Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Multiple Reaction Monitoring-Based Multi-Component Analysis of Bangkeehwangkee-Tang: Method Development, Validation, and Application to Quality Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Marker Compounds for Quality Evaluation of BHT Using UPLC–MS/MS with MRM Detection
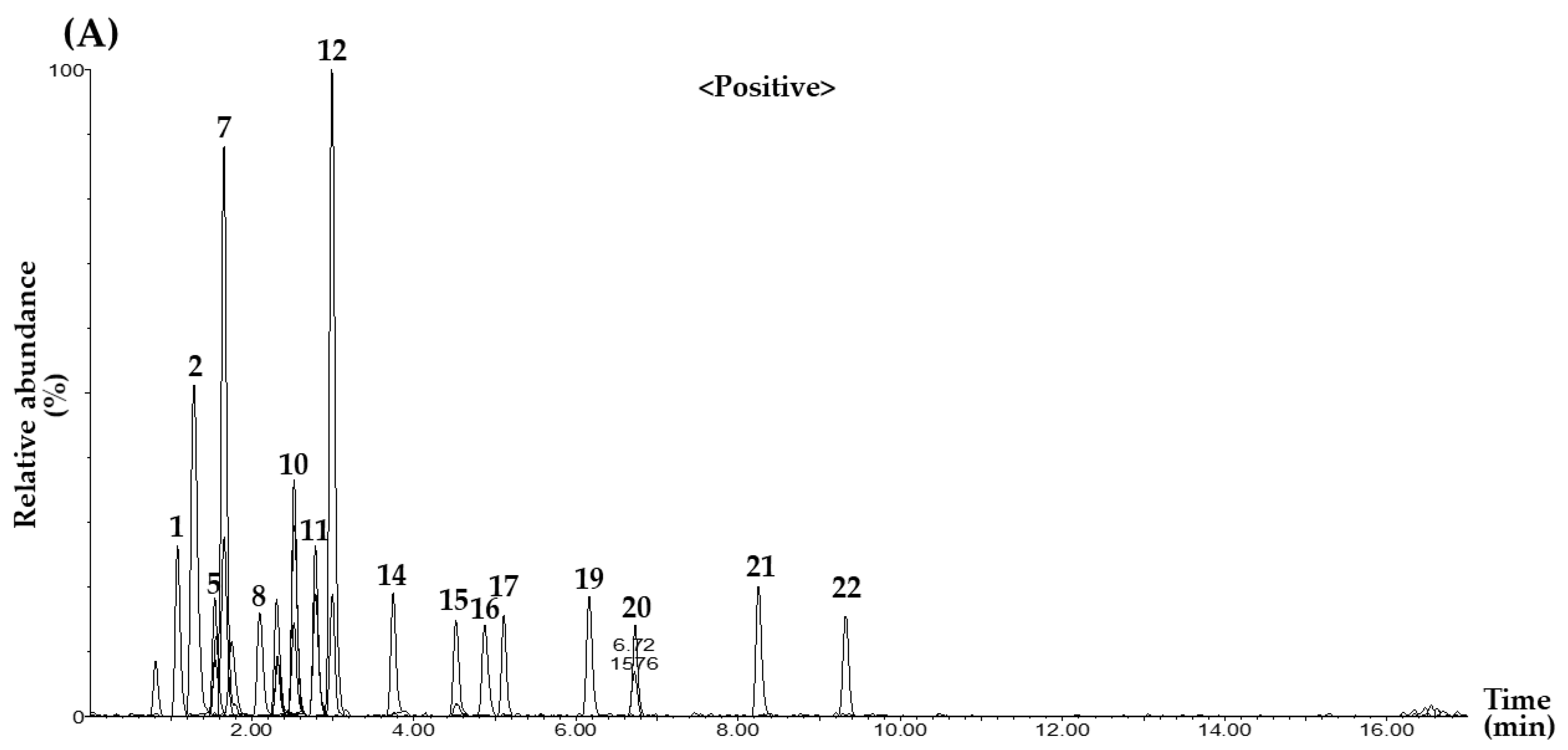
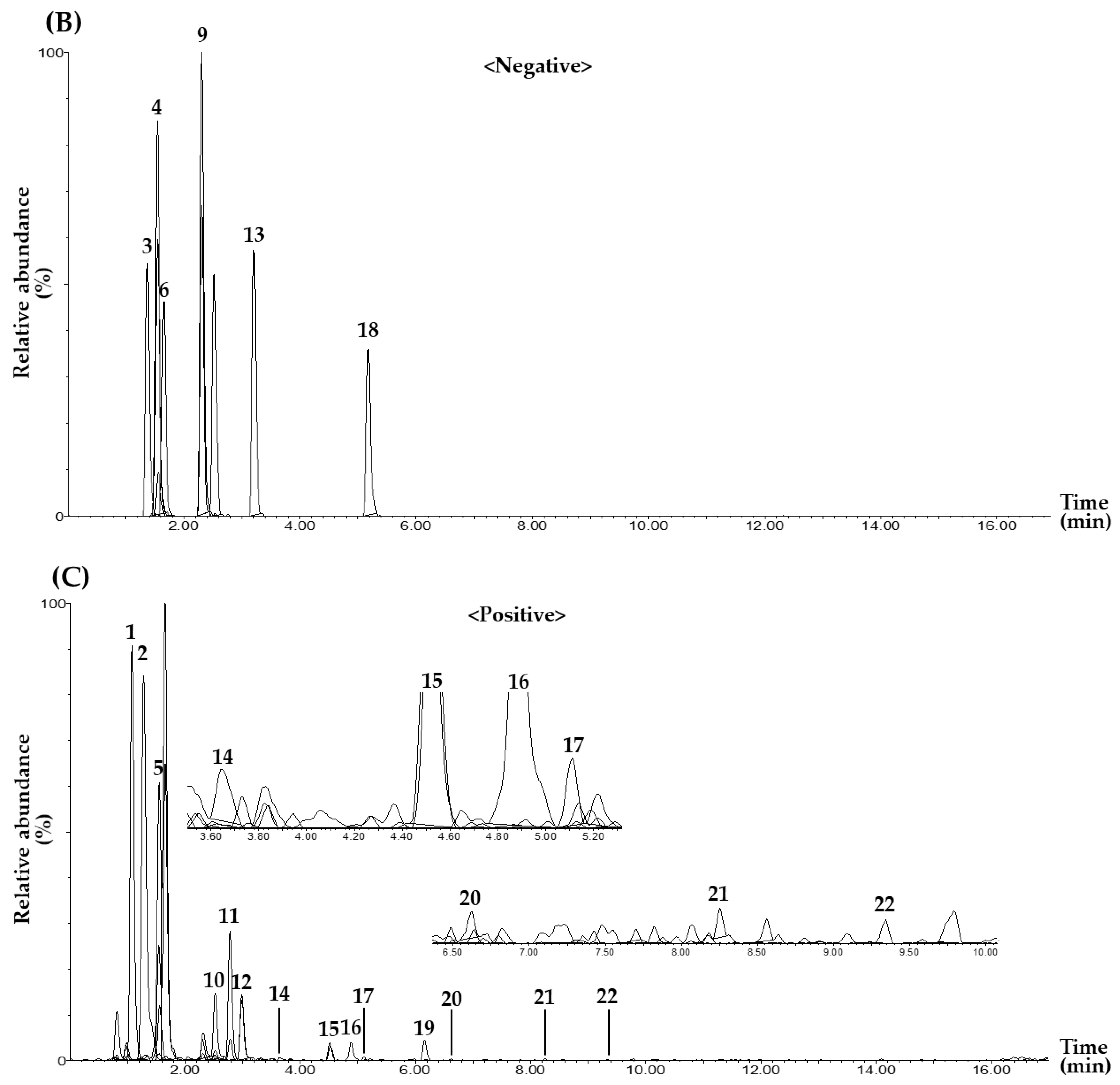
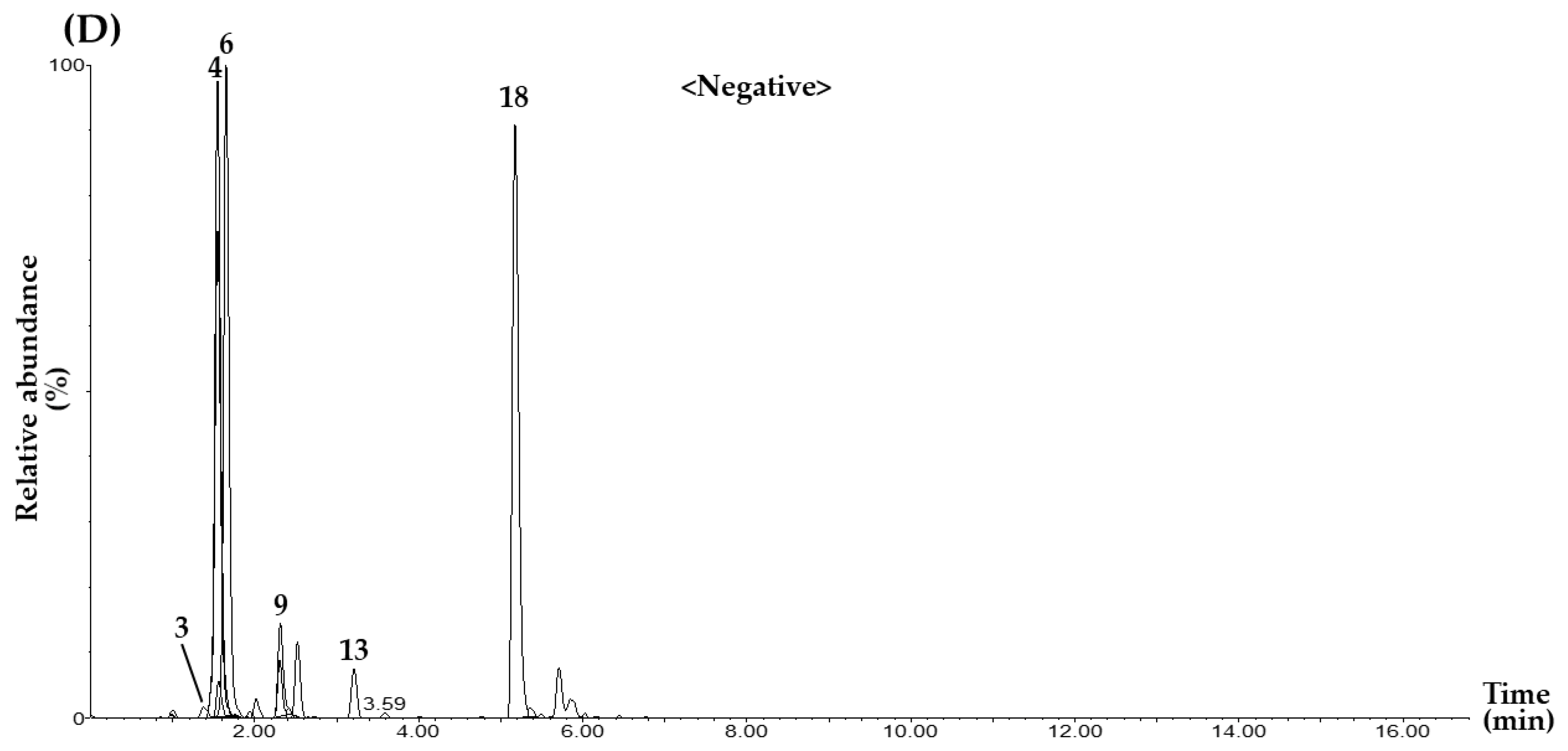
2.2. MRM Conditions for Simultaneous Determination of 22 Marker Compounds
2.3. Method Validation of the Developed UPLC–MS/MS Assay
2.3.1. Selectivity
2.3.2. Linearity
2.3.3. Sensitivity
2.3.4. System Stability
2.3.5. Accuracy
2.3.6. Precision
2.4. Simultaneous Determination of the 22 Marker Compounds in a BHT Sample by the UPLC–MS/MS MRM Method
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Preparation of BHT Sample
3.4. UPLC–MS/MS Analytical Conditions and Preparation of Standard and Sample Solutions
3.5. Validation of the Developed UPLC–MS/MS Method
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujitsuka, N.; Tamai, M.; Tsuchiya, K.; Iizuka, S.; Tsuchiya, N.; Makino, B.; Hattori, T.; Kase, Y.; Isohama, Y. Boiogito, a Kampo medicine, improves hydrarthrosis in a rat model of knee osteoarthritis. BMC Complement. Altern. Med. 2015, 15, 451. [Google Scholar] [CrossRef]
- Kobayashi, K.; Matsuyama, W.; Arai, Y.; Koizumi, S.; Shimizu, T.; Tomioka, R.; Sasaki, K. Boiogito increases the metabolism of fatty acids in proximal tubular cells through proliferators-activated receptor (PPAR) α agonistic activity. Biol. Pharm. Bull. 2016, 39, 143–147. [Google Scholar] [CrossRef][Green Version]
- Guo, S.; Yang, L.; Zhang, Q.; Zhang, L.; Li, A. Metabolomics combined with serum pharmacochemistry discovering the potential effective compounds of Fangji Huangqi Tang against nephrotic syndrome. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2023, 1214, 123532. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.S.; Leung, K.N. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 2007, 113, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.W.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and oxidative stress. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Li, J.M.; Yao, Y.D.; Luo, J.F.; Liu, J.X.; Lu, L.L.; Liu, Z.Q.; Dong, Y.; Xie, Y.; Zhou, H. Pharmacological mechanisms of sinomenine in anti-inflammatory immunity and osteoprotection in rheumatoid arthritis: A systematic review. Phytomedicine 2023, 121, 155114. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Liao, J.; Gong, Z.; Chai, X.; Lyu, H. Towards better sinomenine-type drugs to treat rheumatoid. Molecules 2022, 27, 8645. [Google Scholar] [CrossRef]
