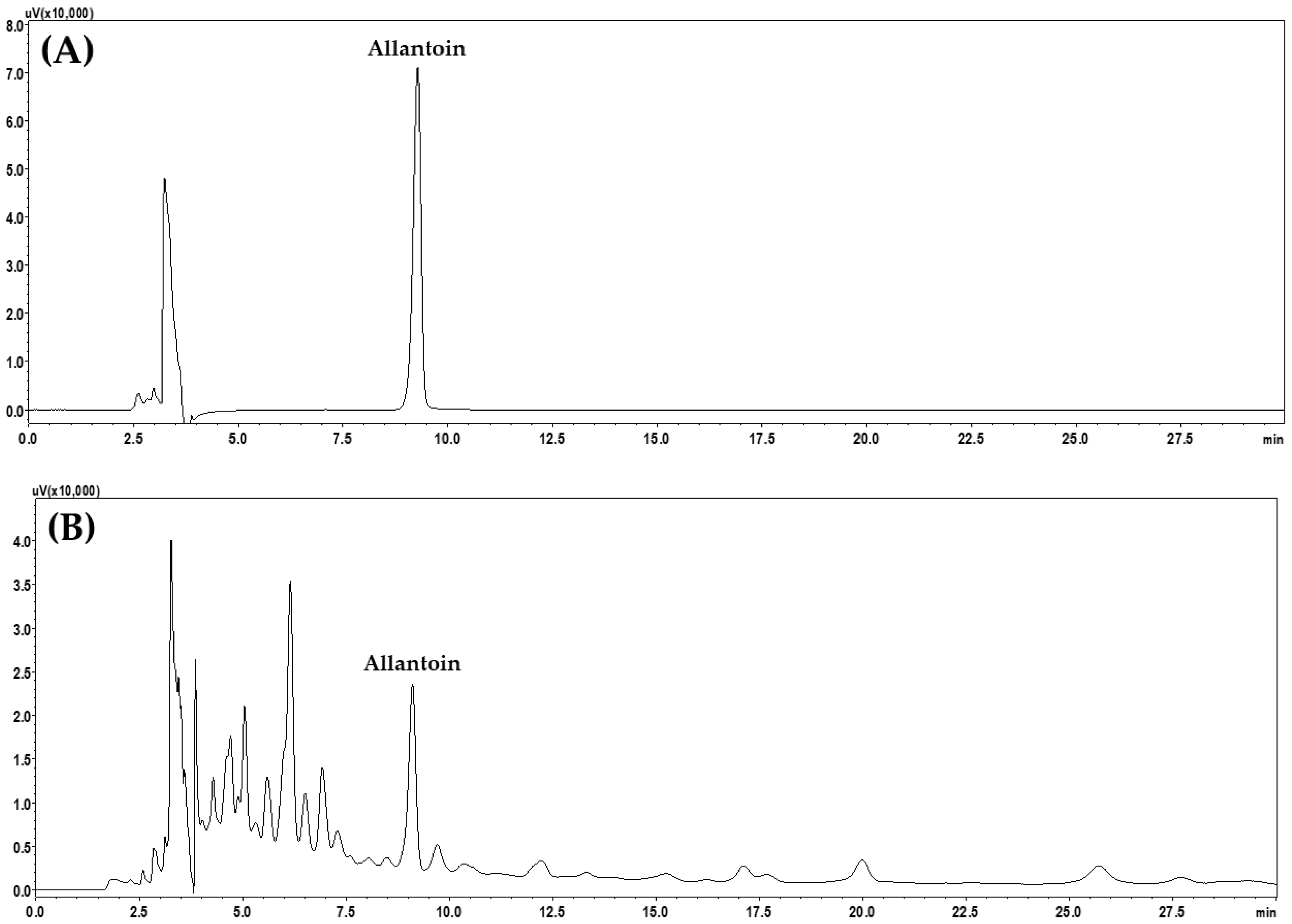
2.4. Stability Test
2.5. System Suitability Evaluation
2.6. Simultaneous Quantitative Analysis of Nine Marker Compounds in JGE Samples
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Preparation of JGE Water Extract Sample
3.4. Preparation of Standard and Sample Solutions for the Simultaneous Quantification by the HPLC–PDA Assay
3.5. Instrumental and Operating Conditions for Simultaneous HPLC–PDA Analysis
3.6. Analytical Method Validation of the Developed HPLC–PDA Assay
3.7. Stability Test
3.8. System Suitability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seo, C.S.; Lee, M.Y. Method development and validation for simultaneous analysis of eleven components for quality control of Geumgwesingihwan using HPLC–DAD and UPLC–MS/MS. Separations 2022, 9, 213. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chang, F.R.; Liou, J.R.; Lo, I.W.; Chung, T.C.; Lee, L.Y.; Chi, C.C.; Du, Y.C.; Wong, M.H.; Juo, S.H.H.; et al. Rapid HPLC quantification approach for detection of active constituent in modern combinatorial formula, San-Huang-Xie-Xin-Tang (SHXXT). Front. Pharmacol. 2016, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ren, Y.; Li, Z.; Xu, H. TCMM: A unified database for traditional Chinese medicine modernization and therapeutic innovations. Comput. Struct. Biotechnol. J. 2024, 23, 1619–1630. [Google Scholar]
- Gao, Y.; Liang, A.; Fan, X.; Hu, L.; Hao, F.; Li, Y. Safety research in traditional Chinese medicine: Methods, applications, and outlook. Engineering 2019, 5, 76–82. [Google Scholar]
- Kono, T.; Shimada, M.; Yamamoto, M.; Kaneko, A.; Oomiya, Y.; Kubota, K.; Kase, Y.; Lee, K.; Uezono, Y. Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: Analysis of daikenchuto. Front. Pharmacol. 2015, 6, 159. [Google Scholar] [CrossRef]
- Han, B.H.; Yu, J.K.; Jang, Y.J.; Kim, H.Y.; Yoon, J.J.; Cho, N.G.; Lee, H.S.; Kang, D.G. The protective effect of Oryeongsan, Geumgwe-sinkihwan, and Jwagwieum on renal injury in rats with unilateral ureteral obstruction. Herb. Formula Sci. 2023, 31, 133–144. [Google Scholar]
- Na, S.W.; Jang, Y.J.; Hong, M.H.; Yoon, J.J.; Lee, H.S.; Kim, H.Y.; Kang, D.G. Protective effect of Joa-Gui Em through the improvement of the NLRP3 and TLR4/ NF-κb signaling by ischemia/reperfusion-induced acute renal failure rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 7178868. [Google Scholar] [PubMed]