- Devi, S. Advancements in quantitative and qualitative methods for quality control of herbal drugs: A comprehensive review. Pharmacogn. Res. 2025, 17, 411–415. [Google Scholar] [CrossRef]
- Wei, X.C.; Cao, B.; Luo, C.H.; Huang, H.Z.; Tan, P.; Xu, X.R.; Xu, R.C. Recent advances of novel technologies for quality consistency assessment of natural herbal medicines and preparations. Chin. Med. 2020, 15, 56. [Google Scholar] [CrossRef]
- He, M.; Li, S. Chemometrics in quality control of traditional Chinese medicines. In Quality Control of Chinese Medicines; Li, S., Zhao, J., Eds.; Springer Nature: Singapore, 2024; pp. 837–881. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Yan, G.; Han, Y.; Sun, H. UHPLC–MS for the analytical characterization of traditional Chinese medicines. TrAC-Trends Anal. Chem. 2014, 63, 180–187. [Google Scholar] [CrossRef]
- Tu, Y.; Li, L.; Wang, Z.; Yang, L. Advances in analytical techniques and quality control of traditional Chinese medicine injections. J. Pharm. Biomed. Anal. 2021, 206, 114353. [Google Scholar] [CrossRef]
- Sim, H.J.; Kim, J.H.; Lee, K.R.; Hong, J.K. Simultaneous determination of structurally diverse compounds in different fangchi species by UHPLC–DAD and UHPLC–ESI–MS/MS. Molecules 2013, 18, 5235–5250. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; He, F.; Huang, Y.F.; Ma, H.L.; Wang, Y.P.; Cheng, C.S.; Cheng, J.L.; Lao, C.C.; Chen, D.A.; Zhang, Z.F.; et al. Discovery of chemical markers for identifying species, growth mode and production area of Astragali Radix by using ultra-high-performance liquid chromatography coupled to triple quadrupole mass spectrometry. Phytomedicine 2020, 67, 153155. [Google Scholar] [CrossRef]
- Zhan, C.; Wang, H.; Wang, Y. Quality evaluation of Atractylodis macrocephalae rhizome through fingerprint qualitative analysis and quantitative analysis of multi-components by single marker. J. Pharm. Biomed. Anal. 2022, 219, 114899. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Shakeel, F.; Yusufoglu, H.S.; Ross, S.A.; Alam, P. Simultaneous determination of 6-shogaol and 6-gingerol in various ginger (Zingiber officinale Roscoe) extracts and commercial formulations using a green RP-HPTLC-densitometry method. Foods 2020, 9, 1136. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, C.; Qiao, X.; Ye, M. Chemical analysis of the Chinese herbal medicine licorice (Gan-Cao): An update review. J. Ethnopharmacol. 2022, 299, 115686. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.; Tang, Y.; Su, S.; Shang, E.; Ni, S.; Qian, D. High-performance liquid chromatography–two wavelength detection of triterpenoid acids from the fruits of Ziziphus jujuba containing various cultivars in different regions and classification using chemometric analysis. J. Pharm. Biomed. Anal. 2009, 49, 1296–1302. [Google Scholar] [CrossRef]
- Aydogan, C. Recent advances and applications in LC-HRMS for food and plant natural products: A critical review. Anal. Bioanal. Chem. 2020, 412, 1973–1991. [Google Scholar] [CrossRef]
- International Council for Harmonisation. Validation of analytical procedures: Text and Methodology Q2(R1). In Proceedings of the International Conference on Harmonisation, Geneva, Switzerland, 1–13 January 2005. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry: Analytical Procedures and Methods Validation for Drugs and Biologics; New Hampshire Avenue; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2015. [Google Scholar]
- Ministry of Food and Drug Safety. Guidelines on Establishment of Chemical Profiles for Herbal Medicinal Products; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2023. [Google Scholar]
- Jiang, Z.; Wang, Y.; Zheng, Y.; Yang, J.; Zhang, L. Ultra high performance liquid chromatography coupled with triple quadrupole mass spectrometry and chemometric analysis of licorice based on the simultaneous determination of saponin and flavonoids. J. Sep. Sci. 2016, 39, 2928–2940. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Yu, J.G.; Wang, M.; Ma, Y.J.; Li, C.L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising Jujube (Ziziphus jujuba Mill.) selections. J. Food Sci. 2012, 77, C1218–C1225. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, H.; Liu, Q.; Zhao, Y.; Cui, X.; Guo, S.; Zhang, L.; Ho, C.T.; Bai, N. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef]
- Huang, Y.F.; He, F.; Wang, C.J.; Xie, Y.; Zhang, Y.Y.; Sang, Z.; Qiu, P.; Luo, P.; Xiao, S.Y.; Li, J.; et al. Discovery of chemical markers for improving the quality and safety control of Sinomenium acutum stem by the simultaneous determination of multiple alkaloids using UHPLC–QQQ–MS/MS. Sci. Rep. 2020, 10, 14182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC–MS/MS. J. Pharm. Anal. 2019, 9, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, G.; Li, Q.; Cai, B.; Hu, S. Quantitative evaluation main of the components in Paeoniae Radix Alba–Atractylodis Macrocephalae Rhizoma herbal pair by high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 239–246. [Google Scholar] [CrossRef]