- Hwang, S.Y.; Hwang, B.Y.; Choi, W.H.; Jung, H.J.; Huh, J.D.; Lee, K.S.; Ro, J.S. Quantitative determination of 5-hydroxymethyl-2-furaldehyde in the Rehmanniae Radix Preparata samples at various processing stages. Kor. J. Pharmacogn. 2001, 32, 116–120. [Google Scholar]
- Seo, C.S.; Huang, D.S.; Lee, J.K.; Ha, H.K.; Kim, H.K.; Seo, Y.B.; Shin, H.K. Quantitative determination of the marker constituent of Dioscoreae Rhizoma by the packaging methods. Kor. J. Herbol. 2008, 23, 45–49. [Google Scholar]
- Zhao, B.T.; Jeong, S.Y.; Hwangbo, K.; Moon, E.C.; Seo, E.K.; Lee, D.; Lee, J.H.; Min, B.S.; Ma, E.S.; Son, J.K.; et al. Quantitative analysis of betaine in Lycii fructus by HILIC-ELSD. Arch. Pharm. Res. 2013, 36, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, H.; Wang, L.; Zou, J.; Zheng, X.; Liu, Z. Quality evaluation of polar and active components in crude and processed Fructus corni by quantitative analysis of multicomponents with single marker. J. Anal. Methods Chem. 2016, 2016, 6496840. [Google Scholar] [PubMed]
- Jeong, E.J.; Kim, T.B.; Yang, H.; Kang, S.Y.; Kim, S.Y.; Sung, S.H.; Kim, Y.C. Neuroprotective iridoid glycosides from Cornus officinalis fruits against glutamate-induced toxicity in HT22 hippocampal cells. Phytomedicine 2012, 19, 317–321. [Google Scholar] [PubMed]
- Li, G.; Xu, M.L.; Lee, J.S.; Woo, M.H.; Chang, H.W.; Son, J.K. Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch. Pharm. Res. 2004, 27, 829–833. [Google Scholar] [PubMed]
- Zhou, S.; Cao, J.; Qiu, F.; Kong, W.; Yang, S.; Yang, M. Simultaneous determination of five bioactive components in Radix Glycyrrhizae by pressurized liquid extraction combined with UPLC–PDA and UPLC/ESI–QTOF–MS confirmation. Phytochem. Anal. 2013, 24, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Meng, X.S.; Bao, Y.R.; Wang, S.; Kang, T.G. Simultaneous quantitative determination of nine active chemical compositions in traditional Chinese medicine Glycyrrhiza by RP-HPLC with full-time five-wavelength fusion method. Am. J. Chin. Med. 2013, 41, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S. Simultaneous quantification of nine target compounds in traditional Korean medicine, Bopyeo-tang, using high-performance liquid chromatography–photodiode array detector and ultra-performance liquid chromatography–tandem mass spectrometery. Molecules 2024, 29, 1171. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Baek, S.H. Ultra-high performance liquid chromatography–tandem mass spectrometry assay for simultaneous determination of 22 marker compounds in traditional herbal medicine Ojeoksan. J. Pharm. Biomed. Anal. 2024, 246, 116193. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Go, S.H.; Ku, S.; Kim, M.K.; Jang, Y.P. Development and validation of simultaneous HPLC–PDA analysis method for quality control of Hwang-ryeon-hae-dok-tang: An analytical quality by design approach. J. Liq. Chromatogr. Relat. Technol. 2024, 47, 14–25. [Google Scholar] [CrossRef]
- Kiang, M.; Cao, J.; Zhang, C.; Su, B.; Wang, S.; Ning, N.; Lei, T.; Li, P. A comprehensive strategy for quality evaluation of Wushe Zhiyang Pills by integrating UPLC–DAD fingerprint and multi-ingredients rapid quantitation with UPLC–MS/MS technology. J. Pharm. Biomed. Anal. 2022, 210, 114556. [Google Scholar]
- International Conference on Harmonisation. Guidance for Industry, Q2B, Validation of Analytical Procedures: Methodology; Food and Drug Administration: Rockville, MD, USA, 1996. [Google Scholar]
- Bose, A. HPLC calibration process parameters in terms of system suitability test. Austin Chromatogr. 2014, 1, 1–4. [Google Scholar]

| Analyte a | Detection Wavelength (nm) | Linear Range (μg/mL) | Regression Equation | r2 c | LOD d (μg/mL) | LOQ e (μg/mL) | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| 1 | 270 | 0.16–10.00 | y = 53,334.63x + 144.75 | 1.0000 | 0.004 | 0.011 | 5.99 |
| 2 | 280 | 0.31–20.00 | y = 144,135.94x − 18,031.79 | 0.9997 | 0.041 | 0.125 | 8.34 |
| 3 | 240 | 0.16–10.00 | y = 92,740.24x + 2003.38 | 1.0000 | 0.004 | 0.013 | 12.42 |
| 4 | 235 | 0.16–10.00 | y = 56,300.13x + 1261.95 | 1.0000 | 0.006 | 0.020 | 14.71 |
| 5 | 275 | 0.47–30.00 | y = 38,748.93x + 3593.01 | 1.0000 | 0.026 | 0.079 | 18.39 |
| 6 | 275 | 0.16–10.00 | y = 50,734.87x + 1491.47 | 1.0000 | 0.003 | 0.010 | 18.79 |
| 7 | 250 | 0.16–10.00 | y = 39,806.34x − 3769.30 | 0.9980 | 0.009 | 0.027 | 23.96 |
| 8 | 250 | 1.56–100.00 | y = 8363.50x + 2067.17 | 1.0000 | 0.071 | 0.216 | 36.18 |
| 9 b | 210 | 3.13–100.00 | y = 9179.35x − 8.21 | 1.0000 | 0.764 | 2.315 | 9.27 |
| Analyte | Original Amount (μg/mL) | Spiked Amount (μg/mL) | Detected Amount (μg/mL) | Recovery (%) a | SD b | RSD (%) c |
|---|---|---|---|---|---|---|
| 1 | 2.14 | 1.00 | 3.16 | 102.65 | 1.52 | 1.48 |
| 2.00 | 4.17 | 101.79 | 1.17 | 1.15 | ||
| 4.00 | 6.18 | 101.01 | 0.60 | 0.60 | ||