- Nishidono, Y.; Saifudin, A.; Nishizawa, M.; Fujita, T.; Nakamoto, M.; Tanaka, K. Identification of the chemical constituents in ginger (Zingiber officinale) responsible for thermogenesis. Nat. Prod. Commun. 2018, 13, 869–873. [Google Scholar] [CrossRef]
- Dilek, H.; Doyuk, F. Determination of phytochemical content by chromatographic methods and antioxidant capacity in methanolic extract of Jujube (Zizyphus jujuba Mill.) and oleaster (Elaeagnus angustifolia L.). Int. J. Fruit Sci. 2020, 20, S1876–S1890. [Google Scholar] [CrossRef]
- Zhou, S.; Cao, J.; Qiu, F.; Kong, W.; Yang, S.; Yang, M. Simultaneous determination of five bioactive components in Radix Glycyrrhizae by pressurized liquid extraction combined with UPLC–PDA and UPLC/ESI–QTOF-MS confirmation. Phytochem. Anal. 2013, 24, 527–533. [Google Scholar] [CrossRef]
- Seo, C.S.; Shin, H.K. Ultra-performance liquid chromatography with tandem mass spectrometry for simultaneous analysis of 22 analytes of Oncheong-eum, a traditional Korean herbal formula. Processes 2023, 11, 2906. [Google Scholar] [CrossRef]
| Analyte | Ion Mode | Exact Mass (Da) | Precursor Ion (m/z) | Production Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|---|
| SIN | Positive | 329.16 | 330.3 | 181.1 | 30 | 35 |
| MAG | Positive | 342.17 | 342.4 | 297.2 | 30 | 20 |
| RUT | Negative | 610.15 | 609.3 | 300.0 | 45 | 30 |
| LIQA | Negative | 550.17 | 549.3 | 255.0 | 45 | 30 |
| CALG | Positive | 446.12 | 447.4 | 285.2 | 30 | 20 |
| LIQ | Negative | 418.13 | 417.4 | 255.2 | 30 | 15 |
| FAN | Positive | 608.29 | 609.5 | 367.3 | 30 | 40 |
| TET | Positive | 622.30 | 623.5 | 381.0 | 30 | 30 |
| ILIQA | Negative | 550.17 | 549.3 | 255.1 | 30 | 30 |
| ILIQ | Positive | 418.13 | 419.3 | 257.0 | 35 | 15 |
| ONO | Positive | 430.13 | 431.3 | 269.0 | 25 | 15 |
| LIQG | Positive | 256.07 | 257.2 | 137.0 | 35 | 25 |
| CAL | Negative | 284.07 | 283.3 | 268.1 | 30 | 20 |
| CINA | Positive | 148.05 | 149.1 | 131.0 | 20 | 10 |
| ILIQG | Positive | 256.07 | 257.2 | 137.0 | 15 | 20 |
| FOR | Positive | 268.07 | 269.1 | 253.0 | 40 | 25 |
| AST IV | Positive | 784.46 | 785.4 | 143.0 | 15 | 20 |
| GLY | Negative | 822.40 | 821.9 | 351.2 | 45 | 40 |
| GIN | Positive | 294.18 | 295.3 | 177.1 | 13 | 10 |
| ATR III | Positive | 248.14 | 249.3 | 231.2 | 25 | 10 |
| ATR II | Positive | 232.15 | 233.3 | 187.1 | 35 | 15 |
| ATR I | Positive | 230.13 | 231.2 | 185.1 | 35 | 20 |
| Analyte | Retention Time (min) | Linear Range (μg/L) | Regression Equation 1 | r2 | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|---|
| SIN | 1.08 | 1000–16,000 | y = 3.37x − 1383.43 | 0.9976 | 6.22 | 18.65 |
| MAG | 1.16 | 250–4000 | y = 1.97x + 45.57 | 0.9962 | 1.35 | 4.06 |
| RUT | 1.16 | 250–4000 | y = 1.93x + 252.49 | 0.9922 | 1.35 | 4.06 |
| LIQA | 1.29 | 1000–16,000 | y = 7.45x + 7228.74 | 0.9958 | 3.16 | 9.47 |
| CALG | 1.39 | 50–800 | y = 3.45x − 39.60 | 0.9952 | 0.47 | 1.41 |
| LIQ | 1.55 | 250–4000 | y = 4.77x − 609.61 | 0.9953 | 22.73 | 68.20 |
| FAN | 1.58 | 100–1600 | y = 7.27x − 345.76 | 0.9965 | 0.91 | 2.72 |
| TET | 1.66 | 500–8000 | y = 3.62x − 496.01 | 0.9954 | 0.54 | 1.61 |
| ILIQA | 1.77 | 1000–16,000 | y = 1.00x − 1051.84 | 0.9919 | 154.58 | 463.73 |
| ILIQ | 2.11 | 1000–16,000 | y = 1.22x − 1476.09 | 0.9913 | 326.58 | 979.75 |
| ONO | 2.32 | 250–4000 | y = 5.21x − 393.22 | 0.9950 | 19.27 | 57.80 |
| LIQG | 2.51 | 500–8000 | y = 1.38x − 64.59 | 0.9951 | 3.81 | 11.43 |
| CAL | 2.80 | 250–4000 | y = 23.32x − 1153.02 | 0.9952 | 0.36 | 1.08 |
| CINA | 3.00 | 250–4000 | y = 10.88x − 974.76 | 0.9976 | 1.26 | 3.78 |
| ILIQG | 3.21 | 100–1600 | y = 8.00x − 376.07 | 0.9955 | 1.14 | 3.42 |
| FOR | 3.75 | 25–400 | y = 10.73x − 30.91 | 0.9980 | 4.99 | 14.98 |
| AST IV | 4.53 | 25–400 | y = 17.13x − 240.51 | 0.9954 | 1.39 | 4.16 |
| GLY | 4.88 | 50–800 | y = 14.48x + 561.13 | 0.9973 | 2.94 | 8.81 |
| GIN | 5.11 | 100–1600 | y = 3.83x − 150.78 | 0.9951 | 0.51 | 1.53 |
| ATR III | 5.17 | 1000–16,000 | y = 1.31x − 303.46 | 0.9952 | 2.45 | 7.36 |
| ATR II | 6.16 | 100–1600 | y = 2.47x − 130.97 | 0.9954 | 2.87 | 8.60 |
| ATR I | 6.72 | 250–4000 | y = 9.33x − 853.56 | 0.9953 | 3.45 | 10.35 |
| Analyte | Retention Time (min) | Peak Area | ||||
|---|---|---|---|---|---|---|
| Mean | SD 1 | RSD (%) 2 | Mean | SD | RSD (%) | |
| SIN | 1.08 | 0.01 | 1.36 | 139,394.06 | 13,122.04 | 9.41 |
| MAG | 1.29 | 0.01 | 0.65 | 125,943.83 | 9596.52 | 7.62 |
| RUT | 1.39 | 0.01 | 0.88 | 184.97 | 7.36 | 3.98 |
| LIQA | 1.55 | 0.02 | 1.06 | 41,775.71 | 2484.52 | 5.95 |
| CALG | 1.58 | 0.03 | 1.77 | 4600.14 | 311.14 | 6.76 |
| LIQ | 1.66 | 0.01 | 0.74 | 5383.68 | 299.81 | 5.57 |