| 2 | 8.13 | 2.00 | 10.18 | 102.26 | 1.12 | 1.09 |
| 4.00 | 12.21 | 101.95 | 1.01 | 0.99 | ||
| 8.00 | 16.09 | 99.41 | 0.64 | 0.65 | ||
| 3 | 4.14 | 1.00 | 5.18 | 104.66 | 0.67 | 0.64 |
| 2.00 | 6.07 | 96.63 | 0.43 | 0.45 | ||
| 4.00 | 8.38 | 106.20 | 0.19 | 0.18 | ||
| 4 | 3.50 | 1.00 | 4.55 | 105.01 | 1.00 | 0.96 |
| 2.00 | 5.57 | 103.50 | 0.29 | 0.28 | ||
| 4.00 | 7.78 | 106.95 | 0.30 | 0.28 | ||
| 5 | 7.42 | 2.00 | 9.38 | 98.20 | 0.48 | 0.49 |
| 4.00 | 11.68 | 106.49 | 0.63 | 0.59 | ||
| 8.00 | 15.56 | 101.83 | 0.44 | 0.43 | ||
| 6 | 1.52 | 1.00 | 2.50 | 98.19 | 2.45 | 2.50 |
| 2.00 | 3.56 | 102.21 | 0.58 | 0.57 | ||
| 4.00 | 5.37 | 96.36 | 0.74 | 0.76 | ||
| 7 | 1.50 | 1.00 | 2.50 | 100.37 | 0.57 | 0.57 |
| 2.00 | 3.50 | 99.73 | 0.21 | 0.21 | ||
| 4.00 | 5.64 | 103.56 | 0.10 | 0.10 | ||
| 8 | 37.02 | 7.00 | 44.37 | 105.00 | 0.22 | 0.21 |
| 17.50 | 54.57 | 100.28 | 0.21 | 0.21 | ||
| 35.00 | 74.54 | 107.21 | 0.08 | 0.08 | ||
| 9 | 23.85 | 6.00 | 29.90 | 100.77 | 0.50 | 0.49 |
| 15.00 | 39.62 | 105.10 | 1.39 | 1.32 | ||
| 30.00 | 54.20 | 101.15 | 0.99 | 0.98 |
| Analyte | Conc. (μg/mL) | Intraday (n = 5) | Interday (n = 15) | ||||
|---|---|---|---|---|---|---|---|
| Observed Conc. (μg/mL) | Precision (RSD, %) | Accuracy (%) | Observed Conc. (μg/mL) | Precision (RSD, %) | Accuracy (%) | ||
| 1 | 2.5 | 2.50 | 0.37 | 99.86 | 2.47 | 1.19 | 98.90 |
| 5.0 | 4.95 | 0.67 | 99.04 | 4.93 | 0.66 | 98.70 | |
| 10.0 | 9.88 | 0.12 | 98.78 | 9.82 | 0.64 | 98.18 | |
| 2 | 5.0 | 4.99 | 0.43 | 99.77 | 5.03 | 0.96 | 100.55 |
| 10.0 | 10.02 | 0.72 | 100.15 | 10.07 | 0.75 | 100.72 | |
| 20.0 | 20.11 | 0.32 | 100.55 | 20.30 | 0.92 | 101.48 | |
| 3 | 2.5 | 2.53 | 0.44 | 101.18 | 2.54 | 0.67 | 101.74 |
| 5.0 | 5.04 | 0.34 | 100.85 | 5.09 | 1.08 | 101.87 | |
| 10.0 | 10.03 | 0.33 | 100.26 | 10.10 | 0.77 | 100.95 | |
| 4 | 2.5 | 2.50 | 0.60 | 99.96 | 2.52 | 0.99 | 100.71 |
| 5.0 | 5.00 | 0.41 | 100.04 | 5.11 | 1.01 | 102.21 | |
| 10.0 | 10.00 | 0.51 | 100.04 | 10.07 | 0.77 | 100.70 | |
| 5 | 7.5 | 7.62 | 0.57 | 101.58 | 7.66 | 0.71 | 102.12 |
| 15.0 | 15.14 | 0.50 | 100.91 | 15.30 | 1.11 | 101.98 | |
| 30.0 | 30.05 | 0.35 | 100.18 | 30.26 | 0.78 | 100.87 | |
| 6 | 2.5 | 2.54 | 0.53 | 101.63 | 2.55 | 0.73 | 102.13 |
| 5.0 | 5.05 | 0.58 | 100.94 | 5.10 | 1.12 | 102.01 | |
| 10.0 | 10.02 | 0.33 | 100.21 | 10.09 | 0.77 | 100.90 | |
| 7 | 2.5 | 2.53 | 0.61 | 101.13 | 2.54 | 0.68 | 101.66 |