| FAN | 1.77 | 0.06 | 3.43 | 2355.67 | 191.05 | 8.11 |
| TET | 2.11 | 0.04 | 1.83 | 4006.18 | 188.50 | 4.71 |
| ILIQA | 2.32 | 0.03 | 1.41 | 6746.28 | 518.66 | 7.69 |
| ILIQ | 2.51 | 0.02 | 0.62 | 264.68 | 2.58 | 0.98 |
| ONO | 2.80 | 0.02 | 0.58 | 17,323.28 | 1522.39 | 8.79 |
| LIQG | 3.00 | 0.01 | 0.40 | 2815.17 | 252.23 | 8.96 |
| CAL | 3.21 | 0.01 | 0.23 | 1391.32 | 103.60 | 7.45 |
| CINA | 3.75 | 0.01 | 0.28 | 473.03 | 32.87 | 6.95 |
| ILIQG | 4.53 | 0.02 | 0.43 | 272.82 | 21.94 | 8.04 |
| FOR | 4.88 | 0.02 | 0.42 | 1514.02 | 62.55 | 4.13 |
| AST IV | 5.11 | 0.01 | 0.16 | 468.15 | 43.25 | 9.24 |
| GLY | 5.17 | 0.00 | 0.08 | 18,298.43 | 1756.04 | 9.60 |
| GIN | 6.16 | 0.01 | 0.16 | 309.83 | 30.59 | 9.87 |
| ATR III | 6.72 | 0.01 | 0.12 | 2877.97 | 277.48 | 9.64 |
| ATR II | 8.26 | 0.01 | 0.14 | 461.48 | 21.65 | 4.69 |
| ATR I | 9.32 | 0.01 | 0.10 | 416.88 | 33.70 | 8.08 |
| Analyte | Original Amount (μg/L) | Spiked Amount (μg/L) | Found Amount (μg/L) | Recovery (n = 5) | Precision (RSD, %) | ||
|---|---|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Intra-Day (n = 5) | Inter-Day (n = 15) | ||||
| SIN | 10,822.20 | 2000 | 12,760.32 | 99.52 | 2.16 | 0.89 | 1.37 |
| 4000 | 15,116.86 | 101.99 | 1.60 | 3.54 | 2.02 | ||
| 8000 | 19,979.72 | 106.15 | 0.96 | 4.74 | 2.70 | ||
| MAG | 4771.20 | 2000 | 6853.28 | 101.22 | 2.14 | 1.92 | 2.24 |
| 4000 | 8771.97 | 100.01 | 1.02 | 3.20 | 2.22 | ||
| 8000 | 13,894.42 | 108.80 | 1.66 | 4.18 | 2.86 | ||
| RUT | 54.41 | 100 | 151.98 | 98.69 | 8.38 | 12.46 | 8.86 |
| 200 | 271.16 | 106.76 | 6.52 | 13.82 | 9.95 | ||
| 400 | 474.52 | 104.52 | 2.67 | 9.92 | 7.55 | ||
| LIQA | 1823.60 | 500 | 2319.57 | 99.85 | 2.23 | 2.64 | 1.88 |
| 1000 | 2902.25 | 102.81 | 3.57 | 1.40 | 2.55 | ||
| 2000 | 4047.21 | 105.86 | 2.22 | 0.83 | 2.25 | ||
| CALG | 1386.59 | 200 | 1563.87 | 98.60 | 1.29 | 0.92 | 1.09 |
| 400 | 1781.05 | 99.72 | 3.06 | 1.70 | 2.03 | ||
| 800 | 2207.05 | 100.96 | 1.45 | 2.68 | 1.81 | ||
| LIQ | 2378.15 | 1000 | 3458.97 | 102.40 | 2.35 | 1.11 | 2.00 |
| 2000 | 4516.76 | 103.17 | 3.20 | 2.28 | 3.15 | ||
| 4000 | 6554.79 | 102.77 | 1.47 | 2.22 | 2.84 | ||
| ILIQA | 2669.20 | 500 | 3220.16 | 101.61 | 0.55 | 1.76 | 1.19 |
| 1000 | 3786.34 | 103.20 | 1.94 | 3.87 | 2.57 | ||
| 2000 | 4839.43 | 103.65 | 2.78 | 1.57 | 1.78 | ||
| ILIQ | 4355.81 | 1000 | 5207.48 | 97.25 | 1.00 | 3.24 | 1.82 |
| 2000 | 6023.68 | 94.79 | 2.18 | 2.43 | 2.67 | ||
| 4000 | 7549.93 | 90.36 | 3.37 | 3.23 | 3.82 | ||
| ONO | 1519.69 | 500 | 2052.02 | 101.64 | 1.88 | 3.64 | 2.22 |
| 1000 | 2582.57 | 102.52 | 2.15 | 5.99 | 3.29 | ||
| 2000 | 3616.14 | 102.76 | 1.04 | 1.57 | 1.85 | ||
| LIQG | 236.40 | 500 | 772.83 | 105.00 | 3.77 | 13.16 | 6.61 |
| 1000 | 1381.49 | 111.77 | 2.91 | 9.59 | 5.37 | ||
| 2000 | 2351.34 | 105.16 | 4.40 | 5.62 | 4.59 | ||
| CAL | 254.19 | 200 | 480.45 | 105.83 | 1.61 | 8.03 | 4.73 |
| 400 | 680.64 | 104.07 | 5.53 | 6.90 | 5.40 | ||
| 800 | 1066.18 | 101.16 | 1.77 | 5.42 | 3.82 | ||
| CINA | 52.60 | 50 | 109.57 | 107.42 | 1.94 | 8.03 | 6.14 |
| 100 | 161.03 | 105.94 | 3.72 | 11.25 | 7.30 | ||
| 200 | 255.29 | 101.31 | 5.38 | 7.43 | 7.28 | ||
| ILIQG | ≤LOQ | 50 | 54.40 | 108.80 | 0.56 | 14.09 | 7.62 |
| 100 | 101.62 | 101.62 | 10.28 | 12.28 | 11.85 | ||
| 200 | 200.23 | 100.12 | 10.25 | 8.49 | 10.82 | ||
| FOR | 145.80 | 100 | 233.71 | 95.39 | 4.42 | 7.39 | 5.47 |
| 200 | 329.69 | 95.56 | 2.54 | 5.47 | 4.80 | ||
| 400 | 517.32 | 94.92 | 3.80 | 5.27 | 5.61 | ||
| AST IV | 202.70 | 200 | 425.06 | 105.74 | 1.93 | 2.89 | 2.01 |
| 400 | 598.90 | 99.48 | 9.17 | 10.55 | 8.85 | ||
| 800 | 1092.25 | 109.01 | 2.59 | 7.89 | 5.58 | ||
| GLY | 3366.50 | 2000 | 5545.27 | 103.34 | 3.37 | 4.34 | 3.68 |
| 4000 | 7627.82 | 103.55 | 5.65 | 4.04 | 4.91 | ||
| 8000 | 12,343.69 | 108.60 | 2.77 | 4.24 | 2.63 | ||
| GIN | 208.97 | 200 | 393.50 | 96.45 | 6.78 | 8.22 | 6.55 |
| 400 | 619.37 | 101.87 | 7.42 | 6.46 | 6.76 | ||
| 800 | 1075.39 | 106.69 | 3.70 | 8.33 | 5.76 | ||
| ATR III | 480.20 | 500 | 1028.93 | 104.99 | 2.76 | 4.88 | 3.28 |
| 1000 | 1556.79 | 105.19 | 5.74 | 11.70 | 8.50 | ||
| 2000 | 2431.67 | 98.05 | 6.49 | 4.81 | 5.24 | ||
| ATR II | 108.31 | 100 | 203.79 | 97.98 | 7.07 | 8.33 | 7.88 |
| 200 | 326.53 | 106.02 | 3.28 | 10.56 | 7.91 | ||
| 400 | 495.38 | 97.51 | 3.92 | 6.27 | 6.33 | ||
| ATR I | 31.60 | 50 | 84.92 | 104.84 | 3.58 | 4.09 | 4.60 |
| 100 | 132.59 | 101.22 | 6.89 | 13.94 | 9.12 | ||
| 200 | 219.21 | 94.89 | 2.38 | 4.96 | 3.37 | ||
| Analyte | BHT–1 1 | BHT–2 | BHT–3 | Source 2 | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD (mg/g) | RSD (%) | Mean ± SD (mg/g) | RSD (%) | Mean ± SD (mg/g) | RSD (%) | ||
| SIN | 22.90 ± 2.16 | 9.42 | 2.39 ± 0.06 | 2.41 | 1.60 ± 0.16 | 9.77 | SCR |
| MAG | 9.42 ± 0.76 | 8.01 | 7.65 ± 0.19 | 2.52 | 1.77 ± 0.15 | 8.60 | SCR, ZF |
| RUT | 0.01 ± 0.001 | 8.31 | 0.02 ± 0.001 | 6.00 | 0.01 ± 0.001 | 7.75 | ZF |
| LIQA | 3.66 ± 0.25 | 6.95 | 0.63 ± 0.01 | 2.03 | 0.80 ±0.02 | 1.96 | GRR |