| 5.0 | 5.01 | 0.38 | 100.24 | 5.07 | 1.11 | 101.32 | |
| 10.0 | 10.18 | 0.37 | 101.75 | 10.25 | 0.80 | 102.49 | |
| 8 | 25.0 | 25.40 | 0.53 | 101.58 | 25.51 | 0.65 | 102.04 |
| 50.0 | 50.45 | 0.43 | 100.90 | 50.93 | 0.99 | 101.86 | |
| 100.0 | 100.28 | 0.39 | 100.28 | 100.96 | 0.77 | 100.96 | |
| 9 | 25.0 | 24.99 | 0.47 | 99.97 | 24.89 | 0.45 | 99.58 |
| 50.0 | 49.88 | 0.26 | 99.75 | 49.99 | 0.63 | 99.98 | |
| 100.0 | 98.08 | 0.10 | 98.08 | 98.17 | 0.44 | 98.17 | |
| Analyte | JGE–1 | JGE–2 | JGE–3 | |||
|---|---|---|---|---|---|---|
| Mean (mg/g) ± SD (×10–2) | RSD (%) | Mean (mg/g) ± SD (×10–2) | RSD (%) | Mean (mg/g) ± SD (×10–2) | RSD (%) | |
| 1 | 0.21 ± 0.10 | 0.47 | 0.21 ± 0.25 | 1.23 | 0.21 ± 0.24 | 1.16 |
| 2 | 0.81 ± 0.17 | 0.21 | 0.82 ± 0.08 | 0.10 | 0.82 ± 0.08 | 0.09 |
| 3 | 0.41 ± 0.02 | 0.04 | 0.41 ± 0.01 | 0.02 | 0.41 ± 0.03 | 0.06 |
| 4 | 0.35 ± 0.10 | 0.29 | 0.35 ± 0.01 | 0.04 | 0.35 ± 0.04 | 0.13 |
| 5 | 0.74 ± 0.24 | 0.32 | 0.74 ± 0.06 | 0.08 | 0.75 ± 0.14 | 0.19 |
| 6 | 0.15 ± 0.23 | 1.50 | 0.15 ± 0.04 | 0.30 | 0.15 ± 0.18 | 1.17 |
| 7 | 0.15 ± 0.10 | 0.69 | 0.15 ± 0.01 | 0.08 | 0.15 ± 0.08 | 0.52 |
| 8 | 3.69 ± 0.45 | 0.12 | 3.68 ± 0.17 | 0.05 | 3.67 ± 0.24 | 0.06 |
| 9 | 2.94 ± 0.62 | 0.21 | 2.92 ± 0.95 | 0.33 | 2.88 ± 0.16 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, C.-S.; Jung, J.; Shin, S. Development and Validation of an HPLC–PDA Method for Quality Control of Jwagwieum, an Herbal Medicine Prescription: Simultaneous Analysis of Nine Marker Compounds. Pharmaceuticals 2025, 18, 481. https://doi.org/10.3390/ph18040481
Seo C-S, Jung J, Shin S. Development and Validation of an HPLC–PDA Method for Quality Control of Jwagwieum, an Herbal Medicine Prescription: Simultaneous Analysis of Nine Marker Compounds. Pharmaceuticals. 2025; 18(4):481. https://doi.org/10.3390/ph18040481
Chicago/Turabian StyleSeo, Chang-Seob, Jeeyoun Jung, and Sarah Shin. 2025. "Development and Validation of an HPLC–PDA Method for Quality Control of Jwagwieum, an Herbal Medicine Prescription: Simultaneous Analysis of Nine Marker Compounds" Pharmaceuticals 18, no. 4: 481. https://doi.org/10.3390/ph18040481
APA StyleSeo, C.-S., Jung, J., & Shin, S. (2025). Development and Validation of an HPLC–PDA Method for Quality Control of Jwagwieum, an Herbal Medicine Prescription: Simultaneous Analysis of Nine Marker Compounds. Pharmaceuticals, 18(4), 481. https://doi.org/10.3390/ph18040481