| CALG | 0.25 ± 0.02 | 9.92 | 0.12 ± 0.003 | 2.71 | 0.12 ± 0.01 | 5.01 | AR |
| LIQ | 0.44 ± 0.01 | 3.23 | 0.78 ± 0.01 | 1.64 | 1.08 ± 0.11 | 9.88 | GRR |
| FAN | ND 3 | – | ND | – | ND | – | SCR |
| TET | ND | – | ND | – | ND | – | SCR |
| ILIQA | 0.47 ± 0.04 | 8.62 | 0.09 ± 0.002 | 2.13 | 0.11 ± 0.01 | 5.51 | GRR |
| ILIQ | 0.80 ± 0.03 | 4.31 | 0.10 ± 0.01 | 7.46 | 0.14 ± 0.01 | 4.46 | GRR |
| ONO | 0.26 ± 0.03 | 9.45 | 0.09 ± 0.002 | 2.27 | 0.10 ± 0.01 | 6.73 | AR, GRR |
| LIQG | 0.04 ± 0.004 | 9.19 | 0.10 ± 0.01 | 8.74 | 0.09 ± 0.01 | 8.37 | GRR |
| CAL | 0.05 ± 0.002 | 5.32 | 0.04 ± 0.004 | 9.04 | 0.03 ± 0.001 | 4.38 | AR |
| CINA | 0.01 ± 0.001 | 6.96 | ≤LOQ | – | ≤LOQ | – | ZF |
| ILIQG | ≤LOQ | – | ≤LOQ | – | ≤LOQ | – | GRR |
| FOR | 0.03 ± 0.002 | 7.09 | 0.02 ± 0.001 | 4.75 | 0.01 ± 0.001 | 9.34 | AR |
| AST IV | 0.04 ± 0.004 | 9.90 | ≤LOQ | – | 0.03 ± 0.002 | 5.81 | AR |
| GLY | 6.44 ± 0.46 | 7.09 | 2.33 ± 0.07 | 3.13 | 2.86 ± 0.14 | 5.00 | GRR |
| GIN | 0.04 ± 0.03 | 8.05 | 0.14 ± 0.01 | 6.14 | 0.08 ± 0.01 | 8.01 | ZRR |
| ATR III | 0.08 ± 0.01 | 9.55 | ≤LOQ | – | 0.12 ± 0.004 | 3.24 | ARA |
| ATR II | 0.02 ± 0.002 | 9.13 | ≤LOQ | – | 0.08 ± 0.002 | 2.92 | ARA |
| ATR I | 0.01 ± 0.0002 | 3.64 | ≤LOQ | – | 0.01 ± 0.001 | 9.14 | ARA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, C.-S. Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Multiple Reaction Monitoring-Based Multi-Component Analysis of Bangkeehwangkee-Tang: Method Development, Validation, and Application to Quality Evaluation. Pharmaceuticals 2025, 18, 1474. https://doi.org/10.3390/ph18101474
Seo C-S. Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Multiple Reaction Monitoring-Based Multi-Component Analysis of Bangkeehwangkee-Tang: Method Development, Validation, and Application to Quality Evaluation. Pharmaceuticals. 2025; 18(10):1474. https://doi.org/10.3390/ph18101474
Chicago/Turabian StyleSeo, Chang-Seob. 2025. "Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Multiple Reaction Monitoring-Based Multi-Component Analysis of Bangkeehwangkee-Tang: Method Development, Validation, and Application to Quality Evaluation" Pharmaceuticals 18, no. 10: 1474. https://doi.org/10.3390/ph18101474
APA StyleSeo, C.-S. (2025). Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Multiple Reaction Monitoring-Based Multi-Component Analysis of Bangkeehwangkee-Tang: Method Development, Validation, and Application to Quality Evaluation. Pharmaceuticals, 18(10), 1474. https://doi.org/10.3390/ph18101474





